Abstract
Fluorescent labeling is a mainstream technology for detecting molecular binding. Despite the importance, few studies have been devoted to quantitatively examine the effect of labeling on the molecular binding processes. Here we present a quantitative study on the binding kinetics of fluorescent-labeled and un-labeled molecules (lectin proteins) with glycoproteins on the membrane of cells using surface plasmon resonance imaging (SPRi) technique. The study shows that fluorescent labeling has a significant influence on the binding behaviors of proteins, especially the association processes, and the influence depends sensitively on the charge of fluorescent labels. It further shows that the labels also affect the local distribution of probe proteins, due to the inhomogeneous surface charge distribution of the cell membrane. Our work indicates that fluorescent labeling in general affects the binding behaviors, but proper design of the label will help to minimize its effect.
Keywords: fluorescent labeling, binding kinetics, surface plasmon resonance imaging, wheat germ agglutinin (WGA), lectin-glycoprotein interactions
1. Introduction
Fluorescence detection is perhaps the most popular imaging and detection technology for studying molecular interactions (Giepmans, Adams et al. 2006, Kerppola 2006). Because most molecules, including proteins, are not intrinsically fluorescent, one must attach a fluorescent label to a probe molecule in order to detect its binding to a target molecule(Zhang, Campbell et al. 2002, Marks and Nolan 2006, Resch-Genger, Grabolle et al. 2008). This practice raises the questions if and how the presence of the fluorescent label alters the interaction of the probe molecule with the target molecule (Jones and Thornton 1996, Nguyen, DeFina et al. 2002). Such effects could be responsible for the inconsistent and even contradictory results of similar molecular interactions studied with different labeling methods (Kasnavia, Vu et al. 1999, Neu, Swerhone et al. 2001, Hoffmann, Leroy-Dudal et al. 2008). It is thus necessary to study and quantify the effect of fluorescent labels on the native binding properties of biomolecules. Such information is valuable not only for correct interpretation of results obtained with the fluorescent labeling technology, but also for designing better fluorescent labels.
The effect of fluorescent labels has been investigated by mass spectrometry(Salih and Zenobi 1998), X-ray crystallography(Ianeselli, Zhang et al. 2010), electrophoresis(Bao, Krylova et al. 2011) and molecular dynamics simulation(Unruh, Gokulrangan et al. 2005). These methods are suitable for evaluating the effect of the labels on the structural or other static properties of the molecules, but they do not provide direct kinetic information that is necessary for quantifying interactions between molecules. Surface plasmon resonance (SPR) is a label-free detection technology for studying molecular binding kinetics(Liljeblad, Lundblad et al. 2002, Homola 2008), which can evaluate the effect of fluorescent labels in real time (Sun, Landry et al. 2008).
However, traditional SPR typically studies the binding of probe molecules with target molecules (e.g., proteins) immobilized on a SPR sensor surface. The protocol involves the extraction, purification and immobilization of the proteins, which could be problematic, especially for membrane proteins whose conformations are sensitive to the local membrane environment in an intact cell. We recently developed a surface plasmon resonance imaging (SPRi) approach to measure the binding kinetics of membrane proteins in intact cells (Wang, Yang et al. 2012).
Here we present a quantitative study on the effect of fluorescent labeling on the binding of proteins (lectin) with their membrane protein receptors (glycoproteins) in intact cells with SPRi technique. The study shows that fluorescent labeling has a significant influence on the binding of the proteins, compared to that of the unlabeled lectin. To further examine the origin of the effect, we labeled the lectin with different fluorescent labels and obtained the binding kinetics of labeled lectins in phosphate-buffered saline (PBS) solution with different ionic strengths. The result shows that the effect depends sensitively on the charge of the fluorescent labels. Additionally, it is also found that the binding kinetics is inhomogeneous within a cell, which is due to the local surface charge distribution of the cell membrane.
2. Materials and Methods
2.1 Materials
N-acetylglucosamine (GlcNAc), unlabeled wheat-germ agglutinin (WGA) and Fluorescein isothiocyanate conjugate of WGA, WGA/FITC(1−), were obtained from Sigma-Aldrich (St Louis, MO). WGA conjugated with Alexa Fluor 488, WGA/Alexa-488(2−) or tetramethylrhodamine, WGA/TMR(1+), were purchased from Invitrogen (Carlsbad, California). The stock solution of each WGA conjugate was prepared by dissolving the sample in PBS to achieve a WGA concentration of 1 mg/mL. By further diluting the stock solution with PBS, solutions of different WGA concentrations were prepared.
