Abstract
Background. Vaginal infections are common, frequently recur, and may increase women's risk for sexually transmitted infections (STIs). We tested the efficacy of a novel regimen to prevent recurrent vaginal infections.
Methods. Human immunodeficiency virus (HIV)–negative women 18–45 years old with 1 or more vaginal infections, including bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), or Trichomonas vaginalis (TV), were randomly assigned to receive vaginal suppositories containing metronidazole 750 mg plus miconazole 200 mg or matching placebo for 5 consecutive nights each month for 12 months. Primary endpoints, evaluated every 2 months, were BV (Gram stain) and VVC (positive wet mount and culture).
Results. Participants (N = 234) were randomly assigned to the intervention (N = 118) or placebo (N = 116) arm. Two hundred seventeen (93%) women completed an end-of-study evaluation. The intervention reduced the proportion of visits with BV compared to placebo (21.2% vs 32.5%; relative risk [RR] 0.65, 95% confidence interval [CI] .48–.87). In contrast, the proportion of visits with VVC was similar in the intervention (10.4%) versus placebo (11.3%) arms (RR 0.92, 95% CI .62–1.37).
Conclusions. Monthly treatment with intravaginal metronidazole plus miconazole reduced the proportion of visits with BV during 12 months of follow-up. Further study will be important to determine whether this intervention can reduce women's risk of STIs.
Keywords: bacterial vaginosis, metronidazole, miconazole, periodic presumptive treatment, Trichomonas vaginalis, vulvovaginal candidiasis
Vaginal infections, including bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), and Trichomonas vaginalis (TV), are common, and have been associated with increased risk for acquisition of human immunodeficiency virus (HIV) [1–9]. Bacterial vaginosis has also been associated with herpes simplex virus type-2 (HSV-2) [10–12], human papilloma virus (HPV) [13], Neisseria gonorrhoeae [14], Chlamydia trachomatis [14, 15], and TV [8, 15–17]. It is not known whether prevention of vaginal infections can reduce women's risk of acquiring sexually transmitted infections (STIs), although 1 small trial has demonstrated promising results [18].
Treatment of BV and VVC can be challenging due to frequent recurrences. This has led to interest in suppressive regimens. Oral and intravaginal metronidazole have been used to reduce BV recurrences [19–21]. Notably, treatment of BV with metronidazole has been associated with increased risk for symptomatic VVC [19, 22]. Weekly oral fluconazole has been used to reduce the incidence of VVC in women with frequent symptomatic recurrences [23]. There remains a need for safe, efficacious, well-tolerated regimens that reduce the incidence of vaginal infections over prolonged periods.
METHODS
This study evaluated the effect of monthly periodic presumptive treatment (PPT) using topical metronidazole 750 mg with miconazole 200 mg intravaginal suppositories versus matching placebo nightly for 5 consecutive nights each month for 12 months for reducing the rates of BV and VVC, including both symptomatic and asymptomatic cases, in a randomized, double-blind, placebo-controlled trial. Intravaginal application was chosen to allow for delivery of higher doses and longer courses of medication compared to oral administration, while minimizing side effects. The trial included parallel arms with participants allocated in a 1-to-1 ratio.
Participants
Participants were recruited from 4 research clinics, including 1 in Mombasa and 2 in Nairobi, Kenya, and 1 in Birmingham, Alabama. The clinic in Mombasa and 1 clinic in Nairobi recruited women who reported transactional sex. The other 2 clinics recruited general-population women.
Inclusion criteria included age 18–45 years old, HIV seronegative, sexually active (≥4 episodes of intercourse with a male partner during the past month), willing to comply with the visit schedule, to abstain from sex or use nonlatex condoms for 24 hours following vaginal insertion of study product, and to abstain from alcohol for 48 hours following use of study product. Women had to have a vaginal infection at screening, which could include 1 or more of BV (Nugent's score ≥7) [24], VVC (fungal elements on wet mount and positive culture on Sabouraud agar) [25], or TV (motile trichomonads on vaginal saline wet preparation). In addition, women had to agree not to concurrently participate in other research involving drugs, medical devices, or vaginal products.
Women were excluded if they were currently pregnant, breastfeeding, or within the first 3 months postpartum. Those who were menstruating were invited to enroll after completion of menses. Women with ≥4 episodes of treatment for symptomatic vaginal infections during the past year were excluded because of their expected frequent need for open-label treatment. We also excluded those with a history of adverse reaction to the study medications or current use of medications or devices that would interact with the study drug. Women currently using oral or intravaginal antifungal medication, metronidazole, tinidazole, or clindamycin were also excluded. Finally, women could be excluded at the discretion of the investigator if a medical condition or situation was present, such that trial participation was not advisable.
