Abstract
The first two highly enantioselective palladium-catalyzed allylic alkylations with benzylic nucleophiles activated with Cr(CO)3 have been developed. These methods enable the enantioselective synthesis of “α-2-propenyl benzyl” motifs, which are important scaffolds in natural products and pharmaceuticals. A variety of cyclic and acyclic allylic carbonates are competent electrophilic partners furnishing the products in excellent enantioselectivity (up to 99% ee and 92% yield). This approach was employed to prepare a nonsteroidal anti-inflammatory drug analogue.
Keywords: palladium, asymmetric allylic alkylation, (η6-Arene) complexes, chromium
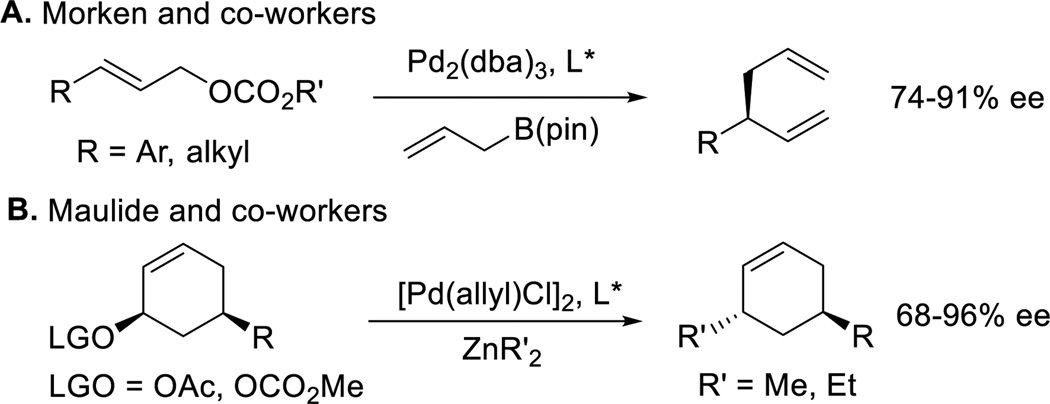
Transition-metal-catalyzed allylic substitution reactions have emerged as a powerful method to construct C−C bonds.[1] Of these, the palladium-catalyzed asymmetric allylic alkylation (AAA) reaction has attracted the greatest interest due to its applications in total synthesis and the preparation of bioactive compounds.[2] A wide variety of stabilized or “soft” nucleophiles (originally defined as pronucleophiles with pKa < 25[3]) have been successfully employed in palladium-catalyzed AAA reactions, including malonates,[4] imides[5] and many others.[6] In contrast, few palladium-catalyzed allylic substitution reactions with “hard” nucleophiles (generally defined as pronucleophiles with pKa > 25) have materialized.[1a] Recent advances in palladium-catalyzed AAA with “hard” nucleophiles have been reported by Morken and co-workers with allylboronates in allyl-allyl coupling reactions[7] (Scheme 1A) and Maulide and co-workers with the use of dialkylzinc nucleophiles[8] (Scheme 1B).
Scheme 1.
Palladium-catalyzed AAA reaction with “hard” nucleophiles.
The most significant difference between “soft” and “hard” nucleophile classes in the Tsuji-Trost allylic substitution is their reaction pathways: “soft” nucleophiles react via a double inversion mechanism whereas “hard” nucleophiles are proposed to undergo single inversion,[9] wherein the nucleophile transmetallates to the palladium catalyst. Both reactions in Scheme 1 were shown to proceed by the “hard” nucleophile pathway. As a valuable complement, Fletcher and co-workers reported copper-catalyzed AAA reaction of alkylzirconium reagents (generated in situ from alkenes by hydrometallation) via dynamic kinetic asymmetric transformation.[10] The scope of this reaction, however, does not include simple benzylic nucleophiles.
