Abstract
Background. In treating malaria in Uganda, artemether-lumefantrine (AL) has been associated with a lower risk of recurrent parasitemia, compared with artesunate-amodiaquine (AS/AQ), but changing treatment practices may have altered parasite susceptibility.
Methods. We enrolled 602 children aged 6–59 months with uncomplicated falciparum malaria from 3 health centers in 2013–2014 and randomly assigned them to receive treatment with AS/AQ or AL. Primary outcomes were risks of recurrent parasitemia within 28 days, with or without adjustment to distinguish recrudescence from new infection. Drug safety and tolerability and Plasmodium falciparum resistance–mediating polymorphisms were assessed.
Results. Of enrolled patients, 594 (98.7%) completed the 28-day study. Risks of recurrent parasitemia were lower with AS/AQ at all 3 sites (overall, 28.6% vs 44.6%; P < .001). Recrudescences were uncommon, and all occurred after AL treatment (0% vs 2.5%; P = .006). Recovery of the hemoglobin level was greater with AS/AQ (1.73 vs 1.39 g/dL; P = .04). Both regimens were well tolerated; serious adverse events were uncommon (1.7% in the AS/AQ group and 1.0% in the AL group). AS/AQ selected for mutant pfcrt/pfmdr1 polymorphisms and AL for wild-type pfcrt/pfmdr1 polymorphisms associated with altered drug susceptibility.
Conclusions. AS/AQ treatment was followed by fewer recurrences than AL treatment, contrasting with older data. Each regimen selected for polymorphisms associated with decreased treatment response. Research should consider multiple or rotating regimens to maintain treatment efficacies.
Keywords: malaria, Uganda, artemether-lumefantrine, amodiaquine-artesunate, drug resistance
Malaria, particularly infection with Plasmodium falciparum, remains one of the most important infectious diseases in the world [1]. With older therapies limited by widespread drug resistance, standard therapy for uncomplicated falciparum malaria is now artemisinin-based combination therapy (ACT). In sub-Saharan Africa, nearly all malaria-endemic countries recommend artemether/lumefantrine (AL) or artesunate/amodiaquine (AS/AQ) to treat uncomplicated malaria [2]. In Uganda and the rest of East Africa, the most widely recommended first-line regimen is AL.
Multiple clinical trials have shown both AL and AS/AQ to offer excellent efficacy for the treatment of malaria in Africa [3]. Artemisinin resistance, manifested by delayed parasite clearance after treatment, is a growing concern in southeast Asia, but delayed parasite clearance and parasite markers of artemisinin resistance have not been clearly documented in Africa [4–7]. However, the efficacy of ACTs requires activity of both components of the combination regimen [8]. AS/AQ is limited by widespread P. falciparum resistance to chloroquine. Cross-resistance between chloroquine and amodiaquine is common, although AS/AQ is often effective against chloroquine-resistant infections, presumably because of the superior potency of amodiaquine and some differences in mediators of altered susceptibility to the 2 drugs [9]. In contrast, resistance to lumefantrine does not yet appear to be a problem. Consistent with this understanding, in trials in East Africa, AL consistently outperformed AS/AQ [10–14]. In other regions of Africa, AL and AS/AQ have generally shown equivalent efficacy [3, 15–18].
In Uganda, standard treatment for uncomplicated malaria changed from chloroquine to chloroquine plus sulfadoxine/pyrimethamine in 2000 and then to AL in 2004, with implementation taking several years in each case. The proportion of children aged <5 years with a fever treated with ACTs improved between 2009 and 2014, with values of 14% in 2009, 44% in 2011, and 67% in 2014 [19, 20]. Although presumptive treatment of all fevers as malaria is no longer policy, these statistics indicate increasing access to ACTs for the treatment of malaria. While this improved access is welcome, of concern is potential selection of parasites with decreased drug susceptibility. Resistance to amodiaquine is mediated principally by mutations in 2 putative drug transporter genes, encoded by pfcrt and pfmdr1 [9], and these mutations are selected in infections that emerge soon after treatment with AS/AQ [21, 22]. Interestingly, lumefantrine exerts the opposite selective pressure, with emergent infections after treatment with AL showing selection of wild-type sequences at the pfcrt K76T and pfmdr1 N86Y and D1246Y alleles [23–26]. Recently, coincident with changing malaria treatment practices, P. falciparum isolates in Uganda have shown decreasing lumefantrine susceptibility [27] and increasing prevalence of wild-type pfcrt K76 and pfmdr1 N86 and D1246 genotypes [26, 28]. With this background, it was of interest to compare the contemporary antimalarial efficacies of AS/AQ and AL and to assess the impacts of the 2 therapies on the selection of resistance-mediating genetic polymorphisms in the parasite.
METHODS
Study Design, Sites, and Participants
The study was a randomized trial conducted at 3 health centers in the Northern (Aduku Health Center, Apac District), Central (Mubende Health Center, Mubende District), and Western (Kihihi Health Center, Kanungu District) regions of Uganda from May 2013 to June 2014. These districts all experience perennial malaria transmission, with transmission intensity previously very high in Apac, but decreased due to recent intensive indoor residual spraying of insecticide, and moderate in Mubende and Kanungu [29]. Consecutive patients presenting to the health centers with symptoms suggestive of malaria and a positive thick blood smear were referred to study physicians for assessment. Patients were enrolled if they fulfilled the following selection criteria: (1) no prior enrollment in the study, (2) age 6–59 months, (2) weight ≥5 kg, (4) fever (≥37.5°C axillary) or history of fever in the previous 24 hours, (5) no history of serious side effects to study medications, (6) no evidence of a concomitant febrile illness, (7) provision of informed consent by a parent or guardian and agreement to follow-up for 28 days, (8) no evidence of severe malaria or danger signs, (9) absence of repeated vomiting of study medications on day 0, (10) no history of hypersensitivity or contraindications to study drugs, and (11) P. falciparum monoinfection with a parasite density of 2000–200 000 parasites/µL of blood. Because laboratory results were generally not available until the following day, patients could be excluded after randomization if laboratory results were outside selection criteria.
