Abstract
White matter disturbance in the ventral prefrontal cortex (vPFC) in major depressive disorder (MDD) has been noted with diffusion tensor imaging (DTI). However, the cellular and molecular pathology of prefrontal white matter in MDD and potential influence of antidepressant medications is not fully understood. Oligodendrocyte morphometry and myelin-related mRNA and protein expression was examined in the white matter of the vPFC in MDD. Sections of deep and gyral white matter from the vPFC were collected from 20 subjects with MDD and 16 control subjects. Density and size of CNPase-immunoreactive (−IR) oligodendrocytes were estimated using 3-dimensional cell counting. While neither density nor soma size of oligodendrocytes was significantly affected in deep white matter, soma size was significantly decreased in the gyral white matter in MDD. In rhesus monkeys treated chronically with fluoxetine there was no significant effect on oligodendrocyte morphometry. Using quantitative RTPCR to measure oligodendrocyte-related mRNA for CNPase, PLP1, MBP, MOG, MOBP, Olig1 and Olig2, in MDD there was a significantly reduced expression of PLP1 mRNA (which positively correlated with smaller sizes) and increased expression of mRNA for CNPase, OLIG1 and MOG. The expression of CNPase protein was significantly decreased in MDD. Altered expression of four myelin genes and CNPase protein suggests a mechanism for the degeneration of cortical axons and dysfunctional maturation of oligodendrocytes in MDD. The change in oligodendrocyte morphology in gyral white matter may parallel altered axonal integrity as revealed by DTI.
Keywords: postmortem, myelin, CNPase, MOG, PLP1, OLIG1
1. Introduction
Ventral prefrontal cortex (vPFC) is involved in the control of emotional, motivational and social behavior (Koenigs & Grafman, 2009; Kringelbach, 2005; Kringelbach and Rolls, 2004; Rolls and Grabenhorst, 2008), functions that are abnormal in patients with major depressive disorder (MDD) (DSM-5, American Psychiatric Association, 2013). Neuroimaging and neuroanatomical studies strongly support the involvement of this cortical region and its connections to the orbital and medial prefrontal cortical network in the pathophysiology of MDD (Drevets et al., 2008; Koolschijn et al., 2009; Miguel-Hidalgo et al., 2010; Myers-Schulz and Koenigs, 2012; Price and Drevets, 2010; Rajkowska et al., 1999; 2005; 2013).
Diffusion tensor imaging (DTI) studies in MDD reveal white matter pathology in frontal cortex and its ventral part, including reductions in fractional anisotropy, an index of axonal integrity (Murphy and Frodl, 2011; Nobuhara et al., 2006; Sexton et al., 2009; Shimony et al., 2009; Tham et al., 2011). Reductions in fractional anisotropy and other DTI parameters in MDD suggest altered orientation of white matter axons and pathology of axonal membranes or oligodendrocyte-derived myelin sheaths (Du and Ongur, 2013; Song et al., 2002, 2003). Not all DTI studies, however, find white matter alterations in MDD (Abe et al., 2010; Choi et al., 2014). In a recent DTI study optimizing imaging acquisition, white matter integrity in wide-spread cortical areas in many medication-free patients with MDD was not significantly different from controls (Choi et al., 2014). The discrepancy in results between DTI studies may be related to the clinical heterogeneity, location and direction of the finding, cohort size as well as acquisition and analytic methods (Choi et al., 2014). Thus, neuroimaging studies do not consistently reveal disrupted white matter integrity in MDD.
There are no studies in vPFC white matter at the microscopic level indicating which cellular and molecular substrates may underlie a reduction in fractional anisotropy in MDD. Of four studies using a Nissl stain to examine oligodendrocyte density in prefrontal white matter, three reported no significant changes in oligodendrocyte density in MDD in the dorsolateral prefrontal, anterior cingulate or subgenual cingulate cortex, while a fourth reported a significant decrease in the density of oligodendrocytes in similar regions (Mosebach et al., 2013; Sibille et al., 2009; Uranova et al., 2004; Williams et al., 2013).
Morphometry of selectively-labeled oligodendrocytes in vPFC white matter has not been evaluated in MDD, nor is it known if there is pathology related to gene expression for oligodendrocytes or the structural components of myelin in MDD.