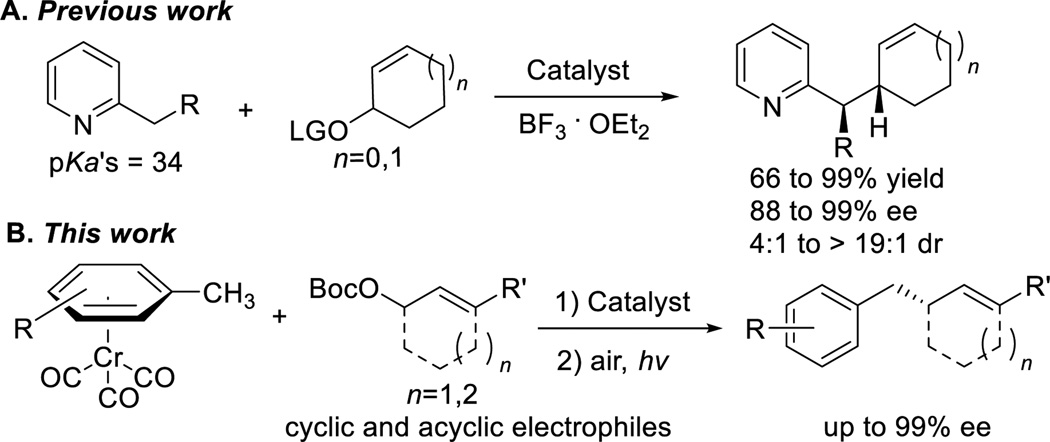
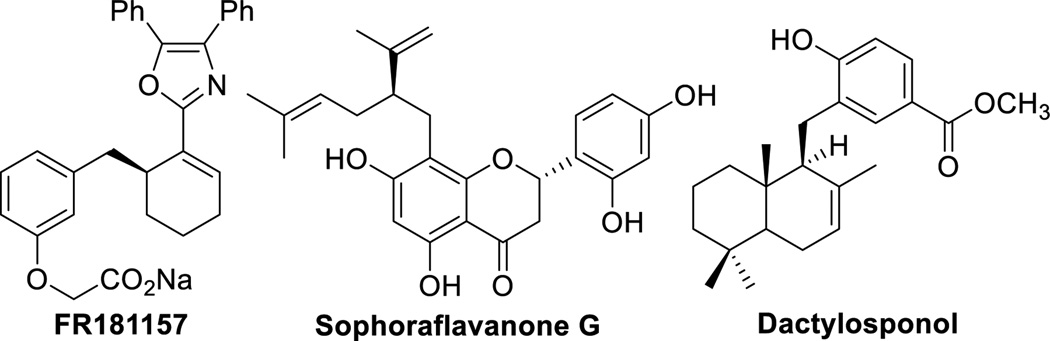
To broaden the synthetic utility of the palladium-catalyzed AAA, researchers have focused on expanding the classes of nucleophiles that can undergo the more controllable double inversion or “soft” nucleophile reaction pathway. This approach entails “softening” of hard nucleophiles by addition of activating agents to stabilize the resulting anionic charge. In pioneering studies, Trost and co-workers reported the highly enantioselective palladium-catalyzed AAA with 2-methylpyridine derivatives (Scheme 2A, LG=leaving group).[11] Key to success of this approach was addition of 1.3 equiv BF3 to bind the nitrogen and acidify the sp3-hybridized C-H’s of 2-methylpyridine (pKa ~ 34[12]). A different strategy is necessary for pronucleophiles bearing less acidic C–H’s in the absence of Lewis basic heteroatoms, such as toluene derivatives (pKa ~ 43[13]). AAA with benzyl anion nucleophiles could potentially furnish benzylated substructures, which are important scaffolds in natural products and pharmaceuticals, such as FR181157,[14] Sophoraflavanone G,[15] and Dactylosponol[16] (Figure 1).
Scheme 2.
Palladium-catalyzed AAA reactions with softened “hard” pronucleophiles
Figure 1.
Selected natural product containing “α-2-propenyl benzyl” motifs.
Herein we report the first palladium-catalyzed AAA reactions of toluene-based pronucleophiles. By activation of toluene derivatives with η6-tricarbonylchromium, both cyclic and acyclic allylic carbonates are benzylated with enantioselectivities as high as 96 and 99%, respectively (Scheme 2B).
