Abstract
Brain metastases occur in 20 to 40% of patients with metastatic breast cancer. The process is complex and depends on successful cancer cell evasion from the primary tumor, distribution and survival within the blood stream and cerebral microvasculature, penetration of the blood brain barrier and proliferation within the brain microenvironment. The initial steps of brain colonization are difficult to study in vivo. Therefore, in vitro assays have been developed to mimic this process. Most commonly, in vitro studies of brain colonization focus on tumor cell adhesion to brain endothelial cells and transendothelial migration. We previously investigated breast cancer brain colonization from the blood stream in vivo and defined the time and process of brain entry for five different cancer cell lines in a mouse model. We now investigated if in vitro approaches can reliably emulate the initial steps that determine successful brain colonization in vivo. To this end, we optimized an in vitro model of the vascular blood brain barrier and compared the brain invasion properties of the in vivo characterized cell models with their ability to interact with and penetrate the blood brain barrier model in vitro. Our results show that the in vitro findings correlate only poorly with the vivo results. The limitations of the in vitro approaches are discussed in light of the in vivo processes. We conclude that investigation of mechanisms supporting the earliest steps of breast cancer brain metastasis from the blood stream will depend on in vivo analyses.
Keywords: Brain metastasis, Breast cancer, In vitro, In vivo, Blood brain barrier, Transendothelial migration, Extravasation, Brain metastasis model
Introduction
Brain metastases develop in 20 to 40% of patients with metastatic breast cancer, mostly late during disease progression [1–4]. The incidence is increasing, likely due to improved treatments for extracranial disease and longer patient survival. Current therapies include surgical resection combined with whole-brain radiation and/or chemotherapy, and stereotactic radiosurgery (gamma knife). Irrespective of these treatments, the prognosis is poor with a median survival time of 7–16 months after diagnosis [2, 5–7]. To develop better treatments and/or preventive measures, experimental models are needed that adequately recapitulate the complex process of brain metastasis.
Upon primary tumor evasion, cancer cells within the blood stream have to survive, attach to target organ vessels, extravasate and resume proliferation to establish metastatic lesions. This process depends on a number of sequential and rate-limiting steps that are difficult to dissect in vivo due to high complexity and experimental limitations. In vitro assays have been developed in an attempt to mimic individual steps of the metastatic cascade. Among these are cancer cell adhesion assays to endothelial monolayers, their matrix, and transendothelial migration. In vitro models use endothelial monolayers grown on permeable porous filters to mimic the vessel wall and allow transmigration of lymphocytes and cancer cells across the endothelial barrier.
In vivo studies using animal models to analyze cancer cell colonization of the brain are very scarce. However, they clearly demonstrate that extravasation into the brain takes significantly longer than extravasation into other organs [8–11]. This is likely because the blood–brain barrier (BBB) is significantly tighter than blood-organ barriers in other tissues [12–20]. Only brain endothelial cells are supported by astrocyte end-feet, which contribute to the tightness of the BBB. Furthermore, brain endothelial cells have prominent tight junctions and metabolic barriers that strongly restrict the passage of cells and molecules, unlike vessels in other organs. All of these characteristics must be considered when establishing a model for studying extravasation of cancer cells through the BBB. Different in vitro models of the BBB have been reported using endothelial cells of murine, rat, bovine, porcine and human origin [19, 21, 22]. The majority of the human models use human umbilical vein endothelial cells. Due to the specific nature and differentiation state of these cells, their monolayers achieve only limited tightness and cannot truly mimic the characteristics of the BBB. Furthermore, endothelial cells display organ specific markers and functional determinants [12–14]. Therefore, it is important to use brain derived endothelial cells when studying tumor cell extravasation into the brain. Current models of the human BBB are based on primary cells from the human brain and often have limited reproducibility because tissue samples vary. In this study, we used commercially available primary human brain microvascular endothelial cells (HBMEC) from donor pools. We optimized the culture conditions to increase endothelial monolayer tightness, a distinctive characteristic of the BBB in vivo. We defined cell culture parameters that result in a highly reproducible BBB model which expresses both endothelial and brain-specific markers, achieves a high level of tightness, and is not hampered by lack of material. We used this model to study attachment and extravasation of five different breast cancer cell lines. We compared the in vitro findings to our previous results from studies of the same cell lines in vivo [11]. We show that there is very poor correlation between the ability of breast cancer cells to colonize the brain in vivo and to adhere to endothelial cells or their subendothelial matrix, and cross the optimized BBB model in vitro. Thus, our results suggest that in vitro data for malignant brain colonization must be interpreted with caution, as they do not reliably correlate with in vivo data.