Expression of oligodendrocyte and myelin-related genes has been examined in mixtures of gray and white matter in cerebral cortex in MDD (Aston et al., 2005, Kim and Webster, 2010; Klempan et al., 2009). Significant reductions in the expression of transcripts of myelin- and oligodendrocyte-related genes were noted in MDD in temporal, pregenual anterior cingulate and lateral prefrontal cortex, although the vPFC was not examined.
The present study examined the packing density and soma size of oligodendrocytes immunoreactive for 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) and the expression of mRNA and protein for myelin-related markers expressed by oligodendrocytes in the vPFC white matter in MDD. The morphometry of oligodendrocytes was investigated in two subregions of the human vPFC: deep white matter (association, projection and commissural fibers) and gyral white matter (short association (U) fibers). Morphometry of oligodendrocytes was also examined in the vPFC white matter in rhesus monkeys treated chronically with fluoxetine to determine the effects of antidepressants on oligodendrocytes.
2. Methods
2.1. Human Subjects
The protocol for recruitment, tissue collection, and interviews was approved by the Institutional Review Boards of University Hospitals Case Medical Center, Cleveland, OH, and University of Mississippi Medical Center. Written informed consent was obtained from legally-defined next-of-kin for tissue collection and informant-based retrospective diagnostic interviews. Brain samples from the left prefrontal cortex were collected from 36 subjects at autopsy at the Cuyahoga County Medical Examiner’s Office (Cleveland, OH). All subjects underwent retrospective assessment according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed.)(DSM-IV; APA, 1994) by a trained interviewer using the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1995). Lifetime and recent Axis I psychopathology, with the aid of medical records, were determined by consensus diagnosis. Information was also collected from informants or medical records about psychoactive substance use and medication history. No subjects had evidence of head trauma, neurologic or neuropathological disease. Twenty subjects met criteria for MDD in the last month of life except one for whom the disorder was in full remission. Eight of the MDD subjects were comorbid for another Axis I disorder (Table 1). None of the 16 control subjects currently met criteria for a DSM-IV diagnosis, although one subject met criteria for alcohol abuse only at 30 years prior to death. Fifteen of the 20 MDD subjects died by suicide (Table 1). Postmortem samples of urine and blood were examined by the medical examiner for the presence of psychotropic medication or psychoactive substances. Ten of the 20 MDD subjects had a prescription for an antidepressant medication within the last month of life, however, an antidepressant drug was only detected postmortem in five subjects (Table 1). Control and MDD subjects were matched for age, gender, postmortem interval (PMI), tissue pH and storage time in freezer (TF)(Table 1). These 20 MDD and 16 control subjects were used for morphometric analyses of oligodendrocytes. Due to limited tissue availability, 11–14 MDD and 11–14 control subjects were used for determining mRNA and protein expression.
