Abstract
Recent pertussis resurgence represents a major public health concern. Currently, there are no effective treatments for critical pertussis in infants. Recent data have demonstrated the potential of sphingosine-1-phosphate receptor (S1PR) agonism in the treatment of infectious diseases. We used the murine Bordetella pertussis model to test the hypothesis that treatment with S1PR agonist AAL-R reduces pulmonary inflammation during infection. AAL-R treatment resulted in reduced expression of inflammatory cytokines and chemokines and attenuated lung pathology in infected mice. These results demonstrate a role for sphingosine-1-phosphate (S1P) signaling in B. pertussis–mediated pathology and highlight the possibility of host-targeted therapy for pertussis.
Keywords: AAL-R, bordetella, host-directed, pertussis, sphingosine, sphingosine-1-phosphate
Recent years have seen resurgence in pertussis (whooping cough) incidence despite the successful implementation of mass vaccination programs [1]. This represents a major public health concern, as critical pertussis is a significant cause of neonate hospitalization and mortality [2]. Currently, there are no effective treatments for critical pertussis in infants or for pertussis cough in any infected individuals. Bordetella pertussis, the etiologic agent of pertussis, uses an array of virulence factors to manipulate host immune responses [3]. One such factor, pertussis toxin (PT), is associated with exacerbated airway inflammation during infection in mouse models, increasing the duration and severity of lung pathology [4]. PT ADP-ribosylates the α subunit of heterotrimeric Gi proteins, resulting in ablated G protein–coupled receptor (GPCR) signaling [5].
Sphingosine-1-phosphate (S1P) is a sphingolipid that regulates many cellular processes important to health and disease [6] and signals through 5 GPCRs that couple to PT-sensitive Gi proteins [7]. S1P signaling enhances pulmonary vascular endothelial barrier integrity [8], and protects against airway inflammation induced by lipopolysaccharide-mediated acute lung injury [9] or by influenza virus–mediated cytokine storm [10]. Therefore, B. pertussis infection, through the actions of PT, may inhibit S1P signaling, reducing pulmonary vascular endothelial barrier function and exacerbating lung inflammation. On the other hand, stimulation of S1P receptor signaling may have beneficial effects for the host during B. pertussis infection. In this report, we tested the hypothesis that S1P receptor agonism will reduce pathology in the lungs of B. pertussis–infected mice.
MATERIALS AND METHODS
Bacterial Strains
The B. pertussis strain used here, WT, is a streptomycin- and nalidixic acid-resistant derivative of Tohama I [11]. B. pertussis was grown on Bordet-Gengou (BG) agar plates supplemented with 10% defibrinated sheep blood and 200 µg/mL streptomycin.
Mouse Infections
Six- to 8-week-old C57BL/6 mice (Charles River Laboratories or in-house bred) were used in accordance with the University of Maryland, Baltimore (UMB), Institutional Animal Care and Use Committee. Bacterial inoculum was prepared in a phosphate-buffered saline (PBS) suspension following 48 hours growth on BG agar. Mice were anesthetized with isoflurane, and the inoculum was administered intranasally in a final volume of 50 µL. AAL-R [12] (kindly donated by Dr Hugh Rosen, The Scripps Institute) was prepared in sterile water at a concentration of 0.5 mg/mL and administered intranasally at a final concentration of 0.5 mg/kg. Sterile water was used as a volume-matched vehicle control. For organ harvest, animals were euthanized by carbon dioxide inhalation followed by thoracotomy. The lungs and trachea were removed for bacterial counts, histology, and RNA purification. For analysis of cytokine protein levels, bronchoalveolar lavage fluid (BAL) was isolated as described previously [11].
RNA Isolation and Processing
Lung tissue was snap-frozen upon harvest using a dry ice-isopropanol bath. RNA was extracted using RNA Stat60 (TelTest, Inc) as per manufacturer's instructions. In brief, samples were homogenized in RNA Stat60 using an Omni TH mixer (Omni, Inc), phase separated with the addition of chloroform, and precipitated with isopropanol. RNA was quantified, and 1 µg was reverse transcribed using a reverse transcription system (Promega). Quantitative real-time polymerase chain reaction was performed with Maxima SYBR green/ROX quantitative PCR (qPCR) master mix (Thermo Scientific) in an Applied Biosystems 7500 Fast real-time PCR system. The hypoxanthine phosphoribosyltransferase (HPRT) gene was used as an internal housekeeping control gene, with all other genes normalized to the HPRT gene and expression calculated as fold change compared with PBS-inoculated control animal levels (calculated by the 2(−ΔΔCT) method).
