Abstract
Objective
Pepducins are membrane-tethered, cell-penetrating lipopeptides that target the cytoplasmic surface of their cognate receptor. Here, we report the first human use of a protease-activated receptor-1–based pepducin, which is intended as an antiplatelet agent to prevent ischemic complications of percutaneous coronary interventions.
Approach and Results
PZ-128 was administered by 1 to 2 hours continuous intravenous infusion (0.01–2 mg/kg) to 31 subjects with coronary artery disease or multiple coronary artery disease risk factors. Safety, antiplatelet efficacy, and pharmacokinetics were assessed at baseline and 0.5, 1, 2, 6, 24 hours, and 7 to 10 days postdosing. The inhibitory effects of PZ-128 on platelet aggregation stimulated by the protease-activated receptor-1 agonist SFLLRN (8 μmol/L) at 30 minutes to 6 hours were dose dependent with 20% to 40% inhibition at 0.3 mg/kg, 40% to 60% at 0.5 mg/kg, and ≥80% to 100% at 1 to 2 mg/kg. The subgroup receiving aspirin in the 0.5 and 1-mg/kg dose cohorts had 65% to 100% inhibition of final aggregation to SFLLRN at 30 minutes to 2 hours and 95% to 100% inhibition by 6 hours. The inhibitory effects of 0.5 mg/kg PZ-128 were reversible with 50% recovery of aggregation to SFLLRN by 24 hours. There were no significant effects of PZ-128 on aggregation induced by AYPGKF, ADP, or collagen, indicating that the observed effects were specific to protease-activated receptor-1. The plasma half-life was 1.3 to 1.8 hours, and PZ-128 was nondetectable in urine. There were no effects on bleeding, coagulation, clinical chemistry, or ECG parameters.
Conclusions
PZ-128 is a promising antiplatelet agent that provides rapid, specific, dose dependent, and reversible inhibition of platelet protease-activated receptor-1 through a novel intracellular mechanism.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01806077.
Keywords: aspirin, collagen, coronary artery disease, lipopeptides, risk factors
Coronary thrombosis during acute coronary syndromes (ACS) and percutaneous coronary interventions (PCIs) is dependent on reactive platelets.1,2 Antiplatelet therapy plays a central role in preventing stent thrombosis and recurrent myocardial infarction. Platelet activation involves multiple signaling pathways activated by thrombin, thromboxane A2, ADP, and collagen.3 Blockade of more than one of these pathways has proven superior in attenuating thrombotic event occurrence than mono-blockade. However, it remains unclear which pathway is central to the generation of thrombotic events and thrombin activation of the protease-activated receptors (PARs) and platelet-dependent thrombin generation may vary greatly from patient-to-patient.4–6 Glycoprotein IIb–IIIa inhibitors (abciximab, eptifibatide, and tirofiban) that block the final common pathway of platelet clot formation exhibit potent antiplatelet activity in patients with PCI, but also significantly increase the risk of bleeding proportional to their potency.7,8 Upstream inhibition of thrombin with bivalirudin provides significant protection from thrombin-induced platelet aggregation in the PCI setting, but by design also directly inhibits coagulation leading to a commensurate increase in bleeding risk.5,9,10
The antiplatelet effect of the most widely used P2Y12 inhibitor, clopidogrel, is slow in onset, variable and irreversible. Newer, more potent oral P2Y12 inhibitors, such as ticagrelor, have a faster peak onset of action than clopidogrel11; however, the pharmacodynamic effect of the new P2Y12 inhibitors may be delayed in subjects with ACS.12 A relation exists between fast and effective on-treatment platelet reactivity and ischemic event occurrence in patients with PCI.12–14 In addition, the slow offset of P2Y12 inhibition and residual bleeding risk by all currently approved oral agents may be problematic in patients requiring coronary artery bypass graft surgery.15
PAR1 or PAR4 inhibition is an emerging strategy to target thrombin-induced platelet activation.4,16 Two orally active PAR1 inhibitors, vorapaxar17 and atopaxar,18 have been studied in phase 2 trials and have been associated with a reduction in ischemic event occurrence without an increase in major bleeding. However, acute and then chronic administration of vorapaxar in patients with non–ST elevation ACS in the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) trial did not reduce the composite occurrence of cardiovascular death, myocardial infarction, stroke, hospitalization for ischemia, or urgent revascularization but increased major bleeding and intracranial hemorrhage.19 The ability to reversibly inhibit PAR1 signaling by a parenteral strategy may reduce bleeding in the high-risk patient undergoing PCI. Whether rapid, short-term PAR1 blockade improves outcomes in patients undergoing PCI remains unknown.
Pepducins are lipidated peptides, which specifically target the cytoplasmic surface of their cognate receptor and can act as either allosteric agonists or antagonists.20–22 PZ-128 is a cell-penetrating lipopeptide pepducin that selectively inhibits PAR1-G protein signaling on the inner leaflet of the lipid bilayer.9,23,24 PZ-128 is being developed for prevention of acute thrombotic complications of PCI. In this study, we assessed the pharmacokinetics, pharmacodynamics, safety, and tolerability associated with single ascending intravenous doses of PZ-128 in subjects with multiple risk factors for coronary artery disease (CAD).
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Demographics
This phase 1 study of the antiplatelet efficacy, pharmacokinetics and safety of a pepducin was conducted in both male and female subjects with equal race distribution. The age range was 43 to 74 years. Twenty-two percent of subjects had documented CAD and all subjects had multiple risk factors for coronary disease, including smoking, hypertension, diabetes mellitus, and dyslipidemia (Table 1). Subjects were concurrently taking medications for the treatment of cardiovascular disease, including aspirin (63%), antihypertensives (66%), statins and other lipid-lowering drugs (56%), and agents to control blood glucose (22%). Sixty-nine subjects were screened and 31 were given a dose of PZ-128. One enrolled patient developed hypertension after pretreatment with an antihistamine and was not administered study drug. All enrolled patients completed 30-day follow-up (Figure 1).
Table 1.