Table 1.
Characteristics of Control and MDD subjects.
| Parameter | Controls (n=16) | MDD (n=20) |
|---|---|---|
| Age (years) (range) | 52 ± 4 (27 – 80) | 55 ± 4 (20 – 87) |
|
| ||
| Gender (F:M) | 6:10 | 9:11 |
|
| ||
| PMI (hrs) (range) | 20.0 ± 1.4 (10 – 29) | 24.5 ± 1.7 (10 – 44) |
|
| ||
| Tissue pH (range) | 6.73 ± 0.07 (6.2 – 7.1) | 6.51 ± 0.07 (5.6 – 6.9) |
|
| ||
| RNA purity# (range) | 1.99 ± 0.03 (1.74 – 2.15) | 1.97 ± 0.03 (1.79 – 2.08) |
|
| ||
| TF (months) (range) | 97.9 ± 8.7 (34 – 133) | 92.8 ± 9.3 (19 – 141) |
| Cause of death | Cardiovascular disease (14); hemorrhagic pancreatitis (1); accidental CO asphyxiation (1) | Suicide (15); firearm (4); CO poisoning (5); hanging (3); drowning (1); fall from height (1); hyperkalemia (1) Other causes: cardiovascular disease (5) |
|
| ||
| Diagnosis | none | MDD (20); Comorbid Diagnoses (8): GAD (2); alcohol & cannabis abuse (1); anxiolytic abuse (1); anxiety disorder NOS (1); sexual disorder NOS (1); panic disorder with agoraphobia (1); pathological gambling (1) |
| Duration of MDD (years) (range) | not applicable | 12.5 ± 3.4 (0.2 – 62 years) |
| AD Medication Status* | none | 10 |
|
| ||
| Toxicology (Antidepressant drugs) | none | n=5 (fluoxetine 1; paroxetine 1; sertraline 2; venlafaxine 1) |
Data represent the mean ± S.E.M. AD – antidepressant; CO-carbon monoxide; GAD – generalized anxiety disorder; MDD-major depressive disorder; NOS – not otherwise specified; PMI-postmortem interval; TF-time in freezer;
RNA purity was determined by Nanodrop Spectrophotometry at multiple wavelengths, 260, 280 and 230. Ratio of 260/280 represents RNA purity. The standard for RNA purity is ratio of 2;
Prescription for antidepressants within 4 weeks prior to death. The average age (t=2.591, df=34, p=0.56), PMI (t=1.980, df=34, p=0.06) and TF (t=0.381, df=32, p=0.71) of subjects with MDD were not statistically different from the control subjects. However, brain tissue pH was significantly lower in depressed subjects (t=2.441, df=33, p=0.02).
2.2. Tissue preparation
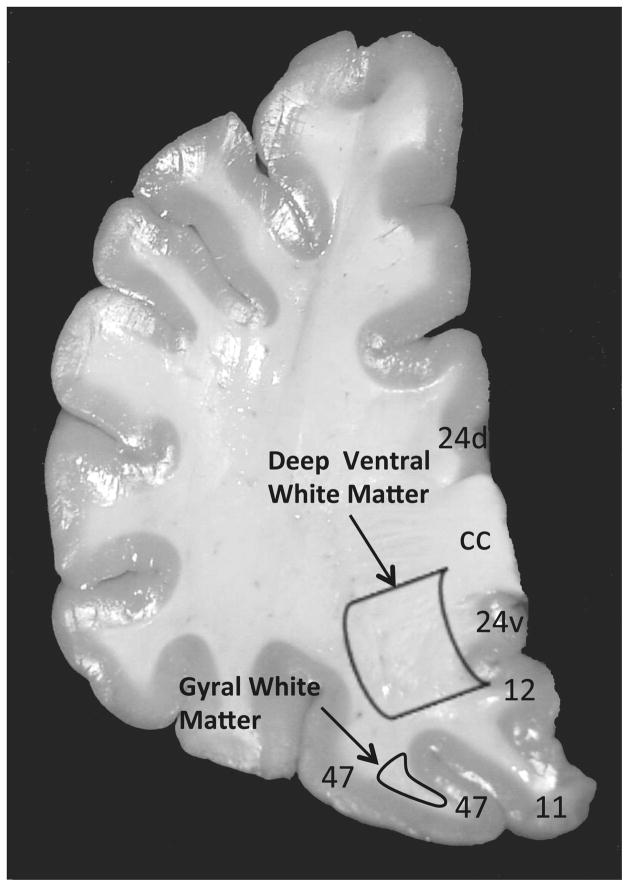
Frozen 20μm sections were cut from the ventral half of the prefrontal cortex in all subjects. Two regions of the vPFC white matter were examined, including deep white matter and gyral white matter (Fig. 1). Frozen sections adjacent to the ones labeled for CNPase immunohistochemistry were cut at a thickness of 50μm and punches of tissue including both deep and gyral white matter of the vPFC were collected for mRNA expression and western blotting experiments.
Figure 1.
Coronal section of the human prefrontal cortex indicating location of two regions where oligodendrocyte density and soma size were measured. A larger contour was placed in the deep part of the ventral prefrontal white matter, while a smaller contour was placed inside the gyral ventral prefrontal white matter underlying Brodmann area 47. Numbers refer to Brodmann areas.
Abbreviations: cc, corpus callosum; d, dorsal; v, ventral.