Materials and methods
Cells
MDA-MB-435, MDA-MB-231, MDA-MB-231/brain [23], 4T1, and MCF-7 cells were grown as described [11]. Culturing of primary human brain microvascular endothelial cells (HBMEC) and astrocytes is detailed in supplementary material.
Animal models of brain metastasis
Animal models of brain metastasis were previously described [11].
In vitro model of the human blood brain barrier
To optimize the in vitro BBB model, HBMEC were cultured on Boyden chamber inserts with or without astrocytes on the porous filter underside. Exact culture conditions, ECM matrix, media, and measurement of the trans-endothelial electrical resistance (TEER) are described in Supplementary material.
Immunocytochemistry and immunofluorescence microscopy
Antibodies used were anti-ZO-1 (Zymed), anti-GFAP (Promega), anti-GLUT-1 (R&D Systems), anti-vWF mAb 2.2.9 [24, 25], anti-p-selectin (BD Pharmingen), and anti-ICAM-1 (BD Pharmingen). Secondary antibodies were conjugated with AlexaFluor594, FITC, or APC (Molecular Probes and Jacksons Immunoresearch). Detailed protocols are provided in Supplementary material.
Trans-endothelial migration assay and adhesions assay
Breast cancer cells labeled with CellTracker Orange (Cambrex) were seeded onto HBMEC monolayers and migration stopped after 24 h by fixation with 4% PFA. Transmigrated cells were counted within the entire area of the filter underside. Details are given in Supplementary material.
Results and discussion
Establishment of an in vitro blood brain barrier model
High impermeability is an important characteristic of the blood–brain barrier (BBB) in vivo, and distinguishes brain endothelial cells from those of other tissues [17, 19]. This impermeability is thought to be a major hurdle for tumor cell entry into the brain. To optimize our BBB model, we therefore used transendothelial electrical resistance (TEER) as a reliable measure of monolayer tightness, to define growth conditions under which primary human brain microvascular endothelial cells (HBMEC) maintain their in vivo characteristics and barrier properties.
An important component of the vessel wall and determinant of vascular integrity is the subendothelial basement membrane. In the brain, it consists mainly of fibronectin, laminin and collagen [26, 27]. We therefore investigated the influence of the individual matrix components on the tightness of brain endothelial monolayers in vitro. HBMEC were grown on matrix protein-coated tissue culture inserts, and TEER was measured on days 2, 3, and 4 (Fig. 1a). Barrier tightness was best supported by fibronectin (TEER of 30 Ω cm2), followed by astrocyte-derived ECM (attachment factor, ScinCell), collagen I, and laminin. Fibronectin was therefore used in all further experiments.
Fig. 1.
In-vitro model of the human blood–brain barrier (BBB). a Effects of extracellular matrix proteins on the transendothelial resistance (TEER) of human brain microvascular endothelial cell (HBMEC) monolayers. Porous filters of Boyden chamber tissue culture inserts were coated with the indicated extracellular matrix proteins before seeding the endothelial cells. TEER was measured daily for 4 days. Attachment factor: astrocyte matrix (commercially available from Cell Systems, Kirkland). b Comparison of HBMEC monocultures and HBMEC co-cultures with primary human astrocytes in different cell culture media. HBMEC alone or together with astrocytes (grown at the underside of the culture inserts) were cultured in the indicated media for 7 days. CSC− CS-C Complete Serum-Free Medium (Cell Systems), CSC + CS-C Complete Medium with 10% serum (Cell Systems), F12, ECM: Endothelial Cell Medium (ScienCell), EGM-MV EGM-MV Microvascular Endothelial Cell Medium (Clonetics). c HBMEC (in the absence of astrocytes) were grown on culture inserts in ECM alone or in ECM containing 50% astrocyte-conditioned medium (ACM). The latter significantly increased TEER of the endothelial monolayers. Further increase of TEER was achieved by adding cAMP-elevating agents (250 μM CPT-cAMP + 35 μM RO-20-1724) to cultures containing 50% ACM on day 7
In addition to brain endothelial cells, astrocytes are part of the BBB. Reportedly, endothelial monolayer tightness increases by co-culture of endothelial cells and astrocytes or co-incubation with astrocyte-conditioned medium [20, 22, 28, 29]. To find the best conditions for our model, HBMECs were grown in different media, with or without astrocytes at the filter underside of the culture inserts. Primary human astrocytes were used, and tested positive for GFAP expression by immunocytochemistry and Western blot (data not shown). Figure 1b shows that the tightness of the BBB model varied significantly depending on the culture media. Notably, astrocytes supported increased barrier tightness in some media (CSC- and F12), but had the opposite effect in others (CSC+, ECM, and EGM-MV). The tightest monolayer was obtained in ECM medium without astrocytes (40 Ω cm2). Therefore, these conditions were used for further optimization.