Ethical Considerations
The study protocol was approved by the Makerere University Research and Ethics Committee, the Uganda National Council of Science and Technology, the World Health Organization (WHO) Research Ethics Committee, and the University of California, San Francisco Committee for Human Research. The study is registered at the Australian New Zealand Clinical Trials Registry (ACTRN12613000408785).
Randomization
A randomization list was computer generated in blocks of 4 for each of the 3 study sites by an off-site investigator. Sequentially numbered, sealed, opaque envelopes containing treatment assignments were prepared from the list and secured in a locked cabinet accessible only to the study nurse. The nurse administered the assigned treatment after opening an envelope to learn the treatment number. Only the study nurse, who was not involved in assessing treatment outcomes, was aware of treatment assignments; all other study personnel were blinded. Patients were not informed of their treatment regimen.
Interventions
A nurse administered study medications orally with water according to weight-based guidelines as follows: AL (Coartem, Novartis, Basel, Switzerland; 20 mg artemether/120 mg lumefantrine), 1 (patient weight, 5–14 kg), 2 (15–24 kg), or 3 (25–34 kg) tablets twice daily for 3 days; AS/AQ (ASAQ Winthrop, Sanofi, Paris, France), 1 tablet of 25 mg artesunate/67.5 mg amodiaquine (5–9 kg), 50 mg/135 mg (9–18 kg), or 100 mg/270 mg (18–36 kg) once daily for 3 days. The AS/AQ group received lactose placebo tablets to simulate the AL evening dose, but placebo tablets did not match the color or taste of study drugs. All treatment was directly observed by the study nurse; participants waited at the clinic or returned for the evening dose (transportation was provided). Children were observed for 30 minutes after each dose, and if vomiting occurred the dose was readministered. Children were provided with paracetamol for treatment of febrile symptoms. Those with a hemoglobin level of <10 g/dL were treated according to Integrated Management of Childhood Illness guidelines with ferrous sulfate and antihelmintics. Patients were asked to return on days 1, 2, 3, 7, 14, 21, and 28, as well as any other day that they felt ill, for a standardized history and physical examination and blood sampling by finger prick for thick blood smears and storage on filter paper. On the first 3 days, a second blood sample was obtained by finger prick for blood smear and storage on filter paper, at the time of the evening medication dose. Hemoglobin level was measured on day 0, day 28, and at the time of recurrent malaria. Patients who did not return for follow-up were visited at home. Treatment failures were treated with dihydroartemisinin-piperaquine. Patients with evidence of severe malaria (including those with a hemoglobin level of < 5 g/dL) or danger signs (convulsions, lethargy, inability to drink or breast feed, repeated vomiting, inability to stand/sit due to weakness) were referred for treatment with parenteral artesunate or quinine. Patients were excluded during follow-up for use of antimalarial drugs outside of the study, serious adverse events requiring a change in treatment, withdrawal of informed consent, or loss to follow-up (not located within 24 hours days 1–3 or 48 hours days 4–28).
Laboratory Procedures
Thick blood smears for screening were stained with 10% Giemsa for 10 minutes, and follow-up thick and thin smears were stained with 2% Giemsa for 30 minutes. Expert microscopists determined parasite densities as previously reported [13] and examined thick blood smears for gametocytes and thin blood smears for nonfalciparum infections. A second microscopist, blinded to initial readings, reread all slides, and a third resolved discrepant readings. Hemoglobin level was measured with a portable spectrophotometer (HemoCue, Ängelholm, Sweden). Parasite DNA was extracted from filter paper, using Chelex (Biorad, Hercules, California). For recurrent infections, molecular genotyping was used to distinguish recrudescent from new infections, as previously described [30]. In brief, paired samples were genotyped with capillary electrophoresis in a stepwise fashion, using msp-2, msp-1, and 4 microsatellites. Recrudescence was defined as the presence of at least 1 matched allele at every locus; if at least 1 locus showed only unmatched alleles, the outcome was classified as a new infection. P. falciparum genetic polymorphisms were characterized using a ligase chain reaction fluorescent microsphere assay, as previously described [26, 31].
Outcomes
Primary outcomes were parasitemia, assessed by microscopy, within 28 days of treatment with study drug, either unadjusted or adjusted to distinguish recrudescence from new infection. Secondary outcomes were clinical outcomes based on WHO guidelines [32], resolution of fever, parasite clearance time (defined as the duration from treatment initiation to the first of 2 consecutive parasite-negative blood smears), change in hemoglobin concentration, presence of gametocytes during follow-up, the occurrence of adverse events, and prevalence of P. falciparum pfcrt and pfmdr1 polymorphisms associated with altered drug susceptibility. At each follow-up visit, study clinicians assessed patients for adverse events and graded them according to scales from the WHO and National Institutes of Health. Adverse events were defined on the basis of International Conference on Harmonization guidelines as untoward medical occurrences, and serious adverse events were defined as experiences resulting in death, life-threatening experience, inpatient hospitalization, persistent or significant incapacity, or medical or surgical intervention to prevent serious outcomes.
Sample Size
We hypothesized, based on prior data, that the risk of recurrent parasitemia within 28 days would be greater after AS/AQ. With an estimated risk of recurrent parasitemia after AS/AQ of 53% at high and 33% at medium transmission sites, we needed to enroll 100 patients per arm (assuming 90% follow-up) to test the hypothesis that risks of recurrent parasitemia after AL were ≤32% at the high-transmission site and ≤15% at the medium-transmission sites (80% power; 2-sided α of 0.05).
