Abstract
Circulating T cells that specifically target normal self-proteins expressed by regulatory immune cells were first described in patients with cancer, but can also be detected in healthy individuals. The adaptive immune system is distinguished for its ability to differentiate between self-antigens and foreign antigens. Thus, it was remarkable to discover T cells that apparently lacked tolerance to important self-proteins, eg, IDO, PD-L1, and FoxP3, expressed in regulatory immune cells. The ability of self-reactive T cells to react to and eliminate regulatory immune cells can influence general immune reactions. This suggests that they may be involved in immune homeostasis. It is here proposed that these T cells should be termed antiregulatory T cells (anti-Tregs). The role of anti-Tregs in immune-regulatory networks may be diverse. For example, pro-inflammatory self-reactive T cells that react to regulatory immune cells may enhance local inflammation and inhibit local immune suppression. Further exploration is warranted to investigate their potential role under different malignant conditions and the therapeutic possibilities they possess. Utilizing anti-Tregs for anticancer immunotherapy implies the direct targeting of cancer cells in addition to regulatory immune cells. Anti-Tregs provide the immune system with yet another level of immune regulation and contradict the notion that immune cells involved in the adjustment of immune responses only act as suppressor cells.
The immune system is a complex network of cells and molecules that protect the organism by eliminating elements judged to be harmful, without reacting to normal cells. Many regulatory mechanisms control the termination of immune responses to ensure unresponsiveness or tolerance to self-antigens. However, the very immune regulation mechanisms that prevent autoimmunity may be harnessed by cancer cells to accomplish immune escape. This phenomenon was highlighted in the recently updated version of The Hallmarks of Cancer by Hanahan and Weinberg; now, “evasion of immune destruction” is listed as an emerging hallmark of cancer (1).
Cancer cells can directly suppress anticancer immune mechanisms. In addition, cancer cells attract and/or convert immune-competent cells to generate and uphold an immune-permissive microenvironment. For example, tumor cells can escape from immune surveillance by usurping local regulatory T cells (Tregs), dendritic cell subtypes, myeloid-derived suppressor cells, and M2 or tumor-associated macrophages. Under normal physiological conditions, these immune cells are involved in maintaining immune homeostasis (2), but in cancerous conditions, they become involved in the creation of an immunosuppressive microenvironment around tumors. Detailed knowledge of the factors responsible for protecting cancer cells from immune destruction is crucial for the development of novel, immune-based anticancer treatment modalities (3). Indeed, impressive clinical responses have been achieved by characterizing inhibitory T cell pathways and targeting them with monoclonal antibodies against specific membrane proteins (eg, CTLA-4, PD-1, or PD-L1) (4–6).
It has been described that TCRβ-specific T cells may be involved in recovery from antigen-induced autoimmune disease (7,8). Thus, recognition of disease-causing T cells by TCR-specific T cells may be a mechanism of controlling or limiting autoimmune reactions. We recently reported that the immune system apparently has established a mechanism to counteract the many different immune-suppressive feedback signals by creating auto-reactive, antigen-specific, pro-inflammatory T cells that target immune-suppressive cells. We characterized self-reactive T cells that specifically recognized human leukocyte antigen (HLA)–restricted epitopes derived from proteins expressed in regulatory immune cells, eg, indoleamine 2,3-dioxygenase (IDO), IDO2, tryptophan 2,3-dioxygenase (TDO), programmed death-ligand 1 (PD-L1), heme-oxygenase-1 (HO-1), forkhead box P3 (Foxp3), and FoxO3 (9–25). Because of the ability of these T cells to react against regulatory immune cells, it is here proposed that these cells should be termed antiregulatory T cells (anti-Tregs). The preservation of self-tolerance is secured in the thymus (26). Circulating CD34+ hematopoietic stem cells exist in the bone marrow and develop into T cell precursors, which seed the thymus. These progenitors express clonally well-defined αβ T cell receptors (TCRs), and their fates depend on whether their TCRs react against self-peptides presented by HLA molecules. Cells that do not express TCRs and cells that express TCRs that do not react with target complexes are neglected, and they die. Cells that express TCRs with low affinity towards the target peptide/HLA complex undergo positive selection and develop into “normal” CD4+ or CD8+ T cells. In contrast, cells that harbor TCRs with high affinity to a target/HLA complex undergo clonal deletion to maintain self-tolerance. However, recent studies have described several distinct subpopulations of self-reactive lymphocytes, which are not removed in the thymus (27). These cells have been assigned to immune regulation and immune homeostasis. These regulatory self-reactive lymphocytes include natural Tregs (nTregs) and natural T helper 17 (nTh17) cells. The close association between immune regulation and anti-Tregs could indicate that anti-Tregs likewise may avoid deletion in the thymus. In addition, a few other self-reactive, regular, gamma-delta CD8+ or CD4+ T cells have been detected in healthy individuals. A recent study showed that most (>80%) healthy individuals harbored T cells specific to the transcription factor OCT4, which is critical for pluripotency in different human stem cells. Hence, there seems to be a lack of tolerance to this normal self-protein. Furthermore, it was demonstrated that healthy individuals harbored immune responses against other self-proteins, including p53, cyclin B1, and carcinoembryonic antigen (28–30).
Self-Reactive Anti-Tregs
In recent years, we have identified spontaneous T-lymphocyte responses against regulatory immune cells and characterized their role in patients with malignant diseases. First, we examined the natural recognition of different metabolic enzymes. L-tryptophan is an essential amino acid required for the synthesis of proteins. The degradation of L- (and D-) tryptophan to N-formylkynurenine is catalyzed by the heme dioxygenases IDO, IDO2, and TDO. IDO expression is upregulated by inflammatory cytokines, like type I and II interferons (IFNs); therefore, IDO is thought to be an important counter-regulatory enzyme, which controls disproportionate immune responses (31). Although they act with distinct mechanisms, both TDO and IDO catalyze the first, rate-limiting step of tryptophan oxidation, which yields kynurenine (32). The impact of tryptophan metabolism on immune responses is well established. When T cells sense low levels of tryptophan via the serine/threonine-protein kinase GCN2, they undergo proliferative arrest (33).