Recently, our team introduced a strategy to employ (η6-C6H5CH2R)Cr(CO)3 complexes as cross-coupling partners to produce di- and triarylmethanes[17] and enantioenriched diarylmethylamines[18] via direct arylations.[19] Using these pronucleophiles, we also explored palladium-catalyzed allylic substitution of diverse cyclic and acyclic electrophiles to give racemic products.[20] Despite significant progress in palladium-catalyzed Tsuji-Trost reactions, highly enantioselective processes with benzylic nucleophiles (toluene derivatives) remain a limitation of this method. Our prior demonstration of a double inversion reaction pathway with Cr(CO)3 activated benzylic nucleophiles to afford racemic products inspired us to pursue palladium-catalyzed AAA reactions.[20]
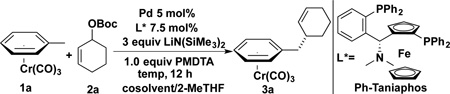
Given the known difficulty of identifying chiral ligands that can moderate all of the steps of a complex catalytic cycle and provide useful enantioselectivity, we initially examined over 140 diverse enantioenriched mono- and bidentate phosphine ligands (see Supporting Information for full ligand structures and results). We employed 1 equiv (η6-C6H5CH3)Cr(CO)3 (1a) as the pronucleophile, 2 equiv tert-butyl cyclohex-2-enyl carbonate (2a), 3 equiv LiN(SiMe3)2, 1 equiv PMDTA (pentamethyldiethylenetriamine) additive,[21] 10 mol % Pd(COD)Cl2, and 10 mol % chiral bidentate ligand or 20 mol % monodentate phosphine in THF at room temperature for 12 h (Table 1). Surprisingly, only the Ph-Taniaphos ligand[22] out of 140 ligands, provided significant turnover and high enantioselectivity, highlighting the challenging nature of this reaction. Translation of this lead to laboratory scale with Ph-Taniaphos at 0 °C afforded 3a in 50% assay yield (AY), with 85% ee (entry 1). Solvent 2-MeTHF resulted in improvement to 61% AY, while maintaining the ee (85%, entry 2). Next we screened the impact of the temperature on the reactivity and enantioselectivity in 2-MeTHF. Conducting the reaction at room temperature resulted in a drop in AY (48%), and ee (79%, entry 3). As the temperature was decreased from 0 °C to −20 °C, the AY increased to 67% at −10 °C (86% ee), then decreased to 56% at −20 °C (87% ee) (entries 4–5). We next examined the solvent composition.[18] Use of 30% toluene as cosolvent resulted in an increase in AY to 79% (86% ee, entry 6). Changing the ratio of the toluene to 2-MeTHF had a dramatic impact on the yield but maintained the ee (see Supporting Information for details). Increasing the concentration from 0.1 M to 0.2 M had a detrimental impact (entry 7) while decreasing to 0.05 M led to an increase in assay yield (84%, 86% ee, entry 8).
Table 1.
Optimization of the Reaction Parametersa
 | ||||||
|---|---|---|---|---|---|---|
| entry | cosolvent [%]b |
T [°C] |
Pd | conc [M] |
yield [%]c |
ee [%]d |
| 1 | THF | 0 | Pd(COD)Cl2 | 0.1 | 50 | 85 |
| 2 | 2-MeTHF | 0 | Pd(COD)Cl2 | 0.1 | 61 | 85 |
| 3 | 2-MeTHF | 25 | Pd(COD)Cl2 | 0.1 | 48 | 79 |
| 4 | 2-MeTHF | −10 | Pd(COD)Cl2 | 0.1 | 67 | 86 |
| 5 | 2-MeTHF | −20 | Pd(COD)Cl2 | 0.1 | 56 | 87 |
| 6 | Tol (30) | −10 | Pd(COD)Cl2 | 0.1 | 79 | 86 |
| 7 | Tol (30) | −10 | Pd(COD)Cl2 | 0.2 | 76 | 84 |
| 8 | Tol (30) | −10 | Pd(COD)Cl2 | 0.05 | 84 | 86 |
| 9 | Tol (30) | −10 | Pd(OAc)2 | 0.05 | 79 | 89 |
| 10 | Tol (30) | −10 | Pd(NCPh)2Cl2 | 0.05 | 64 | 86 |
| 11 | Tol (30) | −10 | Pd(dba)2 | 0.05 | 72 | 87 |
| 12 | Tol (30) | −10 | [Pd(ally)Cl]2 | 0.05 | 72 | 88 |
| 13 | Tol (30) | −20 | Pd(OAc)2 | 0.05 | 84 | 89 |
| 14 | Tol (30) | −30 | Pd(OAc)2 | 0.05 | 87 | 91 |
| 15 | Tol (30) | −40 | Pd(OAc)2 | 0.05 | 80 | 91 |
| 16 | Tol (30) | −30 | Pd(OAc)2 | 0.05 | 63 | 91e |
| 17 | Tol (30) | −30 | Pd(OAc)2 | 0.05 | 92 | 92f |
Reactions performed using 1.0 equiv. of 1a and 2 equiv. of 2a on a 0.1 mmol scale.
Cosolvent indicates 30% toluene by volume in 2-MeTHF
Yields determined by 1H NMR analysis of crude mixtures with CH2Br2 as internal standard.
The ee was determined by chiral SFC
0.5 equiv. PMDTA was used.
1.5 equiv. PMDTA was used.