2.3. Immunohistochemistry
Frozen sections were mounted on slides, dried for 20 min, washed five min in PBS, fixed 30 min in 4% paraformaldehyde and washed in Tris-HCl buffer saline solution (TBS, pH 7.6). Sections were incubated overnight with a mouse monoclonal anti-oligodendrocyte antibody which specifically recognizes CNPase in oligodendrocytes (Watanabe et al., 2006) (1:1000 dilution; Millipore/Chemicon MAB 1580, Billerica, MA). Binding of the primary antibody was detected with a secondary anti-mouse antibody (1:200; ABC kit, Vector Laboratories, Burlingame, CA). To minimize inter-assay variability in the intensity of staining, each experiment included yoked sections from both MDD and control subjects. The specificity of the anti-CNPase antibody was tested by performing parallel immunohistochemical assays where either the primary antibody or the secondary antibody was omitted and where the primary antibody was blocked with the immunogen from Millipore/Chemicon. In all cases above, there was no immunoreactivity.
2.4. Morphometric analyses
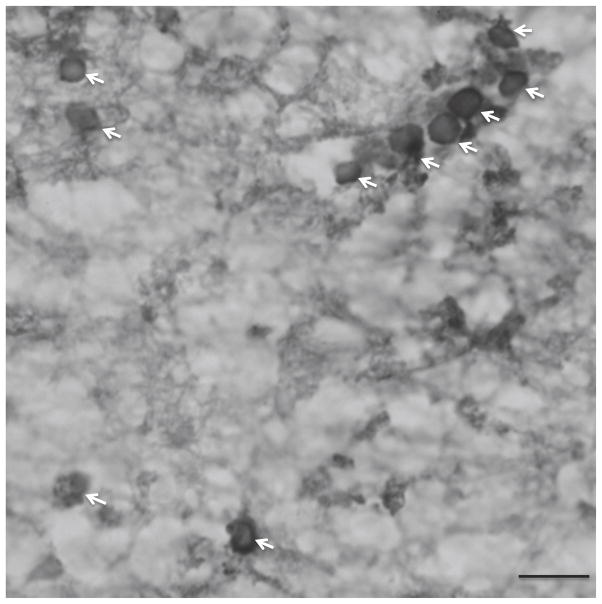
Cell packing density and soma size (cell volume) of CNPase-IR oligodendrocytes (Fig. 2). was estimated in triplicate sections with a 3-dimensional cell counting method (StereoInvestigator software, v.10; MBF Bioscience, Williston, VT). For cell counting and cell size measurements of oligodendrocytes in deep white matter, a contour was drawn enclosing the ventral part of the prefrontal white matter, but excluding the white matter within gyri (Fig. 1). This portion of the deep white matter underlies cortical Brodmann areas 47, 11, 12 and the ventral part of area 24 (Fig. 1) (Uylings et al., 2010). For cell measurements in the gyral white matter, a contour was drawn immediately adjacent to the gray matter of the middle orbital gyrus (Brodmann area 47) (Fig. 1). Random counting boxes (50 X 50 X 5.2 μm) were systematically placed within each contour. Within each counting box, oligodendrocytes were examined with a 100X objective (na=1.40). The optical fractionator probe was used to estimate cell packing density and the nucleator probe was used to measure soma size.
Figure 2.
Photomicrograph showing human oligodendrocytes immunoreactive for 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase)(see arrows) in the deep ventral prefrontal white matter. The packing density and soma size of these glial cells were estimated in the deep and gyral parts of the ventral prefrontal white matter in major depressive disorder (MDD) and control subjects. Scale bar = 10μm.
2.5. Non-human primates
Fifteen of the subjects with MDD either had a history of treatment with antidepressant medication or the drug was present in postmortem fluids. Therefore, brain tissue was also examined in 6 male rhesus monkeys treated daily for 39 weeks with the antidepressant drug fluoxetine (3 mg/kg/day) and 6 male monkeys treated with vehicle. Details on drug administration, measurement of plasma concentrations of fluoxetine and norfluoxetine and euthanasia are outlined in Szewczyk et al. (2010). Plasma levels of fluoxetine and its metabolite approximated clinically relevant values. Triplicate sections per monkey from area 14 (Paxinos et al., 2000), analogous to vPFC in human brain (Mackey and Petrides, 2010), were processed for CNPase-immunohistochemistry. The density and size of CNPase-IR oligodendrocytes from white matter located in the narrow region between the medial and orbital part of area 14 were measured using the same methods outlined above for human tissue.
