Abstract
Background. Antibody responses to seasonal influenza vaccines are defective during older age and human immunodeficiency virus (HIV) infection. The effect of HIV on immune function in aging is relatively unknown.
Methods. HIV-infected and HIV-uninfected young women (age, 19–54 years) and older women (age, >55 years) were evaluated for B-cell and T-cell responses before and 4 weeks after influenza vaccination.
Results. Frequencies of seroprotection pre-vaccination and vaccine responsiveness (≥4-fold increase in antibody titer) were lower in HIV-infected participants than in age-matched HIV-uninfected participants. A subgroup of vaccine nonresponders were compared to responders and found to have reduced frequencies of memory B cells and antigen-specific antibody-secreting cells after vaccination. Frequencies of peripheral T-follicular helper (pTfh) cells correlated with memory B-cell function and influenza A(H1N1) antibody titers. Serologic and immunologic deficits were most frequent in older HIV-infected participants. Underlying CD4+ T-cell immune activation and inflammation correlated negatively with antibody titers and B-cell function, which was not enhanced by exogenous interleukin 21 supplementation in HIV-infected, older vaccine nonresponders.
Conclusions. Immune activation associated with HIV infection and impaired pTfh function heighten deficiencies in antibody responses to influenza vaccine in older individuals. Strategies to reduce immune activation or augment pTfh function may enhance antibody responses in the aging HIV-infected population.
Keywords: seasonal influenza vaccination, aging, HIV infection, memory B cells, peripheral T follicular helper cells, IL-21, CD4 T-cell immune activation, inflammation
The aging human immunodeficiency virus (HIV)-infected population is rapidly increasing as a result of survival gains due to improved combination antiretroviral therapy (cART) [1] and a rising incidence of HIV infections in people >50 years of age [2]. The immune dysfunction associated with HIV infection bears many similarities with the immune dysfunction attributable to aging [3–5], and the term “immunosenescence” [6] is used to refer such dysfunction in both settings. Features common to aging and HIV infection include an increased risk for influenza virus infection [7, 8], an impaired serologic response to influenza vaccination [9], and a heightened state of inflammation and immune activation [6, 10, 11]. Our recent studies indicate that HIV infection and advanced age together could have a more deleterious effect on immunity than advanced age alone, based on poorer serologic responses to influenza vaccine in postmenopausal women with, versus those without, HIV infection [5, 12].
The present study was designed to ascertain how HIV infection exacerbates the immune defects associated with aging and to understand the mechanism of the impaired antibody (Ab) response to influenza vaccination. Ab generation by B cells is dependent upon cognate help from T-follicular helper (Tfh) cells, a specialized CD4+ T-helper cell subset in lymphoid germinal centers [13, 14]. Tfh cells play a critical role in the class-switch recombination and somatic hypermutation required for the development of memory B cells for secretion of high-affinity Ab [15, 16]. A CXCR5+ memory subset of peripheral blood CD4+ T cells with a Tfh-like helper function for B cells are known as peripheral Tfh (pTfh) cells [17, 18]. In a cohort of younger HIV-infected persons, we previously reported that influenza vaccine nonresponsiveness was associated with impaired expansion of pTfh cells, reduced plasma levels of interleukin 21 (IL-21), and lower expression of IL-21 receptor (IL-21R) on memory B cells [18]. Here, we show that, among HIV-infected and HIV-uninfected young and older women, the older HIV-infected group had the poorest serologic responses and the worst cellular functions associated with antibody production. An important role of baseline immune activation and inflammatory cytokines for inhibiting pTfh helper function for memory B-cell function was identified.
MATERIALS AND METHODS
Study Population and Samples
We conducted a study to evaluate the effect of HIV infection and aging on immune responses to influenza vaccine during the 2011–2012 influenza season. Women were considered eligible if they were >18 years of age, had no active malignancies, and were not receiving hormonal replacement therapy. Women classified as older were >55 years of age, amenorrheic for ≥12 months, and did not have a history of premenopausal oophorectomy. Women classified as young were <45 years of age and were not amenorrheic. The older participants were matched for age and for time to menopause. HIV-infected participants had been receiving cART for >6 months with virologic suppression (HIV RNA load, <100 copies/mL) [5, 12]. Participants received a single intramuscular dose (15 mg) of inactivated influenza vaccine (Fluarix, GSK, United Kingdom) containing the strains A/California/7/2009(H1N1), A/Perth/16/2009(H3N2), and B/Brisbane/60/2008 at the special immunology clinic at the University of Miami. The study was approved by the university's institutional review board, and participants were enrolled after they provided informed consent. Peripheral blood specimens were collected before vaccination and 4 weeks after vaccination; processed within 6 hours of collection for isolation of peripheral blood mononuclear cells (PBMCs), using Ficoll-Hypaque gradients; and cryopreserved in liquid nitrogen [19]. Plasma specimens were stored in aliquots at −80°C. Serologic analysis for influenza virus Ab was performed in 70 women (Table 1), and a subgroup of 48 participants underwent immunologic assessments (Supplementary Table 1).
Table 1.