Statistical Methods
Data were entered and verified using Epi Info, version 6.04 (Centers for Disease Control and Prevention) and analyzed using Stata, version 8.0 (Stata). Efficacy and safety data were evaluated using a modified intention-to-treat analysis that included all patients who fulfilled enrollment criteria and considered all data up to the time of exclusion. Patients who were randomized to therapy but not enrolled due to laboratory results outside the range of selection criteria were not included in the analysis. Cumulative risks of recurrent parasitemia (unadjusted and adjusted by genotyping) were estimated using the Kaplan–Meier product limit formula. For outcomes adjusted by genotyping, data were censored for loss of follow-up and new infections. Comparisons of cumulative risks of recurrent parasitemia were made using the nonparametric log-rank test. Categorical variables were compared using χ2 analysis or the Fisher exact test, and continuous variables were compared using the independent-samples t test. P values were 2 sided without adjustment for multiple testing and were considered statistically significant if P < .05.
RESULTS
Trial Subjects
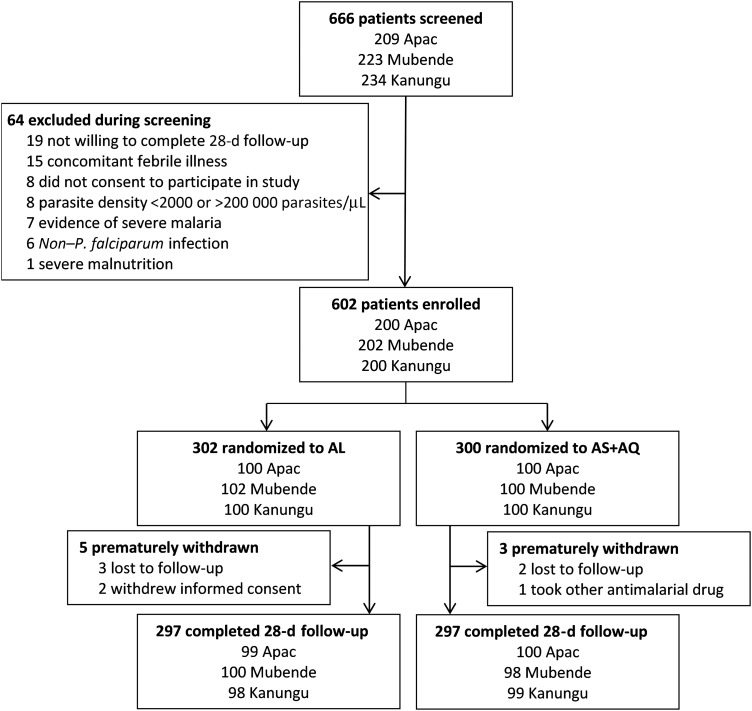
Of 666 patients screened, 64 were excluded during screening, and 602 were enrolled and randomized to therapy with AL or AS/AQ (Figure 1). Eight patients were withdrawn prior to completion; 98.7% completed the trial and were assigned outcomes. No patients experienced repeated vomiting of study medications. Baseline characteristics were the same for children assigned to the 2 study arms (Table 1).
Figure 1.
Trial profile. Abbreviations: AL, artemether-lumefantrine; AS/AQ, artesunate-amodiaquine.
Table 1.
Baseline Characteristics of Study Patients, According to Treatment Arm
| Characteristic, Location | Treatment Arm |
|
|---|---|---|
| AL | AS + AQ | |
| Female sex, % | ||
| Apac | 46.0 | 57.0 |
| Mubende | 46.1 | 54.0 |
| Kanungu | 48.0 | 47.0 |
| Age, y, median (IQR) | ||
| Apac | 2.8 (1.8–4.0) | 3.0 (2.0–4.0) |
| Mubende | 2.5 (1.4–3.7) | 2.4 (1.5–3.3) |
| Kanungu | 2.9 (1.6–3.5) | 3.0 (1.7–3.6) |
| Temperature, °C, mean ± SD | ||
| Apac | 37.5 (1.2) | 37.6 (1.2) |
| Mubende | 37.9 (1.4) | 37.5 (1.3) |
| Kanungu | 37.7 (1.3) | 37.7 (1.4) |
| Parasite density, parasites/µL, geometric mean (range) | ||
| Apac | 21 616 (2200–333 280) | 22 614 (2000–156 420) |
| Mubende | 35 153 (2710–193 780) | 30 864 (2260–165 680) |
| Kanungu | 14 264 (2020–189 500) | 12 827 (2020–125 000) |
| Gametocytes present, % | ||
| Apac | 18.0 | 14.0 |
| Mubende | 2.9 | 11.0 |
| Kanungu | 7.0 | 5.0 |
| Hemoglobin level, g/dL, mean ± SD | ||
| Apac | 10.2 (1.7) | 10.1 (1.6) |
| Mubende | 10.0 (1.5) | 10.1 (1.7) |
| Kanungu | 9.9 (1.6) | 9.8 (1.5) |
Abbreviations: AL, artemether-lumefantrine; AS/AQ, artesunate-amodiaquine; IQR, interquartile range; SD, standard deviation.