Baseline Demographics and Medical History
| All Subjects (n=32) | |
|---|---|
| Age, y | 57±8 |
| Range | 43–74 |
| Male, n (%) | 19 (59) |
| Race, n (%) | |
| White | 16 (50) |
| Black | 15 (47) |
| Other | 1 (3) |
| Weight, kg | 91±17 |
| Range | 63–127 |
| Body mass index, kg/m2 | 31±6 |
| Range | 20–43 |
| Vascular disease, n (%) | 7 (22) |
| Coronary artery disease | 6 (19) |
| Previous myocardial infarction | 5 (16) |
| Previous percutaneous coronary intervention | 4 (13) |
| Previous coronary artery bypass-grafting | 4 (13) |
| Coronary artery disease risk factors, n (%) | |
| Dyslipidemia | 26 (81) |
| Hypertension | 22 (69) |
| Obesity* | 17 (53) |
| Smoking history | 13 (41) |
| Diabetes mellitus or prediabetes | 11 (34) |
| Age† | 25 (78) |
| Baseline medications, n (%) | |
| Blood pressure | 21 (66) |
| ACE inhibitor, ARB | 14 (44) |
| β-Blocker | 7 (22) |
| Calcium channel blocker | 7 (22) |
| Diuretic | 5 (16) |
| Aspirin | 20 (63) |
| Lipid lowering | 18 (56) |
| HMG-CoA reductase inhibitor | 18 (56) |
| Other (fibrate, absorption inhibitor) | 3 (9) |
| Diabetes mellitus (insulin, oral hypoglycemic, and incretin mimetic) | 7 (22) |
Data represent mean±SD where indicated. ACE indicates angiotensin-converting enzyme; ARB, angiotensin receptor blocker; and HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A.
Defined as body mass index ≥30 kg/m2.
Males >45 years of age, females >55 years of age.
Figure 1.
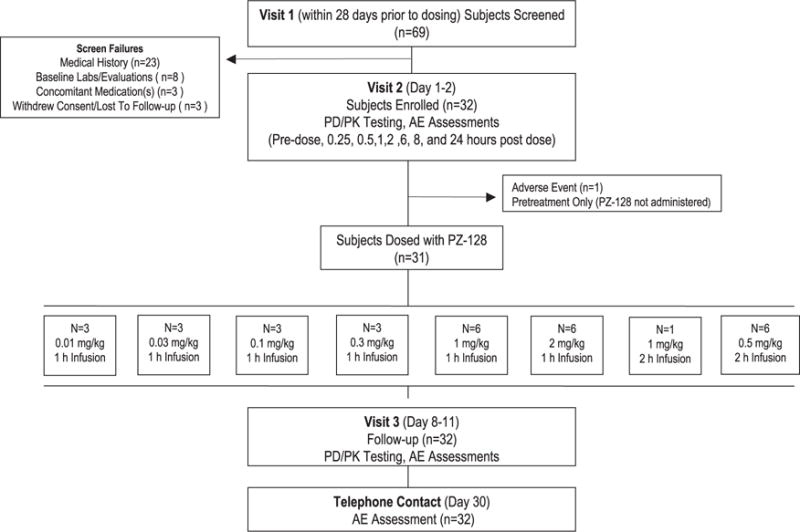
Disposition of subjects. A total of 32 volunteers with vascular disease or coronary artery disease risk factors were prospectively assigned to 8 sequential dose levels and all subjects completed the study protocol. One subject became ineligible to receive the study drug after the administration of the pretreatment regimen in the predose setting. PD indicates pharmacodynamics; and PK, pharmacokinetics.
Pharmacodynamics
PZ-128 (P1pal-7) is a cell-penetrating, membrane-tethered lipopeptide, which closely resembles the off-state of the corresponding juxtamembrane region of the PAR1 third-intracellular loop and TM6 region (Figure 2A). PZ-128 targets PAR1-G protein signaling, which can be assayed ex vivo in platelets as a pharmacodynamic marker of drug activity and specificity.9 Accordingly, the ability of PZ-128 to block maximal and final platelet aggregation to the PAR1 agonist SFLLRN versus agonists for other platelet receptors (PAR4, ADP, and collagen) was determined at several time points (0, 30 minutes, 1, 2, 6, 24, and 192 hours). There were no significant effects of PZ-128 on platelet aggregation at the lowest doses (0.01–0.1 mg/kg) for all agonists. PZ-128 rapidly inhibited mean PAR1 platelet aggregation at the 0.5-mg/kg dose (2-hour infusion) with 40% to 50% inhibition of final aggregation to 8 μmol/L SFLLRN at 30 minutes to 2 hours (Figure 2B). By the 6-hour time point, PZ-128 significantly inhibited aggregation induced by 8 μmol/L SFLLRN in the 0.5-mg/kg dose cohort (Table 2). At 24 hours, aggregation induced by 8 μmol/L SFLLRN recovered by 50%, with near complete recovery by the 192-hour time point (Figure 2B). The inhibitory effects of 0.5-mg/kg PZ-128 were reversed by higher concentrations of PAR1 agonist (20 μmol/L SFLLRN) at 30 minutes to 24 hours with no significant inhibition observed (Table I in the online-only Data Supplement).
Figure 2.