IDO expression has been repeatedly described in cancer (31,34). Tumor cells transfected with IDO become resistant to immune eradication (35). Thus, IDO has been the focus of much attention. We recently described spontaneous T cell–mediated immune reactivity against IDO, IDO2, and TDO in patients with cancer (9–14). First, we identified HLA-restricted peptides within the IDO protein that stimulated spontaneous T cell reactivity in direct, ex vivo assays performed on samples from patients with unrelated tumor types, ie, renal cell carcinoma, melanoma, and breast cancer. These IDO-reactive CD8+ T cells were peptide-specific, cytotoxic effector cells. Thus, IDO-specific anti-Tregs effectively lysed IDO+ cancer cell lines of different origins, including ex vivo enriched leukemia cells. Likewise, IDO2- and TDO-specific anti-Tregs could recognize malignant cells of different origins. IDO-driven immune suppression is a common mechanism that has been described in diverse human cancers. However, even more distinctive was our finding that IDO-specific anti-Tregs recognized and killed IDO+ dendritic cells. This finding demonstrated that IDO-specific anti-Tregs could also react against nonmalignant immune cells.
The immune system also controls T cell overactivity by stimulating the programmed death 1 (PD-1) protein, which inhibits T cells by making them functionally silent against antigens. In particular, chronic antigen exposure that takes place in chronic infections and cancer can lead to high levels of persistent PD1 expression, which induces a state of exhaustion or anergy in cognate antigen-specific T cells. This state seems to be partially reversible by PD1-pathway blockade (36). The ligand for PD-1, PD-L1 (B7-H1), is expressed on antigen-presenting cells (APCs), placental cells, and nonhematopoietic cells. PD-L1 expression can be induced by IFNs, which are found in inflammatory microenvironments. Hence, PD-1 and its ligands play a central role in maintaining peripheral tolerance and preventing autoimmunity. In multiple cancers high PD-L1 expression has been described both on malignant cells as well as other cells in the tumor microenvironment (37,38). Thus, cancer cells exploit this system to create an immune suppressive microenvironment, which is protecting them from immune surveillance. PD-L1 expression was first described as an indicator of tumor aggressiveness in renal cell carcinoma (39). In addition, PD-L1 expression on tumor cells has been suggested as a prognostic factor in a number of solid cancers including ovarian cancer and pancreatic cancer (40,41). Additionally, surface expression of PD-L1 on cancer cells has been described in several hematological cancers (42–47).
Natural PD-L1–reactive anti-Tregs were readily isolated from the peripheral blood of patients with cancer (17,19). PD-L1–specific anti-Tregs could recognize both nonmalignant and malignant cells that expressed PD-L1, and they reacted in a PD-L1 concentration–dependent manner. Thus, PD-L1–specific anti-Tregs are another example of the ability of the immune system to react directly against immune-suppressive mechanisms that have been adopted by cancerous cells. Of note, humoral recognition of PD-L1 has also been described in rheumatoid arthritis (48).
Foxp3 expression has been strongly associated with Tregs (49). We recently reported measurements of natural CD8+ T cell reactivity to FoxP3 in humans (23). Those FoxP3–specific anti-Tregs could recognize both Tregs and malignant T cells that expressed high levels of FoxP3. That finding suggested that a vaccination against FoxP3 could be a valuable treatment for patients with FoxP3+ malignant T cell lymphoma. Recently, it was shown by Nair et al. that FoxP3-specific anti-Tregs in addition are able to lyse FoxP3-expressing breast cancer cells (50). In a previous study conducted in a mouse model, a vaccination that stimulated a Foxp3-specific, cytotoxic T cell response led to the elimination of Foxp3+ Tregs and improved anticancer immunity (51). Of note, because activated T cells express FoxP3, this may result in presentation of FoxP3-specific peptide/HLA ligands on the surface with simultaneous self-recognition and fratricide as a result. This may indeed be the situation under certain conditions. However, the detectable numbers of FoxP3-specific anti-Tregs in the periphery of cancer patients suggest that FoxP3-specific anti-Tregs can expand in vivo. This was confirmed by the data from Gilboa and colleagues (51). This may simply be because of a lower expression of FoxP3 in activated effector cells compared with Tregs or it may be because of a different antigen processing and presentation of FoxP3 epitopes.
Another study showed that tumor-associated dendritic cells (TADCs) played an essential role in suppressing tumor-specific immunity (52,53). This TADC-induced T cell tolerance was recently shown to be mediated by FoxO3 (54,55). Like FoxP3, FoxO3 was also shown to be a natural target for anti-Tregs isolated from patients with cancer (25).
Presence of Specific Immunity in the Periphery of Healthy Individuals
It is quite surprising that immune responses to proteins like IDO, PD-L1, and FoxP3 are frequently detected in patients with cancer, in view of the fact that these antigens are abundantly expressed in normal immune cells. Under healthy conditions, we expect the T cell arm of the immune system to be effectively tolerized to inflammation-induced proteins. For example, Foxp3 should be recognized as a self-protein, because it is expressed in the thymus, both in thymocytes destined to become Tregs and in thymic stromal cells (56,57). We speculated that anti-Tregs may somehow escape depletion in the thymus, via an unknown mechanism, to allow their involvement in regulating the immune system. Thus, we assumed that anti-Tregs may be involved in immune homeostasis as depicted in Figure 1. To characterize these cells further, we continued our search for anti-Tregs in healthy donors. First, we found that circulating IDO-specific CD8+ anti-Tregs indeed were present in healthy donors, although detection was not as frequent as detection in patients with cancer (10). We likewise observed small numbers of IDO-specific CD4+ anti-Tregs in healthy individuals (12,14). Furthermore, we detected specific reactivity to other enzymes involved in tryptophan catabolism in healthy individuals, ie, IDO2 and TDO. An important difference between the IDO- and TDO/IDO2-specific immunities was that the latter were detected as frequently in healthy donors as in patients with malignant disease (10,12).