Comparative Efficacies of Trial Regimens
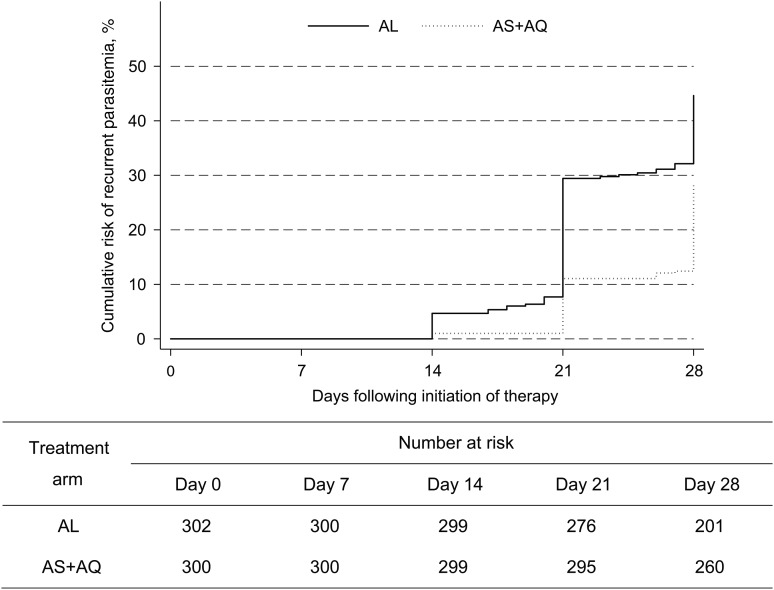
At all 3 sites, 28-day outcomes demonstrated lower risks of recurrent parasitemia and recrudescence after treatment with AS/AQ, compared with AL (Table 2 and Figure 2). Considering standard WHO clinical outcomes, there were no early treatment failures, and risks of late clinical failure and late parasitological failure were lower in the AS/AQ arm at all 3 sites (Table 2). Combining results from the 3 sites, the risk of recurrent parasitemia was significantly lower in children treated with AS/AQ as compared to AL (28.6% vs 44.6%; P < .0001). Recrudescences (confirmed by genotyping) were uncommon, but all occurred in the AL treatment arm (0% for AS/AQ vs 2.5% for AL; P = .006). Secondary outcomes, including parasite clearance after treatment and proportion of subjects with circulating gametocytes or fever after therapy, did not differ between treatment groups (Table 2). The mean increase in hemoglobin level 28 days after therapy (or on day of clinical failure) was greater in the AS/AQ group than in the AL group (1.73 vs 1.39 g/dL; P = .04).
Table 2.
Primary and Secondary Efficacy Outcomes
| Outcome | All 3 Sites |
Apac |
Mubende |
Kanungu |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AL (n = 302) | AS + AQ (n = 300) | P Value | AL (n = 100) | AS + AQ (n = 100) | P Value | AL (n = 102) | AS + AQ (n = 100) | P Value | AL (n = 100) | AS + AQ (n = 100) | P Value | |
| Proportion with parasitemia | ||||||||||||
| Day 1 | 275 (91.1) | 268 (89.3) | .48 | 90 (90.0) | 91 (91.0) | .81 | 94 (92.2) | 86 (86.0) | .16 | 91 (91.0) | 91 (91.0) | 1.0 |
| Day 2 | 57 (18.9) | 46 (15.4) | .25 | 19 (19.0) | 17 (17.0) | .71 | 24 (23.5) | 20 (20.0) | .54 | 14 (14.1) | 9 (9.1) | .27 |
| Day 3 | 0 | 1 (0.3) | .32 | 0 | 1 (1.0) | .32 | 0 | 0 | 0 | 0 | ||
| Parasite clearance time, h, mean ± SD | 39.3 ± 12.1 | 38.1 ± 11.7 | .23 | 37.0 ± 12.5 | 37.6 ± 12.8 | .74 | 42.6 ± 12.3 | 39.4 ± 12.1 | .06 | 38.3 ± 10.8 | 37.5 ± 9.9 | .57 |
| Proportion with gametocytes | ||||||||||||
| Day 1 | 42 (13.9) | 38 (12.7) | .67 | 24 (24.0) | 16 (16.2) | .17 | 9 (8.8) | 14 (14.0) | .25 | 9 (9.0) | 8 (8.0) | .80 |
| Day 2 | 39 (13.0) | 38 (12.7) | .93 | 24 (24.0) | 18 (18.0) | .30 | 4 (3.9) | 9 (9.0) | .14 | 11 (11.1) | 11 (11.1) | 1.0 |
| Day 3 | 35 (11.7) | 32 (10.7) | .71 | 22 (22.0) | 15 (15.0) | .20 | 2 (2.0) | 8 (8.0) | .05 | 11 (11.1) | 9 (9.1) | .64 |
| Days 4–28 | 26 (8.7) | 28 (9.4) | .77 | 19 (19.0) | 15 (15.0) | .45 | 2 (2.0) | 6 (6.0) | .15 | 5 (5.1) | 7 (7.1) | .55 |
| Proportion with fevera | ||||||||||||
| Day 1 | 176 (58.5) | 168 (56.2) | .57 | 62 (62.0) | 66 (66.0) | .56 | 54 (53.5) | 44 (44.0) | .21 | 60 (60.0) | 58 (58.0) | .77 |
| Day 2 | 62 (20.7) | 74 (24.8) | .23 | 35 (35.0) | 37 (37.0) | .77 | 6 (5.9) | 6 (6.1) | .97 | 21 (21.4) | 31 (31.3) | .12 |
| Day 3 | 34 (11.4) | 30 (10.1) | .61 | 11 (11.0) | 10 (10.0) | .82 | 5 (5.0) | 3 (3.0) | .48 | 18 (18.4) | 17 (17.2) | .83 |
| Hemoglobin recovery, g/dL, mean ± SDb | 1.39 ± 1.86 | 1.73 ± 1.73 | .04 | 1.74 ± 1.85 | 1.99 ± 1.69 | .35 | 1.33 ± 1.62 | 1.65 ± 2.01 | .30 | 1.09 ± 2.00 | 1.52 ± 1.46 | .10 |
| WHO treatment outcome | ||||||||||||
| No outcome | 5 (1.7) | 3 (1.0) | 1 (1.0) | 0 | 2 (2.0) | 2 (2.0) | 2 (2.0) | 1 (1.0) | ||||
| Early treatment failure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Late clinical failure | 64 (21.1) | 31 (10.3) | 13 (13.0) | 3 (3.0) | 27 (26.5) | 11 (11.0) | 24 (24.0) | 17 (17.0) | ||||
| Late parasitological failure | 69 (22.9) | 54 (18.0) | 18 (18.0) | 12 (12.0) | 27 (26.5) | 22 (22.0) | 24 (24.0) | 20 (20.0) | ||||
| Adequate response | 164 (54.3) | 212 (70.7) | 68 (68.0) | 85 (85.0) | 46 (45.1) | 65 (65.0) | 50 (50.0) | 62 (62.0) | ||||
| Cumulative risk of recurrent parasitemia, % | 44.6 | 28.6 | <.0001 | 31.2 | 15.0 | .004 | 53.7 | 33.6 | .0008 | 49.0 | 37.4 | .03 |
| Cumulative risk of recrudescence, % | 2.5 | 0 | .006 | 2.1 | 0 | .15 | 4.3 | 0 | .04 | 1.1 | 0 | .30 |
Data are no. (%) of patients, unless otherwise indicated.