Palladium precursors can impact both activity and enantioselectivity in AAA.[3] Varying the palladium source from Pd(COD)Cl2 to Pd(OAc)2, Pd(dba)2, Pd(NCPh)2Cl2, [Pd(ally)Cl]2 (entries 9–12) revealed that Pd(OAc)2 resulted in the highest enantioselectivity (89% ee, 79% AY, entry 9). With Pd(OAc)2 we revisited the reaction temperature. As the temperature was decreased from −10 °C to −40 °C, the enantioselectivity increased to 91% at −30 °C (87% AY, entry 14). When the additive PMDTA was decreased to 0.5 equiv at −30 °C, the AY dropped to 63% (91% ee, entry 16). In contrast, the AY increased to 92% (92% ee) with 1.5 equiv. PMDTA (entry 17). The role of the PMDTA is likely to decrease the aggregation state of the LiN(SiMe3)2, facilitating the deprotonation of the arene complex.[23] The optimized reaction conditions in entry 17 afforded the AAA product in 92% yield and 92% ee, favoring the R enantiomer.[24]
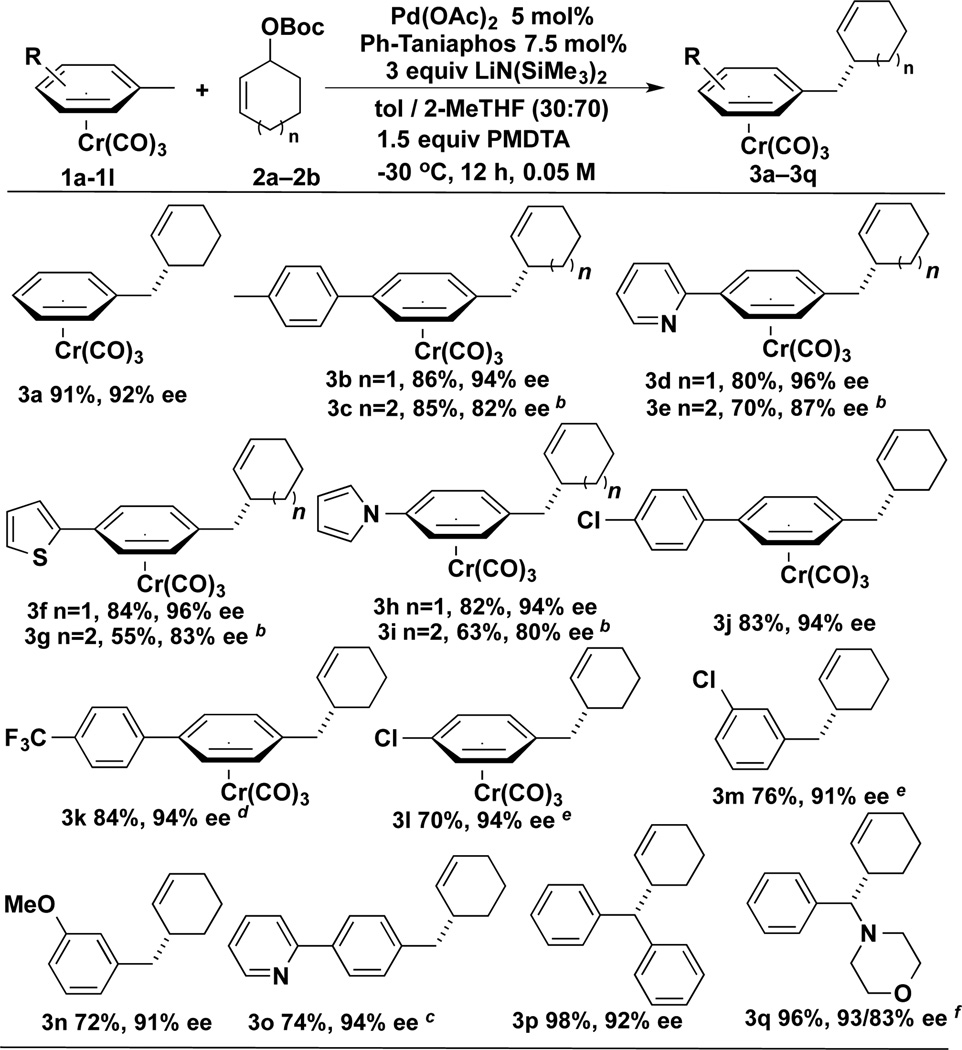
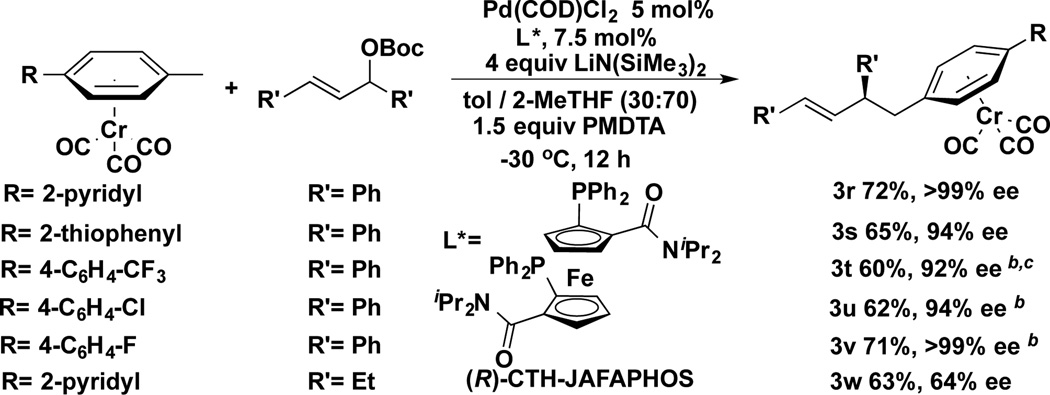
With the optimized reaction conditions in hand, we next examined the scope of the nucleophiles in the AAA with cyclohexenyl-OBoc and cycloheptenyl-OBoc (Scheme 3). In addition to the toluene complex (3a), a variety of substituents on the η6-arene were well tolerated. To compare the selectivity of the (η6-C6H5CH3)Cr(CO)3 with an unactivated tolyl group, the 4,4’-dimethylbiphenyl complex was examined. The AAA °Ccurred exclusively at the chromium-activated position giving the product in 86% yield with 94% ee (3b). Substrates bearing 2-pyridyl, 2-thiophene, and N-pyrrolyl groups on the 4-position of the η6-arene underwent AAA reaction affording the desired product in 80–84% yield with excellent enantioselectivities (94–96%, 3d, 3f, and 3h). The aryl chloride containing substrate was also a good partner, giving the desired product in 83% yield and 94% ee (3j). The trifluoromethyl containing biaryl substrate was compatible with this AAA reaction, furnishing the product with 84% yield and 94% ee (3k). Chromium complexes of aryl chlorides exclusively give the AAA products in excellent enantioselectivity (94% ee, 3l).[25] In addition to the cyclohexenyl-OBoc derivative, seven-membered allylic carbonate (Scheme 3, n=2) was also a competent electrophile. With the same set of pronucleophiles, the corresponding products were generated in 55–85% yields with 80–87% ee (3c, 3e, 3g, and 3i).