Characteristics of Women Who Received Influenza Vaccination, by Age Group and Human Immunodeficiency Virus (HIV) Status
| Characteristic | Young Women |
Older Women |
||
|---|---|---|---|---|
| HIV Uninfected (n = 15) | HIV Infected (n = 15) | HIV Uninfected (n = 20) | HIV Infected (n = 20) | |
| Age, y | 32.9 ± 4.4 | 37.9 ± 4.7 | 65 ± 4.9 | 61.5 ± 3 |
| Vaccine responder, % | 60 | 47 | 30 | 35 |
| Vaccine nonresponder, % | 40 | 53 | 70 | 65 |
| CD4+ T-cell count, cells/µL | ND | 654.3 ± 313.8 | ND | 602.7 ± 273.6 |
| Seroprotection | ||||
| Before vaccination, % | 68.6 | 56.6 | 50 | 25 |
| 4 wks after vaccination, % | 100 | 93.3 | 75 | 55 |
| HAI titer | ||||
| Before vaccination | 64 ± 44.2a,b | 36 ± 22.6a | 58 ± 59.2a | 22.5 ± 10.6 |
| 4 wks after vaccination | 328 ± 338.8a,b,c | 100 ± 53.9a | 96.5 ± 83.9a | 56 ± 48.08 |
Data are mean value ± SD or percentage of subjects.
Abbreviations: HAI, hemagglutination inhibition; ND, not determined; SD, standard deviation.
a Statistically significantly different from older HIV-infected women.
b Statistically significantly different from young HIV-infected women.
c Statistically significantly different from older HIV-uninfected women.
Serologic Assessments
Influenza virus Ab titers for H1N1/09 vaccine antigen (H1N1 A/California/07/2009 antigen, a gift from Novartis Vaccines and Diagnostics) were determined in plasma by a hemagglutination inhibition assay (HAI) [20]. Seroprotection was defined as an HAI titer of ≥1:40. Participants with a postvaccination titer of ≥1:40 and a ≥4-fold increase from the prevaccination titer were classified as vaccine responders.
Phenotypic and Functional Characterization of pTfh and B Cells
The following monoclonal Abs (mAbs) were used: CD3-AmCyan, Ki-67–PerCPCy5.5, CXCR5-AF647, CXCR3-PeCy5, CD20-APCCy7, CD21-PeCy5, IL-21R–APC (BD Biosciences), CD4-QDot655, CD8-QDot605, CD10-QDot605, biotinylated anti-human immunoglobulin G (IgG) and Live/Dead Fixable Violet Dead cell stain (ViViD, Life Technologies) kit, CD27-AF700, immunoglobulin D (IgD)–FITC, inducible T-cell costimulator (ICOS)–PeCy7, HLA-DR–PerCP, CD38-AF700, streptavidin (Biolegend), CD45RO-ECD (Beckman Coulter), and IL-21–PE (eBiosciences) [21].
For all assays, cryopreserved PBMCs were thawed and rested overnight prior to further processing for phenotype or function. For flow cytometry, stained fixed cells were acquired on an LSRII Fortessa and analyzed using FlowJo (TreeStar). The pTfh cells were defined as CD4+CD45RO+CXCR5+ cells by gating on live CD3+CD4+ T cells; they were further gated to determine ICOS and programmed cell death-1 (PD-1) expression on pTfh. For B-cell characterization, live CD3−CD20+ cells were sequentially gated on CD21, CD27, and CD10 to identify resting memory B cells (CD20+CD21hiCD27+CD10−), together with frequencies of IL-21R+ resting memory B cells.
For functional characterization of cell subsets, PBMCs were stimulated with 5 µg/mL H1N1/09 vaccine antigen plus anti-CD28 mAb (1 µg/mL) for 12 hours at 37°C, and brefeldin A (10 µg/mL) was added for the last 7 hours of incubation. Cells were stained with ViViD and pTfh surface markers, fixed, permeabilized, and stained for intracellular IL-21. In other experiments, PBMCs were stimulated as above for 5 days with H1N1 antigen plus anti-CD28 mAb in the presence or absence of 50 ng/mL exogenous IL-21 (Life Technologies) [18]. The cells were analyzed for Ab-secreting cells in enzyme-linked immunosorbent spot (ELIspot) assays.
ELIspot Assays
PBMCs from the 5-day cultures were plated in wells coated with goat anti-human IgG (2 µg/mL, Jackson Immunoresearch) at 100 000 cells/well for 4 hours at 37°C and assayed for H1N1-specific IgG. Data are expressed as Ab-secreting cells/million PBMCs [21, 22].
Cytokine Measurements
The cytokines tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) were determined in appropriate dilutions of plasma, using Milliplex cytokine magnetic bead panel in the Magpix instrument (Luminex) [12]. Mean fluorescent intensities (MFIs) were analyzed with Milliplex Analyst Software (EMD Millipore), and cytokine levels are expressed as picograms per milliliter.
Statistical Analysis
Data were analyzed with Spearman correlation, linear regression, 1-way analysis of variance, and linear mixed models. Analysis of variance results are presented as means and standard deviations for planned comparisons among groups. The data are presented as scatter plots with regression lines and Spearman correlation coefficients with P values. Box and whisker plots are presented with the P values for planned comparisons of mean values before and after vaccination within groups and among groups within times before and after vaccination. The 2-tailed 0.05 level was used to determine statistical significance. GraphPad Prism (GraphPad Software) and SAS 9.3 (SAS Institute) were used for all analyses.