Abbreviations: AL, artemether-lumefantrine; AS/AQ, artesunate-amodiaquine; SD, standard deviation; WHO, World Health Organization.
a Temperature of ≥37.5°C or history of fever during the previous 24 hours.
b Change in hemoglobin level from day 0 to day 28 or day of clinical failure.
Figure 2.
Cumulative risk of treatment failure in the 2 treatment arms. Risks of treatment failure, due either to recrudescence or new infection after therapy, are shown for the artesunate/amodiaquine (AS/AQ) and artemether/lumefantrine (AL) treatment groups.
Adverse Events
Adverse events were common, with the most frequent events being cough and coryza, and no differences observed between the study arms (Table 3). Serious adverse events were uncommon (7 subjects had bronchopneumonia, and 1 had a traumatic corneal tear) and did not differ between the study arms (1.7% for AS/AQ vs 1.0% for AL; P = .47); none of these events were judged as related to study medications.
Table 3.
Safety and Tolerability Outcomes
| Adverse Event | All 3 Sites |
Apac |
Mubende |
Kanungu |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AL | AS + AQ | P Value | AL | AS + AQ | P Value | AL | AS + AQ | P Value | AL | AS + AQ | P Value | |
| Overall | 180 (59.6) | 180 (60.0) | .92 | 75 (75.0) | 76 (76.0) | .87 | 39 (38.2) | 40 (40.0) | .80 | 66 (66.0) | 64 (64.0) | .77 |
| Specific events of any severitya | ||||||||||||
| Cough | 80 (26.5) | 90 (30.0) | .34 | 40 (40.0) | 42 (42.0) | .77 | 22 (21.6) | 21 (21.0) | .92 | 18 (18.0) | 27 (27.0) | .13 |
| Coryzab | 53 (26.5) | 63 (31.5) | .27 | 35 (35.0) | 44 (44.0) | .19 | 18 (18.0) | 19 (19.0) | .87 | |||
| Abdominal pain | 19 (13.3) | 17 (11.6) | .66 | 4 (8.0) | 5 (8.8) | .89 | 3 (7.0) | 4 (10.5) | .57 | 12 (24.0) | 8 (15.4) | .27 |
| Diarrhea | 36 (11.9) | 34 (11.3) | .82 | 14 (14.0) | 13 (13.0) | .84 | 8 (7.8) | 7 (7.0) | .82 | 14 (14.0) | 14 (14.0) | 1.0 |
| Anorexia | 30 (9.9) | 36 (12.0) | .42 | 11 (11.0) | 17 (17.0) | .22 | 3 (2.9) | 3 (3.0) | .98 | 16 (16.0) | 16 (16.0) | 1.0 |
| Vomiting | 22 (7.3) | 26 (8.7) | .53 | 3 (3.0) | 8 (8.0) | .12 | 10 (9.8) | 7 (7.0) | .47 | 9 (9.0) | 11 (11.0) | .64 |
| Pallor | 14 (4.6) | 19 (6.3) | .36 | 6 (6.0) | 5 (5.0) | .76 | 0 | 0 | 1.0 | 8 (8.0) | 14 (14.0) | .18 |
| Headache | 11 (7.8) | 4 (2.8) | .07 | 6 (12.5) | 1 (1.9) | .04 | 2 (4.7) | 0 | .18 | 3 (5.9) | 3 (5.9) | 1.0 |
| Any | 3 (1.0) | 5 (1.7) | .47 | 1 (1.0) | 2 (2.0) | .56 | 0 | 0 | 1.0 | 2 (2.0) | 3 (3.0) | .65 |
Data are no. (%) of patients.
Abbreviations: AL, artemether-lumefantrine; AS/AQ, artesunate-amodiaquine.
a Unable to assess in all study participants.
b Not assessed in Mubende.