2.2 Cell Culture
SH-EP1 cell line were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and cultured according to their specifications. (See Supplementary Materials Section 1.1 for details).
2.3 SPRi Setup
Both prism-based and objective-based SPRi configurations were used in the present study. The prism-based configuration provided 10X magnification, allowing for the statistical analysis to tens of individual cells. The objective-based configuration had 60X magnification and enabled subcellular analysis. (Details for the setups and the preparation of cell-based sensor chip in Supplementary Materials Section 1.2).
2.4 Flow system
A gravity-based multichannel drug perfusion system (SF-77B, Warner Instruments, CT) was used to control the local solution that surrounded the target cell. The typical transition time between different solutions was about 1–2 seconds. Note that the flow rate of this flow system is 350 μL/min. It was found that the mass transport effect does not influence the binding kinetics at such a high flow rate.
3. Results and discussions
3.1 Measuring binding kinetics of fluorescent labeled and unlabeled molecules
Figure 1a is a schematic illustration of the experimental setup for SPR imaging of single cells. The cells were cultured on a gold-coated glass slide, and the slide was placed either on a prism (low magnification) or on an objective lens (high magnification) for SPR imaging (see Supplementary Materials Section 1.2 for details). The binding of unlabeled and fluorescent-labeled lectin molecules to the corresponding glycoproteins on the membrane of single cells was studied by flowing buffer solution containing lectin over the cells. The lectin molecules interacted with and bound to the GlcNAc sugar units of the glycoproteins in the cell membrane. The lectin binding led to an increase in the SPR intensity during the association process. The subsequent washing of the cell by flowing pure buffer solution allowed dissociation of WGA from the membrane surface, leading to a dissociation curve. Analysis of the association and dissociation curves could determine the binding constants and provide quantitative characterization of the molecular interactions. Note that the binding kinetics was quantified by a label-free detection technology in the present work, so that the binding kinetics of un-labeled lectin could be obtained and compared with the results of fluorescence-labeled lectins. Note also that we did not evaluate the binding kinetics with the fluorescence approach as it measures the fluorescent intensity of labels, which significantly depends on the stoichiometry of lectin-dye conjugates as well as the efficiency of fluorescence emission. Unlike the fluorescence approach, the SPR method detects the change of mass density of bound lectins during the binding process, thus it is insensitive to the instability of fluorescent labels and valid for comparing the binding kinetics of different fluorophore-labeled proteins.
Figure 1.
SPRi study on the binding kinetics of fluorescent labeled and unlabeled molecules. (a) Schematic illustration on the interaction of fluorescent labeled WGA with membrane proteins of single cells by SPRi. (b) Molecular structures of tags in the fluorescent labeled WGA. The three dyes share similar structure except the R1, R2, R3, R4, R5 groups as indicated by the red dashed square. X indicates the reaction moiety to conjugate with the amine group of WGA, which is removed after the reaction. (c) Typical SPR image of cells adhered onto gold-coated glass chip by the prism setup. Scale bar: 50μm. (d) The average SPR sensorgrams for unlabeled and three Fluorescent-labeled WGA. Fifty cells were examined in each case and the variation for each curve was shown in Figure S2. (e) Illustration of two-component kinetic model. Fifty cells were examined with variations shown in gray background. Black and red curves are original and fitted sensorgrams, respectively. The magenta dash line represented the slow binding process and the blue one is for the fast part. (f) Steady state intensity for the fast binding part derived from the kinetic model. The values are 65.2±19.5, 116.8±31.1, 55.9±16.4, 62.0±11.0mDeg, respectively, from left to right (n = 50).
3.2 Selection of fluorescent labels
In the present work, we selected dyes, Alexa flour-488, tetramethylrhodamine (TMR) and fluorescein isothiocyanate (FITC, Figure 1b) for study because they are the most popular fluorescent labels used for protein labeling and cell staining. Another reason for studying these labels is that they share the same 9-(2-carboxyphenyl)xantheneskeleton as marked by the red dashed square in Figure 1b. Under experimental condition (pH=7.4), both FITC and Alexa-488 are negatively charged with net values −1.02 and −2.00, respectively. In contrast, TMR has a net positive charge of +0.99. The calculation details are provided in Supplementary Materials Section 2. In this paper, we denote lectin labeled with these three dyes as WGA/TMR(1+), WGA/FITC(1−) and WGA/Alexa-488(2−), respectively.