Pathology
When harvesting lung tissue for pathology, lungs were intracardially perfused with PBS before removal into 10% (w/v) buffered formalin (Sigma). Slide preparation and staining with hematoxylin and eosin was performed by the Pathology, EM and Histology Laboratory (UMB core facility). Histopathology was scored on a scale of 0 to 3, with 3 being the most severe for each of (1) the degree of inflammation at the site of the bronchovascular bundle (BVB), (2) the percentage of BVB involved, and (3) the degree of pleuritis observed, with a total maximum possible score of 9 [11].
Statistical Analysis
Graphs were plotted and data analyzed using GraphPad Prism software. Fold changes, for real-time PCR, were calculated per mouse compared to the average value obtained for the respective PBS/water-inoculated group. All plots represent the mean value ± standard deviation. Significance was determined by Student t test using the GraphPad Prism software.
RESULTS
S1P Receptor Agonism Reduces Inflammatory Cytokine Expression/Production Following B. pertussis Infection
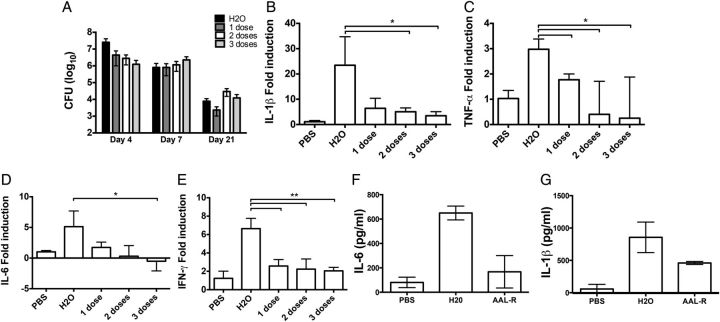
Treatment with AAL-R, an agonist for S1P receptors 1, 3, 4, and 5, dampened cytokine storm elicited by influenza virus infection and reduced mortality rates in mice [10]. Therefore, we hypothesized that AAL-R treatment could abate inflammation in the lungs of B. pertussis–infected animals. One potential risk of immune modulation in the setting of bacterial infection is uncontrolled bacterial growth. To assess this risk, pulmonary bacterial burden was measured in mice at various time points postinoculation with B. pertussis (Figure 1A). At day 4 postinoculation, no significant difference in bacterial loads was observed between animals that received 1 dose of AAL-R (at 1 hour postinoculation) and those that received vehicle control. Two (1 and 6 hours postinoculation) and 3 (1, 6, and 24 hours postinoculation) doses of AAL-R led to slight, statistically significant decreases in colony-forming units (P = .04 and P = .03, respectively) at day 4. No differences in bacterial burden were noticed at day 7 or 21 postinoculation between groups of mice receiving AAL-R and water.
Figure 1.
Effect of sphingosine-1-phosphate (S1P) agonism on cytokine production. A, Bacterial burden in the lungs of B. pertussis–infected mice that were either untreated (black bars) or treated with 1 (1 hour postinoculation) (dark gray), 2 (1 and 6 hours postinoculation) (white), or 3 (1, 6, and 24 hours postinoculation) (light gray) doses of AAL-R was determined. B–E, Real-time PCR was used to determine the transcriptional IL-1β (B), TNF-α (C), IL-6 (D), and IFN-γ (E) responses from lung homogenates at day 4 postinoculation. Protein levels of IL-6 (F) and IL-1β (G) in these lung homogenates were also assayed. Results are presented as mean ± SD. *P ≤ .05; **P ≤ .01. Abbreviations: CFU, colony-forming units; IFN, interferon; IL, interleukin; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; TNF, tumor necrosis factor.
Cytokine gene expression in these mice was monitored by quantitative real-time PCR performed on samples derived from lung homogenate at 4 days postinoculation. Treatment with a single dose of AAL-R resulted in significant reduction in expression of tumor necrosis factor (TNF)–α (Figure 1C; P = .01), interferon (IFN)–γ (Figure 1E; P < 0.01), C-X-C motif chemokine 10 (CXCL10; P < 0.01), and chemokine (C-C motif) ligand 5 (CCL5; P < .01) (chemokine data not shown). Following 2 doses of AAL-R, IL-1β (Figure 1B; P < .01) and chemokine (C-X-C motif) ligand 2 (CXCL2, not shown; P = .04) gene expression was also significantly reduced (P < .01). A third dose of AAL-R led to interleukin (IL)–6 gene expression also being significantly reduced (Figure 1D; P = .03). To determine if these transcriptional differences resulted in lower protein production, IL-6 and IL-1β levels were measured by enzyme-linked immunosorbent assay. Both cytokines were reduced in the BAL fluid of animals receiving AAL-R versus those receiving water (Figure 1F and 1G). However, only the reduction in IL-6 was found to be statistically significant (P = .04). Therefore, we conclude that AAL-R treatment significantly reduces expression of the inflammatory cytokines (TNF-α, IFN-γ, IL-1β, and IL-6) and chemokines (CCL5, CXCL2, and CXCL10) during B. pertussis infection.