Selection of P. falciparum Genetic Polymorphisms
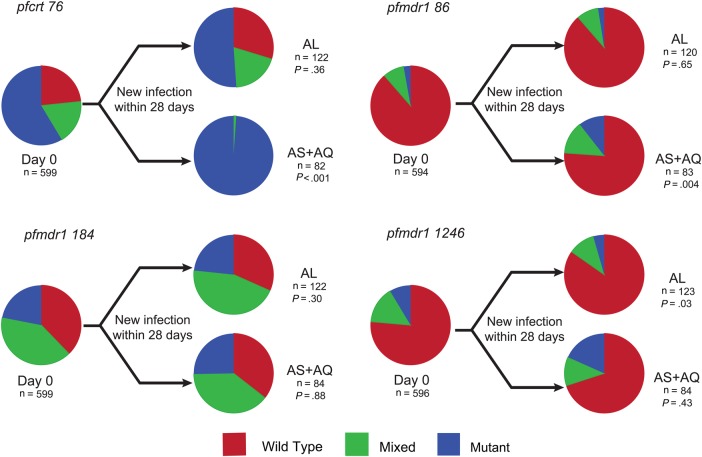
We characterized P. falciparum genetic polymorphisms that have been associated with drug susceptibilities in isolates collected at enrollment and upon new infections after therapy. Baseline prevalences were similar to those recently reported from Tororo, in eastern Uganda [27], with a high prevalence of the pfcrt 76T mutation (58.6% pure mutant), albeit lower than in older surveys [22, 33], and a low prevalence of pfmdr1 86Y and 1246Y mutations (<10% pure mutant). Considering new isolates that emerged after therapy, treatment with AS/AQ selected for mutant genotypes at pfcrt 76T and pfmdr1 86Y and 1246Y, and AL selected for wild type at these alleles (Figure 3). Overall, in new isolates the prevalence of pure wild type at each of these alleles was significantly greater in the AL group, compared with the AS/AQ treatment group (pfmdr1 N86, 90% vs 77% [P = .017]; pfmdr1 D1246, 85% vs 73% [P = .032]; and pfcrt K76, 27% vs 0% [P < .001]). Most notably, the prevalence of a wild-type or mixed genotype at pfcrt 76 was 41.4% at baseline, compared with 1.2% in new infections following AS/AQ therapy (P < .001).
Figure 3.
Impact of treatment arm on Plasmodium falciparum genotypes. Parasite genotypes were characterized at the time of presentation with malaria (day 0) and for new infections that emerged within 28 days of treatment with artemether/lumefantrine (AL) or artesunate/amodiaquine (AS/AQ). n values represent the number of samples analyzed for each category. Wild-type, mixed, and mutant genotypes are indicated based on the 3D7 reference strain for the alleles pfcrt K76T and pfmdr1 N86Y, Y184F, and D1246Y. P values are based on comparison of values for wild-type and mixed versus mutant genotypes, using the Fisher exact test.
DISCUSSION
We conducted a randomized trial comparing the antimalarial efficacies of the 2 ACTs that are widely used in Africa. Prior studies from East Africa showed superior efficacy of AL over AS/AQ, consistent with known resistance to amodiaquine but not lumefantrine in this area [10–14]. Our new results demonstrate an important change, with fewer episodes of recurrent parasitemia after treatment with AS/AQ, compared with AL, at 3 different sites in Uganda. This change is consistent with recent changes in the genetics of P. falciparum in Uganda [26–28, 33], with an increased prevalence of polymorphisms predicted to improve responsiveness to amodiaquine and decrease responsiveness to lumefantrine. However, treatment with AS/AQ selected strongly in subsequent infections for mutations expected to decrease the response to amodiaquine. Thus, AL, the current national treatment regimen in Uganda, may not offer the best available therapy, but the improved performance of AS/AQ may not be durable, suggesting that novel treatment strategies may be warranted.
ACTs have offered a profound improvement in the treatment and control of malaria [8]. Nearly all countries in sub-Saharan Africa recommend AL or AS/AQ (or list both) for the treatment of uncomplicated malaria [2]. In Uganda, the recommended regimen is AL, and trials demonstrated a lower risk of recurrent parasitemia after treatment with AL, compared with AS/AQ, in Tororo in 2004–2005 (risk of recurrent infection, 51% for AL vs 66% for AS/AQ) [12] and Kampala in 2004–2006 (7% vs 17%) [13]. Elsewhere in East Africa, risks of recurrent parasitemia following treatment with AL and AS/AQ were 1% and 11%, respectively, in Muheza, Tanzania, in 2002–2004 [10]; 23% and 44%, respectively, in Zanzibar in 2002–2003 [11]; and 13% and 37%, respectively, in southern Tanzania in 2004 [14]. In all of these studies, risks of recrudescence following treatment were low. Most studies elsewhere in Africa showed similar performance for AL and AS/AQ [3, 15–18, 34], although some in West Africa showed superiority of AS/AQ over AL [3, 35]. These results are consistent with the longer half-life of amodiaquine as compared to lumefantrine and the lower levels of amodiaquine resistance in West Africa as compared to other regions. Importantly, our new results demonstrate a major change, with decreased recurrences after AS/AQ, compared with AL, in Uganda.
Polymorphisms in 2 P. falciparum genes, pfcrt and pfmdr1, are associated with altered susceptibility to many drugs, and these are selected by drug pressure [9]. Importantly, AS/AQ and AL select in opposite directions: for the key alleles at which mutants are selected by AS/AQ, wild-type parasites are selected by AL. Of interest in our new findings was the extent of selection in the context of current polymorphism prevalences. Parasites at the 3 study sites were similar to those recently characterized in Tororo [26, 28], with increasing prevalence of wild-type pfcrt K76 and pfmdr1 N86 and D1246 sequences as compared to historical data [28, 33], consistent with the selective pressure of decreasing use of chloroquine and increasing use of AL. Interestingly, the artemether component of AL may contribute to selection of wild-type pfmdr1 alleles, as older studies showed decreased susceptibility to artemisinin in parasites with wild-type rather than mutant alleles at positions 1034, 1042, and 1246; the key pfmdr-1 86 locus has not been studied in this regard [36, 37]. In our study, treatment with AL exerted modest additional selection, as the pfmdr1 alleles most affected by this regimen were already mostly wild type, and AS/AQ selected strongly for the mutant pfcrt 76T and pfmdr1 86Y alleles. In Apac, compared to a baseline prevalence of wild-type and mixed pfcrt 76 genotypes of 73%, the prevalence in new infections following AS/AQ therapy was 0%.