Scheme 3.
Scope of Pronucleophiles in AAA Reactionsa
[a] Isolated yields with ee’s determined by chiral HPLC. [b] Reactions performed using 1.0 equiv. of 1, 3 equiv. of 2b and 4 equiv LiN(SiNe3)2. [c] A two-step one pot procedure was employed to afford the chromium-free product with only 2% ee erosion. [d] Reaction time was 4 h. [e] Reaction time was 2.5 h. [f] The dr value was 55 : 45.
To demonstrate the versatility of this approach, a two-step one-pot procedure to afford the chromium-free products was explored. [η6-(3-Chlorotoluene)]Cr(CO)3 gave the demetallated product in 76% yield and 91% ee (3m).[25] The (η6-3-methylanisole)Cr(CO)3 complex gave 72% yield with 91% ee (3n). The 4-(2-pyridyl) containing toluene complex was also a good substrate, providing the product in 74% isolated yield with 94% ee (3o). Comparison of metallated (3d) and demetallated (3o) products indicates that demetallation °Ccurs with loss of 6% yield and only 2% erosion in the ee. Diarylmethane derivatives are important motifs in pharmaceuticals and have found wide application in material science.[26] The diphenylmethane complex (η6-C6H5CH2Ph)Cr(CO)3 gave the AAA/demetalated product in 98% yield with 92% ee (3p). We also examined the benzyl amine complex, which afforded the allylation product as ~ 1:1 ratio of diastereomers in 96% yield with 83 and 93% ee (3q). These different ee’s suggest that the AAA products (3q) do not epimerize under the reaction conditions.
We next investigated palladium-catalyzed allylic substitutions with acyclic allylic substrates, starting with (E)-tert-butyl (1,3-diphenylallyl) carbonate (Scheme 4). Given the significant difference between acyclic and cyclic substrates, it is not surprising that Ph-Taniaphos did not give high enantioselectivity. After rescreening a subset of ligands (see Supporting Information) we found that (R)-CTH-JAFAPHOS[27] (Scheme 4) was the leading hit. (E)-tert-Butyl (1,3-diphenylallyl) carbonate underwent AAA to furnish the corresponding products 3r (72% yield, >99% ee) and 3s (65% yield, 94% ee). Substrates containing 4-CF3, 4-Cl and 4-F were also compatible with the AAA affording the allylated products with excellent enantioselectivities (3t–3v, 92 to >99% ee). (E)-tert-Butyl (1,3-diethylallyl) carbonate exhibited lower enantioselectivity (3w, 63% yield, 64% ee).
Scheme 4.