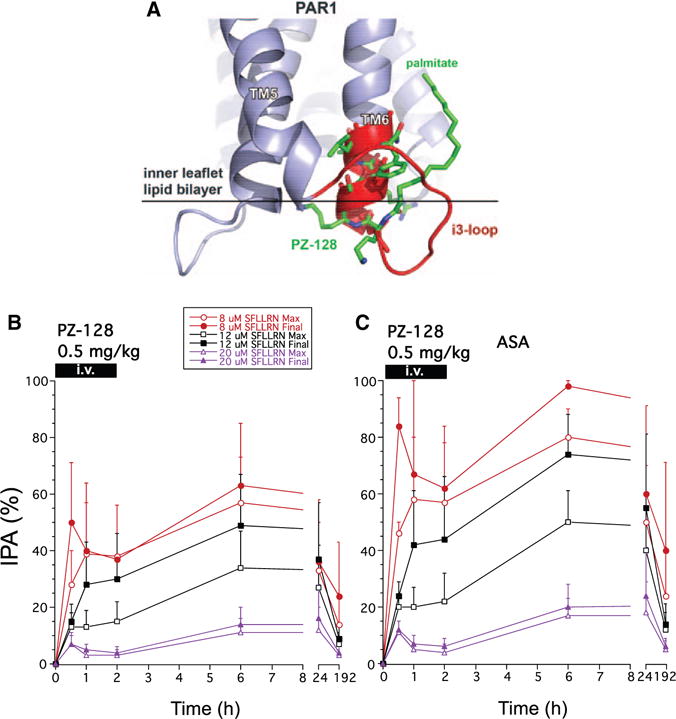
Inhibition of platelet aggregation (IPA) in the 0.5 mg/kg PZ-128 dose cohort to 8, 12, and 20 μmol/L protease-activated receptor-1 (PAR1) agonist peptide, SFLLRN. A, The structure of the PZ-128 pepducin9 (green) aligns closely (backbone root mean square deviation 1.4 Å) with the i3-loop/cytoplasmic α-helical extension of TM6 based on the rhodopsin25,26 (red), and PAR1-vorapaxar27 (blue) x-ray structures. Ex vivo aggregation was conducted in platelet-rich plasma by standard light transmission aggregometry and maximal and final (at 6–7 minutes) aggregation (mean±SD) normalized to baseline (t=0) for each agonist for (B) all subjects, and the subset receiving (C) aspirin (acetylsalicylic acid [ASA]).
Table 2.
Inhibitory Effects of PZ-128 on PAR1 Light Transmission Aggregometry (Maximal and Final) in Subjects With CAD Risk Factors
| Dose Level | Time, h | % Inhibition of PAR1 Platelet Aggregation
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 8 μmol/L SFLLRN Max
|
8 μmol/L SFLLRN Final
|
12 μmol/L SFLLRN Max
|
12 μmol/L SFLLRN Final
|
||||||
| All | +ASA | All | +ASA | All | +ASA | All | +ASA | ||
| 0.5 mg/kg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| All (n=6) | 0.5 | 28 | 46 | 50 | 84† | 13 | 20 | 15 | 24 |
| +ASA (n=4) | 1 | 39 | 58† | 40 | 67 | 13 | 20 | 28 | 42 |
| 2 | 38 | 57† | 37 | 62 | 15 | 22 | 30 | 44 | |
| 6 | 57* | 80** | 63 | 98* | 34* | 50* | 49† | 74* | |
| 24 | 33 | 50 | 36 | 60 | 27 | 40* | 37 | 55 | |
| 192 | 14 | 24 | 24 | 40 | 7 | 12 | 9 | 14 | |
| 1 mg/kg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| All (n=6) | 0.5 | 40† | 40† | 84** | 84*** | 16 | 16 | 40 | 40 |
| +ASA (n=5) | 1 | 54** | 54** | 88** | 88*** | 25 | 25 | 44 | 44 |
| 2 | 66*** | 64** | 100*** | 100*** | 23 | 22 | 39 | 40 | |
| 6 | 48* | 58** | 75** | 94*** | 18 | 22 | 47 | 57 | |
| 24 | 34 | 28 | 80** | 75** | 25 | 19 | 42 | 31 | |
| 192 | 35 | 42* | 80** | 100*** | 14 | 16 | 34 | 40 | |
| 2 mg/kg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| All (n=6) | 0.5 | 36 | 21 | 24 | 24 | 10 | 7 | 19 | 19 |
| +ASA (n=4) | 1 | 54** | 45† | 96* | 96* | 19 | 21* | 50* | 60* |
| 2 | 47* | 50* | 98* | 98* | 12 | 13 | 21 | 23 | |
| 6 | 29 | 20 | 15 | 15 | 9 | ≤0 | 21 | 2 | |
| 24 | ≤0 | ≤0 | 21 | 21 | ≤0 | ≤0 | ≤0 | ≤0 | |
| 192 | 18 | 5 | 19 | 19 | 12 | 3 | 16 | 4 | |
Data are presented as mean platelet inhibition (%) of maximal and final (6 min) light transmission aggregometry (LTA) in response to 8–20 μmol/L PAR1 agonist SFLLRN. ANOVA analyses of the effects of PZ-128 on platelet inhibition over time for each agonist concentration within each dose cohort was performed with the Dunnett post-test correction using the predose (0) time point as the control. A subgroup analysis was done among subjects taking concomitant aspirin. Average SEM was 11. ASA indicates acetylsalicylic acid (aspirin); CAD, coronary artery disease; LTA, light transmission aggregometry; and PAR1, protease-activated receptor-1.
0.050<P<0.099,
P≤0.05,
P≤0.01,
P≤0.001.
Analysis of the subgroup of subjects on concomitant aspirin (acetylsalicylic acid) revealed stronger apparent effects of PZ-128 on inhibition of maximal and final aggregation to 8 and 12 μmol/L SFLLRN in the 0.5-mg/kg dose cohort. The subjects also receiving acetylsalicylic acid in the 0.5-mg/kg dose cohort had 60% to 80% average inhibition of final aggregation to 8 μmol/L SFLLRN at 30 minutes to 2 hours and 98% inhibition by 6 hours (Table 2; Figure 2C). There was 40% recovery of aggregation induced by 8-μmol/L PAR1 agonist by 24 hours and 60% recovery by 192 hours. Higher concentrations of PAR1 agonist (20 μmol/L SFLLRN) showed 80% recovery of aggregation by 24 hours and 95% by 192 hours in the 0.5-mg/kg dose cohort receiving concomitant acetylsalicylic acid. At the higher dose of 1 mg/kg, PZ-128 gave rapid and sustained ≈80% to 100% inhibition at 30 minutes to 6-hour time points regardless of whether the subject was taking concomitant acetylsalicylic acid (Figure 3). Similar 96% to 98% inhibitory effects on final aggregation to 8 μmol/L SFLLRN were also seen at the highest dose of 2 mg/kg at 1 to 2-hour time points, however, recovery of platelet aggregation was faster at the 6 to 192-hour time points at the highest dose with only 15% to 21% inhibition observed (Table 2).