3.3 Influences of fluorescent labels on the binding kinetics
Figure 1c is a low magnification SPR image of cells obtained with the prism setup, showing individual cells as bright spots. The binding kinetics measurement started with flowing 1×PBS buffer over the cells for 120s to stabilize the SPRi system. The buffer solution was then switched to WGA solution (100 μg/mL WGA in 1×PBS) to allow the binding of WGA to the sugar groups on the membrane surface of the cells for another 600s. Finally, the WGA solution was switched back to 1×PBS buffer for 1400s to study the dissociation of WGA from the cell membrane. After obtaining the association and dissociation curves, we regenerated the surface for the next binding kinetics study, which was descried in Supplementary Materials Section 3.
Using the experimental procedure described above, the binding kinetics of both unlabeled and fluorescent labeled WGA molecules were measured, as shown in Figure 1d. Compared to unlabeled WGA (blue curve), fluorescent labeling of WGA significantly altered the kinetics and the amount of WGA binding to cell membrane. Among them, WGA/TMR(1+)(red curve) exhibited the largest alteration.
In order to quantify the effect of the fluorescent labeling, we analyzed the binding kinetic of fluorescent labeled and un-labeled WGA with a two-component model, as shown in Figure 1e (Srisa-Art, Dyson et al. 2008). In this model, the recorded sensorgram is considered as the superposition of two independent components that follow first order kinetics. One component describes the fast binding part (blue dashed line), and the other one is for slow binding (magenta dashed line). The model was applied to fit each sensorgrams of unlabeled or fluorescent labeled WGA with fitting parameters, R1, ka1, kd1, KD1, R2, ka2, ka2, and KD2. Note that R1 is the SPR response of the fast component at equilibrium which indicates the amount of bound WGA molecules contributed by fast binding process. ka1, kd1, and KD1 describe the association rate, dissociation rate, and equilibrium constants for the fast component, respectively. Similarly, R2, ka2, kd2, and KD2 describe the counterparts for slow binding component. The detail of this model and the fitting results are shown in Supplementary Materials Section 4.
Compared to unlabeled WGA, the labeled WGA conjugates showed quite different kinetics features, indicating that the fluorescent labels indeed altered the binding of WGA with the same glycoproteins in cell membrane. As shown in Figure 1f, WGA/TMR(1+) presented the largest R1, suggesting the largest amount of fast binding of WGA/TMR(1+) to the cell membrane. This observation was attributed to the positive charge of TMR(1+), which interacted favorably with the negatively charged membrane surface (Yeung, Gilbert et al. 2008) due to the electrostatic attraction. The intrinsically negative charge of cell membrane arises from the sialic acid residues in the membrane proteins (Fuster and Esko 2005). In contrast, WGA/Alexa-488(2−) shows the smallest R1, which is consistent with its negative charge.
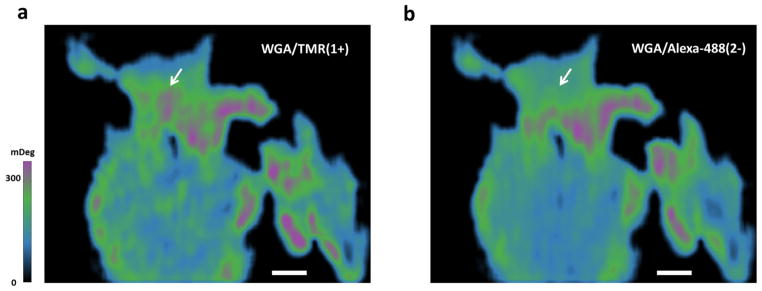
The spatial distribution of fluorescent-labeled lectins was often used to demonstrate the subcellular distribution of lectin-binding sites in single cell staining. We thus studied whether the type of fluorescent label would affect the resulted spatial distributions of WGA-binding sites, which can be obtained by subtracting the original SPR image of a single cell from the one recorded at the end of dissociation. We found that the distributions of WGA-binding sites varied among different fluorescent labeled probe proteins, especially for WGA/Alexa-488(2−)and WGA/TMR(1+). Figure 2a–b show the representative distribution maps when using WGA/TMR(1+) and WGA/Alexa-488(2−), respectively. It is clear that their binding distributions are significantly different at certain subcellular locations, especially where indicated by white arrows. We believe the difference was related with the inhomogeneous distribution of surface charge of cell membranes. Note that the reproducible binding maps can be obtained for the same type of WGA with successive binding-regeneration cycles (See Supplementary Materials Section 5).