Palladium/(R)-CTH-JAFAPHOS catalyzed AAA with acyclic electrophiles.a
[a] Isolated yields with ee’s determined by chiral HPLC or SFC. [b] 10 mol% catalysis was used. [c] Reaction time was 4h.
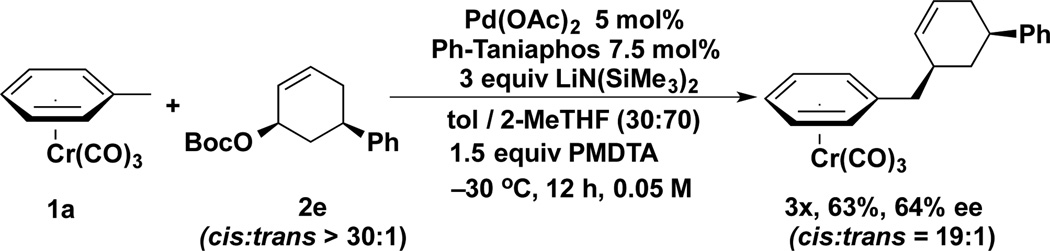
To determine if our organolithium nucleophiles react via the “double inversion” pathway, the toluene complex was coupled with the cis-disubstituted stereoprobe (Scheme 5). The product (3x) was obtained in 64% yield with 64% ee (unoptimized). Comparison of the 1H NMR coupling constants with related compounds led to the conclusion that this reaction proceeded by the “soft” nucleophile pathway.[20]
Scheme 5.
AAA reaction and mechanistic study with Ph-Taniaphos.
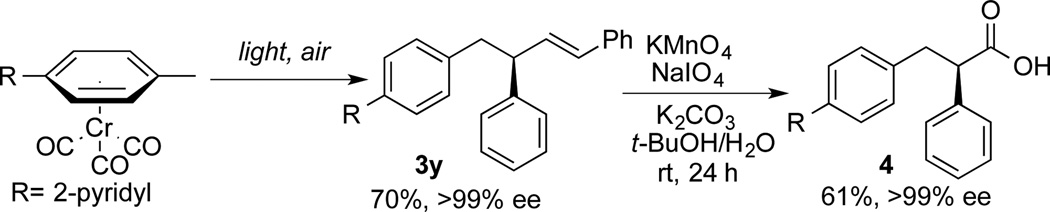
To demonstrate the utility of our protocol, a nonsteroidal anti-inflammatory drug analogue (NSAIDs)[28] was prepared in two steps (Scheme 6). Beginning with the pyridyl-containing toluene complex 1c, AAA/demetalation as outlined in Scheme 3 gave compound 3y in >99% ee. The allylated product 3y was then converted to enantioenriched α-arylalkanoic acid 4 in 61% yield with >99% ee (Scheme 6).
Scheme 6.
Further transformations of the allylated product.
In conclusion, we have successfully developed the first two catalysts for the palladium-catalyzed AAA employing toluene-derived pronucleophiles. This method provides ready access to the enantioenriched “α-2-propenyl benzyl” motifs and expands the classes of nucleophiles that can be employed in AAA reactions. It is noteworthy that the optimized catalysts for both AAA reactions advanced herein, and for our palladium(Cy-Mandyphos) catalyzed enantioselective arylation of (η6-C6H5CH2NR2)Cr(CO)3 complexes,[18] all contain strongly coordinating tertiary amine or amide groups on the ligands that can serve to bind the lithium counterions of the nucleophiles. This observation suggests a possible design feature for related enantioselective processes with strongly basic lithiated nucleophiles.
Supplementary Material
Acknowledgments
We thank the National Science Foundation (CHE-1464744) and National Institutes of Health (NIGMS 104349) for financial support.
Contributor Information
Dr. Jianyou Mao, Department of Applied Chemistry, China Agricultural University, 2 West Yuanmingyuan Road, Beijing 100193, P. R. China Roy and Diana Vagelos Laboratories, Department of Chemistry. University of Pennsylvania, 231 South 34th Street, Philadelphia, Pennsylvania 19104-6323, United States.
Dr. Jiadi Zhang, Roy and Diana Vagelos Laboratories, Department of Chemistry. University of Pennsylvania, 231 South 34th Street, Philadelphia, Pennsylvania 19104-6323, United States
Hui Jiang, Department of Applied Chemistry, China Agricultural University, 2 West Yuanmingyuan Road, Beijing 100193, P. R. China; Roy and Diana Vagelos Laboratories, Department of Chemistry. University of Pennsylvania, 231 South 34th Street, Philadelphia, Pennsylvania 19104-6323, United States.