Figure 3.
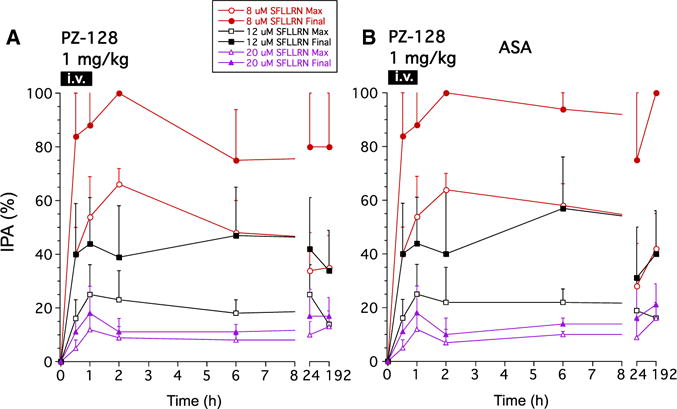
Inhibition of platelet aggregation (IPA) in the 1 mg/kg PZ-128 dose cohort to 8, 12, and 20 μmol/L protease-activated receptor-1 agonist peptide, SFLLRN. Ex vivo aggregation was conducted in platelet-rich plasma by light transmission aggregometry and maximal and final (at 6 minutes) aggregation (mean±SD) normalized to baseline (t=0) for each agonist for (A) all subjects, and the subset receiving (B) aspirin (acetylsalicylic acid [ASA]).
The inhibitory effects of PZ-128 on PAR1 platelet aggregation (8 μmol/L SFLLRN, final aggregation) were dose dependent with 20% to 40% mean inhibition at 0.3 mg/kg, 40% to 60% at 0.5 mg/kg, and ≤80% to 100% at 1 to 2 mg/kg (Figure 4). PZ-128 was specific to PAR1 with no significant effects on aggregation induced by any other platelet agonists, including 160-μmol/L AYPGKF, 5-μmol/L ADP, 20-μmol/L ADP, 4-μg/mL collagen, or 20-μg/mL collagen in any dose cohort (Figure I in the online-only Data Supplement). As anticipated, there were nonsignificant inhibitory effects of PZ-128 on aggregation induced by collagen (≈10% to 20% inhibition at 0.5 to 2-mg/kg doses), consistent with suppressing a previously described collagen–MMP1 (matrix metalloprotease-1)–PAR1 mechanism.28
Figure 4.
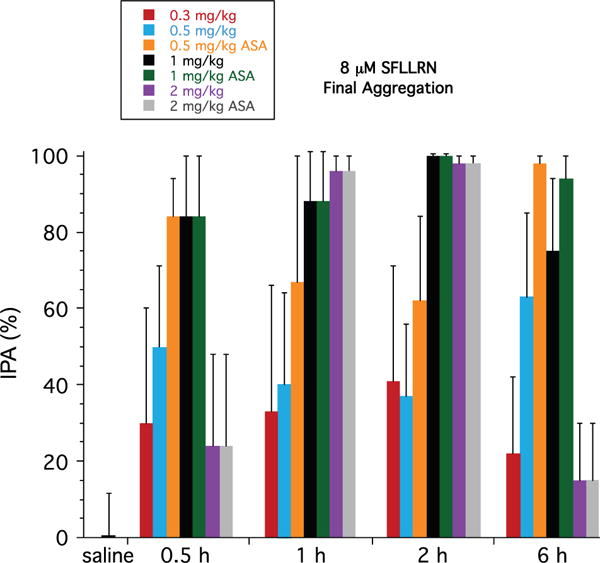
Dose dependence of mean inhibition of platelet aggregation (IPA) in the 0.3 to 2 mg/kg PZ-128 dose cohorts to 8 μmol/L protease-activated receptor-1 agonist peptide, SFLLRN. Ex vivo aggregation was conducted in platelet-rich plasma by light transmission aggregometry and maximal and final (at 6–7 minutes) aggregation (mean±SD) normalized to baseline (t=0) for each agonist for either all subjects or the subset receiving aspirin (acetylsalicylic acid) as indicated. Saline was the IPA response at 30 minutes for subject TMC-27 who received a 1-hour infusion of saline only.
Pharmacokinetics
Plasma drug levels of the pepducin peaked (Cmax) at the end of the 1- or 2-hour infusion in all dose cohorts with a terminal t1/2 of elimination of 1.3 to 1.8 hours (Figure 5; Table II in the online-only Data Supplement). Drug was undetectable in plasma at 24 to 192 hours in all subjects. There was little or no PZ-128 detected in urine at any time point in all subjects. The mean volume of distribution of PZ-128 was 0.11 to 0.17 L/kg indicating that the palmitoylated peptide was not distributed in the water compartments (intracellular and extracellular) that constitute ≈60% of body volume (eg, volume of distribution at steady state-0.6 L/kg for a 100 kg subject).
Figure 5.
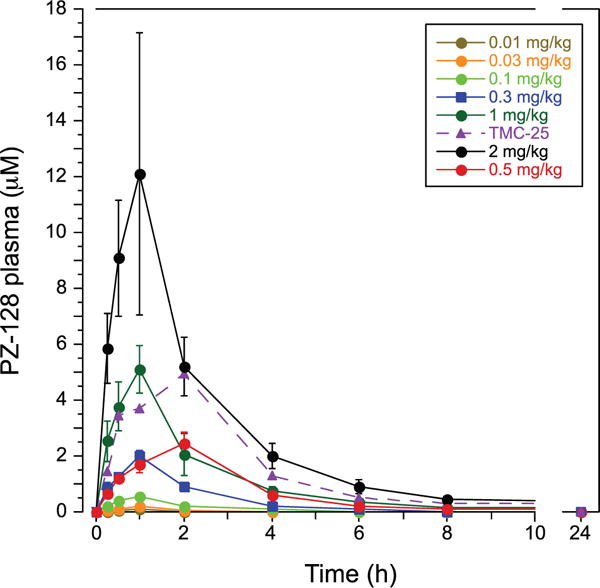
Pharmacokinetics of PZ-128 (μmol/L) in plasma for 0.01 to 2 mg/kg dose cohorts.