Ethical Approvals and Consent for Participation
This study was approved by the human subjects research committees at Kenyatta National Hospital, the University of Washington, and the University of Alabama at Birmingham. All participants completed written informed consent prior to screening, and completed a second written informed consent if they were eligible and agreed to enroll.
Procedures
During screening, standardized questionnaires were completed, a pelvic speculum examination with collection of genital specimens was performed, and blood was collected for HIV-1 testing. A pregnancy test was completed using a rapid urine β-human chorionic gonadotropin assay following standard procedures at each site. Women were invited to return after 7 days for results of their laboratory tests. Those with symptomatic vaginal infections were treated at the screening visit, and TV infections were treated regardless of symptoms. Women remained eligible to enroll any time between 7 and 28 days following the screening visit.
At enrollment, a face-to-face interview was conducted using a standardized questionnaire to collect information on demographic characteristics, and medical and sexual history. A physical examination and pelvic speculum examination were performed. Swabs of vaginal and cervical secretions were collected for laboratory diagnosis of genital tract infections. The examining clinician measured vaginal pH using a test strip (ColorpHast, EMD Chemicals), then applied vaginal secretions to a microscope slide and tested for the release of an amine odor after adding a drop of 10% potassium hydroxide. A urine pregnancy test was performed to confirm nonpregnant status.
Participants were randomized to the intervention or placebo arm by assigning them the next sequentially numbered study product kit. Each kit included a 1-year supply of active or placebo product according to a computer-generated block randomization scheme with blocks of 2, 4, and 6 participants, stratified by site. The randomization was generated by the unblinded statistician (SL). Participants and all other investigators remained blinded to treatment allocation until after completion of all participant follow-up. A 1-month supply of study product was dispensed at each monthly visit. Remaining product was retained by the study team for room-temperature storage. Active product consisted of vaginal suppositories with 750 mg metronidazole plus 200 mg miconazole (Embil Pharmaceutical Company). Placebo suppositories were identical in appearance, and included the excipient vehicle Witepsol S55 with no drug.
Participants were instructed to insert 1 vaginal suppository each night, just prior to going to sleep, for 5 consecutive nights each month. They were encouraged to begin using suppositories on the same night they were dispensed. If needed, a pelvic model was used to demonstrate insertion. Use during menses was allowed. Participants were asked to abstain from intercourse or use nonlatex condoms (LifeStyles, SKYN) provided by the study for 24 hours following insertion of each vaginal suppository, because the oil-based excipient could weaken latex. This is consistent with labeling in Kenya and across 37 additional countries in Europe, Asia, and Africa where the product is marketed. If doses were missed, participants were instructed to continue using the remaining suppositories to complete 5 doses each month. Participants were asked to avoid using suppositories before performing intravaginal practices such as douching or vaginal washing. They were instructed to abstain from alcohol during product use and for 48 hours after treatment to avoid a possible disulfiram-like reaction.
After enrollment, participants were scheduled to return monthly for 12 months. At each visit, study staff assessed adherence, provided adherence counseling, reviewed correct product use, and provided free condoms. Adverse events (AEs) were assessed at each visit. A urine pregnancy test was performed, and an additional 1-month supply of study product was dispensed. Participants who became pregnant were retained in follow-up, but did not receive study product during pregnancy. Participants with symptomatic vulvovaginitis, vaginal discharge, or itching were treated syndromically with open-label oral metronidazole 400 mg or 500 mg twice daily for 7 days plus single-dose oral fluconazole 150 mg. This syndromic approach facilitated provision of study product together with open-label medication, ensuring that treatment was provided to symptomatic women while minimizing interruptions of study product use. Asymptomatic participants with a laboratory diagnosis of BV or VVC were not treated. There is currently no indication for treatment of these conditions in asymptomatic, nonpregnant women.
During follow-up at months 2, 4, 6, 8, 10, and 12, participants had a physical examination, including pelvic speculum examination with collection of genital swabs for diagnosis of vaginal infections. Counseling and testing for HIV infection were repeated at the end-of-study evaluation.
Laboratory
Serological testing for HIV infection was performed using parallel testing with either 2 rapid tests or 2 laboratory-based enzyme-linked immunoassays (ELISAs) according to standard procedures at each study site. Testing for HSV-2 was performed using an ELISA (HerpeSelect, Focus Technologies). An optical density (OD) ≥2.1 was considered positive for women in Kenya [26]. Specimens from the United States were tested using kits that produced a dichotomous positive versus negative result.