Dr. Ana Bellomo, Roy and Diana Vagelos Laboratories, Department of Chemistry. University of Pennsylvania, 231 South 34th Street, Philadelphia, Pennsylvania 19104-6323, United States
Mengnan Zhang, Roy and Diana Vagelos Laboratories, Department of Chemistry. University of Pennsylvania, 231 South 34th Street, Philadelphia, Pennsylvania 19104-6323, United States.
Zidong Gao, Department of Applied Chemistry, China Agricultural University, 2 West Yuanmingyuan Road, Beijing 100193, P. R. China.
Dr. Spencer D. Dreher, Department of Process Chemistry, Merck Research Laboratories, P.O. Box 2000, Rahway, New Jersey 07065, United States
Prof. Patrick J. Walsh, Roy and Diana Vagelos Laboratories, Department of Chemistry. University of Pennsylvania, 231 South 34th Street, Philadelphia, Pennsylvania 19104-6323, United States
References
- 1.a) Trost BM, Van Vranken DL. Chem. Rev. 1996;96:395–422. doi: 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]; b) Trost BM. Chem. Pharm. Bull. 2002;50:1–14. doi: 10.1248/cpb.50.1. [DOI] [PubMed] [Google Scholar]; c) Graening T, Schmalz H-G. Angew. Chem. 2003;115:2684–2688. doi: 10.1002/anie.200301644. Angew. Chem., Int. Ed. 2003, 42, 2580–2584. [DOI] [PubMed] [Google Scholar]
- 2.a) Trost BM, Dirat O, Dudash J, Jr, Hembre EJ. Angew. Chem. 2001;113:3770–3772. doi: 10.1002/1521-3773(20011001)40:19<3658::aid-anie3658>3.0.co;2-2. Angew. Chem., Int. Ed. 2001, 40, 3658–3660. [DOI] [PubMed] [Google Scholar]; b) Trost BM, Dudash J, Jr, Hembre EJ. Chem. Eur. J. 2001;7:1619–1629. doi: 10.1002/1521-3765(20010417)7:8<1619::aid-chem16190>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Hartwig JF. Organotransition Metal Chemistry: From Bonding to Catalysis. Sausalito: University Science Books; 2011. [Google Scholar]
- 4.a) Dawson GJ, Frost CG, Williams JMJ, Coote SJ. Tetrahedron Lett. 1993;34:3149–3150. [Google Scholar]; b) Sprinz J, Helmchen G. Tetrahedron Lett. 1993;34:1769–1772. [Google Scholar]; c) Trost BM, Bunt RC. J Am. Chem. Soc. 1994;116:4089–4090. [Google Scholar]
- 5.a) Trost BM, Krueger AC, Bunt RC, Zambrano J. J Am. Chem. Soc. 1996;118:6520–6521. [Google Scholar]; b) Trost BM, Patterson DE. Chem. - Eur. J. 1999;5:3279–3284. [Google Scholar]
- 6.Trost BM, Crawley ML. Chem. Rev. 2003;103:2921–2944. doi: 10.1021/cr020027w. [DOI] [PubMed] [Google Scholar]
- 7.a) Ardolino MJ, Morken JP. J Am. Chem. Soc. 2014;136:7092–7100. doi: 10.1021/ja502280w. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang P, Brozek LA, Morken JP. J Am. Chem. Soc. 2010;132:10686–10688. doi: 10.1021/ja105161f. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhang P, Le H, Kyne RE, Morken JP. J Am. Chem. Soc. 2011;133:9716–9719. doi: 10.1021/ja2039248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Misale A, Niyomchon S, Luparia M, Maulide N. Angew. Chem. 2014;126:7188–7193. doi: 10.1002/anie.201309074. Angew. Chem., Int. Ed. 2014, 53, 7068–7073. [DOI] [PubMed] [Google Scholar]; b) Niyomchon S, Audisio D, Luparia M, Maulide N. Org. Lett. 2013;15:2318–2321. doi: 10.1021/ol401033g. [DOI] [PubMed] [Google Scholar]; c) Audisio D, Gopakumar G, Xie L-G, Alves LG, Wirtz C, Martins AM, Thiel W, Fares C, Maulide N. Angew. Chem. 2013;125:6434–6438. doi: 10.1002/anie.201301034. Angew. Chem., Int. Ed. 2013, 52, 6313–6316. [DOI] [PubMed] [Google Scholar]
- 9.a) Trost BM, Verhoeven TR. J Org. Chem. 1976;41:3215–3216. [Google Scholar]; b) Matsushita H, Negishi E. J Chem. Soc., Chem. Commun. 1982:160–161. [Google Scholar]