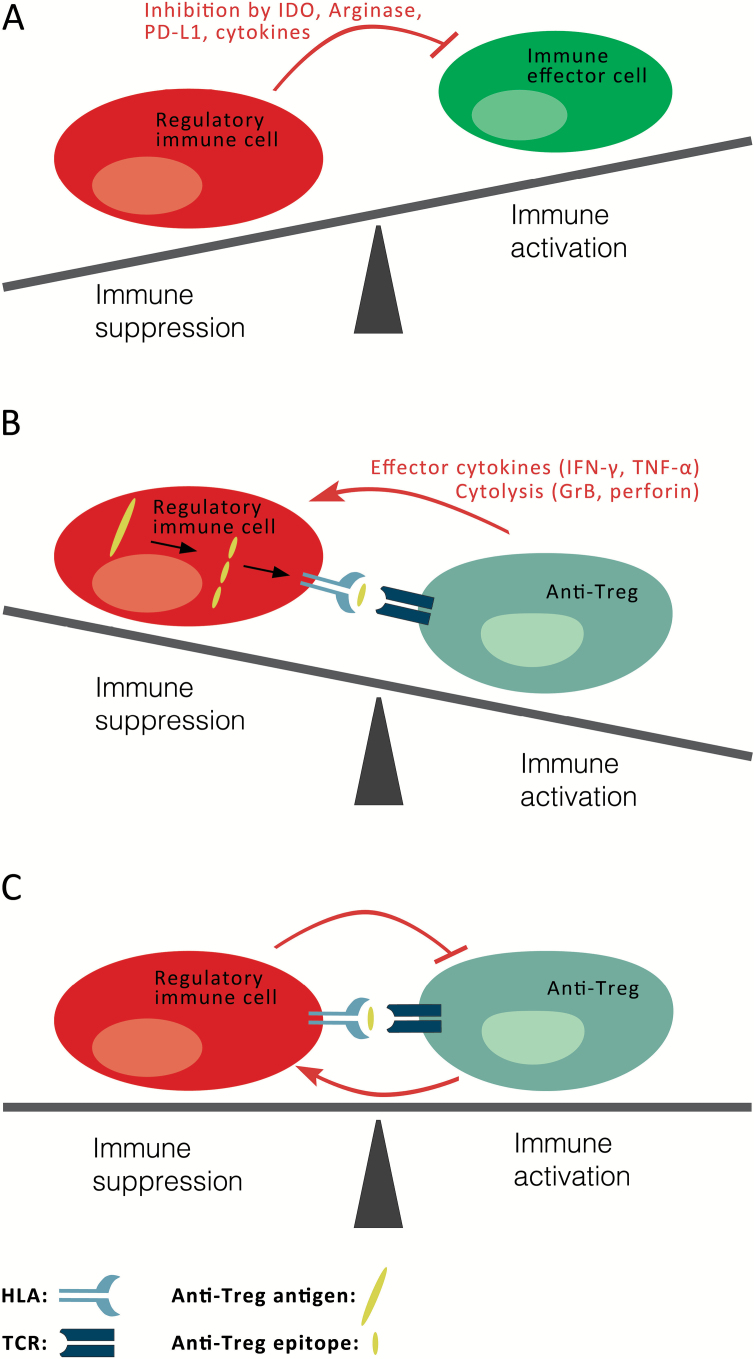
Figure 1.
Proposed model for the involvement of anti-Tregs in immune homeostasis. A) The immune system consists of both immune effector cells (green), eg, T cells, B cells, and natural killer (NK) cells, which are responsible for eliminating elements injurious to the organism, and regulatory immune cells (red), eg, regulatory T cells, different dendritic cell subtypes, myeloid derived suppressor cells, and M2 macrophages, which control or terminate the immune response. The regulatory arm secures the unresponsiveness or tolerance to self-antigens. Regulatory immune cells suppress immunity through a number of different cellular and extracellular factors (red arrow), including the stimulation of inhibitory T cell pathways (eg, PD-1 and CTLA-4); the release of immune suppressive cytokines, like TGF-β and IL-10; and the expression of metabolic enzymes, like IDO and Arginase. These immune-tolerance mechanisms may also be exploited by cancer cells to achieve immune escape, which becomes more pronounced with disease progression. Hence, many of the mechanisms considered helpful in autoimmune settings are used by tumors to suppress immune responses towards malignant cells in cancerous settings. A detailed understanding of the factors involved in immune evasion in malignant conditions is essential for the development of novel, immune-therapeutic treatment modalities in cancer. B) Regulatory immune cells (red) express normal self-proteins (large yellow), which are subsequently processed into peptides (small yellow) and presented on the cell surface by HLA molecules, where they are recognized by anti-Tregs (blue-gray). Hence, anti-Tregs can promote local immune suppression by the secretion of effector cytokines or by directly eliminating regulatory immune cells (red arrow). Similarly, they can eliminate malignant cells that express their cognate targets. Open questions remain of how and when these anti-Tregs are induced or become activated and whether they play a role in the pathogenesis and development of autoimmune diseases. C) Self-reactive anti-Tregs (blue-gray) may avoid thymic selection and peripheral tolerance and are able to react to and even eliminate regulatory immune cells (red), thereby influencing general immune reactions. It must be assumed that anti-Tregs themselves are hampered by the suppressive effects of their targets. Hence, under normal physiological conditions equilibrium between immune activation and suppression may indeed be necessary to maintain immune homeostasis. The role of self-reactive effector and suppressor cells in immune-regulatory networks may thus be miscellaneous. GrB = granzyme B; HLA = human leucocyte antigen; IDO = indoleamine 2,3-dioxygenase; IFN-γ = interferon gamma; PD-L1 = programmed death-ligand 1; TCR = αβ T cell receptor; TNF-α = tumor necrosis factor alpha.