2.6. mRNA expression experiments
RNA isolation and quantitative PCR
RNA was isolated using Ambion RNA Aqueous kit and cleaned up with Qiagen RNEasy kit. RNA (1μg) was reverse-transcribed into cDNA using dT primers and reverse-transcriptase. RNA was then hydrolyzed, cDNA precipitated (linear acrylamide, Ambion) and re-suspended in nuclease-free water. Gene specific primers were designed and tested for efficiency and specificity by serial dilutions and melt curve analysis. Sybr Green mix (Quantitect, Qiagen) was used to amplify cDNA in the Mastercycler ep Gradient S (Eppendorf) real time PCR machine. Four housekeeping genes, cyclophilin, GAPDH, β-actin and β-tubulin were tested for amplification efficiency and suitability as genes for normalization. Gene regulation was calculated by normalizing target gene expression to cyclophilin and β-actin. Quantitative PCR analysis was conducted to examine relative mRNA expression levels of CNPase, proteolipid protein 1 (PLP1), myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG), myelin-associated oligodendrocyte basic protein (MOBP) and oligodendrocyte transcription factors 1 and 2 (Olig1 and Olig2).
2.7. Western blotting experiment
The expression of CNPase protein was examined by western blotting as described (Karolewicz et al., 2010). Expression of other myelin-related proteins was not determined due to a lack of tissue. Tissue sample homogenates were centrifuged at 900 × g and supernatants were mixed with sample buffer. Solubilized protein (5 μg per lane) was subjected to 10% Criterion Precast Tris-HCl gel electrophoresis (Bio-Rad Laboratories, Hercules, CA, USA) and transferred to nitrocellulose membranes (Hybond ECL; GE HealthCare Life Sciences, Pittsburgh, PA, USA). Blots were incubated overnight (at 4º C) with mouse anti-CNPase monoclonal antibody (Clone SMI-91; diluted 1:3000; Covance, CA, USA; no.SMI-92R). As a control for transfer and loading, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was detected on each blot using rabbit polyclonal anti-GAPDH antibody (Abcam Inc., Cambridge, MA, USA; no. AB36840; diluted 1:1000). Membranes were washed in TBS buffer and incubated with peroxidase-conjugated anti-mouse (diluted 1:3000; GE HealthCare Life Sciences, Pittsburgh, PA, USA, no. NA931) or anti-rabbit secondary antibodies (GE HealthCare Life Sciences, Pittsburgh, PA, USA, no. NA934; diluted 1:3000). Immunoreactive bands were visualized by chemi-luminescence detection (ECL; PerkinElmer Life Sciences, Inc., Boston, MA, USA) with photographic film (Hyperfilm-ECL, GE HealthCare Life Sciences, Pittsburgh, PA, USA). The anti-CNPase monoclonal antibody reacts with the 46 and 48 kDa subunits of the CNPase dimer, whereas the anti-GAPDH antibody reacts with a band at 36 kDa.
Pairs of MDD and control subjects were matched for age, gender, post-mortem interval, tissue pH, and storage time in freezer. A maximum of five pairs of control and MDD subjects were run on the same gel with duplicates run on separate gels. There was a linear relationship between optical density values and protein concentrations for all antibodies used. In order to minimize inter-blot variability and to aid in quantifying blots, each gel was loaded with 3 concentrations of the same cortical tissue standard (dissected from a normal control subject) consisting of 2.5, 5 and 10 μg of total protein. Protein band densities were analyzed using MCID Elite 7.0 (Imaging Research, St. Catherines, Ontario, Canada). Relative optical density values were converted to arbitrary protein units using a standard curve. The data were normalized to GAPDH immunoreactivity detected on the same blot.