RESULTS
Ab Response to Influenza Vaccination Is Diminished With Aging and HIV Infection
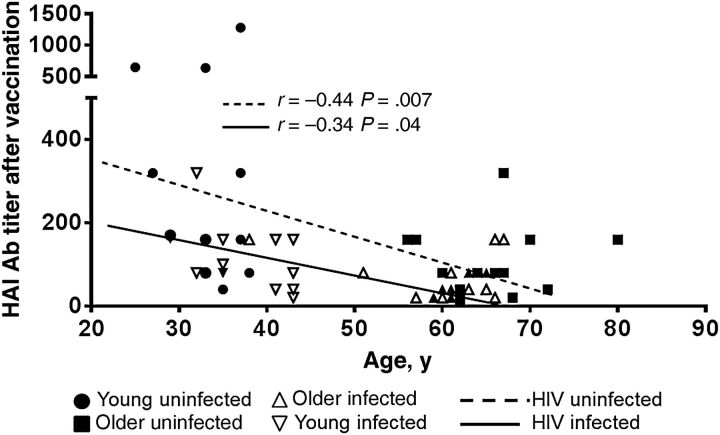
Before and after vaccination, older and young HIV-infected participants had lower Ab titers than older and young HIV-uninfected participants, respectively, with the older HIV-infected participants exhibiting the lowest titers among all groups (Table 1). Only 25% of older HIV-infected had titers of ≥1:40 before vaccination, compared with 50%, 57%, and 69% of older uninfected, young infected, and young uninfected participants, respectively. Although seroprotection rates after vaccination were 55%, 75%, 93%, and 100% in older infected, older uninfected, young infected, and young uninfected participants, respectively (Table 1), frequencies of response were equivalent in HIV-infected and HIV-uninfected older participants, highest in young uninfected participants, and intermediate in young infected participants. HAI influenza virus Ab titers after vaccination correlated inversely with age in both HIV-uninfected (P = .007) and HIV-infected (P = .04) groups (Figure 1).
Figure 1.
Age and human immunodeficiency virus (HIV) infection status contribute to impaired antibody (Ab) responses to influenza vaccination. Linear correlation between hemagglutination inhibition (HAI) Ab titers after vaccination and age in HIV-uninfected and HIV-infected young and older women (Table 1). P values were calculated from planned comparisons of general linear mixed model mean values. Correlation between HIV-infected subjects is indicated by the continuous line. The dashed line shows the correlation between the 2 variables when only the HIV-uninfected participants were taken into account.
For immunologic investigations, we selected equal numbers of participants (ie, 12) in the 4 groups, with roughly even distribution of responders and nonresponders (Supplementary Table 1). Cellular determinants of Ab responses constituted by CD4+ T-cell and B-cell subsets and inflammatory cytokines were investigated in participants in each of the 4 groups.
Frequencies and Function of Memory B Cells Are Impaired With Aging and HIV Infection
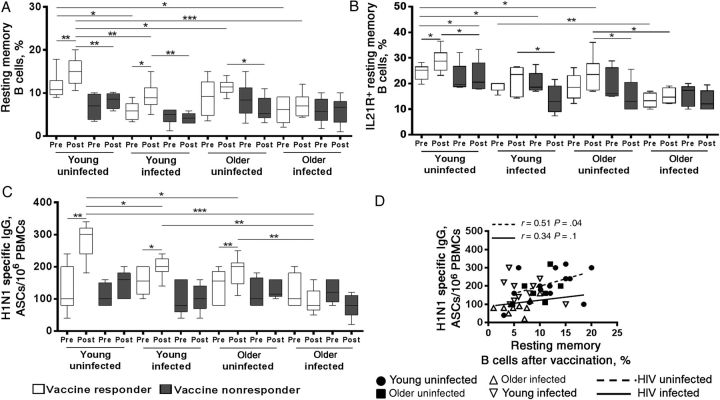
Among participants classified as responders, only the young HIV-uninfected women showed a consistent increase in frequencies of total and IL-21R+ resting memory B cells (Figure 2A and 2B), as well as in H1N1 antigen–specific Ab-secreting cells from before to after vaccination (Figure 2C). Young HIV-infected responders showed an increase in 2 of 3 characteristics: total resting memory B cells and H1N1-specific Ab-secreting cells. The older HIV-uninfected responders had a significant increase only in the H1N1-specific Ab-secreting cells. The older HIV-infected responders did not show any of these changes, despite their responder classification. The nonresponders in all 4 groups did not show these B-cell changes from before to after vaccination. Frequencies of IL-21R+ memory B cells and their Ab-secreting cell responses were similar between the older HIV-uninfected and young HIV-infected groups (Figure 2B and 2C). When responders and nonresponders were combined, after vaccination the young HIV-uninfected group showed consistent expansion of total resting memory and IL-21R+ memory B cells, as well as H1N1-specific Ab-secreting cells, while the older participants did not show changes in these B-cell characteristics (Supplementary Figure 1A–C). In the HIV-uninfected group, the frequencies of resting memory B cells after vaccination were correlated with H1N1-specific Ab-secreting cells (Figure 2D).
Figure 2.
Impaired memory B-cell responses to influenza vaccination in aging and human immunodeficiency virus (HIV) infection. Cryopreserved peripheral blood mononuclear cells (PBMCs) from HIV-uninfected and HIV-infected responders (7 young uninfected participants, 5 young infected participants, 6 older uninfected participants, and 6 older infected participants) and nonresponders (5 young uninfected participants, 7 young infected participants, 6 older uninfected participants, and 6 older infected participants) before and after vaccination were thawed and rested overnight followed by staining with monoclonal antibody (Ab). Frequencies of resting memory (RM) B cells (CD20+CD21hiCD27+CD10−; A) and interleukin 21 receptor–expressing (IL-21R+) RM B cells (B) before vaccination (Pre) and after vaccination (Post). C, H1N1-specific memory B-cell responses measured by an enzyme-linked immunosorbent spot assay in PBMCs stimulated with H1N1 antigen for 5 days. D, Linear correlation between H1N1-specific Ab-secreting cell (ASC) and RM B-cell frequencies after vaccination in HIV-uninfected and HIV-infected participants. Box plots include median with 25th and 75th percentile borders, and error bars represent 10th and 90th percentiles. *P < .05, **P < .01, and ***P < .001. Abbreviation: IgG, immunoglobulin G.