The TDO-specific T cell responses appeared to have different functional phenotypes in health and disease. In healthy subjects, TDO-reactive CD4+ anti-Tregs predominately comprised Th1 cells that produced IFN-γ and tumor necrosis factor (TNF)–α. In contrast, in patients with cancer, the TDO-reactive CD4+ anti-Tregs were more differentiated; in addition to IFN-γ and TNF-α, they also released interleukin (IL)-17 and IL-10 in response to TDO-derived class II HLA-restricted peptides. Hence, in healthy donors, a Th1 helper response was predominant, but in patients with cancer, the CD4+ T cell responses were skewed towards a regulatory T cell (Treg) response. Hence, the functional phenotype of TDO-specific T cell responses differed, depending on conditions in the host.
Next, we identified the frequent, natural occurrence of PD-L1–specific CD8+ and CD4+ anti-Tregs among peripheral blood lymphocytes in healthy donors. PD-L1–specific T cell responses were readily detectable ex vivo in blood samples (17,18). In contrast, immune responses to the FoxO3 transcription factor seemed to be detected only in patients with a malignant disease, not in healthy individuals (25). Thus, the frequency of specific T cells in healthy conditions appeared to be—perhaps not surprisingly—target dependent.
Anti-Tregs Impact Immunity
To characterize the significance of anti-Tregs in immune reactions, we examined their effects on other adaptive immune cells. We found that, by reacting to IDO+ cells, IDO-specific anti-Tregs enhanced other T cell responses (10). For example, co-activation of IDO-specific, cytotoxic anti-Tregs boosted T cell immunity towards viral and tumor-associated antigens (Figure 2). This “supportive” effect on T cell immunity by IDO-specific anti-Tregs was mediated in several direct and indirect manners. First of all, IDO-specific anti-Tregs were capable of killing IDO-expressing regulatory cells, thereby directly targeting the IDO-dependent counter-regulatory pathway. Thus, when IDO-specific anti-Tregs were activated, the IDO activity decreased, the level of tryptophan was elevated, and T cell activities were enhanced. Furthermore, IDO-specific anti-Tregs caused increases in the overall production of pro-inflammatory cytokines, like TNF-α and IL-6, but a decrease in IL-10. The metabolites of tryptophan are known to be toxic to CD8+ T cells and CD4+ Th1 cells (58), but not to Th2 cells. Hence, increased IDO activity appears to favor the polarization of helper T cells toward a Th2 phenotype (59). Conversely, activation of IDO-specific cytotoxic anti-Tregs may drive T-helper polarization in the Th1-direction. Moreover, IDO produces kynurenine, which may effectively hamper the immune response by binding the aryl hydrocarbon receptor, which favors the local formation of Tregs. Hence, targeting IDO-positive cells should decrease the number of Tregs. Indeed, the frequency of Tregs decreased when IDO-specific anti-Tregs were activated (Figure 2).
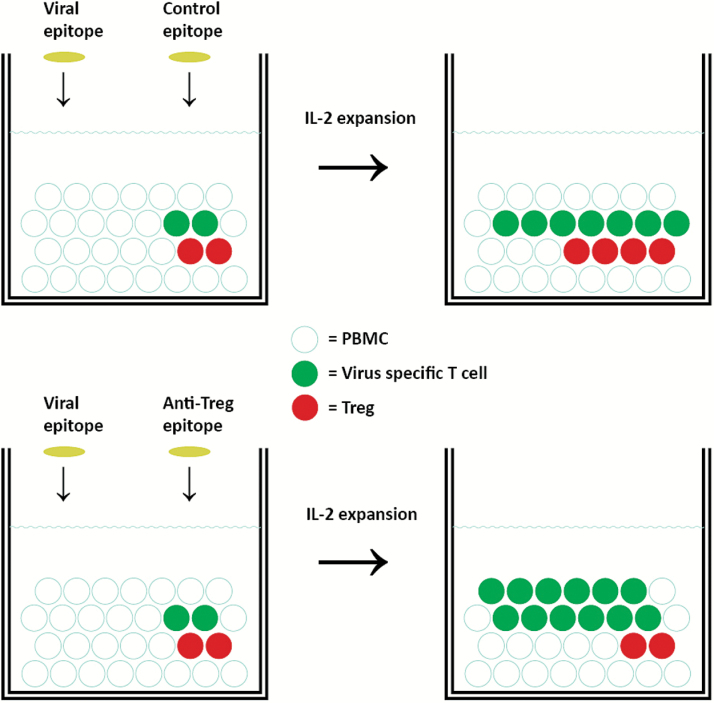
Figure 2.
Activation of anti-Tregs boosts immunity in vitro. Anti-Tregs are able to boost specific immunity against virus or tumor antigens in human peripheral blood mononuclear cells (PBMC). When stimulating PBMC with a known HLA-restricted, viral T cell epitope and interleukin-2 (IL-2), virus-specific T cells (green) begin to expand. The activation of anti-Tregs by the costimulation with an anti-Treg epitope (bottom), eg, programmed death-ligand 1 (PD-L1) or indoleamine 2,3-dioxygenase (IDO) peptides, facilitates further expansion of virus-specific T cells and a decrease in the numbers of Tregs (red), compared with cultures costimulated with an irrelevant control peptide, eg, an HIV peptide epitope (top). This “supportive” effect of anti-Tregs on immune effector cells may well be mediated in several direct and indirect manners, which may depend on the anti-Treg antigen. Of note, IDO+ cells may well be immune suppressive by other means than by the expression of IDO. Hence, the IDO+ cells may in addition express, eg, Arginase, PD-L1, and immune regulatory cytokines (eg, interleukin-10 and transforming growth factor (TGF)–β). IDO-specific anti-Tregs may therefore not only reduce IDO-mediated suppression directly but in addition further immune suppression mediated by IDO+ regulatory cells.