This study had limitations. First, although it was a randomized trial, the treatment regimens differed in taste and appearance, and placebos used to match second-daily doses of AL in the AS/AQ group differed in appearance from study drugs. Second, the study included only 28 days of follow-up; risks of recurrence or properties of parasites beyond this time frame may have differed between treatment arms, but this information was not captured. Third, genotyping to distinguish new and recrudescent parasites after therapy is inexact, and so some misclassification of outcomes may have occurred. Fourth, studied parasite polymorphisms were limited to 4 well-characterized alleles. These are arguably most relevant, as they influence antimalarial activities of both amodiaquine and lumefantrine, but a more in-depth characterization of the genetics of parasites under ACT pressure would be of interest. Of note, key polymorphisms in the K13 gene [38] and other candidate loci that may influence susceptibility to ACT components [39, 40] have been described recently.
Although both AL and AS/AQ performed well for the treatment of uncomplicated malaria, with few recrudescences after treatment, changes in the relative performance of the 2 regimens are important, especially considering the very high incidence of malaria in Uganda. Remarkably, nearly half of children treated with AL experienced recurrent malaria within 1 month of therapy. This risk was 16% lower in children treated with AS/AQ, suggesting important benefits of this regimen. However, our data and prior results [21, 22] suggest that widespread use of AS/AQ will rapidly select for parasites with decreased susceptibility. Indeed, an optimal strategy may be to take advantage of the opposite selective pressures of amodiaquine and lumefantrine. Thus, as has been previously suggested [41], research is recommended to explore the value of either wide use of both regimens or rotation of first-line treatments to maintain the efficacies of both regimens. Further, with the very high risk of recurrent malaria after therapy at all study sites, we should consider chemoprevention in high-risk groups, such as with a different ACT, dihydroartemisinin-piperaquine, which recently showed excellent efficacy for the treatment [42, 43] and prevention [44, 45] of malaria in Ugandan children. Finally, our data demonstrate the dynamic nature of antimalarial drug susceptibility in Uganda; continued surveillance of the efficacies of leading treatment regimens and consideration of these data in adjusting treatment policies will be important.
Notes
Acknowledgments. We thank the clinical study team of Stephen Okello, Afizi Kibuuka, Oliver Akello, Christopher Eruaga, Osbert Katuuro, Grace Musimenta, John Bosco Obua, Martha Adongo, Josephine Zawedde, Sally Salome Opus, and Ronald Bampinga; Moses Kiggundu, for training the laboratory staff and providing laboratory quality control; the health workers and administrators at each health center, for allowing us to conduct these studies and working alongside study teams; Michelle Verghese and Christine Sheridan, for assistance with laboratory studies; Josephine Nambooze and Marian Warsame at the World Health Organization (WHO), for support in designing the study; and the children who participated in this study, as well as their parents and guardians. This paper is dedicated to Dr. Peter Okui, who sadly passed away after this work was accepted for publication.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC), the Department of Health and Human Services, or the US government.
Financial support. This work was supported by the US President's Malaria Initiative and the US Agency for International Development, under an interagency agreement with the CDC; and the Bill and Melinda Gates Foundation, through the WHO.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet 2014; 383:723–35. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. World malaria report 2014. Geneva: World Health Organization, 2104. [Google Scholar]
- 3.Four Artemisinin-Based Combinations Study G. A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med 2011; 8:e1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley EA, Dhorda M, Fairhurst RM et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2014; 371:411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conrad MD, Bigira V, Kapisi J et al. Polymorphisms in K13 and falcipain-2 associated with artemisinin resistance are not prevalent in Plasmodium falciparum isolated from Ugandan children. PLoS One 2014; 9:e105690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor SM, Parobek CM, DeConti DK et al. Absence of putative artemisinin resistance mutations among Plasmodium falciparum in sub-Saharan Africa: a molecular epidemiologic study. J Infect Dis 2015; 211:680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper RA, Conrad MD, Watson QD et al. Lack of artemisinin resistance in Plasmodium falciparum in Uganda based on parasitological and molecular assays. Antimicrob Agents Chemother 2015; 59:5061–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg 2007; 77(6 suppl):181–92. [PubMed] [Google Scholar]
- 9.Rosenthal PJ. The interplay between drug resistance and fitness in malaria parasites. Mol Microbiol 2013; 89:1025–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutabingwa TK, Anthony D, Heller A et al. Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet 2005; 365:1474–80. [DOI] [PubMed] [Google Scholar]
- 11.Martensson A, Stromberg J, Sisowath C et al. Efficacy of artesunate plus amodiaquine versus that of artemether-lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clin Infect Dis 2005; 41:1079–86. [DOI] [PubMed] [Google Scholar]
- 12.Bukirwa H, Yeka A, Kamya MR et al. Artemisinin combination therapies for treatment of uncomplicated malaria in Uganda. PLoS Clin Trials 2006; 1:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorsey G, Staedke S, Clark TD et al. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA 2007; 297:2210–9. [DOI] [PubMed] [Google Scholar]
- 14.Kabanywanyi AM, Mwita A, Sumari D, Mandike R, Mugittu K, Abdulla S. Efficacy and safety of artemisinin-based antimalarial in the treatment of uncomplicated malaria in children in southern Tanzania. Malar J 2007; 6:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adjei GO, Kurtzhals JA, Rodrigues OP et al. Amodiaquine-artesunate vs artemether-lumefantrine for uncomplicated malaria in Ghanaian children: a randomized efficacy and safety trial with one year follow-up. Malar J 2008; 7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falade CO, Ogundele AO, Yusuf BO, Ademowo OG, Ladipo SM. High efficacy of two artemisinin-based combinations (artemether-lumefantrine and artesunate plus amodiaquine) for acute uncomplicated malaria in Ibadan, Nigeria. Trop Med Int Health 2008; 13:635–43. [DOI] [PubMed] [Google Scholar]
- 17.Faye B, Offianan AT, Ndiaye JL et al. Efficacy and tolerability of artesunate-amodiaquine (Camoquin plus) versus artemether-lumefantrine (Coartem) against uncomplicated Plasmodium falciparum malaria: multisite trial in Senegal and Ivory Coast. Trop Med Int Health 2010; 15:608–13. [DOI] [PubMed] [Google Scholar]
- 18.Michael OS, Gbotosho GO, Folarin OA et al. Early variations in plasmodium falciparum dynamics in Nigerian children after treatment with two artemisinin-based combinations: implications on delayed parasite clearance. Malar J 2010; 9:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.President's Malaria Initiative. Uganda Malaria Operational Plan FY 2014. 2014.