Aging and HIV Infection Are Associated With Reduced Frequencies of pTfh, Impaired Antigen-Specific pTfh Function, and Impaired Induction of ICOS on pTfh
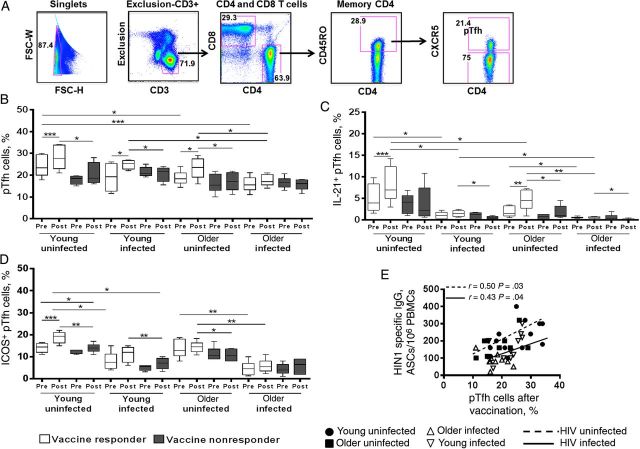
Similar to findings for resting memory B cells, among responders, only young HIV-uninfected participants showed a consistent increase in frequencies of total pTfh cells (Figure 3A and 3B) and IL-21+ pTfh cells (Figure 3C), as well as in ICOS+ pTfh cells (Figure 3D), from before to after vaccination, whereas young HIV-infected responders showed increases only in frequencies of pTfh cells. Older HIV-uninfected responders showed increases in pTfh cells and IL-21+ pTfh cells, whereas older HIV-infected responders did not show changes in any of these pTfh-cell characteristics, despite their responder classification. The nonresponders in all 4 groups failed to show pTfh-cell changes from before to after vaccination. Frequencies of IL-21+ pTfh cells and ICOS+ pTfh cells were significantly lower in HIV-infected responders, compared with HIV-uninfected responders, both before and after vaccination. After vaccination, frequencies of pTfh cells correlated with H1N1-specific Ab-secreting cells (Figure 3E), and frequencies of ICOS+ pTfh correlated with IL-21+ pTfh cells (data not shown). Collectively, the HIV-uninfected participants showed expansion of total pTfh cells, IL-21+ pTfh cells, and ICOS+ pTfh cells after vaccination, with IL21+ pTfh cells and ICOS+ pTfh cells being significantly higher in HIV-uninfected participants, compared with HIV-infected participants, before and after vaccination (Supplementary Figure 1D–F).
Figure 3.
Association of impaired antibody (Ab) response with defective peripheral T-follicular helper (pTfh)–cell frequency and function. A, Flow cytometric dot plots showing the gating strategy of pTfh cells (CD3+CD4+CD45RO+CXCR5+) from live (ViViD−) CD3+ cells. B, Frequencies of pTfh cells before and after vaccination in human immunodeficiency virus (HIV)-uninfected and HIV-infected young and older responders and nonresponders. B, Frequencies of interleukin 21–expressing (IL-21+) pTfh cells in responders and nonresponders following H1N1 antigen stimulation for 12 hours and intracellular staining. D, Frequencies of inducible T-cell costimulatory–expressing (ICOS+) pTfh cells in vaccine responders and nonresponders. E, Correlation between H1N1-specific Ab-secreting cell (ASC) responses with frequencies of pTfh cells after vaccination. Abbreviation: IgG, immunoglobulin G.
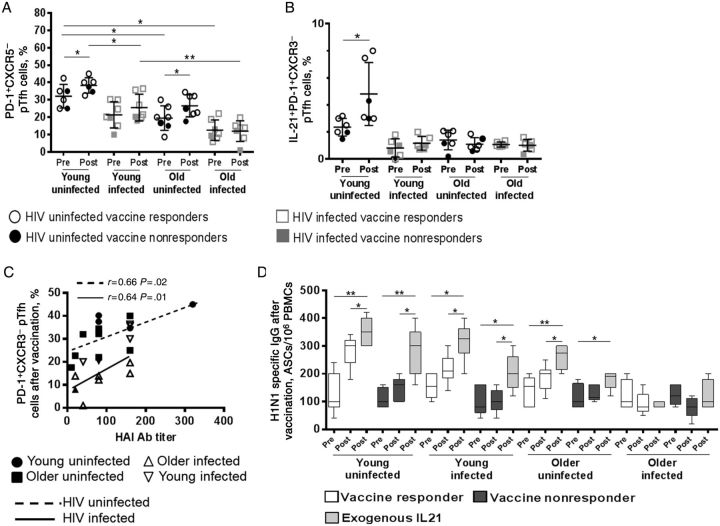
In a subset of participants, we also investigated frequencies of PD-1+CXCR3−CXCR5+ memory pTfh cells (Figure 4), a subset recently described by Locci et al to represent germinal center–derived Tfh cells that correlate with broadly neutralizing HIV Ab responses [23]. The frequencies of this pTfh-cell subset were highest in young HIV-uninfected participants before and after vaccination and lowest in older HIV-infected participants (Figure 4A), with the young HIV-uninfected participants also showing the greatest expansion of this subset by phenotype and antigen-induced IL-21 expression after vaccination (Figure 4B). This subset also correlated with HAI Ab titer (Figure 4C).
Figure 4.