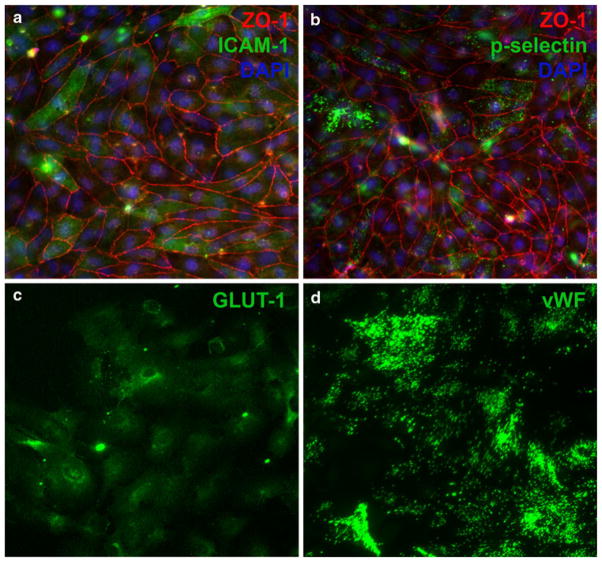
Since direct contact between astrocytes and endothelial cells did not enhance barrier function in our model, we tested the role of soluble astrocytes-derived factors. We found that 50% astrocyte-conditioned medium significantly increased endothelial monolayer tightness (Fig. 1c), reaching a TEER value of 100 Ω cm2 on culture day 9. Finally, increased cAMP reportedly enhances endothelial monolayer tightness in vitro [29–31]. In our model, cAMP-elevating agents CPT-cAMP and RO-20-1724, added on day 7, increased TEER to 120 Ω cm2 (Fig. 1c). The brain-endothelial character of HBMECs, cultured under these conditions, was confirmed by expression of endothelial cell markers ZO-1, von Willebrand factor (vWF), and P-selectin, and brain-specific marker GLUT-1 (Fig. 2) [19]. Without stimulation, only a minority of the endothelial cells expressed ICAM-1, indicating minimal activation (Fig. 2a). ICAM-1 expression was up-regulated upon incubation with TNF-α, mimicking inflammatory conditions in vivo and documenting endothelial responsiveness (not shown). TNF-α was not used in the BBB model because it is known to compromise endothelial barrier integrity. Otherwise, the above-optimized conditions were applied to generate the in vitro BBB model for analyses of breast cancer cell adhesion and transendothelial migration.
Fig. 2.