Vaginal secretions were Gram stained and evaluated for BV according to Nugent's criteria [24]. A vaginal saline wet mount was examined at 40× magnification to identify motile TV organisms, clue cells, and fungal elements. A drop of 10% potassium hydroxide was then added to the slide, and it was examined again for budding yeast, hyphae, or both. All laboratory staff passed the Microbicide Trials Network vaginal wet preparation proficiency test prior to study initiation, and annually thereafter. Culture for vaginal yeast was performed on Sabouraud agar. Nucleic acid amplification testing (NAAT) for N. gonorrhoeae and C. trachomatis was performed at enrollment (APTIMA Combo-2, Hologic/Gen-Probe). Samples from all examinations were batched and tested for TV by NAAT (APTIMA TV, Hologic/Gen-Probe) after completion of follow-up.
Sample Size and Statistical Analyses
The primary outcomes were the proportions of visits with BV or VVC, regardless of whether symptoms were present or absent. Bacterial vaginosis was considered to be present if the Nugent's score was ≥7 [24]. Vulvovaginal candidiasis was diagnosed based on the presence of both yeast forms on wet mount microscopy and a positive yeast culture [25]. Requiring both culture and wet mount positivity increases diagnostic specificity. Notably, a positive wet mount is associated with higher yeast concentrations and greater likelihood of symptoms and signs [27]. There were 2 secondary endpoints. One was a combined “any vaginal infection” endpoint, including 1 or more of BV, VVC, and TV by NAAT. The second was BV by Amsel's criteria [28], which was considered positive if women had ≥3 of 4 clinical signs: homogeneous vaginal discharge, vaginal pH >4.5, amine odor, and ≥20% clue cells on vaginal saline wet mount. We conducted 2 ancillary analyses. The first investigated the effect of the intervention on the proportion of visits with TV by NAAT. Trichomonas was not included as a primary or secondary endpoint because it was anticipated that the frequency of infection would not provide sufficient power for a robust statistical comparison. The second evaluated the effect of the intervention on the proportion of visits with abnormal vaginal microbiota by Gram stain (Nugent score ≥4).
To calculate sample size, the overall 2-sided significance level of .05 was split between the 2 primary endpoints, BV and VVC. Sample size was driven by VVC, because this was predicted to be less frequent. We assumed the prevalence of VVC at visits in the control arm would be 14%, and the correlation between observations within individual was estimated at 0.13 [21]. We hypothesized a relative risk of 0.5 for VVC in the intervention versus placebo arm. Assuming 90% retention at 1 year, α = 0.02, and power = 80%, we required 117 participants per study arm. This sample size was expected to provide 99% power for detecting a relative risk of 0.8 for BV, assuming α = 0.03, 47% prevalence in controls, and within-individual correlation of 0.34 [21].
Baseline characteristics of intervention and placebo participants were compared using Mantel-Haenszel tests stratified by study site for binary data and analysis of variance tests for continuous data, adjusted for study site. The primary analysis population for efficacy and safety was the intent-to-treat (ITT) population, which included all randomized participants. Confirmatory analyses were performed with the per protocol (PP) population, defined as all randomized participants who completed 12 months of follow-up, had genital swabs for endpoint analysis on ≥4 of 6 possible visits, and reported using ≥48 (80%) of 60 doses of study product.
For the analyses of primary endpoints, each participant within a study arm was considered a cluster, with observations at a maximum of 6 visits. For each infection of interest (BV, VVC), the proportion of visits at which the infection was detected was compared between the intervention versus placebo arm using a χ2 statistic adjusted for clustering [29]. To evaluate differences in the intervention effect by study site and use of intravaginal practices, we tested the statistical significance of interaction terms for each of the 2 primary endpoints. Relative risks (RRs) were generated using generalized estimating equations with a log link and exchangeable correlation structure. Similar analyses were conducted for secondary and ancillary endpoints. The proportions of participants achieving ≥80% medication adherence, participants experiencing AEs, and visits at which open-label treatment for symptomatic vaginal infections were dispensed in the intervention versus placebo arms were compared using stratified Mantel-Haenszel tests. This trial was registered with ClinicalTrials.gov (NCT01230814; http://clinicaltrials.gov).