To evaluate the efficiency and safety of IDO-based vaccinations we conducted a phase I first-in-human clinical phase I vaccination trial. The study comprised 15 patients with advanced non–small cell lung cancer (NSCLC) that were vaccinated with an IDO-derived peptide in Montanide adjuvant (www.clinicaltrials.gov, NCT01219348) (60). In the study the median overall survival (OS) was longer than two years, which was higher than expected for this patient group and is underlined by the fact that six of the seven of the stable disease (SD) patients are still alive (60). In one patient with liver metastasis an objective response (PR) was observed. The patient had continuous tumor regression on vaccine treatment for one year before qualifying as a partial response. He is still enrolled in the trial along with one additional patient. The vaccine comprised an HLA-A2–restricted epitope from IDO. Hence, prior to inclusion in the study, HLA tissue typing was performed. Therefore, it was possible to compare the OS of the HLA-A2+ patients who were vaccinated with the patients who were otherwise eligible for the study but were excluded because they did not express HLA-A2. The HLA-A2+ patients who were vaccinated had a median survival of 25.9 months (778 days), demonstrating statistically significantly longer OS (P = .03) when compared with the HLA-A2–, vaccine-untreated group of patients who had a median OS of 7.7 months (237 days). Of note, the clinical significance of HLA phenotype in cancer patients has been widely investigated. Importantly, it was recently described in a large study that expression of HLA-A2 was an unfavorable prognostic factor in stage I NSCLC patients (61). This study underlines the potential importance of the substantially longer OS observed in vaccinated HLA-A2 patients compared with unvaccinated HLA-A2–negative NSCLC patients, although these data need confirmation in large clinical trials.
Immune monitoring revealed that IDO-specific anti-Tregs were indeed detectable in all patients, thus not only in patients who seem to benefit clinically. In two SD patients an IDO-specific anti-Treg response was detected during one year of treatment, suggesting sustained long-term IDO reactivity. Interestingly, we observed a marked reduction in the Treg population after the sixth vaccine in all treated patients (60). Taken together, both the preclinical and clinical results indicate that the activation of IDO-specific anti-Tregs influences adaptive immune responses by suppressing the effects of IDO activity (10).
Immune responses to several widespread viruses are frequently detectable in healthy individuals. The most common response is the CD8+ T cell response to cytomegalovirus, which typically engages a considerable fraction of the CD8+ T cell repertoire (62). We further characterized the effects of anti-Tregs on the adaptive immune response by examining their effects under conditions of a viral infection. We added PD-L1–specific anti-Tregs to cultured peripheral blood mononuclear cells (PBMCs) one week after stimulating with viral epitopes. The result was an immense increase in the number of virus-specific CD8+ T cells in vitro (20). This effect was confirmed in other costimulation assays. For example, we observed a substantial increase in the numbers of virus-specific T cells in cultures that had been costimulated with a known HLA-restricted PD-L1 peptide epitope, compared with cultures costimulated with an irrelevant HIV epitope (21). These results suggested that PD-L1–specific anti-Tregs may support the effector phase of an immune response by removing PD-L1–expressing regulatory immune cells that inhibit PD-1+ effector T cells. The major role of the PD1 pathway is believed to be the regulation of effector T cell responses to control tissue damage. Thus, this protective pathway is more important after activation, rather than at the initial T cell activation stage (36). Accordingly, the presence of PD-L1–specific anti-Tregs during the activation phase of an immune response may not increase or support the antiviral response. Thus, the effect of the addition of PD-L1–specific anti-Tregs may depend on the timing. Indeed, virus stimulation in the presence of PD-L1–specific anti-Tregs resulted in decreased numbers of viral-specific T cells after two weeks of culture (20); this decrease may have been because of the expression of PD-L1 on APCs or resting T cells. Thus, the effects of PD-L1–specific anti-Tregs might vary, depending on the expression of both PD-1 and PD-L1, ie, because of the microenvironment and the state of the immune response.
Finally, it should be considered that anti-Tregs may influence immune regulatory pathways other than those directly mediated by their targets. Thus, immune-suppressive cells can inhibit immune responses by utilizing a number of different immune-suppressive mechanisms at the same time, including arginase, IDO, PD-L1, and the secretion of immune-regulatory cytokines, like IL-10 and transforming growth factor–β (TGF-β). Noticeably, in addition to restraining the immune-regulatory effects of PD-L1, PD-L1-specific anti-Tregs also inhibit other routes of immune suppression mediated by PD-L1+ target cells.
Involvement in Immune Surveillance
The sizable reactivity to self-antigens observed in normal individuals may contribute to immune surveillance against cancer. It was recently suggested that T cells specific to the self-protein OCT4 may be involved in immune surveillance, because these T cells were reduced in patients with germ-cell cancers compared with healthy individuals. Interestingly, the risk of developing those cancers is increased in patients with immunodeficiencies (63). Furthermore, it was reported that chemotherapy led to the induction of anti-OCT4 immunity in patients with cancer. Again, that finding underlined the apparent lack of tolerance to this antigen.
We showed that the cytotoxicity of circulating IDO-specific anti-Tregs towards IDO-expressing malignant cells was similar for IDO-specific anti-Tregs isolated from healthy individuals and those isolated from patients with cancer (10). Furthermore, a direct link between IDO expression in PBMCs and the presence of IDO-specific anti-Tregs has been demonstrated, because the addition of known IDO-inducers like IFN-γ and CpG oligodeoxynucleotides caused the expansion of IDO-specific anti-Tregs among PBMCs without any other stimulation (10). Finally, in the phase I IDO-vaccination trial described above patients with SD showed statistically significantly higher levels of IDO-specific anti-Tregs in the blood at pretreatment compared with patients who progressed at the time of the first evaluation (60). However, in contrast to finding a decrease in OCT4-specific T cells in patients compared with healthy individuals, we found that patients with cancer had increased levels of anti-Tregs that specifically recognized self-antigens expressed in immune cells, compared with healthy individuals. Thus, IDO-, PD-L1-, and Foxp3-specific anti-Tregs were more frequently detected in patients with cancer than in healthy individuals. Apparently, the frequency of TDO-specific T cell reactivity was similar between healthy donors and patients with cancer. However, we have observed that, when patients with cancer hosted a TDO-specific IL-17 response, they showed a trend towards improved overall survival, and survival was impaired in patients with IL-10 producing, TDO-reactive CD4+ T cells (16). Nevertheless, the role of anti-Tregs in immune surveillance for cancer is currently only speculative.