Figure 2.
Binding distribution of (a) WGA/TMR(1+) and (b) WGA/Alexa-488(2−). Each map was generated by subtracting the SPR image before association from the one at the end of dissociation. Note that they are differential SPR images, directly indicating the massing density of bound proteins on the cell membrane. Scale bar: 10μm
3.4 Charge effect
In order to further clarify how the surface charge of fluorescent label affects WGA-glycoprotein interactions, we studied the binding kinetics in buffers with different ionic strengths. If electrostatic interaction was indeed important, the binding would depend on the ionic strength because of ionic screening of electrostatic interactions. Figures 3a–b show SPR sensorgrams of WGA/TMR(1+)and WGA/Alexa-488(2−) in different media with increasing ionic strength(i.e. 0.2X, 0.5X, 1X, and 2X PBS), respectively. Interestingly, the two WGA conjugates exhibited opposite dependences on the ionic strength of medium. For positively charged WGA/TMR(1+), the SPR intensity at steady state due to fast binding (R1) and slow binding (R2) decreases with the ionic strength, while negatively charged WGA/Alexa-488(2−) shows an opposite trend, as shown in Figures 3c–d respectively. This observation is consistent with the surface charge hypothesis. For positively charged WGA/TMR(1+), the binding to the negatively charged membrane is facilitated by the electrostatic attraction. As the ionic strength increases, the effective positive charge of WGA/TMR(1+) decreases due to increased ionic screening, leading to a reduced electrostatic attraction effect. In contrast, for negatively charged WGA/Alexa-488(2−), higher ionic strength reduces the electrostatic repulsion, and thus increases the binding intensity. Additionally, WGA/FITC(1−) exhibits similar dependence on the ionic strength as WGA/Alexa-488(2−), which is consistent with its charge property (Supplementary Materials Section 6).
Figure 3.
Effect of ionic strength. The sensorgrams of (a) WGA/TMR(1+) and (b) WGA/Alexa-488(2−) interactions with glycoproteins on cell membrane surfaces in 0.2×PBS, 0.5×PBS, 1×PBS and 2×PBS buffer. Dependence of binding intensity on the ionic strength for (c) WGA/TMR(1+) and (d) WGA/Alexa-488(2−). Dependence of the fast and the slow association rate constants on ionic strength for (e) WGA/TMR(1+) and (f) WGA/Alexa-488(2−). Fifty cells were examined for the sensorgrams in 1×PBS, and ten cells were done for that in other PBS concentrations. The values and the variations are listed in Supplementary Materials Section 7.
The kinetics constants are also found to depend on the ionic strength of the buffer solution. For WGA/TMR (1+), association rate constants for both the fast and the slow binding processes decrease with the ionic strength (Figure 3e). In contrast, for WGA/Alexa-488(2−), association rate constants increase with the ionic strength (Figure 3f). These observations indicate that the charge of a fluorescence label affects not only the amount of protein binding, but also the association rate constants of the binding processes. This may be attributed to the electrostatic forces, which affects the local concentration of the probe molecules (WGA) near the cell surface, and thus alters the association rate constants. However, we did not observe obvious dependence of the dissociation rate constants on the ionic strength, suggesting that dissociation rate is independent of electrostatic interactions. This is because a dissociation process is mainly determined by short-range interactions, e.g. hydrophobic bonds and van der Waals interactions, rather than electrostatic interactions(Selzer, Albeck et al. 2000). Besides, we also observed the difference on the binding kinetics between WGA/TMR(1+) and WGA/Alexa-488(2−) decreased in higher concentration PBS as it provides better charge screening effect, as shown in Supplementary Materials Section 8.
4. Conclusion
We have studied the influence of fluorescent labeling on the binding kinetics of WGA with glycoprotein on the membrane of intact cells. The fluorescent labels were found to significantly affect the amount of protein binding, binding kinetic constants, and spatial distribution of probe protein. The effect arises from the charge of the fluorescent labels, which enhances or weakens the binding due to electrostatic interaction between the fluorescent-labeled probe molecule and the target molecule on the negatively charged membrane surface. But the electrostatic interaction does not take effect during the dissociation process, as it is dominated by short-range interactions. This work shows that results from fluorescence labeling techniques can introduce substantial errors in protein binding studies on the cell membrane, and provides important information for proper design of fluorescence tags to minimize such errors. It also underscores the importance of studying membrane proteins in their native membrane environment.