Drug concentrations were highly linearly correlated with increasing dose at 0.25 to 8-hour time points with R values of 0.980 to 0.998 (Figure II in the online-only Data Supplement). Likewise, area under the curve and Cmax of PZ-128 in plasma were linearly correlated with dose (Figure III in the online-only Data Supplement). Supra-therapeutic drug concentrations were achieved at the 1- and 2-mg/kg dose levels with mean peak PZ-128 levels reaching 5 and 12 μmol/L, respectively (Figure 5). Accordingly, PZ-128 blocked 85% to 100% of aggregation to 8 μmol/L SFLLRN agonist at the 1 to 2-hour time points in the 1- to 2-mg/kg dose cohorts. At 0.3- to 0.5-mg/kg doses where anti-PAR1 effects on platelet aggregation were observed at 30 minutes to 2 hours, drug levels of PZ-128 achieved therapeutic concentrations of 1 to 3 μmol/L. In agreement with preclinical studies in nonhuman primates,9 persistent antiplatelet effects of 60% to 100% inhibition were still observed at the 6-hour time point with the 0.5-mg/kg dose where plasma PZ-128 drug levels had fallen to <0.5 μmol/L. This is consistent with the mechanism of action of the pepducin, which flips across the cell membrane where it remains associated with its cognate receptor on the inner leaflet of the lipid bilayer to give prolonged inhibition of PAR1 activity.24,29,30
Safety and Effects of PZ-128 on Hemostasis Parameters
PZ-128 was well tolerated in the 0.01-, 0.03-, 0.1-, and 0.3-mg/kg dose cohorts using a 1-hour intravenous infusion. At higher doses, adverse events were transient in nature and resolved by 24 hours after administration of the study drug (Table III in the online-only Data Supplement). Transient tingling or a numb sensation in the skin was commonly reported at the moderate to high dose cohorts (0.5–2 mg/kg). The most important adverse event was acute allergic reactions occurring in several subjects receiving the highest doses of PZ-128. The occurrence of drug allergic reactions at the high (1–2 mg/kg) doses using a 1-hour infusion time necessitated dropping the dose to 0.5 mg/kg and extending the infusion time to 2 hours. This strategy along with premedication mitigated the allergic reaction and resulted in a tolerated and efficacious dose of 0.5 mg/kg.
No significant effects on coagulation, hemostasis parameters, or bleeding were evident at any dose, despite the fact that 63% of the subjects (20/32) were also taking aspirin (Figure 6). There were no significant changes in heart rate or any ECG parameter including RR, PR, QRS, QT, QTc, or QTd intervals or pulmonary function with PZ-128 dose. Likewise, all other laboratory outcomes including clinical chemistry and hematology, and urinalysis were not significantly affected by PZ-128.
Figure 6.
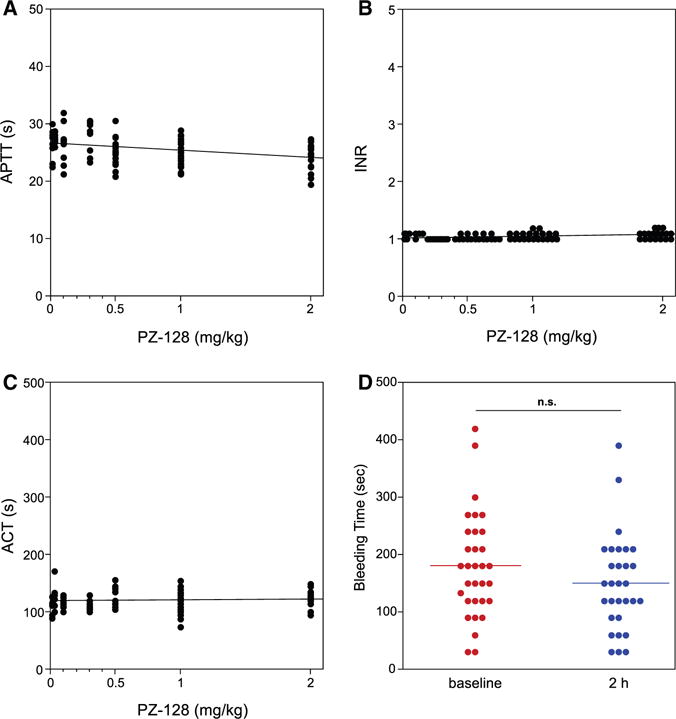
Hemostasis parameters: Effect of PZ-128 dose on (A) activated partial thromboplastin time (APTT), (B) international normalized ratio (INR), (C) activated clotting time (ACT), and (D) bleeding time for all subjects. Hemostasis parameter (at all time points after drug infusion was initiated) versus dose for all subjects TMC1-32 was analyzed with PZ-128 dose as a continuous variable and correlations and P values for each slope were not significant (P>0.01) using repeated measures, mixed effects models, based on a Bonferroni post hoc test correction. Changes in bleeding time between baseline and 2 hours for all individuals in D was also analyzed for significance by 2-tailed, paired t test, with the mean bleeding times shown as horizontal lines.
Discussion
This study describes the first-in-man effects of PZ-128, a cell-penetrating pepducin specific for the PAR1 receptor. We demonstrated that the platelet inhibitory effects of PZ-128 were: (1) dose dependent and rapid in onset with moderate to high levels of platelet inhibition stimulated by 8 μmol/L SFLLRN observed with doses ≥0.5 mg/kg, (2) sustained for ≥6 hours after the start of the infusion, (3) reversible, with recovery of platelet function by 24 hours at the 0.5-mg dose and 80% to 100% aggregation achieved by 20 μmol/L SFLLRN at all time points, and (4) specific for the PAR1 receptor with no significant effects on aggregation stimulated by a PAR4 agonist, ADP, or collagen. There were no effects on bleeding time, PTT, international normalized ratio, activated clotting time, pulmonary function, heart rate, or other laboratory or ECG parameters.