Expression of endothelial and brain-specific markers by HBMEC monolayers. During their culture on Boyden chamber culture inserts, HBMEC monolayers expressed endothelial markers ZO-1 (a, b), p-selectin (b), and von Willebrand Factor (vWF) (d), as well as brain specific marker GLUT-1 (c). Without stimulation, ICAM-1 (a) was expressed by only few cells and at a low level, shown on Day 8 in culture
Breast cancer brain colonization in vivo and blood brain barrier interaction in vitro
We previously characterized the brain colonization properties of five breast cancer cell lines after carotid artery injection in experimental mice. We followed BBB penetration, initial proliferation and host cell responses by detailed histology [11]. Thus, the investigations focused on initial steps of brain metastasis including tumor cell arrest within the cerebral microvasculature and extravasation into the brain parenchyma. By non-invasive bioluminescence imaging, firefly-luciferase tagged MCF-7, MDA-MB-231 cells and their in vivo selected brain-homing variant (231 brain), MDA-MB-435, and 4T1 cells, all showed significant signal in the brain 15 min after carotid artery injection. However, histology revealed that only MDA-MB-231 brain, MDA-MB-435, and 4T1 cells successfully extravasated, and that this occurred between day 3 and 5 [11]. In the long-term, only MDA-MB-435 (435), MDA-MB-231/brain (231/brain) and 4T1 cells successfully colonized the brain in 100% of the animals after injecting 1 × 104 or 1 × 105 cells (Fig. 3a). 231 parental cells colonized the brain poorly, producing brain lesions in only 10% of the animals (1 × 105 cells injected). The non-metastatic cell line MCF-7 failed to colonize the brain completely. From the three cell lines that successfully entered the brain, 4T1 cells grew the fastest while 435 and 231/brain cells displayed a lag phase of 7–10 days, before resuming growth within the brain tissue (Fig. 3b) [11].
Fig. 3.
Comparison of in vivo brain colonization and in vitro tumor cell interaction with HBMEC monolayers. a Ability of MDA-MB 435, 4T1, MDA-MB 231brain, and MCF7 breast cancer cells to colonize the brain in the mouse model. Percentage of animals with brain metastasis after injection of 1×105 cancer cells into the carotid artery. b Intracranial growth of 4T1, MDA-MB 231brain and MDA-MB 435 breast cancer cells after carotid artery injection. Tumor cell growth was quantified by non-invasive bioluminescence imaging of the animals following the signal from the F-luc tagged cancer cells. c Adhesion of the cancer cells used in (a) to human brain microvascular endothelial (HBMEC) monolayers in vitro. d Transendothelial migration of the cancer cells in vitro. Monolayers used in c and d had achieved the highest possible tightness, as shown in Fig. 1c, before addition of the tumor cells. e Adhesion of cancer cells to subendothelial matrix of HBMEC monolayers
To investigate if and to what degree in vivo brain colonization is mirrored by the ability of the tumor cells to attach to and invade monolayers of cultured brain microvascular endothelial cells, we analyzed the in vivo studied breast cancer cell models in our BBB model in vitro. This comparison of in vivo and in vitro properties is important, because in vitro assays of tumor cell adhesion to endothelial monolayers and transendothelial migration are often performed to analyze molecular functions required for brain metastasis. However, the in vitro data are rarely validated in vivo.
We first analyzed adhesion of the in vivo validated cancer cells to monolayers of primary brain microvascular endothelial cells grown under conditions that gave the tightest brain endothelial monolayer (Fig. 3c). 4T1 cells colonized the brain most aggressively in vivo, and showed the highest adhesion rate to endothelial cells in vitro (12%). 231/brain cells showed a lower adhesion rate than parental 231 cells (4% versus 6.5%), followed by MCF-7 (3.5%) and 435 cells (2%). Together, brain colonization in vivo correlated only partially with cancer cell adhesion to endothelial monolayers in vitro. Notably, in vivo all breast cancer cell lines initially showed high signal in the brain [11]. This might be due to specific tumor cell adhesion to cerebral endothelial cells, passive trapping within the brain capillaries, or a combination of both. We next examined the ability of the tumor cells to penetrate the BBB model in vitro. 4T1 and 435 cells, which potently colonized the brain in vivo, showed no significant transmigration across brain endothelial monolayers in vitro (0.03% cells penetrating) (Fig. 3d). The same was true for the non-metastatic MCF-7 cells (0.01%). The highest trans-endothelial migration rate in vitro was achieved by parental 231 cells (1.6%), and this rate exceeded the rate achieved by 231/brain cells by ninefold. These findings indicate that there was no correlation between the brain colonization potential of the cancer cells in vivo and their ability to penetrate brain endothelial monolayers in vitro. Even 231 and 231/brain cells, which share a common genetic background, displayed an inverse correlation between the rate of in vitro trans-endothelial migration and in vivo brain colonization. Thus, a correlation between in vitro and in vivo capabilities might be observed for certain cell models but not for others, and can therefore not be expected as a general rule.