Association between CXCR3− peripheral T-follicular helper (pTfh) cells with antibody (Ab) responses to influenza vaccination in human immunodeficiency virus (HIV)-infected and HIV-uninfected participants. Cryopreserved peripheral blood mononuclear cells (PBMCs) from HIV-uninfected participants (7 young participants and 7 older participants) and HIV-infected participants (7 young participants and 7 older participants) before and after vaccination were stained with monoclonal Ab to determine frequencies of the programmed cell death 1–expressing (PD-1+) CXCR3− pTfh-cell subset (gated from CD4+CD45RO+CXCR5+ cells) by flow cytometry (A). B, Frequencies of interleukin 21–expressing (IL-21+) PD-1+CXCR3− pTfh cells at baseline and after vaccination in all groups following H1N1 antigen (5 µg/mL) stimulation. C, Association between frequencies of PD-1+CXCR3− pTfh-cell subset and hemagglutination inhibition (HAI) titers after vaccination. D, PBMCs obtained from participants before and after vaccination were cultured for 5 days with H1N1 antigen with or without recombinant IL-21 (50 ng/mL). After 5 days, cells were analyzed by a B-cell enzyme-linked immunosorbent spot assay. Abbreviation: IgG, immunoglobulin G.
Exogenous IL-21 Supplementation Does Not Enhance B-Cell Function in Older HIV-Infected Nonresponders
Since we observed a correlation between H1N1-specific IL-21 induction in pTfh cells and influenza virus Ab titers, we performed ELIspot assays to investigate whether ex vivo IL-21 supplementation of HIN1 antigen-stimulated PBMC cultures would improve postvaccination B-cell responses. Except for the older HIV-infected participants, increases in H1N1-specific Ab-secreting cells were noted in all responders, as well as in nonresponders (Figure 4D).
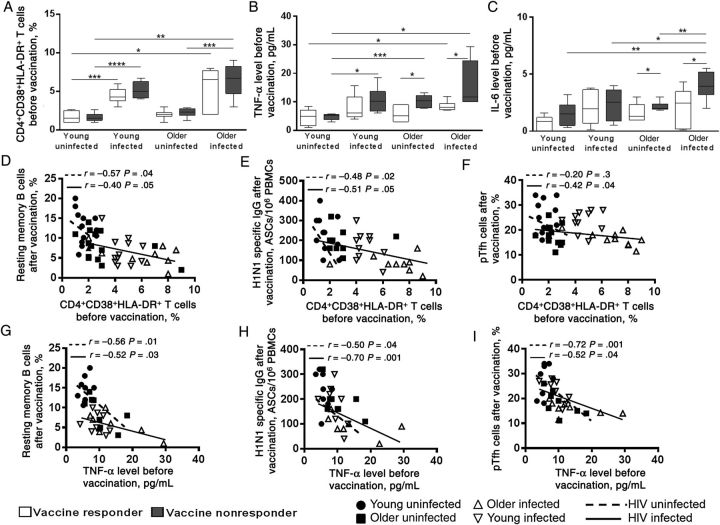
Baseline CD4+ T-Cell Immune Activation and Inflammatory Cytokine Levels Are Higher in Older HIV-Infected Participants and Contribute to Impaired Ab Responses
Next, we examined the basal state of CD4+ T-cell immune activation and inflammation among the groups. Frequencies of activated CD4+ T cells were higher in HIV-infected participants, compared with HIV-uninfected participants (Figure 5A), and collectively were highest in older HIV-infected participants (Supplementary Figure 2A). Plasma levels of TNF-α (Figure 5B) and IL-6 (Figure 5C) were higher in nonresponders, compared with responders, among older HIV-infected and HIV-uninfected participants, and collectively, levels of both cytokines were higher in HIV-infected participants, compared with HIV-uninfected participants (Supplementary Figure 2B and Supplementary Figure 2C). Frequencies of activated CD4+ T cells before vaccination were inversely correlated with the postvaccination frequencies of resting memory B cells (Figure 5D) and H1N1-specific Ab-secreting cells in both HIV-infected and HIV-uninfected participants (Figure 5E) and with pTfh cells in HIV-infected participants (Figure 5F). Plasma TNF-α levels before vaccination correlated inversely with postvaccination frequencies of resting memory B cells (Figure 5G), H1N1-specific Ab-secreting cells (Figure 5H), pTfh cells (Figure 5I), IL-21R+ B cells (Supplementary Figure 2D), and IL21+pTfh cells (Supplementary Figure 2E) and were directly correlated with prevaccination frequencies of activated CD4+ T cells (Supplementary Figure 2F) in both HIV-infected and HIV-uninfected participants.
Figure 5.
Baseline CD4+ T-cell immune activation and the proinflammatory cytokine tumor necrosis factor α (TNF-α) is associated with impaired cellular responses to influenza vaccination. A, Prevaccination frequencies of activated CD4+ T cells (HLA-DR+CD38+) in human immunodeficiency virus (HIV)-uninfected and HIV-infected responders and nonresponders. Plasma levels of TNF-α (B) and interleukin 6 (IL-6; C) before vaccination in young and older responders and nonresponders were estimated using Milliplex beads (Magpix). Linear correlation between frequencies of CD4 immune activation with resting memory B cells (D), antibody-secreting cell (ASC) responses (E), and pTfh frequencies (F) after vaccination. Correlation of baseline plasma TNF-α levels with resting memory B cells (G), ASC responses (H), and pTfh-cell frequencies (I) after vaccination. *P < .05, **P < .01, ***P < .001, and ****P < .0001. Abbreviation: IgG, immunoglobulin G.