RESULTS
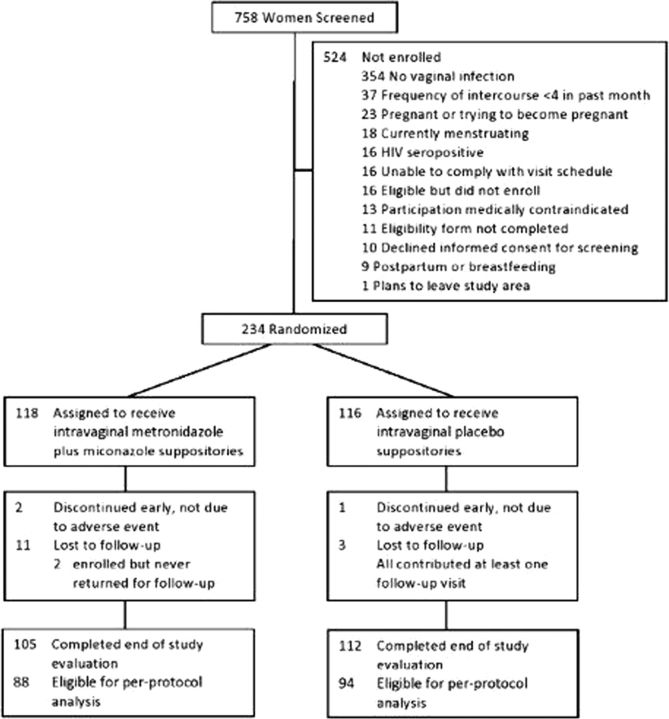
Between April 2011 and August 2012, 758 women were screened, of whom 234 (30.9%) were enrolled with 58–59 participants per site (Figure 1). The most common reason for exclusion was lack of a vaginal infection. Of 234 participants, 118 were randomized to the intervention arm and 116 were randomized to the placebo arm. Two participants did not return for any follow-up visits. Of the remaining 232, 87%–100% of total expected participants returned for each of 12 monthly follow-up visits. Follow-up was completed in August 2013.
Figure 1.
The figure shows the flow of participants from screening through randomization and follow-up to completion of the study. While the study protocol allowed investigators to exclude women if a medical condition or situation was present, such that participation was not advisable, no exclusions were made based on this criterion alone. In the intervention arm, 30 (25.4%) of 118 women were not included in the per-protocol population; 9 (7.6%) completed less than 4 follow-up examination visits and 21 (17.8%) used less than 48 of 60 possible doses of study product. In the placebo arm, 24 (%) of 116 women were not included in the per-protocol population; 6 (%) completed less than 4 follow-up visits and 16 (%) used less than 48 of 60 possible doses of study product.
The study arms were well balanced in terms of demographic, behavioral, obstetrical, medical, and laboratory characteristics with 1 exception; the few women who were not African or African American (N = 5) were all randomized to the control arm (P = .05) (Table 1). At enrollment, 82 (35.0%) participants had BV, 32 (13.7%) had VVC, and 16 (6.8%) had TV infection by NAAT. Eleven (4.7%) participants had more than 1 type of vaginal infection. There were 115 (49.1%) women with no vaginal infection at enrollment, including 46 (19.7%) with intermediate vaginal microbiota (Nugent score 4–6).
Table 1.
Baseline Characteristics of Trial Participants
| Characteristic | Intervention Arma (N = 118) | Placebo Arma (N = 116) |
|---|---|---|
| Age, years | 29 (24, 34) | 29 (23, 35) |
| Years of school completed | 10 (8, 13) | 11 (8, 12) |
| African or African-American raceb | 118 (100) | 111 (96) |
| Nulliparous | 12 (10) | 12 (10) |
| Contraception (any method) | 93 (79) | 100 (86) |
| Oral contraceptive pills | 11 (9) | 14 (12) |
| Depot medroxyprogesterone acetate | 26 (22) | 25 (22) |
| Progestin implant | 6 (5) | 10 (9) |
| Intrauterine device | 4 (3) | 11 (9) |
| Tubal ligation | 10 (8) | 5 (4) |
| Condoms alone | 32 (27) | 31 (27) |
| Vaginal washing in the past week | 38 (32) | 25 (22) |
| Transactional sex in the past week | 45 (38) | 44 (38) |
| Sex partners in past week, number | 1 (1, 2) | 1 (1, 2) |
| Vaginal sex frequency in past week, number | 2 (1, 3) | 2 (1, 3) |
| Unprotected vaginal sex in the past week | 51 (43) | 39 (34) |
| Anal sex in the past week | 0 (0) | 1 (1) |
| Bacterial vaginosis by Nugent's score | 42 (36) | 40 (34) |
| Vulvovaginal candidiasis | 13 (11) | 19 (16) |
| Trichomonas vaginalis infection | 10 (8) | 6 (5) |
| Neisseria gonorrhoeae infection | 3 (3) | 0 (0) |
| Chlamydia trachomatis infection | 9 (8) | 8 (7) |
| Herpes simplex virus type-2 seropositive | 76 (64) | 72 (62) |
a Data are number (percent) or median (interquartile range).
b P = .05 Mantel-Haenszel test, after adjusting for site.
Participants were defined as adherent to PPT if they reported using ≥48 of 60 possible vaginal suppositories. This level of adherence was achieved by 88 (74.6%) participants in the intervention arm and 94 (81.0%) participants in the placebo arm (P = .3). Open-label syndromic treatment for symptomatic vaginal conditions was provided to 24 (36.2%) participants (73 courses of treatment) in the intervention arm versus 49 (42%) participants (85 courses of treatment) in the placebo arm (P = .4).