The vascular basement membrane in the brain is thought to serve as a soil for brain metastasis, since metastatic cancer cells can grow around co-opted brain vessels, and in vitro preferentially adhere to the vascular basement membrane in brain slices [11, 32, 33]. We therefore investigated whether a correlation exists between brain metastasis in vivo and tumor cell adhesion to the vascular basement membrane in vitro. Comparing the above five cancer cell lines, we found that the tumor cell ability to attach to the subendothelial matrix correlated with their adhesion to the endothelial cells (Fig. 3e). Again, the most aggressive cell line 4T1 showed the highest adhesion rate, followed by 231 and 231/brain, MCF-7 and 435 cells. Thus, as for tumor cell attachment to brain microvascular endothelial cells, adhesion to the subendothelial matrix only partially correlated with tumor cell colonization of the brain in vivo.
Mechanisms of brain metastasis from extracranial solid tumors are poorly understood. Yet, cerebral involvement becomes an increasing clinical problem, especially in breast cancer. The desire to prevent brain colonization by circulating tumor cells is therefore high and demands a better understanding of specific functions and molecular determinants that promote breast cancer cell penetration of the BBB and successful brain colonization. Therefore, experimental models that faithfully emulate the initial steps of brain colonization would be of utmost importance. Having characterized the initial events of breast cancer brain colonization in the complexity of the in vivo situation using mouse models, we therefore critically evaluated in vitro models of the BBB as surrogate systems for studying early steps of brain metastasis. Based on a systematic comparison of in vivo and in vitro behavior of five distinct breast cancer cell models, we essentially found no correlation between the ability of breast cancer cells to colonize the brain in vivo and their capacity to adhere to and penetrate a model of the BBB in vitro. In vivo, arrest of circulating cancer cells in brain capillaries is likely due to a combination of trapping within narrow capillaries and receptor-mediated cancer cell adhesion to the endothelium. In our in vivo model, the analyzed breast cancer cell lines differed significantly in their ability to persist within the brain vasculature upon initial arrest. For example, after carotid artery injection the non-metastatic cell line MCF-7 was undetectable in the brain from day 2 on. Unlike in other organs, cancer cells that are arrested within the brain vasculature need to survive within blood vessels for 3–5 days before they can extravasate [10, 11]. It is possible that the ability of cancer cells to adhere to local endothelial cells is important for their permanent arrest. However, we found only a poor correlation between brain metastasis in vivo and cancer cell adhesion to brain microvascular endothelial cells in vitro. Following adhesion, the ability of cancer cells to survive within the cerebral microvasculature for prolonged periods of time before extravasation (3–5 days) is very likely crucial.
Molecular mediators of extravasation in vitro have been previously correlated to the potential of cancer cells to colonize the brain in vivo. For example, Bos et al. [34] recently reported α2,6-sialyltransferase as a possible contributor to breast cancer cell extravasation into the brain. Indicated by gene expression profiling of brain homing variants of breast cancer cell lines and patient samples, knock-down of α2,6 sialyltransferase expression reduced endothelial transmigration in vitro. The in vitro study can be valuable. As indicated by our findings, in vivo analysis of cells expressing or lacking this enzyme should yield definitive results. We found no correlation between trans-endothelial migration in vitro and brain colonization in vivo. Even MDA-MB-231 and MDA-MB-231/brain cells, actually displayed an inverse correlation between in vitro and in vivo parameters. Although our model of the BBB was optimized to obtain controlled, high tightness and expression of endothelial- and brain-specific markers, the in vitro model cannot accurately reflect the in vivo complexity. For example, presence and dynamic interactions of astrocytes and pericytes are missing. Endothelial cells form tubes in vivo but are grown as a monolayer in vitro. Another weakness of the in vitro models is that they can be used only for short-term experiments. This is due to a limited time span during which cultured brain endothelial monolayers maintain their stability. Most studies perform transmigration assays for 6–20 h. We previously determined the time course of breast cancer cell extravasation into the mouse brain in vivo for three different breast cancer cell lines and found no extravasated cells for up to 48 h after carotid artery injection [11]. The cancer cells extravasated only 3–5 days after arresting within the vasculature. Thus, the time course required for extravasation in vivo cannot be recapitulated in vitro due to destabilization of brain endothelial monolayers, and migration of endothelial cells to the lower insert chamber after prolonged periods in culture. Furthermore, the complexity of intravascular host cell types, their release of bioactive factors, and possible contribution of coagulation proteins cannot be fully emulated in vitro.