An apparent enhancement of the antiplatelet effects of PZ-128 by aspirin was observed in the 0.5-mg/kg group. Similar to thrombin, thromboxane A2 also signals through G12/13 to cause the platelet shape change reaction and trigger aggregation. In this regard, aspirin monotherapy has been shown to inhibit PAR1 response in platelets in subjects with PAD.31 Similarly, based on a microchip-based flow chamber system, it was demonstrated that antithrombotic effects of another PAR1 inhibitor, SCH79797, was significantly enhanced in the presence of aspirin and a P2Y12 receptor blocker.32
Analysis of the pharmacokinetic effects demonstrated peak plasma drug levels at the end of the infusion period in all dose cohorts with no detectable drug in plasma at 24 and 192 hours. The absence of PZ-128 in urine demonstrated that the pepducin is not renally cleared. Plasma drug concentrations were highly linearly correlated with dose. At high-drug concentrations of PZ-128 achieved with 1 to 2-mg/kg dosing there was 85% to 100% inhibition of aggregation stimulated by 8 μmol/L SFLLRN regardless of whether the subject received concomitant aspirin. Prolonged antiplatelet efficacy was more prominent at 6 to 192 hours with the 1-mg/kg dose, despite 2-fold higher peak plasma drug levels in the 2-mg/kg dose cohort. The mechanistic basis for this is not clear and could be because of the small sample size; however, it is possible that the high concentrations of PZ-128 (7910–23 300 ng/mL) observed in the 2-mg/kg cohort resulted in more stable pepducin micelle formation with less overall adsorption as monodispersed molecules to the target tissue, namely platelets. At the 0.5 to 1-mg/kg dose cohorts, there was an additional late spike in inhibitory activity evident at 6 to 24 hours. Although speculative, the late spike in inhibition in the higher dose cohorts could also be because of the cell-penetrating pepducin moving from a fatty reservoir/vascularized-compartment (eg, liver, kidney, and bone marrow) into the continuously circulating platelets.
There have been no other previous reports of fast-acting parenteral PAR1 blockade in humans. The oral PAR1 inhibitor, vorapaxar was administered acutely as a loading dose and maintained at a lower dose for a median exposure of 386 days to patients with ACSs in a large phase 3 trial.19 Vorapaxar therapy was associated with a reduction in recurrent myocardial infarction at the expense of more bleeding, including more intracranial bleeding. Vorapaxar gave no appreciable inhibition at 30 minutes, but reached ≥80% inhibition of SFLLRN-induced aggregation at 2 hours after a 40-mg loading dose (≈0.4 mg/kg) in 89% to 96% of PCI and non–ST-segment elevation–ACS patients.17,33 Atopaxar, a second orally active PAR1 inhibitor, was investigated in several phase 2 studies including the Lessons From Antagonizing the Cellular Effect of Thrombin-Coronary Artery Disease Trial (LANCELOT)-ACS trial (n=603),34 and studies in a Japanese ACS (unstable angina and non–ST-segment–elevation myocardial infarction) patient population (J-LANCELOT; n=241),18 and in patients with CAD (LANCELOT-CAD; n=263) on top of standard antiplatelet therapy.35 The LANCELOT-ACS subjects reached maximal platelet inhibition 6 hours after the loading dose of atopaxar. The LANCELOT-ACS trial results demonstrated that atopaxar significantly reduced Holter-detected ischemia without a clear increase in bleeding compared with placebo. Atopaxar resulted in more minor bleeding in the 200-mg daily dose group, and a trend toward fewer ischemic events in patients with CAD.35 However, a transient rise in liver enzymes was observed in 3% to 6% of subjects and prolongation of the QTc interval was observed in some individuals at the higher dose levels, causing the development of atopaxar to be halted.34,35
Unlike the relation of ADP-induced platelet aggregation to clinical thrombotic event occurrence, little is known about whether the magnitude of SFLLRN-induced aggregation is related to or predictive of clinical event occurrence. In the Primary Prevention Parameters Evaluation (PREPARE) POST-STENTING trial, we demonstrated that high thrombin–induced platelet–fibrin clot strength was a marker for recurrent ischemic event occurrence in patients treated with PCI.36 The effects of vorapaxar and PZ-128 on thrombin activation of platelets in patients are also unknown, whereas evidence exists that the direct thrombin inhibitor, bivalirudin inhibits thrombin-induced platelet activation.5 At the 0.5 to 2-mg/kg doses, PZ-128 provided rapid and reversible inhibition of SFLLRN-induced aggregation. Whether a fast-onset of reversible PAR1 blockade induced by PZ-128 provides a better safety profile with preservation of clinical efficacy remains to be determined.
An important adverse event was acute allergic reactions occurring at the highest doses of PZ-128. Anaphylactoid reactions occurred either during the infusion or within 40 minutes from the completion of the infusion with skin manifestations and hemodynamic effects ranging from 10 minutes to 12 hours. None of these acute events were deemed life threatening and all were effectively treated with either stopping the infusion or administering antihistamine(s) or intravenous fluids. The transient hypotension was asymptomatic and without compensatory tachycardia. Of the subjects with allergic reactions, the Cmax levels of PZ-128 were 4.7 to 21.5 μmol/L. Conversely, 50% of the subjects receiving the 2-mg/kg dose over the 1-hour infusion had no hemodynamic effects. To achieve a well-tolerated dose and to mitigate any potential allergic reactions by lowering Cmax, we added the last 0.5-mg/kg dose cohort using a longer 2-hour infusion instead of a 1-hour infusion, along with antiallergic premedication. Accordingly, none of these subjects had an allergic response. The Cmax levels (1.8–2.7 μmol/L) in the 0.5-mg/kg group were all well below the levels reached in the 1 to 2-mg/kg dose cohorts. With the prolonged infusion time of 2 hours, area under the curve levels were still maintained with the 0.5-mg/kg dose, thereby achieving anti-PAR1 pharmacodynamic efficacy especially in the context of concomitant aspirin therapy. On the basis of the above efficacy and safety data, the 0.5-mg/kg dose of PZ-128 infused >2 hours will be used in an upcoming multicenter phase 2 study in PCI/ACS subjects.