We also performed independent correlations of age and vaccine-induced influenza HAI titer with frequencies of various B-cell and T-cell subsets, Ab-secreting cells (by means of ELIspot analysis), T-cell immune activation, and inflammatory cytokines (Table 2). In HIV–uninfected participants, age was positively correlated with activated CD4+ T cells and plasma TNF-α levels before vaccination and negatively correlated with Ab-secreting cell response, frequencies of total and IL-21R+ resting memory B cells, total pTfh cells, IL-21+ pTfh cells, and ICOS+ pTfh cells after vaccination. HAI titers showed negative correlations to the same markers before vaccination, except that the prevaccination pTfh-cell association was not significant and positive correlation to all markers after vaccination. In HIV-infected participants, age correlations were similar to those seen in HIV-uninfected participants, except for the Ab-secreting cell response. HAI titers showed similar negative correlation as the HIV-uninfected participants with prevaccination frequencies of activated CD4+ T cells and plasma TNF-α, positive correlations with postvaccination IL-21R+ resting memory B cells, IL-21+ pTfh cells, and ICOS+ pTfh cells.
Table 2.
Correlation of Age and Hemagglutination Inhibition Assay (HAI) Titer With Various Factors Before and 4 Weeks After Influenza Vaccination Among Young and Older Study Participants, by Human Immunodeficiency Virus (HIV) Status
| Factor, by Time Point |
r2, Uninfected |
r2, Infected |
||
|---|---|---|---|---|
| Age | HAI Titer | Age | HAI Titer | |
| After vaccination | ||||
| RM B-cell frequency (% of CD3−CD20+ B cells) | −0.40a | 0.40a | −0.53a | 0.33 |
| IL-21R expression (% of RM B cells) | −0.56a | 0.48a | −0.45a | 0.46a |
| H1N1 Ab-secreting cell count (cells/106 PBMCs)b | −0.60a | 0.58a | −0.35 | 0.35 |
| pTfh-cell frequency (% of CD3+CD4+ T cells) | −0.62a | 0.45a | −0.51a | 0.35 |
| IL-21 staining (% of pTfh cells) | −0.48a | 0.72a | −0.45a | 0.41a |
| ICOS induction (% of pTfh cells) | −0.52a | 0.40a | −0.50a | 0.50a |
| Before vaccination | ||||
| pTfh frequency (% of CD3+CD4+ T cells) | −0.60a | 0.20 | −0.52a | 0.18 |
| CD38+HLA-DR+CD4+ T-cell frequency (% of CD4+ T cells) | 0.52a | −0.46a | 0.48a | −0.55a |
| TNF-α level (pg/mL)c | 0.43a | −0.50a | 0.68a | −0.63a |
Abbreviations: Ab, antibody; H1N1, influenza A(H1N1); ICOS, inducible T-cell costimulator; IL-21, interleukin 21; IL-21R, interleukin 21 receptor; PBMC, peripheral blood mononuclear cell; pTfh, peripheral T-follicular helper; RM, resting memory; r2, linear correlation coefficient; TNF-α, tumor necrosis factor α.
a P < .05 for the comparison of young and older participants with the specified HIV status.
b Data were enumerated following stimulation of PBMCs with H1N1 antigen for 5 days.
c Values were measured using Milliplex beads (Magpix) in plasma specimens obtained at baseline.
DISCUSSION
Impaired immunity in the aging population and during HIV infection are independently considered to be responsible for suboptimal efficacy of current influenza vaccines [3, 24]. In this study, we used seasonal influenza vaccination to analyze pTfh-cell and B-cell function in HIV-infected and HIV-uninfected older and young women. Seroprotection rates before and after vaccination were higher in the young women, and these participants also demonstrated stronger B-cell and pTfh-cell immune responses, reiterating the negative impact of aging on immunity. Among the various groups, the young HIV-uninfected participants had highest Ab response and best overall cellular functions, while the older HIV-infected participants exhibited the lowest and poorest responses in these assays. The IL21R+ B-cell frequencies and H1N1-specific Ab-secreting cell responses in older HIV-uninfected participants were similar to those in young HIV-infected participants, implying acceleration of immune senescence with HIV infection in the latter group [25]. Interestingly, underlying immune activation was the most prominent feature differentiating HIV-infected from HIV-uninfected nonresponders in both age groups, while decreased IL-21 production by pTfh cells after vaccination was consistently associated with a poor Ab response. These findings point to immune activation and deficient pTfh-cell function as leading factors for impaired responsiveness to influenza vaccines and favor the contention that immunologic decline in aging is hastened by the presence of immune activation associated with HIV infection.
To understand the immunologic basis of impaired Ab responses, we selected roughly equal numbers of young and older HIV-infected and HIV-uninfected vaccine responders and nonresponders and evaluated their B-cell and T-cell responses and plasma biomarkers. We have previously shown that certain B-cell characteristics, such as frequency and function of memory B cells and expression of IL-21R, are correlated with the magnitude of vaccine-induced serologic Ab responses in HIV-infected young adults [21]. Our study shows that, after vaccination, influenza virus HAI titer, frequencies of memory B cells, IL-21R expression, and H1N1 antigen-induced Ab-secreting cell responses, were negatively and independently influenced by age and HIV infection. Although the effect of age dominated, that of HIV infection was also significant. Considered in the context of responder/nonresponder status, positive changes from before to after vaccination in the 3 B-cell characteristics—frequencies of memory B cells, IL-21R expression, and H1N1 antigen-induced Ab-secreting cells—were most clearly evident in young HIV-uninfected responders and less so in young HIV-infected responders and were never seen in nonresponders in either age group, regardless of HIV infection status. Older participants had demonstrable deficiencies in these B-cell features, most evident in the older HIV-infected participants, even in those classified as responders. The cause for the failure of expansion of memory B cells and antigen-induced Ab-secreting cells after vaccination is unclear; recruitment of B cells may be impaired because the naive B-cell pool is depleted as a result of preexisting memory B-cell expansion by repeated prior antigenic stimulation or nonspecific activation by inflammatory cytokines.