Monthly treatment with intravaginal metronidazole plus miconazole reduced the relative risk of BV by Nugent's criteria by 35% compared to placebo (RR 0.65, 95% confidence interval [CI], .48–.87) (Table 2). In contrast, the risk of VVC did not differ in the intervention versus placebo arm (RR 0.92, 95% CI, .62–1.37). Overall, the risk of any vaginal infection (BV, VVC, or TV) was lower in the intervention arm compared to the placebo arm (RR 0.70, 95% CI, .57–.86), reflecting the reduction in BV and a nonsignificant reduction in TV infection. The risk of BV by clinical (Amsel's) criteria did not differ significantly between study arms. Participants receiving the intervention had a lower risk of abnormal vaginal microbiota by Gram stain (Nugent score ≥4) compared to those who received placebo (RR 0.72, 95% CI, .58–.90). Results were similar when restricted to the per-protocol population (Table 3). There was no significant interaction between study site and intervention efficacy for BV by Nugent score (P = .4) or VVC (P = .1), suggesting similar efficacy across the 4 study sites. In addition, there was no significant interaction between vaginal washing status at baseline or as a time-varying exposure for either of the primary outcomes (BV and VVC; both P values > .5).
Table 2.
Number and Proportion of Visits With Vaginal Infections by Study Arm in the Intent-to-treat Population
| Outcome | Intervention Arma 645 Visits Number (%) | Placebo Armb 665 Visits Number (%) | Relative Risk Intervention/Placebo (95% CI) | P Valuec |
|---|---|---|---|---|
| Bacterial vaginosis (Nugent) | 137 (21.2) | 216 (32.5) | 0.65 (.48, .87) | .005 |
| Vulvovaginal candidiasis | 67 (10.4) | 75 (11.3) | 0.92 (.62, 1.37) | .7 |
| Trichomonas vaginalisd | 35 (5.4) | 57 (8.6) | 0.63 (.30, 1.32) | .3 |
| Any vaginal infectione | 210 (32.6) | 309 (46.5) | 0.70 (.57, .86) | .001 |
| Bacterial vaginosis (Amsel) | 93 (14.4) | 121 (18.2) | 0.81 (.55, 1.19) | .2 |
| Abnormal vaginal microbiotad,f | 227 (35.2) | 325 (48.9) | 0.72 (.58, .90) | .004 |
a 116 participants contributed follow-up visits in the intervention arm.
b 116 participants contributed follow-up visits in the placebo arm.
c Based on Donner's clustered χ2 test [29].
d Exploratory ancillary analysis.
e One or more vaginal infections, including bacterial vaginosis by Nugent's criteria [24], vulvovaginal candidiasis by wet prep and culture, and T. vaginalis positive by nucleic acid amplification testing.
f Vaginal Gram stain Nugent's score ≥4 [24].
Abbreviation: CI, confidence interval.
Table 3.
Number and Proportion of Visits With Vaginal Infections by Study Arm in the Per-protocol Population
| Outcome | Intervention Arma 480 Visits Number (%) | Placebo Armb 515 Visits Number (%) | Relative Risk Intervention/Placebo (95% CI) | P Valuec |
|---|---|---|---|---|
| Bacterial vaginosis (Nugent) | 102 (21.3) | 164 (31.8) | 0.67 (.47, .94) | .02 |
| Vulvovaginal candidiasis | 46 (9.6) | 55 (10.7) | 0.90 (.56, 1.44) | .7 |
| Trichomonas vaginalisd | 27 (5.6) | 39 (7.6) | 0.74 (.29, 1.89) | .5 |
| Any vaginal infectione | 151 (31.5) | 236 (45.8) | 0.69 (.54, .88) | .003 |
| Bacterial vaginosis (Amsel) | 68 (14.2) | 99 (19.2) | 0.74 (.47, 1.15) | .2 |
| Abnormal vaginal microbiotad,f | 173 (36.0) | 256 (49.7) | 0.73 (.57, .94) | .01 |
a 80 participants contributed follow-up visits in the intervention arm.
b 86 participants contributed follow-up visits in the placebo arm.
c Based on Donner's clustered χ2 test [29].
d Exploratory ancillary analysis.
e One or more vaginal infections, including bacterial vaginosis by Nugent's criteria [24], vulvovaginal candidiasis by wet prep and culture, and T. vaginalis positive by nucleic acid amplification testing.
f Vaginal Gram stain Nugent's score ≥4 [24].
Abbreviation: CI, confidence interval.