Supplementary Material
Significance.
PZ-128 is a pepducin that modulates platelet function by inhibiting signaling at the receptor–G protein interface. We demonstrated that PZ-128 selectively inhibits the protease-activated receptor-1 receptor in subjects with coronary artery disease or risk factors and at a dose of 0.5 mg/kg appeared to be well tolerated. The property of a rapid pharmacodynamic onset targeting a key platelet activation pathway that is reversible makes PZ-128 an attractive adjunctive agent for percutaneous coronary intervention. Moreover, the reversibility lends promise for enhanced safety. A phase 2 percutaneous coronary intervention/acute coronary syndromes study to further investigate the efficacy and safety of PZ-128 to block protease-activated receptor-1 is underway.
Acknowledgments
We acknowledge the statistical analysis conducted by David Simon.
Sources of Funding
This study was funded by a grant from the National Heart, Lung, and Blood Institute (NHLBI) P50 HL110789 (A. Kuliopulos) and the NHLBI SMARTT program.
Dr Gurbel reports serving as a consultant and/or receiving honoraria from Daiichi Sankyo, Lilly, Bayer, AstraZeneca, Merck, Boehringer, Janssen, and CSL Behring; receiving grants from the National Institutes of Health, Daiichi Sankyo/Lilly, CSL, AstraZeneca, Harvard Clinical Research Institute, Bayer, Haemonetics, Duke Clinical Research Institute, Sinnowa, Coramed, and Accumetrics. Drs Kuliopulos and Covic report serving as consultants for Eli Lilly and as Founders of Oasis Pharmaceuticals. David Simon reports serving as a consultant for Procter and Gamble and Johns Hopkins University.
Nonstandard Abbreviations and Acronyms
- ACS
acute coronary syndromes
- CAD
coronary artery disease
- PAR1
protease-activated receptor-1
- PCI
percutaneous coronary intervention
Footnotes
Disclosures
The other authors report no conflicts.
References
- 1.Gurbel PA, Bliden KP, Zaman KA, Yoho JA, Hayes KM, Tantry US. Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) study. Circulation. 2005;111:1153–1159. doi: 10.1161/01.CIR.0000157138.02645.11. [DOI] [PubMed] [Google Scholar]
- 2.Gurbel PA, Tantry US. Do platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents? platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents. Circulation. 2012;125:1276–1287. doi: 10.1161/CIRCULATIONAHA.111.031195. [DOI] [PubMed] [Google Scholar]
- 3.Gurbel PA, Kuliopulos A, Tantry US. G-protein-coupled receptors signaling pathways in new antiplatelet drug development. Arterioscler Thromb Vasc Biol. 2015;35:500–512. doi: 10.1161/ATVBAHA.114.303412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leger AJ, Covic L, Kuliopulos A. Protease-activated receptors in cardiovascular diseases. Circulation. 2006;114:1070–1077. doi: 10.1161/CIRCULATIONAHA.105.574830. [DOI] [PubMed] [Google Scholar]
- 5.Kimmelstiel C, Zhang P, Kapur NK, Weintraub A, Krishnamurthy B, Castaneda V, Covic L, Kuliopulos A. Bivalirudin is a dual inhibitor of thrombin and collagen-dependent platelet activation in patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2011;4:171–179. doi: 10.1161/CIRCINTERVENTIONS.110.959098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edelstein LC, Simon LM, Montoya RT, Holinstat M, Chen ES, Bergeron A, Kong X, Nagalla S, Mohandas N, Cohen DE, Dong JF, Shaw C, Bray PF. Racial differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376c. Nat Med. 2013;19:1609–1616. doi: 10.1038/nm.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarborough RM, Kleiman NS, Phillips DR. Platelet glycoprotein IIb/IIIa antagonists. What are the relevant issues concerning their pharmacology and clinical use? Circulation. 1999;100:437–444. doi: 10.1161/01.cir.100.4.437. [DOI] [PubMed] [Google Scholar]
- 8.Kimmelstiel C, Badar J, Covic L, Waxman S, Weintraub A, Jacques S, Kuliopulos A. Pharmacodynamics and pharmacokinetics of the platelet GPIIb/IIIa inhibitor tirofiban in patients undergoing percutaneous coronary intervention: implications for adjustment of tirofiban and clopidogrel dosage. Thromb Res. 2005;116:55–66. doi: 10.1016/j.thromres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Gruber A, Kasuda S, Kimmelstiel C, O’Callaghan K, Cox DH, Bohm A, Baleja JD, Covic L, Kuliopulos A. Suppression of arterial thrombosis without affecting hemostatic parameters with a cell-penetrating PAR1 pepducin. Circulation. 2012;126:83–91. doi: 10.1161/CIRCULATIONAHA.112.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander KP, Peterson ED. Minimizing the risks of anticoagulants and platelet inhibitors. Circulation. 2010;121:1960–1970. doi: 10.1161/CIRCULATIONAHA.109.853135. [DOI] [PubMed] [Google Scholar]
- 11.Wallentin L, Becker RC, Budaj A, et al. PLATO Investigators Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 12.Tantry US, Bonello L, Aradi D, et al. Working Group on On-Treatment Platelet Reactivity Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261–2273. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 13.Aradi D, Kirtane A, Bonello L, et al. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J. 2015;36:1762–1771. doi: 10.1093/eurheartj/ehv104. [DOI] [PubMed] [Google Scholar]
- 14.Stone GW, Witzenbichler B, Weisz G, et al. ADAPT-DES Investigators Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013;382:614–623. doi: 10.1016/S0140-6736(13)61170-8. [DOI] [PubMed] [Google Scholar]