Conclusions and Perspectives
In recent years, T cells that specifically recognized self-proteins involved in immune regulation—herein defined as anti-Tregs—have been described in patients with cancer. In addition, anti-Tregs were shown to be present in the natural T cell repertoire of healthy individuals. Anti-Tregs can recognize and react to both malignant cells and normal immune cells. Naturally, the existence of anti-Tregs does not in itself prove their clinical significance. However, as described, the activation of anti-Tregs can strongly influence immunity, by both direct and indirect mechanisms. The expression of proteins like IDO, PD-L1, and Foxp3 can be induced in normal immune cells under different physiological conditions, eg, inflammation and/or stress (50). Therefore, it can be expected that the immune-modulation exerted by anti-Tregs will most likely apply to several other self-proteins expressed in regulatory immune cells.
It remains unknown how anti-Treg responses are elicited in healthy individuals. It may be because of abnormal expression during non-neoplastic events (infections and inflammation), or it may even result from anti-Tregs avoiding deletion in the thymus through a mechanism that allows them to take part in the fine tuning of the immune system. Finn and colleagues have suggested the former explanation. They reported surprising findings: that T cells exhibited self-reactivity towards MUC1 and cyclin B1 in healthy individuals (29). They proposed that the immune system must maintain self-tolerance to the normal expression of these molecules, but that it could respond to abnormal expression brought about by infections or malignant transformation. However, proteins like Foxp3, PD-L1, and IDO are commonly expressed and play vital roles in normal immune cells; thus, this explanation appears to be unlikely for specific T cell reactivity towards these antigens. In this review, we proposed that the role of anti-Tregs in immune regulatory networks might be to suppress the function of regulatory immune cells. Hence, anti-T cells may “support” effector T cells by directly eliminating regulatory cells or by secreting pro-inflammatory cytokines. Presumably, they may even contribute to immune homeostasis.
Research into the different protagonists of the regulatory network and the maintenance of homeostasis in the immune system is moving forward at a rapid pace. Open questions remain of how and when anti-Tregs are induced or become activated during immune responses, the naïve vs memory phenotype of anti-Tregs, the “exhaustion” or activation status, and to what extent they control immune regulation. These matters still await in vivo studies. Amidst these uncertainties, some features of anti-Tregs have become elucidated. Importantly, the finding that anti-Tregs are able to suppress the function of regulatory immune cells makes them interesting targets for future studies that aim to utilize them for clinical applications, especially anticancer immune therapy (Figure 3). Thus, the activation or expansion of anti-Tregs may, in addition to the direct targeting of cancer cells, modulate immune regulation and alter tolerance to tumor antigens. Because regulatory immune cells oppose the aim of therapeutic cancer vaccines, that is, to induce effector anticancer immune response, the addition of anti-Treg–specific antigens should thus be a simple and synergistic approach to improve the effect of such measures. Notably, the first clinical vaccination trial targeting IDO in NSCLC showed interesting clinical results and a substantial decrease in the numbers of Tregs in the periphery during vaccination (60). Likewise, preclinical proof-of-concept of stimulating Foxp3-specific anti-Tregs was provided by the work of Gilboa and colleagues (51). Importantly, it should be stressed that anti-Tregs not only reduce the target protein-mediated immune suppression but general immune-suppressive effects mediated by the target cells. Expression loss of proteins like IDO, PD-L1, or FoxP3 in cells during vaccination therapy as a means of immune escape might save target cells from immune-mediated destruction by vaccine-induced T cells. However, this should lead to the removal of local immune suppression, thereby enabling circulating effector cells to function or to become activated. The activation of anti-Tregs thus represents a new and attractive immune therapeutic approach.
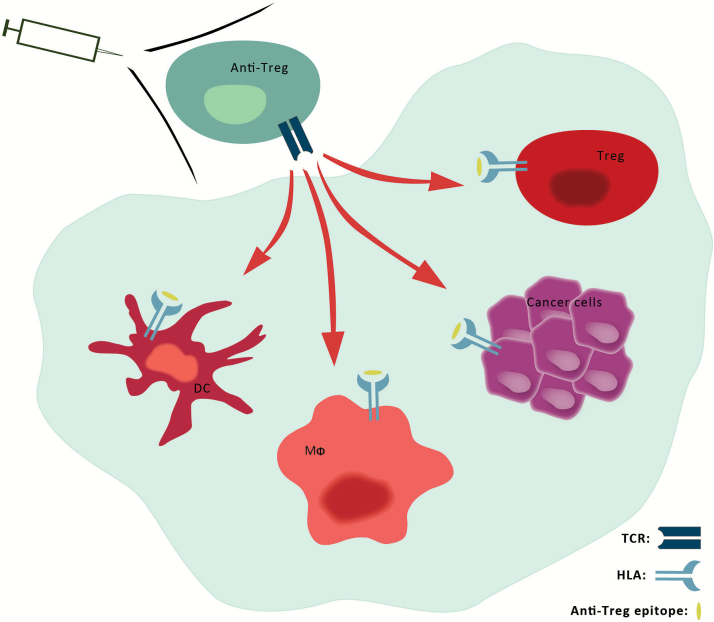
Figure 3.
Exploiting anti-Tregs for anticancer immunotherapy. Cells in the tumor microenvironment (light gray) express multiple proteins, eg, inhibitory cytokines, ligands, and cognate receptors that downmodulate the antitumor activity of immune effector cells including cytotoxic T lymphocytes. Some of these inhibitory proteins are expressed by tumor cells (purple) themselves, whereas others are expressed by tumor-infiltrating suppressive cells including Tregs (red), dendritic cells (DC) (dark red), myeloid cell types (Mφ) like MDSC, and M2 or tumor-associated macrophages (light red). Multiple immune inhibitory and costimulatory pathways in the tumor microenvironment may thus be targeted by therapeutic manipulation of anti-Tregs (green-blue), eg, by therapeutic vaccination. Anti-Tregs recognizing HLA-restricted epitopes (yellow) from antigens like PD-L1, IDO, and FoxP3 are able to eliminate (red arrows) regulatory immune cells as well as cancer cells. Hence, the activation of anti-Tregs by vaccination may directly target immune inhibitory pathways in the tumor microenvironment, modulate immune regulation, and potentially alter tolerance to tumor antigens. Because immune-suppressive cells might antagonize the desired effects of therapeutic cancer vaccines, the addition of anti-Treg antigens would consequently be easily implementable and highly synergistic. FoxP3 = forkhead box P3; HLA = human leucocyte antigen; IDO = indoleamine 2,3-dioxygenase; PD-L1 = programmed death-ligand 1; TCR = αβ T cell receptor.