- 20.Uganda Bureau of Statistics. Uganda Malaria Indicator Survey 2014–15. 2015.
- 21.Humphreys GS, Merinopoulos I, Ahmed J et al. Amodiaquine and artemether-lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob Agents Chemother 2007; 51:991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nsobya SL, Dokomajilar C, Joloba M, Dorsey G, Rosenthal PJ. Resistance-mediating Plasmodium falciparum pfcrt and pfmdr1 alleles after treatment with artesunate-amodiaquine in Uganda. Antimicrob Agents Chemother 2007; 51:3023–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sisowath C, Stromberg J, Martensson A et al. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine (Coartem). J Infect Dis 2005; 191:1014–7. [DOI] [PubMed] [Google Scholar]
- 24.Zongo I, Dorsey G, Rouamba N et al. Artemether-lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: a randomised non-inferiority trial. Lancet 2007; 369:491–8. [DOI] [PubMed] [Google Scholar]
- 25.Baliraine FN, Rosenthal PJ. Prolonged selection of pfmdr1 polymorphisms after treatment of falciparum malaria with artemether-lumefantrine in Uganda. J Infect Dis 2011; 204:1120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conrad MD, LeClair N, Arinaitwe E et al. Comparative impacts over 5 years of artemisinin-based combination therapies on Plasmodium falciparum polymorphisms that modulate drug sensitivity in Ugandan children. J Infect Dis 2014; 210:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tumwebaze P, Conrad MD, Walakira A et al. Impact of antimalarial treatment and chemoprevention on the drug sensitivity of malaria parasites isolated from ugandan children. Antimicrob Agents Chemother 2015; 59:3018–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbogo GW, Nankoberanyi S, Tukwasibwe S et al. Temporal changes in prevalence of molecular markers mediating antimalarial drug resistance in a high malaria transmission setting in Uganda. Am J Trop Med Hyg 2014; 91:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilama M, Smith DL, Hutchinson R et al. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J 2014; 13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenhouse B, Myrick A, Dokomajilar C et al. Validation of microsatellite markers for use in genotyping polyclonal Plasmodium falciparum infections. Am J Trop Med Hyg 2006; 75:836–42. [PMC free article] [PubMed] [Google Scholar]
- 31.Leclair NP, Conrad MD, Baliraine FN, Nsanzabana C, Nsobya SL, Rosenthal PJ. Optimization of a ligase detection reaction fluorescent microsphere assay for the characterization of resistance-mediating polymorphisms in African samples of Plasmodium falciparum. J Clin Microbiol 2013; 51:2564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Methods for surveillance of antimalarial drug efficacy. Geneva: World Health Organization, 2009. [Google Scholar]
- 33.Francis D, Nsobya SL, Talisuna A et al. Geographic differences in antimalarial drug efficacy in Uganda are explained by differences in endemicity and not by known molecular markers of drug resistance. J Infect Dis 2006; 193:978–86. [DOI] [PubMed] [Google Scholar]
- 34.Ndiaye JL, Randrianarivelojosia M, Sagara I et al. Randomized, multicentre assessment of the efficacy and safety of ASAQ--a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria. Malar J 2009; 8:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owusu-Agyei S, Asante KP, Owusu R et al. An open label, randomised trial of artesunate+amodiaquine, artesunate+chlorproguanil-dapsone and artemether-lumefantrine for the treatment of uncomplicated malaria. PLoS One 2008; 3:e2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 2000; 403:906–9. [DOI] [PubMed] [Google Scholar]
- 37.Sidhu AB, Valderramos SG, Fidock DA. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol Microbiol 2005; 5:913–26. [DOI] [PubMed] [Google Scholar]
- 38.Ariey F, Witkowski B, Amaratunga C et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014; 505:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borrmann S, Straimer J, Mwai L et al. Genome-wide screen identifies new candidate genes associated with artemisinin susceptibility in Plasmodium falciparum in Kenya. Sci Rep 2013; 3:3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henriques G, Hallett RL, Beshir KB et al. Directional selection at the pfmdr1, pfcrt, pfubp1, and pfap2mu loci of Plasmodium falciparum in Kenyan children treated with ACT. J Infect Dis 2014; 210:2001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland CJ, Babiker H, Mackinnon MJ, Ranford-Cartwright L, El Sayed BB. Rational deployment of antimalarial drugs in Africa: should first-line combination drugs be reserved for paediatric malaria cases? Parasitology 2011; 138:1459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamya MR, Yeka A, Bukirwa H et al. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trial. PLoS Clin Trials 2007; 2:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeka A, Dorsey G, Kamya MR et al. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treating uncomplicated malaria: a randomized trial to guide policy in Uganda. PLoS One 2008; 3:e2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nankabirwa JI, Wandera B, Amuge P et al. Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in Ugandan schoolchildren: a randomized, placebo-controlled trial. Clin Infect Dis 2014; 58:1404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bigira V, Kapisi J, Clark TD et al. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med 2014; 11:e1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]