S1P Agonism Reduces Pulmonary Pathology During B. pertussis Infection
The murine model of B. pertussis infection is characterized by pulmonary inflammation, typified by dense peribronchiolar cuffing and pleuritis. Previous work demonstrated the immunomodulatory potential of S1P agonism in the context of pulmonary infection [10]. Therefore, we hypothesized that S1P receptor agonism, through administration of AAL-R, during B. pertussis infection would reduce pulmonary inflammatory pathology. B. pertussis–infected animals were treated with 1, 2, or 3 doses of AAL-R, starting at 1 hour postinoculation, and pathology was assessed after 7 days. A quantitative scoring system previously employed by our group [11] was used to assess lung pathology. A significant reduction in pathology was noted following 1 (P < .01), 2 (P < .01), or 3 (P < .01) doses of AAL-R versus water controls (Figure 2). No significant differences were noted between animals receiving 1, 2, or 3 doses of AAL-R. Therefore, we concluded that S1P receptor signaling may represent a host-targeted means of dampening inflammatory pathology following B. pertussis infection.
Figure 2.
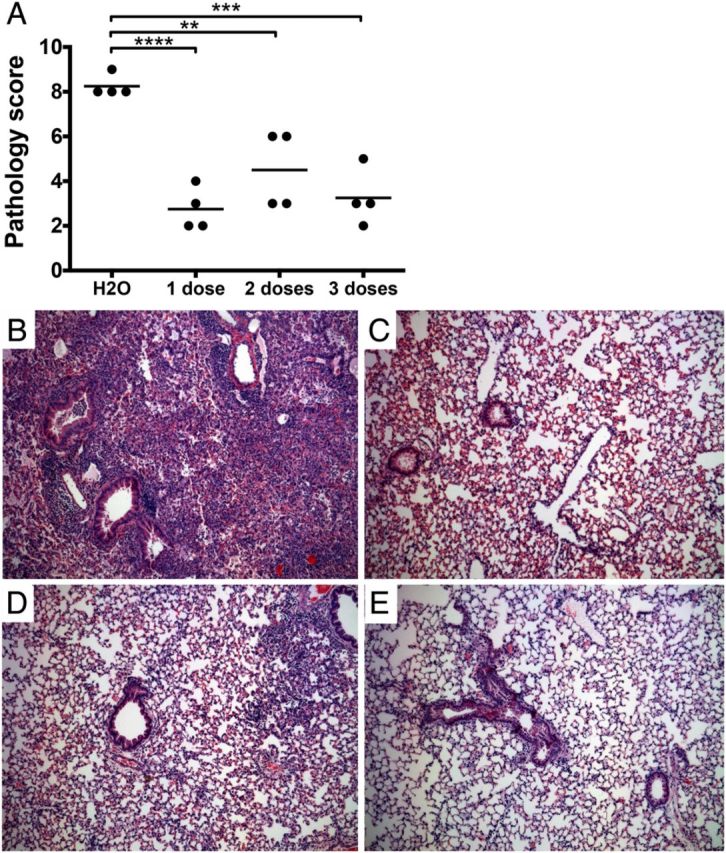
Effect of S1P agonism on pathology. A, Lung pathology was assessed following hematoxylin and eosin (H&E) staining, by 2 independent researchers based on the degree and breadth of bronchovascular bundle (BVB) inflammation and pleuritis. Representative images shown from animals receiving water control (B), or 1 dose (1 hour postinfection) (C), 2 doses (1 and 6 hours) (D), or 3 doses (1, 6, and 24 hours) of AAL-R (E) and harvested 7 days postinoculation. Images are shown at 100× magnification. Results are presented as mean ± SD. **P ≤ .01, ***P ≤ .001; ****P ≤ .0001. Abbreviation: S1P, sphingosine-1-phosphate.
DISCUSSION
In recent years, developed countries have reported a resurgence in pertussis cases, despite widespread vaccine coverage [1, 2]. This is thought to be due to waning of the immunity elicited by acellular vaccination [13]. Multiple approaches exist for limiting the spread of pertussis to infants, including maternal immunization and familial cocooning. However, preventative strategies alone may not be sufficient. The resurgence in infection rates and deaths highlights the need for effective treatments for severe pertussis. Because antibiotic therapies alone are not sufficient in treating critical pertussis, host-directed therapies that reduce inflammation and limit disease are urgently required. Here, we used the S1P analog AAL-R to study the potential of host-directed therapy for reduction of pertussis-associated pathology.
AAL-R treatment significantly reduced expression and production of proinflammatory cytokines and chemokines in the lungs of B. pertussis–infected mice. This was true even with a single dose administered 1 hour postinoculation, although additional doses had further reducing effects for some cytokines. AAL-R treatment slightly reduced bacterial burden at some time points. While this reduction was not sufficiently large to explain the reduction in the inflammatory response, it may be an added benefit of this treatment.