Supplementary Material
Highlights.
Binding kinetics of lectin-dye conjugates with glycoproteins on cells was determined.
Charge property of fluorescent dye greatly alters the binding kinetics of lectin.
Positively-charged fluorescent label promotes the association of lectin onto cells.
This effect arises from electrostatic interaction of charged dye with cell membrane.
Acknowledgments
Financial support from NIH grants (#1R01GM107165-01A1 and #1R44GM106579-01), National Natural Science Foundation of China (NSFC, Grant No. 21327008, 21405080) and Natural Science Foundation of Jiangsu Province (BK20140592) are acknowledged.
Appendix A. Supplementary material
The details for the supporting information as noted in the text can be found in the Supplementary Material.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bao JY, Krylova SM, Reinstein O, Johnson PE, Krylov SN. Analytical Chemistry. 2011;8322:8387–8390. doi: 10.1021/ac2026699. [DOI] [PubMed] [Google Scholar]
- Fuster MM, Esko JD. Nature Reviews Cancer. 2005;57:526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- Giepmans BNG, Adams SR, Ellisman MH, Tsien RY. Science. 2006;3125771:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Leroy-Dudal J, Patel S, Gallet O, Pauthe E. Analytical Biochemistry. 2008;3721:62–71. doi: 10.1016/j.ab.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Homola J. Chemical Reviews. 2008;1082:462–493. doi: 10.1021/cr068107d. [DOI] [PubMed] [Google Scholar]
- Ianeselli L, Zhang FJ, Skoda MWA, Jacobs RMJ, Martin RA, Callow S, Prevost S, Schreiber F. Journal of Physical Chemistry B. 2010;11411:3776–3783. doi: 10.1021/jp9112156. [DOI] [PubMed] [Google Scholar]
- Jones S, Thornton JM. Proceedings of the National Academy of Sciences of the United States of America. 1996;931:13–20. doi: 10.1073/pnas.93.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasnavia T, Vu D, Sabatini DA. Ground Water. 1999;373:376–381. [Google Scholar]
- Kerppola TK. Nature Reviews Molecular Cell Biology. 2006;76:449–456. doi: 10.1038/nrm1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeblad M, Lundblad A, Pahlsson P. Biosensors & Bioelectronics. 2002;1710:883–891. doi: 10.1016/s0956-5663(02)00111-2. [DOI] [PubMed] [Google Scholar]
- Marks KM, Nolan GP. Nature Methods. 2006;38:591–596. doi: 10.1038/nmeth906. [DOI] [PubMed] [Google Scholar]
- Neu TR, Swerhone GDW, Lawrence JR. Microbiology-Sgm. 2001;147:299–313. doi: 10.1099/00221287-147-2-299. [DOI] [PubMed] [Google Scholar]
- Nguyen DH, DeFina SC, Fink WH, Dieckmann T. Journal of the American Chemical Society. 2002;12450:15081–15084. doi: 10.1021/ja027635d. [DOI] [PubMed] [Google Scholar]
- Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Nature Methods. 2008;59:763–775. doi: 10.1038/nmeth.1248. [DOI] [PubMed] [Google Scholar]
- Salih B, Zenobi R. Analytical Chemistry. 1998;708:1536–1543. doi: 10.1021/ac9708506. [DOI] [PubMed] [Google Scholar]
- Selzer T, Albeck S, Schreiber G. Nature Structural Biology. 2000;77:537–541. doi: 10.1038/76744. [DOI] [PubMed] [Google Scholar]
- Srisa-Art M, Dyson EC, Demello AJ, Edel JB. Analytical Chemistry. 2008;8018:7063–7067. doi: 10.1021/ac801199k. [DOI] [PubMed] [Google Scholar]
- Sun YS, Landry JP, Fei YY, Zhu XD. Langmuir. 2008;2423:13399–13405. doi: 10.1021/la802097z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh JR, Gokulrangan G, Lushington GH, Johnson CK, Wilson GS. Biophysical Journal. 2005;885:3455–3465. doi: 10.1529/biophysj.104.054148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang YZ, Wang SP, Nagaraj VJ, Liu Q, Wu J, Tao NJ. Nature Chemistry. 2012;410:846–853. doi: 10.1038/nchem.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Science. 2008;3195860:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- Zhang J, Campbell RE, Ting AY, Tsien RY. Nature Reviews Molecular Cell Biology. 2002;312:906–918. doi: 10.1038/nrm976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.