The mean number of AEs per participant was 6.4 in the intervention arm and 7.2 in the placebo arm (P = .02). The proportions of participants experiencing the most common AEs are shown in Table 4. Adverse events did not differ significantly by study arm except for vaginal discharge and headache, both of which were reported less frequently in the intervention arm compared to the placebo arm. Four women seroconverted for HIV infection at the end-of-study evaluation (3 intervention, 1 placebo). There were 5 serious AEs (4 intervention, 1 placebo), none of which was associated with study product. In the intervention arm, serious AEs included a ruptured ectopic pregnancy, soft tissue injuries in a motor vehicle accident, and hospitalization for malaria and typhoid fever. In the placebo arm, the 1 serious AE was a pelvic fracture in a motor vehicle accident.
Table 4.
Most Commonly Reported Adverse Events by Study Arm
| Adverse Event | Intervention Arm |
Placebo Arm |
P Valuea | ||
|---|---|---|---|---|---|
| Number of Participants (%) N = 116 | Number of Occurrences | Number of Participants (%) N = 116 | Number of Occurrences | ||
| Upper-respiratory-tract infection | 40 (34) | 64 | 36 (31) | 54 | .7 |
| Vulvovaginal pruritis | 37 (31) | 61 | 45 (39) | 79 | .2 |
| Vaginal discharge | 27 (23) | 55 | 43 (37) | 67 | .01 |
| Headache | 24 (20) | 31 | 36 (31) | 53 | .04 |
| Back pain | 21 (18) | 25 | 11 (9) | 13 | .07 |
| Urinary-tract infection | 14 (12) | 24 | 12 (10) | 13 | .7 |
| Vulvovaginitis | 19 (16) | 23 | 19 (16) | 27 | .9 |
| Respiratory-tract infection | 14 (12) | 14 | 18 (16) | 24 | .4 |
a Stratified Mantel-Haenszel test for comparison of proportion of participants with each AE by study arm.
Abbreviation: AE, adverse event.
There were 16 pregnancies in 15 participants in the intervention arm and 11 pregnancies in 11 participants in the placebo arm. There were no complications during pregnancy or labor that required medical attention. Eight normal infants were born (6 intervention, 2 placebo). In the intervention arm, there were 4 spontaneous abortions before 20 weeks gestation, 4 elective abortions, 1 ectopic pregnancy, and 1 birth outcome that was unknown. In the placebo arm, there was 1 spontaneous abortion before 20 weeks gestation, 6 elective abortions, and 2 birth outcomes that were unknown.
DISCUSSION
In this randomized, double-blind, placebo controlled trial, monthly treatment with 5 nights of intravaginal metronidazole 750 mg plus miconazole 200 mg significantly reduced the risk of BV, including both symptomatic and asymptomatic cases, compared to placebo during 1 year of PPT. A significantly lower rate of vaginal discharge was reported in intervention compared to control participants. The intervention appeared to be safe, and incidences of HIV infection and pregnancy were comparable to similar clinical trial and observational cohort populations [30, 31].
This intervention did not produce the hypothesized reduction in VVC, despite including 200 mg of miconazole in each vaginal suppository. Regimens for preventing recurrent symptomatic VCC are typically dosed weekly [23], so the dosing regimen in this trial may have been inadequate for reducing VVC. Another possible explanation is the fact that treatment of BV with metronidazole has been associated with increased risk of symptomatic VVC [19, 22]. In this context, it may be encouraging simply to observe no increase in VVC while using high-dose intravaginal metronidazole.
Earlier trials of interventions for recurrent BV have taken 1 of 2 approaches. The first is to assess the effect of an intervention versus control condition on time to first recurrence after treatment of symptomatic BV. One such trial demonstrated that twice weekly application of 0.75% metronidazole gel reduced recurrence of BV by clinical (Amsel's) criteria during 16 weeks of follow-up (25.5% vs 59.1%, RR 0.43, 95% CI, .25–.73) [19]. Women were more likely to develop VVC using metronidazole gel compared to placebo (43.1% vs 20.5%, P = .02). A recent pilot study of twice-weekly intravaginal metronidazole 750 mg plus miconazole 200 mg (same product as the present study) observed recurrent BV in only 1 of 10 women followed for 12 weeks [32]. These studies address the clinical problem of recurrent symptomatic BV following initial clinical cure, which is quite distinct from the present study.