In conclusion, anti-Tregs provide the immune system with yet another layer of immune regulators, and they contradict the notion that cells involved in immune regulation are only suppressive in nature.
Funding
This work was supported by the Danish Cancer Society, the Danish Council for Independent Research, Lundbeck Foundation, and Herlev Hospital.
The funders did not have a role in the writing of the article or the decision to submit the article for publication. Mads Hald Andersen is an author of three filed patent applications based on the use of PD-L1, TDO, or IDO for vaccination. The rights of the patent applications have been transferred to Copenhagen University Hospital, Herlev/The Capital Region of Denmark, according to the Danish Law of Public Inventions at Public Research Institutions. In addition, the author is a shareholder and board member of the company IO ApS, which intends to develop a commercial IDO vaccine for cancer treatment.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144 (5):646–674. [DOI] [PubMed] [Google Scholar]
- 2. Ostrand-Rosenberg S, Sinha P, Chornoguz O, Ecker C. Regulating the suppressors: apoptosis and inflammation govern the survival of tumor-induced myeloid-derived suppressor cells (MDSC). Cancer Immunol Immunother. 2012;61 (8):1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herreros B, Sanchez-Aguilera A, Piris MA. Lymphoma microenvironment: culprit or innocent? Leukemia. 2008;22 (1):49–58. [DOI] [PubMed] [Google Scholar]
- 4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;19 (8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N Engl J Med. 2012;366 (26):2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012;366 (26):2443–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumar V, Sercarz EE. The involvement of T cell receptor peptide-specific regulatory CD4+ T cells in recovery from antigen-induced autoimmune disease. J Exp Med. 1993;178 (3):909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Broeren CP, Lucassen MA, van Stipdonk MJ, et al. CDR1 T cell receptor beta-chain peptide induces major histocompatibility complex class II-restricted T-T cell interactions. Proc Natl Acad Sci U S A. 1994;91 (13):5997–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sorensen RB, Berge-Hansen L, Junker N, et al. The immune system strikes back: cellular immune responses against indoleamine 2,3-dioxygenase. PLoS One. 2009;4 (9):e6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sorensen RB, Hadrup SR, Svane IM, Hjortso MC, thor Straten P, Andersen MH. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood. 2011;117 (7):2200–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sorensen RB, Kollgaard T, Andersen RS, et al. Spontaneous cytotoxic T-Cell reactivity against indoleamine 2,3-dioxygenase-2. Cancer Res. 2011;71 (6):2038–2044. [DOI] [PubMed] [Google Scholar]
- 12. Munir S, Larsen SK, Iversen TZ, et al. Natural CD4(+) T-Cell Responses against Indoleamine 2,3-Dioxygenase. PLoS One. 2012;7 (4):e34568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersen MH. The specific targeting of immune regulation: T cell responses against Indoleamine 2,3-dioxygenase. Cancer Immunol Immunother. 2012;61 (8):1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andersen MH. CD4 responses against IDO. Oncoimmunology. 2012;1 (7):1211–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Straten PT, Andersen MH. Possible benefits of the targeting of indoleamine 2,3-dioxygenase (IDO) in hepatitis B vaccination. Vaccine. 2011;29 (21):3728. [DOI] [PubMed] [Google Scholar]
- 16. Hjortso MC, Larsen SK, Kongsted P, et al. Tryptophan 2,3-dioxygenase (TDO)-reactive T cells differ in their functional characteristics in health and cancer. Oncoimmunology. 2015;4 (1):e968480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Munir S, Andersen GH, Met O, et al. HLA-restricted cytotoxic T cells that are specific for the immune checkpoint ligand PD-L1 occur with high frequency in cancer patients. Cancer Res. 2013;73 (6):1674–1776. [DOI] [PubMed] [Google Scholar]
- 18. Munir S, Andersen GH, Svane IM, Andersen MH. The immune checkpoint regulator PD-L1 is a specific target for naturally occurring CD4+ T cells. Oncoimmunology. 2013;2 (4):e23991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Munir S, Andersen GH, Woetmann A, Odum N, Becker JC, Andersen MH. Cutaneous T cell lymphoma cells are targets for immune checkpoint ligand PD-L1-specific, cytotoxic T cells. Leukemia. 2013;27 (11):2251–2253. [DOI] [PubMed] [Google Scholar]
- 20. Ahmad SM, Larsen SK, Svane IM, Andersen MH. Harnessing PD-L1-specific cytotoxic T cells for anti-leukemia immunotherapy to defeat mechanisms of immune escape mediated by the PD-1 pathway. Leukemia. 2014;28 (1):236–238. [DOI] [PubMed] [Google Scholar]
- 21. Ahmad SM, Svane IM, Andersen MH. The stimulation of PD-L1-specific cytotoxic T lymphocytes can both directly and indirectly enhance antileukemic immunity. Blood Cancer J. 2014;4:e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andersen MH, Sorensen RB, Brimnes MK, Svane IM, Becker JC, thor Straten P. Identification of heme oxygenase-1-specific regulatory CD8+ T cells in cancer patients. J Clin Invest. 2009;119 (8):2245–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larsen SK, Munir S, Woetmann A, et al. Functional characterization of Foxp3-specific spontaneous immune responses. Leukemia. 2013;27 (12):2332–2340. [DOI] [PubMed] [Google Scholar]
- 24. Andersen MH. FOXP3-specific immunity. Oncoimmunology. 2013;2 (10):e26247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larsen SK, Ahmad SM, Idorn M, et al. Spontaneous presence of FoxO3-specific T cells in cancer patients. Oncoimmunology. 2014;3 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nemazee D. Receptor selection in B and T lymphocytes. Annu Rev Immunol. 2000;18:19–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu Rev Immunol. 2012;30:95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cicinnati VR, Zhang X, Yu Z, et al. Increased frequencies of CD8+ T lymphocytes recognizing wild-type p53-derived epitopes in peripheral blood correlate with presence of epitope loss tumor variants in patients with hepatocellular carcinoma. Int J Cancer. 2006;119 (12):2851–2860. [DOI] [PubMed] [Google Scholar]