- 15.Amsterdam EA, Wenger NK, Brindis RG, et al. American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association for Clinical Chemistry 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Leger AJ, Jacques SL, Badar J, Kaneider NC, Derian CK, Andrade-Gordon P, Covic L, Kuliopulos A. Blocking the protease-activated receptor 1–4 heterodimer in platelet-mediated thrombosis. Circulation. 2006;113:1244–1254. doi: 10.1161/CIRCULATIONAHA.105.587758. [DOI] [PubMed] [Google Scholar]
- 17.Becker RC, Moliterno DJ, Jennings LK, Pieper KS, Pei J, Niederman A, Ziada KM, Berman G, Strony J, Joseph D, Mahaffey KW, Van de Werf F, Veltri E, Harrington RA, TRA-PCI Investigators Safety and tolerability of SCH 530348 in patients undergoing non-urgent percutaneous coronary intervention: a randomised, double-blind, placebo-controlled phase II study. Lancet. 2009;373:919–928. doi: 10.1016/S0140-6736(09)60230-0. [DOI] [PubMed] [Google Scholar]
- 18.Goto S, Ogawa H, Takeuchi M, Flather MD, Bhatt DL, J-LANCELOT (Japanese-Lesson from Antagonizing the Cellular Effect of Thrombin) Investigators Double-blind, placebo-controlled Phase II studies of the protease-activated receptor 1 antagonist E5555 (atopaxar) in Japanese patients with acute coronary syndrome or high-risk coronary artery disease. Eur Heart J. 2010;31:2601–2613. doi: 10.1093/eurheartj/ehq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tricoci P, Huang Z, Held C, et al. TRACER Investigators Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20–33. doi: 10.1056/NEJMoa1109719. [DOI] [PubMed] [Google Scholar]
- 20.Covic L, Misra M, Badar J, Singh C, Kuliopulos A. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat Med. 2002;8:1161–1165. doi: 10.1038/nm760. [DOI] [PubMed] [Google Scholar]
- 21.Kaneider NC, Leger AJ, Agarwal A, Nguyen N, Perides G, Derian C, Covic L, Kuliopulos A. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 2007;8:1303–1312. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, Leger AJ, Baleja JD, Rana R, Corlin T, Nguyen N, Koukos G, Bohm A, Covic L, Kuliopulos A. Allosteric activation of a G protein-coupled receptor with cell-penetrating receptor mimetics. J Biol Chem. 2015;290:15785–15798. doi: 10.1074/jbc.M115.636316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci U S A. 2002;99:643–648. doi: 10.1073/pnas.022460899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Callaghan K, Kuliopulos A, Covic L. Turning receptors on and off with intracellular pepducins: new insights into G-protein-coupled receptor drug development. J Biol Chem. 2012;287:12787–12796. doi: 10.1074/jbc.R112.355461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 26.Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, Srinivasan Y, Arlow DH, Fung JJ, Palmer D, Zheng Y, Green HF, Pandey A, Dror RO, Shaw DE, Weis WI, Coughlin SR, Kobilka BK. High-resolution crystal structure of human protease-activated receptor 1. Nature. 2012;492:387–392. doi: 10.1038/nature11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O’Callaghan K, Covic L, Kuliopulos A. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137:332–343. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tressel SL, Koukos G, Tchernychev B, Jacques SL, Covic L, Kuliopulos A. Pharmacology, biodistribution, and efficacy of GPCR-based pepducins in disease models. Methods Mol Biol. 2011;683:259–275. doi: 10.1007/978-1-60761-919-2_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontanini KB, Janz J, Looby R, Hamilton JA. Rapid binding and transmembrane diffusion of pepducins in phospholipid bilayers. Biophysical J. 2010;98:278a. [Google Scholar]
- 31.van Geffen JP, Kleinegris MC, Verdoold R, Baaten CC, Cosemans JM, Clemetson KJ, Ten Cate H, Roest M, de Laat B, Heemskerk JW. Normal platelet activation profile in patients with peripheral arterial disease on aspirin. Thromb Res. 2015;135:513–520. doi: 10.1016/j.thromres.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 32.Hosokawa K, Ohnishi T, Miura N, Sameshima H, Koide T, Tanaka KA, Maruyama I. Antithrombotic effects of PAR1 and PAR4 antagonists evaluated under flow and static conditions. Thromb Res. 2014;133:66–72. doi: 10.1016/j.thromres.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 33.Storey RF, Kotha J, Smyth SS, et al. Effects of vorapaxar on platelet reactivity and biomarker expression in non-ST-elevation acute coronary syndromes. The TRACER Pharmacodynamic Substudy. Thromb Haemost. 2014;111:883–891. doi: 10.1160/TH13-07-0624. [DOI] [PubMed] [Google Scholar]
- 34.O’Donoghue ML, Bhatt DL, Wiviott SD, et al. Safety and tolerability of atopaxar in the treatment of patients with acute coronary syndromes: the lessons from antagonizing the cellular effects of Thrombin-Acute Coronary Syndromes Trial. Circulation. 2011;123:1843–1853. doi: 10.1161/CIRCULATIONAHA.110.000786. [DOI] [PubMed] [Google Scholar]
- 35.Wiviott SD, Flather MD, O’Donoghue ML, Goto S, Fitzgerald DJ, Cura F, Aylward P, Guetta V, Dudek D, Contant CF, Angiolillo DJ, Bhatt DL, LANCELOT-CAD Investigators Randomized trial of atopaxar in the treatment of patients with coronary artery disease: the lessons from antagonizing the cellular effect of Thrombin–Coronary Artery Disease Trial. Circulation. 2011;123:1854–1863. doi: 10.1161/CIRCULATIONAHA.110.001404. [DOI] [PubMed] [Google Scholar]
- 36.Gurbel PA, Bliden KP, Guyer K, Cho PW, Zaman KA, Kreutz RP, Bassi AK, Tantry US. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol. 2005;46:1820–1826. doi: 10.1016/j.jacc.2005.07.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