It is unclear if reducing inflammatory responses to infection is beneficial or detrimental in the context of active infection. Having determined that B. pertussis proliferation is not increased upon immune modulation by AAL-R (Figure 1A), the effect of treatment on lung pathology was assessed. Animals receiving 1, 2, or 3 doses of AAL-R showed a significant and dramatic reduction in pathology (Figure 2). Further to this, no difference could be detected between animals based on number of doses received, suggesting it may be possible to improve disease outcome with minimal treatment. Reducing pathology, particularly in infants, during infection could lead to a reduction in the growing numbers of fatal pertussis infections. Little is known about the pathology of pertussis in humans, but autopsy studies of fatal pertussis in infants have shown evidence of dramatic inflammation and tissue damage in the lungs [14]. Therefore, the inflammatory pathology in infected mice may represent a model of the severe pulmonary events in hospitalized infants with pertussis. In the absence of experimental human studies and the prohibitive expense of primate studies, the mouse model represents a useful starting point for discovery and preclinical studies of potential therapeutics. The potential of host-directed therapies has been studied with varying success for multiple infectious diseases [10, 15]. Our results add to recent data demonstrating that S1P receptor signaling can dampen influenza virus–mediated cytokine storm [10], reducing morbidity and mortality.
Here, we not only demonstrate a potential use for host-directed therapies in the treatment of pertussis, but also highlight the potential role of inhibition of S1P signaling in pertussis pathogenesis. Future studies will be aimed at further elucidating the mechanisms of S1P signaling in pertussis, as well as the potential of the bacterium to modulate this signaling. AAL-R signals through 4 of the 5 S1P receptors, therefore the specific receptor mediating this phenomenon could not be elucidated in this study, but will be the focus of future studies. One potential advantage of this approach is the current availability of a Food and Drug Administration–approved S1P receptor agonist similar to AAL-R, the multiple sclerosis drug FTY-720 (fingolimod), demonstrating the safety and translational potential of this host-targeted approach. In addition to fingolimod, multiple S1P receptor agonists are currently in clinical trials and may represent safer, more suitable therapeutics for treatment of pertussis.
Notes
Financial support. This work was supported by Public Health Service grant AI-101055 from the National Institute of Allergy and Infectious Diseases.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ausiello CM, Cassone A. Acellular pertussis vaccines and pertussis resurgence: revise or replace? MBio 2014; 5:e01339–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger JT, Carcillo JA, Shanley TP, et al. Critical pertussis illness in children: a multicenter prospective cohort study. Pediatr Crit Care Med 2013; 14:356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fedele G, Bianco M, Ausiello CM. The virulence factors of Bordetella pertussis: talented modulators of host immune response. Arch Immunol Ther Exp (Warsz) 2013; 61:445–57. [DOI] [PubMed] [Google Scholar]
- 4.Connelly CE, Sun Y, Carbonetti NH. Pertussis toxin exacerbates and prolongs airway inflammatory responses during Bordetella pertussis infection. Infect Immun 2012; 80:4317–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katada T, Tamura M, Ui M. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch Biochem Biophys 1983; 224:290–8. [DOI] [PubMed] [Google Scholar]
- 6.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 2012; 22:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem 2004; 92:913–22. [DOI] [PubMed] [Google Scholar]
- 8.Garcia JG, Liu F, Verin AD, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 2001; 108:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng X, Hassoun PM, Sammani S, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 2004; 169:1245–51. [DOI] [PubMed] [Google Scholar]
- 10.Teijaro JR, Walsh KB, Cahalan S, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 2011; 146:980–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scanlon KM, Gau Y, Zhu J, et al. Epithelial anion transporter pendrin contributes to inflammatory lung pathology in mouse models of Bordetella pertussis infection. Infect Immun 2014; 82:4212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Don AS, Martinez-Lamenca C, Webb WR, Proia RL, Roberts E, Rosen H. Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogues. J Biol Chem 2007; 282:15833–42. [DOI] [PubMed] [Google Scholar]
- 13.Witt MA, Arias L, Katz PH, Truong ET, Witt DJ. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis 2013; 56:1248–54. [DOI] [PubMed] [Google Scholar]
- 14.Sawal M, Cohen M, Irazuzta JE, et al. Fulminant pertussis: a multi-center study with new insights into the clinico-pathological mechanisms. Pediatr Pulmonol 2009; 10:970–80. [DOI] [PubMed] [Google Scholar]
- 15.Skerry C, Pinn ML, Bruiners N, Pine R, Gennaro ML, Karakousis PC. Simvastatin increases the in vivo activity of the first-line tuberculosis regimen. J Antimicrob Chemother 2014; 69:2453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]