- 10.You H, Rideau E, Sidera M, Fletcher SP. Nature. 2015;517:351–355. doi: 10.1038/nature14089. [DOI] [PubMed] [Google Scholar]
- 11.a) Trost BM, Thaisrivongs DA. J Am. Chem. Soc. 2008;130:14092–14093. doi: 10.1021/ja806781u. [DOI] [PubMed] [Google Scholar]; b) Trost BM, Thaisrivongs DA. J Am Chem Soc. 2009;131:12056–12057. doi: 10.1021/ja904441a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewick PM. Essentials of Organic Chemistry: For Students of Pharmacy, Medicinal Chemistry and Biological Chemistry. Chichester, West Sussex, UK: John Wiley & Sons, Ltd; 2006. [Google Scholar]
- 13.Bordwell FG. Acc. Chem. Res. 1988;21:456–463. [Google Scholar]
- 14.Tanaka A, Hattori K, Taniguchi K, Okitsu O, Tabuchi S, Nishio M, Nagakura Y, Maeda N, Murai H, Seki J. Bioorg. Med. Chem. Lett. 2006;16:4861–4864. doi: 10.1016/j.bmcl.2006.06.076. [DOI] [PubMed] [Google Scholar]
- 15.Sato M, Tsuchiya H, Takase I, Kureshiro H, Tanigaki S, Iinuma M. Phytother. Res. 1995;9:509–512. [Google Scholar]
- 16.Murray LM, Johnson A, Diaz MC, Crews P. J Org. Chem. 1997;62:5638–5641. [Google Scholar]
- 17. McGrew GI, Temaismithi J, Carroll PJ, Walsh PJ. Angew. Chem. 2010;122:5673–5676. doi: 10.1002/anie.201000957. Angew. Chem., Int. Ed. 2010, 49, 5541–5544. Recently Larrosa reported Cr(CO)3 activated C-H arylation see: Ricci P, Kramer K, Larrosa I. J Am. Chem. Soc. 2014;136:18082–18086. doi: 10.1021/ja510260j.
- 18.McGrew GI, Stanciu C, Zhang J, Carroll PJ, Dreher SD, Walsh PJ. Angew. Chem. 2012;124:11678–11681. doi: 10.1002/anie.201201874. Angew. Chem., Int. Ed. 2012, 51, 11510–11513. [DOI] [PubMed] [Google Scholar]
- 19.Kalinin and co-workers generated [(η6-C6H5CH2Li)Cr(CO)3] and transmetallated this nucleophile to zinc see: Kalinin VN, Cherepanov IA, Moiseev SK. J Organomet. Chem. 1997;536:437–455.
- 20. Zhang J, Stanciu C, Wang B, Hussain MM, Da C-S, Carroll PJ, Dreher SD, Walsh PJ. J Am. Chem. Soc. 2011;133:20552–20560. doi: 10.1021/ja208935u. For allylic substitution with basic carbanions see: Sha S-C, Zhang J, Carroll PJ, Walsh PJ. J Am. Chem. Soc. 2013;135:17602–17609. doi: 10.1021/ja409511n.
- 21.We heve previously demonstrated the use of additives in palladium catalyzed reactions. See: Bellomo A, Zhang J, Trongsiriwat N, Walsh PJ. Chem. Sci. 2013;4:849–857. For use of pentamethyldiethylenetriamine as additive see ref 18.
- 22.a) Ireland T, Grossheimann G, Wieser-Jeunesse C, Knochel P. Angew. Chem. 1999;111:3397–3400. Angew. Chem., Int. Ed. 1999, 38, 3212–3215. [PubMed] [Google Scholar]; b) Ireland T, Tappe K, Grossheimann G, Knochel P. Chem. - Eur. J. 2002;8:843–852. doi: 10.1002/1521-3765(20020215)8:4<843::aid-chem843>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Gessner VH, Daeschlein C, Strohmann C. Chem. Eur. J. 2009;15:3320–3334. doi: 10.1002/chem.200900041. [DOI] [PubMed] [Google Scholar]
- 24.Assignment based on comparison with reported optical rotation values after the chromium was removed. See: Langlois J-B, Alexakis A. Adv. Synth. Catal. 2010;352:447–457.
- 25.Chromium complexes of aryl chlorides exhibit increased susceptibility to reactions see: Uemura M, Nishimura H, Kamikawa K, Nakayama K, Hayashi Y. Tetrahedron Lett. 1994;35:1909–1912.
- 26.Attwood D. J Pharm. Pharmacol. 1976;28:407–409. doi: 10.1111/j.2042-7158.1976.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 27.Jendralla H, Paulus E. Synlett. 1997:471–472. [Google Scholar]
- 28.Landoni MF, Soraci A. Curr. Drug Metab. 2001;2:37–51. doi: 10.2174/1389200013338810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.