The other approach has been to measure the effect of an intervention versus control condition on the incidence of both symptomatic and asymptomatic BV over an extended period. This approach is more directly comparable to the study presented here, and provides data particularly suited to informing the design of strategies to reduce secondary complications of BV, such as STIs. A trial in Malawi demonstrated a modest decrease in BV during 1 year of follow-up in women randomized to intravaginal metronidazole 0.75% gel versus matching placebo for 5 consecutive nights every 3 months (RR 0.84, 95% CI, .75–.94) [20]. Another study, conducted in Kenya, demonstrated that monthly single-dose oral metronidazole 2 g plus fluconazole 150 mg reduced the incidence of BV compared to placebo (hazard ratio [HR] 0.55, 95% CI, .49–.63) [21]. The current trial aimed to deliver a potent monthly regimen while minimizing systemic exposure to metronidazole and its side effects. The dosing schedule, 5 consecutive nights per month, was selected to minimize complexity and drug-taking burden, with the goal of facilitating good adherence.
This study had several strengths. The randomized arms were well balanced, and the clinical trial design provides a strong basis for concluding that PPT reduced BV incidence. There were excellent rates of participant retention, completion, and reported adherence. Inclusion of both high-risk women and general-population women from Africa and North America support the generalizability of the findings.
This study also had limitations. First, adherence was assessed by self-report. Recent studies of HIV prevention interventions have highlighted the fact that self-report may overestimate adherence compared to validation with a biomarker [33]. A second limitation is the fact that the intervention was discontinued when women were pregnant. These women continued to follow up and provide endpoint data, which would attenuate the observed intervention effect. A third limitation was the need to treat symptomatic vaginal conditions in both study arms, which could further attenuate the observed effect of the intervention. Finally, with endpoint assessments every other month, early cure followed by relapse before the next evaluation would be missed.
In conclusion, this study demonstrated that monthly treatment with intravaginal metronidazole 750 mg plus miconazole 200 mg appeared safe and well tolerated, and significantly reduced the prevalence of BV compared to placebo during 12 months of follow-up. While we did not see a reduction in VVC, it is encouraging that there was no increase with the intervention. Further study of vaginal health interventions will be important to determine whether this approach can reduce STIs such as HSV-2, HPV, C. trachomatis, N. gonorrhoeae, and Mycoplasma genitalium. This is a critical question, as there remains a need for effective, female-controlled strategies to reduce women's risk for STIs.
Notes
Acknowldgments. We are grateful to the women who participated in this trial, without whose time and effort the study would not have been possible. We also thank the clinical, laboratory, and administrative staff from each of the research clinics and supporting sites in the United States and Kenya. Special thanks go to the clinical leaders for each site, including Molly Flynn (Birmingham), Jessie Kwatampora (Nairobi – Korogocho), Griffin Manguro (Mombasa), and Geoffrey Ombati (Nairobi – Kangemi). We thank the Mombasa Municipal Council for allowing us to use their clinical facilities and Coast Provincial General Hospital for providing laboratory space. We thank Peter Wolff, Clinical Project Manager at the National Institutes of Health Division of Microbiology and Infectious Disease (DMID) for his many contributions to the study. Finally, we thank Embil Pharmaceutical Company (Istanbul, Turkey) for donation of the active and placebo product used in the trial.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases Contract number HHSN266200400073C through the Sexually Transmitted Infections Clinical Trials Group. Infrastructure and logistical support for the Mombasa site was provided through the University of Washington Center for AIDS Research (P30-AI27757). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. R. S. M. has received honoraria for invited lectures and consulting as well as donated study product for this trial from Embil Pharmaceutical Company. R. S. M. currently receives research funding from Hologic/Gen-Probe. J. E. B. received honoraria from Symbiomix, Inc for consulting and donated reagents from Hologic/Gen-Probe. J. S. has received consultancy payments from Akesis, Hologic, Symbiomix, and Starpharma, and has grants/pending grants from Akesis, BD Diagnostic, Hologic, Cepheid, Quidel, Symbiomix, Starpharma, and Viamet. L. K. is employed by Embil Pharmaceutical Company. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Martin HL, Jr, Nyange PM, Richarson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis 1998; 178:1053–59. [DOI] [PubMed] [Google Scholar]
- 2.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 1998; 12:1699–706. [DOI] [PubMed] [Google Scholar]
- 3.Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis 2005; 192:1372–80. [DOI] [PubMed] [Google Scholar]