Tfh cells provide critical help in the germinal centers for B-cell differentiation and plasma cell generation [13, 26], and defective expansion of pTfh cells after vaccination is an indicator of influenza vaccine nonresponsiveness in HIV-infected persons [18, 21, 27]. In this study, we found that frequencies of pTfh cells and IL-21+ pTfh cells and expression of ICOS were positively correlated with HAI titer and negatively correlated with age. We also observed that pTfh-cell expansion after vaccination correlated with B-cell memory responses in both young and older groups, confirming prior observations that support the requirement for pTfh cells to generate memory B cells following vaccination [18]. Besides age, there was clear evidence of the damaging effect of HIV infection in pTfh cells, with young HIV-infected participants showing significantly lower frequencies of IL-21+ and ICOS+ pTfh cells than young uninfected participants. IL-21 is the signature cytokine produced by Tfh cells that can induce B cells to undergo differentiation, proliferation, and Ab production [27–29]. ICOS is known to regulate IL-21 production and the proliferation and function of Tfh cells [30, 31] and is essential for immunoglobulin secretion from human B cells in vitro [18, 30, 32]. Defects in ICOS expression on CD4+ T cells have been described in individuals with advanced age [33, 34]. The older HIV-infected group had the poorest cellular functions of pTfh cells, as well as of B cells, as discussed above. The severity of their impairment was evident also in the failure of exogenous IL-21 to augment antigen-specific B-cell Ab secretion in ELIspot assays in the older infected nonresponders. These observations add to the increasing body of evidence that aging adversely influences the ability of pTfh cells to expand after immunization, possibly affecting their survival, maintenance, or renewal [35, 36]. Our findings imply that the presence of HIV infection compromises the aging immune system, even when viremia is adequately controlled.
The nature of pTfh cells that are responsible for B-cell help is being intensively investigated [17, 23, 26, 37]. A PD1+CXCR3− memory pTfh-cell subset is believed to originate in germinal centers of lymph nodes and serve as the prime helper pTfh-cell subset [23]. We found a correlation of this pTfh-cell subset with influenza vaccine–induced Ab responses and an inverse correlation with age, similar to total CXCR5+ memory pTfh cells. The role of this and other newly described pTfh-cell subsets [38] in driving the changes in Ab responses needs to be investigated in aging and HIV infection.
Immune activation and inflammation are hallmarks of aging [11, 39], as well as of HIV infection [12], but their role in influencing vaccine-induced responses is less clear. We previously showed that activated CD4+ T cells and elevated levels of the inflammatory cytokine TNF-α were associated with poor Ab responses to vaccination in HIV-infected subjects [5]. In the present study, we observed that the activated CD4+ T cells had an inverse relationship with expansion of antigen-specific resting memory B cells in both HIV-infected and HIV-uninfected participants, implying an independent influence of aging on CD4+ T-cell immune activation and immune response. An elevated TNF-α level compromises B-cell function in vitro both in aged and young mice [40]. Long-term exposure of T cells to TNF-α impairs T-cell proliferation and cytokine production [41, 42], and T cells are rescued by blocking production of this cytokine [43]. Here, we noted that baseline plasma TNF-α levels correlated with activated CD4+ T cells and were negatively associated with expansion of IL-21R+ resting memory B cells, pTfh-cell expansion, and IL-21 production. An increased TNF-α level in nonresponders, compared with responders, in the older infected and older uninfected groups points to the role of inflammation in blunting the vaccine response during aging. IL-6 is known to contribute to inflammation and disease conditions in elderly individuals [44, 45], and here the predominant finding was that of increased IL-6 levels in older infected nonresponders.
We have tried to compensate for the small sample size by including women only, to prevent confounding influence of hormonal differences, with equivalent numbers of young and older participants and of HIV-infected and HIV-uninfected participants and an even distribution of classifiable responders and nonresponders. Despite study limitations, we were able to observe clear differences between the older and young participants within HIV-infected and HIV-uninfected participants, implying that aging and HIV infection together are severely detrimental to the immune system, exceeding the effects of each by itself. Our data point to a similarity between the young HIV-infected and older HIV-uninfected participants in cellular immunologic measures of vaccine-induced immune responses, reinforcing the premise that HIV infection accelerates aging of the immune system [25]. Further, defects attributable to impaired pTfh cells and persistent aberrant immune activation point to these entities as strategic targets for improving influenza vaccine responses in virologically suppressed aging HIV-infected populations. The recent recommendations to use high-dose influenza vaccine in elderly individuals [46, 47] may also be beneficial for the HIV-infected population and warrants investigation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the Miami Center for AIDS Research, University of Miami Miller School of Medicine (Miami, Florida), which is funded by a grant (P30AI073961) from the National Institutes of Health (NIH); Novartis Vaccines and Diagnostics (Siena, Italy), for the H1N1 (A/California/07/2009) vaccine antigen; Daniela Frasca, for providing peripheral blood mononuclear cells from older subjects; Margaret Roach and Maria Celeste Sanchez, for providing technical support; and the volunteers who participated in this study
Financial support. This study was supported by the NIH (grant 1R01AI108472 to S. Pahwa).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012; 60(suppl 1):S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Health Protection Agency UK. HIV in the United Kingdom: 2011 report. http://www.ivecic.com/uploads/7/5/4/1/7541913/hiv_in_the_uk_2011_report.pdf Accessed 14 August 2014.