In summary, our comparison of in vivo brain colonization and in vitro interaction with brain microvascular endothelial cells, their matrix and transendothelial migration of five different breast cancer cell lines suggests that in vitro assays only poorly reflect the ability of breast cancer cells to colonize the brain. Thus, definitive and clinically relevant findings on mechanisms of the earliest steps of breast cancer brain metastasis and their prevention will depend on investigations in animal models.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute of Health (CA112287 to BFH), Department of Defense (W81XWH-08-1-0468 to BFH), California Breast Cancer Research Program (15IB-0050, 16IB-0052 to BFH and 16IB-0030 to ML), and a Susan G. Komen Breast Cancer Foundation fellowship (PDF0707935 to ML).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11060-011-0550-4) contains supplementary material, which is available to authorized users.
References
- 1.Al-Shamy G, Sawaya R. Management of brain metastases: the indispensable role of surgery. J Neurooncol. 2009;92(3):275–282. doi: 10.1007/s11060-009-9839-y. [DOI] [PubMed] [Google Scholar]
- 2.Santarelli JG, Sarkissian V, Hou LC, Veeravagu A, Tse V. Molecular events of brain metastasis. Neurosurg Focus. 2007;22(3):E1. doi: 10.3171/foc.2007.22.3.2. [DOI] [PubMed] [Google Scholar]
- 3.Grewal J, Kesari S. Breast cancer surface receptors predict risk for developing brain metastasis and subsequent prognosis. Breast Cancer Res. 2008;10(2):104. doi: 10.1186/bcr1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grewal J, Zhou H, Factor R, Kesari S. Isolated loss of hormonal receptors in leptomeningeal metastasis from estrogen receptor- and progesterone receptor-positive lobular breast cancer. J Clin Oncol. 2010;28(13):200–202. doi: 10.1200/JCO.2009.25.3518. [DOI] [PubMed] [Google Scholar]
- 5.DeAngelis LM. Treatment of brain metastasis. J Support Oncol. 2008;6(2):87–88. [PubMed] [Google Scholar]
- 6.Gerrard GE, Franks KN. Overview of the diagnosis and management of brain, spine, and meningeal metastases. J Neurol Neurosurg Psychiatry. 2004;75(Suppl2):ii37–ii42. doi: 10.1136/jnnp.2004.040493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norden AD, Wen PY, Kesari S. Brain metastases. Curr Opin Neurol. 2005;18(6):654–661. doi: 10.1097/01.wco.0000191514.37498.2b. [DOI] [PubMed] [Google Scholar]
- 8.Ballinger WE, Jr, Schimpff RD. An experimental model for cerebral metastasis: preliminary light and ultrastructural studies. J Neuropathol Exp Neurol. 1979;38(1):19–34. doi: 10.1097/00005072-197901000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi T, Tobai S, Nakamura K. Extravascular migration of tumor cells in the brain: an electron microscopic study. Invasion Metastasis. 1982;2(1):40–50. [PubMed] [Google Scholar]
- 10.Paku S, Dome B, Toth R, Timar J. Organ-specificity of the extravasation process: an ultrastructural study. Clin Exp Metastasis. 2000;18(6):481–492. doi: 10.1023/a:1011858925376. [DOI] [PubMed] [Google Scholar]
- 11.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176(6):2958–2971. doi: 10.2353/ajpath.2010.090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kieda C. How endothelial cell organo-specificity mediates circulating cell homing. Arch Immunol Ther Exp (Warsz) 2003;51(2):81–89. [PubMed] [Google Scholar]
- 13.Nicolson GL, Menter DG, Herrmann J, Cavanaugh P, Jia L, Hamada J, Yun Z, Nakajima M, Marchetti D. Tumor metastasis to brain: role of endothelial cells, neurotrophins, and paracrine growth factors. Crit Rev Oncog. 1994;5(5):451–471. doi: 10.1615/critrevoncog.v5.i5.20. [DOI] [PubMed] [Google Scholar]