2.8. Statistical analyses
The influence of age, PMI, tissue pH, TF and duration of MDD on the density and size of oligodendrocytes and the mRNA and protein expression of myelin-related genes was evaluated by Pearson correlation analyses (V 5.0b; GraphPad Prism, La Jolla, CA). A significant correlation was noted only between age and density and TF and density of oligodendrocytes in gyral white matter in MDD, and between TF and density of oligodendrocytes in deep white matter in MDD (see Results). The average age, PMI, tissue pH and TF were compared between the MDD and control groups (Table 1). Tissue pH values were significantly lower in MDD vs. controls (t=2.441, df=33, p=0.02) only for subjects used to examine cell density and size, but not for the somewhat fewer control and MDD subjects used for RNA and protein expression. Therefore, in the statistical analyses examining the density and size of oligodendrocytes in human tissue, analysis of covariance (ANCOVA) (either with or without the five subjects with antidepressant medication in postmortem blood) was performed with tissue pH, age and TF as covariates (IBM SPSS, version 22.0; Chicago, IL). The ANCOVA analyses of density and soma size comparing controls and subjects with MDD yielded highly comparable statistical results whether or not the five MDD subjects with antidepressant medication in postmortem blood were included. Statistical analysis using a random effect model was conducted to control for a possible effect of immunostaining batches of sections on different days. Pearson correlation analyses showed no association between mRNA transcripts and potentially confounding variables (Tables 2 and 3). Nevertheless, ANCOVA analyses were performed with PMI, tissue pH, age and TF as covariates to examine the potential effects of these variables on mRNA expression of myelin-related genes between MDD and control subjects. The ANCOVA analyses of mRNA and CNPase protein expression comparing controls and subjects with MDD yielded highly comparable statistical results whether or not the one MDD subject with antidepressant medication in postmortem blood was included. An unpaired, two-tailed Student’s t-test was used to compare oligodendrocyte density and size between fluoxetine-treated and control monkeys. Statistical significance for all tests was set at p ≤ 0.05.
Table 2.
Summary of Pearson correlations between control subjects and those with MDD for mRNA for PLP1 and Olig1 and potentially confounding variables.
| Variable | PLP1 mRNA | Olig1 mRNA | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | MDD | Control | MDD | |||||
| r | p | r | p | r | p | r | p | |
| Age at death | −0.305 | 0.31 | −0.220 | 0.49 | 0.453 | 0.12 | −0.201 | 0.55 |
| PMI | −0.002 | 0.99 | 0.302 | 0.34 | 0.212 | 0.49 | −0.096 | 0.78 |
| Tissue pH | −0.437 | 0.14 | −0.369 | 0.24 | 0.247 | 0.42 | −.0431 | 0.19 |
| TF | 0.310 | 0.30 | 0.195 | 0.54 | −0.448 | 0.12 | 0.356 | 0.28 |
| Duration of depression | - | - | −0.512 | 0.09 | - | - | 0.211 | 0.53 |
MDD – major depressive disorder; Olig1 - oligodendrocyte transcription factors 1; PLP1 -proteolipid protein 1; PMI - postmortem interval; TF - storage time in freezer.
Table 3.
Summary of Pearson correlations between control subjects and those with MDD for mRNA for CNPase and MOG and potentially confounding variables.
| Variable | CNPase mRNA | MOG mRNA | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | MDD | Control | MDD | |||||
| r | p | r | p | r | p | r | p | |
| Age at death | 0.218 | 0.48 | 0.208 | 0.50 | 0.381 | 0.25 | 0.104 | 0.78 |
| PMI | −0.038 | 0.90 | 0.068 | 0.83 | −0.224 | 0.51 | −0.200 | 0.58 |
| Tissue pH | −0.381 | 0.20 | −0.031 | 0.93 | −0.076 | 0.83 | 0.238 | 0.51 |
| TF | 0.370 | 0.21 | −0.372 | 0.21 | −0.474 | 0.14 | −0.348 | 0.32 |
| Duration of depression | - | - | −0.242 | 0.43 | - | - | 0.182 | 0.61 |
CNPase - 2,′3′-cyclic nucleotide 3′-phosphodiesterase; MOG - myelin oligodendrocyte glycoprotein; MDD – major depressive disorder; PMI - postmortem interval; TF - storage time in freezer.