- 29. Vella LA, Yu M, Fuhrmann SR, El-Amine M, Epperson DE, Finn OJ. Healthy individuals have T cell and antibody responses to the tumor antigen cyclin B1 that when elicited in mice protect from cancer. Proc Natl Acad Sci U S A. 2009;106 (33):14010–14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pickford WJ, Watson AJ, Barker RN. Different forms of helper tolerance to carcinoembryonic antigen: ignorance and regulation. Clin Cancer Res. 2007;13(15 Pt 1):4528–4537. [DOI] [PubMed] [Google Scholar]
- 31. Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117 (5):1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Batabyal D, Yeh SR. Human tryptophan dioxygenase: a comparison to indoleamine 2,3-dioxygenase. J Am Chem Soc. 2007;19 (50):15690–15701. [DOI] [PubMed] [Google Scholar]
- 33. Munn DH, Sharma MD, Baban B, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22 (5):633–642. [DOI] [PubMed] [Google Scholar]
- 34. Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27 (28):3889–3900. [DOI] [PubMed] [Google Scholar]
- 35. Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9 (10):1269–1274. [DOI] [PubMed] [Google Scholar]
- 36. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12 (4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andersen MH. The targeting of immunosuppressive mechanisms in hematological malignancies. Leukemia. 2014; In press. [DOI] [PubMed] [Google Scholar]
- 38. Kozako T, Yoshimitsu M, Fujiwara H, et al. PD-1/PD-L1 expression in human T cell leukemia virus type 1 carriers and adult T cell leukemia/lymphoma patients. Leukemia. 2009;23 (2):375–382. [DOI] [PubMed] [Google Scholar]
- 39. Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101 (49):17174–17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104 (9):3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13 (7):2151–2157. [DOI] [PubMed] [Google Scholar]
- 42. Krejsgaard T, Odum N, Geisler C, Wasik MA, Woetmann A. Regulatory T cells and immunodeficiency in mycosis fungoides and Sezary syndrome. Leukemia. 2012;26 (3):424–432. [DOI] [PubMed] [Google Scholar]
- 43. Kollgaard T, Petersen SL, Hadrup SR, et al. Evidence for involvement of clonally expanded CD8+ T cells in anticancer immune responses in CLL patients following nonmyeloablative conditioning and hematopoietic cell transplantation. Leukemia. 2005;19 (12):2273–2280. [DOI] [PubMed] [Google Scholar]
- 44. Ame-Thomas P, Le PJ, Yssel H, et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia. 2012;26 (5):1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van de Donk NW, Kamps S, Mutis T, Lokhorst HM. Monoclonal antibody-based therapy as a new treatment strategy in multiple myeloma. Leukemia. 2012;26 (2):199–213. [DOI] [PubMed] [Google Scholar]
- 46. Tamura H, Ishibashi M, Yamashita T, et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2013;27 (2):464–472. [DOI] [PubMed] [Google Scholar]
- 47. Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121 (5):734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dong H, Strome SE, Matteson EL, et al. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J Clin Invest. 2003;111 (3):363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sakaguchi S. Regulatory T cells. Springer Semin Immunopathol. 2006;28 (1):1–2. [DOI] [PubMed] [Google Scholar]
- 50. Nair S, Aldrich AJ, McDonnell E, et al. Immunologic targeting of FOXP3 in inflammatory breast cancer cells. PLoS One. 2013;8 (1):e53150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67 (1):371–380. [DOI] [PubMed] [Google Scholar]
- 52. Hurwitz AA, Watkins SK. Immune suppression in the tumor microenvironment: a role for dendritic cell-mediated tolerization of T cells. Cancer Immunol Immunother. 2012;61 (2):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhu Z, Singh V, Watkins SK, et al. High-avidity T cells are preferentially tolerized in the tumor microenvironment. Cancer Res. 2013;73 (2):595–604. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54. Watkins SK, Zhu Z, Riboldi E, et al. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121 (4):1361–1372. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55. Watkins SK, Hurwitz AA. FOXO3: A master switch for regulating tolerance and immunity in dendritic cells. Oncoimmunology. 2012;1 (2):252–254. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299 (5609):1057–1061. [DOI] [PubMed] [Google Scholar]
- 57. Chang X, Gao JX, Jiang Q, et al. The Scurfy mutation of FoxP3 in the thymus stroma leads to defective thymopoiesis. J Exp Med. 2005;202 (8):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196 (4):459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu H, Oriss TB, Fei M, et al. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci U S A. 2008;105 (18):6690–6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iversen TZ, Engell-Noerregaard L, Ellebaek E, et al. Long-lasting Disease Stabilization in the Absence of Toxicity in Metastatic Lung Cancer Patients Vaccinated with an Epitope Derived from Indoleamine 2,3 Dioxygenase. Clin Cancer Res. 2014;20 (1):221–232. [DOI] [PubMed] [Google Scholar]
- 61. Nagata Y, Hanagiri T, Mizukami M, et al. Clinical significance of HLA class I alleles on postoperative prognosis of lung cancer patients in Japan. Lung Cancer. 2009;65 (1):91–97. [DOI] [PubMed] [Google Scholar]
- 62. Khan N, Shariff N, Cobbold M, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169 (4):1984–1992. [DOI] [PubMed] [Google Scholar]
- 63. Lim ST, Levine AM. Non-AIDS-Defining Cancers and HIV Infection. Curr Infect Dis Rep. 2005;7 (3):227–234. [DOI] [PubMed] [Google Scholar]