- 4.van de Wijgert JH, Morrison CS, Cornelisse PG, et al. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr 2008; 48:203–10. [DOI] [PubMed] [Google Scholar]
- 5.Kilmarx PH, Limpakarnjanarat K, Mastro TD, et al. HIV-1 seroconverson in a prospective study of female sex workers in northern Thailand: continued high incidence among brothel-based women. AIDS 1998; 12:1889–98. [DOI] [PubMed] [Google Scholar]
- 6.Kapiga SH, Lyamuya EF, Lwihula GK, Hunter DJ. The incidence of HIV infection among women using family planning methods in Dar es Salaam, Tanzania. AIDS 1998; 12:75–84. [DOI] [PubMed] [Google Scholar]
- 7.Laga M, Alary M, Nzila N, et al. Condom promotion, sexually transmitted diseases treatment, and declining incidence of HIV-1 infection in female Zairian sex workers. Lancet 1994; 344:246–8. [DOI] [PubMed] [Google Scholar]
- 8.McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis 2007; 195:698–702. [DOI] [PubMed] [Google Scholar]
- 9.Van Der Pol B, Kwok C, Pierre-Louis B, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis 2008; 197:548–54. [DOI] [PubMed] [Google Scholar]
- 10.Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis 2003; 37:319–25. [DOI] [PubMed] [Google Scholar]
- 11.Gallo MF, Warner L, Macaluso M, et al. Risk factors for incident herpes simplex type 2 virus infection among women attending a sexually transmitted disease clinic. Sex Transm Dis 2008; 35:679–85. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb SL, Douglas JM, Jr, Foster M, et al. Incidence of herpes simplex virus type 2 infection in 5 sexually transmitted disease (STD) clinics and the effect of HIV/STD risk-reduction counseling. J Infect Dis 2004; 190:1059–67. [DOI] [PubMed] [Google Scholar]
- 13.Mao C, Hughes JP, Kiviat N, et al. Clinical findings among young women with genital human papillomavirus infection. Am J Obstet Gynecol 2003; 188:677–84. [DOI] [PubMed] [Google Scholar]
- 14.Gallo MF, Macaluso M, Warner L, et al. Bacterial vaginosis, gonorrhea, and chlamydial infection among women attending a sexually transmitted disease clinic: a longitudinal analysis of possible causal links. Ann Epidemiol 2012; 22:213–20. [DOI] [PubMed] [Google Scholar]
- 15.Brotman RM, Klebanoff MA, Nansel TR, et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis 2010; 202:1907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathod SD, Krupp K, Klausner JD, Arun A, Reingold AL, Madhivanan P. Bacterial vaginosis and risk for Trichomonas vaginalis infection: a longitudinal analysis. Sex Transm Dis 2011; 38:882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV-1-negative women. Sex Transm Dis 2014; 41:123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwebke JR, Desmond R. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. Am J Obstet Gynecol 2007; 196:517 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol 2006; 194:1283–9. [DOI] [PubMed] [Google Scholar]
- 20.Taha TE, Kumwenda NI, Kafulafula G, et al. Intermittent intravaginal antibiotic treatment of bacterial vaginosis in HIV-uninfected and -infected women: a randomized clinical trial. PLOS Clin Trials 2007; 2:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect Dis 2008; 197:1361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006; 193:1478–86. [DOI] [PubMed] [Google Scholar]
- 23.Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004; 351:876–83. [DOI] [PubMed] [Google Scholar]
- 24.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991; 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobel JD. Vulvovaginal candidiasis. In: Holmes KK, Mardh P, Sparling PF, et al., eds. Sexually Transmitted Diseases. 4th ed New York: McGraw-Hill, 2007:823–38. [Google Scholar]
- 26.Mujugira A, Morrow RA, Celum C, et al. Performance of the Focus HerpeSelect-2 enzyme immunoassay for the detection of herpes simplex virus type 2 antibodies in seven African countries. Sex Transm Infect 2011; 87:238–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergman JJ, Berg AO, Schneeweiss R, Heidrich FE. Clinical comparison of microscopic and culture techniques in the diagnosis of Candida vaginitis. J Fam Pract 1984; 18:549–52. [PubMed] [Google Scholar]
- 28.Amsel R, Totten PA, Spiegel CA, Chen KCS, Eschenbach DA, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Intern Med 1983; 74:14–22. [DOI] [PubMed] [Google Scholar]
- 29.Donner A, Klar N. Design and analysis of cluster randomization trials in health research. New York, New York: Oxford University Press, 2000. [Google Scholar]
- 30.Ronen K, McCoy CO, Matsen FA, et al. HIV-1 superinfection occurs less frequently than initial infection in a cohort of high-risk Kenyan women. PLOS Pathog 2013; 9:e1003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mugo NR, Hong T, Celum C, et al. Pregnancy incidence and outcomes among women receiving preexposure prophylaxis for HIV prevention: a randomized clinical trial. JAMA 2014; 312:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguin T, Akins RA, Sobel JD. High-dose vaginal maintenance metronidazole for recurrent bacterial vaginosis: a pilot study. Sex Transm Dis 2014; 41:290–1. [DOI] [PubMed] [Google Scholar]
- 33.Van der Straten A, Brown ER, Marrazzo JM, et al. Divergent adherence estimates with pharmacokinetic and behavioral measures in VOICE (MTN003). In: Conference on Retroviruses and Opportunistic Infections Boston, MA, 2014. [Google Scholar]