- 14.Pauli BU, Lee CL. Organ preference of metastasis. The role of organ-specifically modulated endothelial cells. Lab Invest. 1988;58(4):379–387. [PubMed] [Google Scholar]
- 15.Palmieri D, Chambers AF, Felding-Habermann B, Huang S, Steeg PS. The biology of metastasis to a sanctuary site. Clin Cancer Res. 2007;13(6):1656–1662. doi: 10.1158/1078-0432.CCR-06-2659. [DOI] [PubMed] [Google Scholar]
- 16.Palmieri D, Smith QR, Lockman PR, Bronder J, Gril B, Chambers AF, Weil RJ, Steeg PS. Brain metastases of breast cancer. Breast Dis. 2006;26:139–147. doi: 10.3233/bd-2007-26112. [DOI] [PubMed] [Google Scholar]
- 17.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28(1):5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Lee SW, Kim WJ, Park JA, Choi YK, Kwon YW, Kim KW. Blood-brain barrier interfaces and brain tumors. Arch Pharm Res. 2006;29(4):265–275. doi: 10.1007/BF02968569. [DOI] [PubMed] [Google Scholar]
- 19.Reichel A, Begley DJ, Abbott NJ. An overview of in vitro techniques for blood-brain barrier studies. Methods Mol Med. 2003;89:307–324. doi: 10.1385/1-59259-419-0:307. [DOI] [PubMed] [Google Scholar]
- 20.Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25(1):25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro MM, Castanho MA, Serrano I. In vitro blood-brain barrier models–latest advances and therapeutic applications in a chronological perspective. Mini Rev Med Chem. 2010;10(3):262–270. doi: 10.2174/138955710791185082. [DOI] [PubMed] [Google Scholar]
- 22.Deli MA, Abraham CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25(1):59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PubMed] [Google Scholar]
- 23.Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16(8):1486–1495. doi: 10.1359/jbmr.2001.16.8.1486. [DOI] [PubMed] [Google Scholar]
- 24.Fulcher CA, Zimmerman TS. Characterization of the human factor VIII procoagulant protein with a heterologous precipitating antibody. Proc Natl Acad Sci USA. 1982;79(5):1648–1652. doi: 10.1073/pnas.79.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerman TS, Ruggeri ZM, Fulcher CA. Factor VIII/von Willebrand factor. Prog Hematol. 1983;13:279–309. [PubMed] [Google Scholar]
- 26.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36(6):1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39(2):235–250. doi: 10.1097/00006123-199608000-00001. discussion 250–232. [DOI] [PubMed] [Google Scholar]
- 28.Gaillard PJ, Voorwinden LH, Nielsen JL, Ivanov A, Atsumi R, Engman H, Ringbom C, de Boer AG, Breimer DD. Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur J Pharm Sci. 2001;12(3):215–222. doi: 10.1016/s0928-0987(00)00123-8. [DOI] [PubMed] [Google Scholar]
- 29.Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J, et al. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115(6):1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kis B, Deli MA, Kobayashi H, Abraham CS, Yanagita T, Kaiya H, Isse T, Nishi R, Gotoh S, Kangawa K, Wada A, Greenwood J, Niwa M, Yamashita H, Ueta Y. Adrenomedullin regulates blood-brain barrier functions in vitro. Neuroreport. 2001;12(18):4139–4142. doi: 10.1097/00001756-200112210-00055. [DOI] [PubMed] [Google Scholar]
- 31.Schulze C, Smales C, Rubin LL, Staddon JM. Lysophosphatidic acid increases tight junction permeability in cultured brain endothelial cells. J Neurochem. 1997;68(3):991–1000. doi: 10.1046/j.1471-4159.1997.68030991.x. [DOI] [PubMed] [Google Scholar]
- 32.Carbonell WS, Ansorge O, Sibson N, Muschel R. The vascular basement membrane as “soil” in brain metastasis. PLoS One. 2009;4(6):e5857. doi: 10.1371/journal.pone.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massague J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.