- 3.Lambert ND, Ovsyannikova IG, Pankratz VS, Jacobson RM, Poland GA. Understanding the immune response to seasonal influenza vaccination in older adults: a systems biology approach. Expert Rev Vaccines 2012; 11:985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malaspina A, Moir S, Orsega SM, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis 2005; 191:1442–50. [DOI] [PubMed] [Google Scholar]
- 5.Parmigiani A, Alcaide ML, Freguja R, et al. Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation. PLoS One 2013; 8:e79816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Center for Disease Control and Prevention. People at high-risk of developing flu-like complications. http://www.cdc.gov/flu/about/disease/high_risk.htm Accessed 14 August 2014.
- 8.Sheth AN, Althoff KN, Brooks JT. Influenza susceptibility, severity, and shedding in HIV-infected adults: a review of the literature. Clin Infect Dis 2011; 52:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glezen WP, Simonsen L. Commentary: benefits of influenza vaccine in US elderly--new studies raise questions. Int J Epidemiol 2006; 35:352–3. [DOI] [PubMed] [Google Scholar]
- 10.Jenny NS. Inflammation in aging: cause, effect, or both? Discov Med 2012; 13:451–60. [PubMed] [Google Scholar]
- 11.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med 2009; 17:118–23. [PubMed] [Google Scholar]
- 12.Alcaide ML, Parmigiani A, Pallikkuth S, et al. Immune activation in HIV-infected aging women on antiretrovirals--implications for age-associated comorbidities: a cross-sectional pilot study. PLoS One 2013; 8:e63804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011; 29:621–63. [DOI] [PubMed] [Google Scholar]
- 14.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity 2009; 30:324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L. Molecular programming of B cell memory. Nat Rev Immunol 2011; 12:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oropallo MA, Cerutti A. Germinal center reaction: antigen affinity and presentation explain it all. Trends Immunol 2014; 35:287–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boswell KL, Paris R, Boritz E, et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog 2014; 10:e1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pallikkuth S, Parmigiani A, Silva SY, et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood 2012; 120:985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest 1968; 97:77–89. [PubMed] [Google Scholar]
- 20.Hsiung GD, Fong CKY, Landry ML. Hsiung's diagnostic virology: as illustrated by light and electron microscopy. 4th ed New Haven, CT: Yale University Press, 1994:404. [Google Scholar]
- 21.Pallikkuth S, Pilakka Kanthikeel S, Silva SY, Fischl M, Pahwa R, Pahwa S. Upregulation of IL-21 receptor on B cells and IL-21 secretion distinguishes novel 2009 H1N1 vaccine responders from nonresponders among HIV-infected persons on combination antiretroviral therapy. J Immunol 2011; 186:6173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods 2004; 286:111–22. [DOI] [PubMed] [Google Scholar]
- 23.Locci M, Havenar-Daughton C, Landais E, et al. Human circulating PD-(+)1CXCR3(-)CXCR5(+) memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 2013; 39:758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MMWR. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. http://www.cdc.gov/mmwr.htm Accessed 20 July 2014. [PubMed]
- 25.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol 2012; 24:501–6. [DOI] [PubMed] [Google Scholar]
- 26.Cubas RA, Mudd JC, Savoye AL, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 2013; 19:494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallikkuth S, Parmigiani A, Pahwa S. Role of IL-21 and IL-21 receptor on B cells in HIV infection. Crit Rev Immunol 2012; 32:173–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berglund LJ, Avery DT, Ma CS, et al. IL-21 signalling via STAT3 primes human naive B cells to respond to IL-2 to enhance their differentiation into plasmablasts. Blood 2013; 122:3940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spensieri F, Borgogni E, Zedda L, et al. Human circulating influenza-CD4+ ICOS1+IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proc Natl Acad Sci U S A 2013; 110:14330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi YS, Kageyama R, Eto D, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 2011; 34:932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauquet AT, Jin H, Paterson AM, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol 2009; 10:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt N, Morita R, Bourdery L, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity 2009; 31:158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perkey E, Miller RA, Garcia GG. Ex vivo enzymatic treatment of aged CD4 T cells restores cognate T cell helper function and enhances antibody production in mice. J Immunol 2012; 189:5582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu M, Li G, Lee WW, et al. Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proc Natl Acad Sci U S A 2012; 109:E879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefebvre JS, Haynes L. Vaccine strategies to enhance immune responses in the aged. Curr Opin Immunol 2013; 25:523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Brahmakshatriya V, Swain SL. CD4 T cell defects in the aged: causes, consequences and strategies to circumvent. Exp Gerontol 2014; 54:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herati RS, Reuter MA, Dolfi DV, et al. Circulating CXCR5+PD-1+ response predicts influenza vaccine antibody responses in young adults but not elderly adults. J Immunol 2014; 193:3528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol 2014; 35:436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frasca D, Romero M, Diaz A, et al. A molecular mechanism for TNF-alpha-mediated downregulation of B cell responses. J Immunol 2012; 188:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cope AP, Liblau RS, Yang XD, et al. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med 1997; 185:1573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isomaki P, Panesar M, Annenkov A, et al. Prolonged exposure of T cells to TNF down-regulates TCR zeta and expression of the TCR/CD3 complex at the cell surface. J Immunol 2001; 166:5495–507. [DOI] [PubMed] [Google Scholar]
- 43.Bose F, Raeli L, Garutti C, et al. Dual role of anti-TNF therapy: enhancement of TCR-mediated T cell activation in peripheral blood and inhibition of inflammation in target tissues. Clin Immunol 2011; 139:164–76. [DOI] [PubMed] [Google Scholar]
- 44.Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med 2008; 14:109–19. [DOI] [PubMed] [Google Scholar]
- 45.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci 2006; 61:575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Center for Disease Control and Prevention. Fluzone high-dose seasonal influenza vaccine. http://www.cdc.gov/flu/protect/vaccine/qa_fluzone.htm Accessed 20 November 2014.
- 47.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.