3. Results
3.1. Oligodendrocytes in the white matter of deep vPFC in human brain
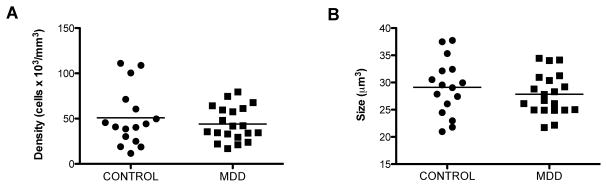
There was no significant difference in the cell packing density of oligodendrocytes in deep white matter when comparing MDD and control subjects (ANCOVA, F(1, 31)=2.498, p=0.12) (Fig. 3A). Similarly, there was no significant difference between MDD and control subjects in the soma size of oligodendrocytes (ANCOVA, F(1, 31)=2.492, p=0.12) (Fig. 3B). There were no significant correlations between cell packing density and age, PMI or pH in either MDD (age: r=0.362, p=0.12; PMI: r=0.346, p=0.14; pH: r=0.362, p=0.12) or control subjects (age: r=0.288, p=0.28; PMI: r=0.095, p=0.73; pH: r=−0.479, p=0.06). However, there was a significant positive correlation between TF and density of oligodendrocytes in MDD (r=0.612, p=0.004) but not in control (r=0.134, p=0.62) subjects. Likewise, no correlation was detected between age, PMI, tissue pH, TF and soma size of oligodendrocytes in either the MDD (age: r=−0.109, p=0.65; PMI: r=0.414, p=;0.07; pH: r=−0.340, p=0.15; TF: r=0.–170, p=0.47) or control subjects (age: r=0.129, p=0.64; PMI: r=−0.209, p=0.45; pH: r=−0.375, p=0.15; TF: r=−0.064, p=0.81). Duration of MDD was not correlated with either the density (r=−0.014, p=0.96) or size (r=−0.031, p=0.91) of oligodendrocytes.
Figure 3.
Morphometric parameters (density and soma size) of CNPase-immunoreactive oligodendrocytes in the deep part of ventral prefrontal white matter in human brain. A, The density of oligodendrocytes was not significantly different between control subjects and those with major depressive disorder (MDD). B, Soma size of oligodendrocytes was not significantly different between control subjects and those with MDD. The horizontal lines represent the mean value of density or size for each diagnostic group.
3.2. Oligodendrocytes in the white matter of gyral vPFC in human brain
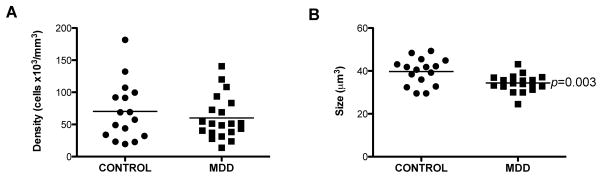
There was no significant difference in the density of oligodendrocytes in gyral white matter between MDD and control subjects (ANCOVA, F(1, 31)=1.276, p=0.27) (Fig. 4A). However, there was a significant positive correlation between packing density and age in MDD (r=0.444, p=0.049) but not in control (r=0.351, p=0.182) subjects and between density and TF in MDD (r=0.441, p=0.05) but not in controls (r=0.036, p=0.89). Factors such as PMI and pH were not correlated with the density of oligodendrocytes in either the MDD (PMI: r=0.127, p=0.59; pH: r=0.341, p=0.14) or the control (PMI: r=−0.133, p=0.62; pH: r=−0.309, p=0.24) groups. The duration of MDD was not correlated with the density of oligodendrocytes (r=0.236, p=0.36).
Figure 4.
Density and soma size of CNPase-immunoreactive oligodendrocytes in the gyral ventral prefrontal white matter in human brain. A, Oligodendrocyte density was not significantly different between control subjects and those with major depressive disorder (MDD). B, There was a statistically significant 13 percent decrease in soma size in subjects with major depressive disorder (MDD), as compared to control subjects. The horizontal lines represent the mean value of density or soma size for each diagnostic group.
In contrast to packing density, the size of oligodendrocyte cell bodies differed significantly between the MDD and control groups. Sizes were 13 percent significantly smaller in the MDD vs. the control group (ANCOVA, F(1, 31)=10.290, p=0.003) (Fig. 4B). After controlling for (very modest) baseline differences in the intensity of immunohistochemistry between days, there was still a significant difference in oligodendrocyte soma size between the control and MDD groups [p-value=0.0134, 95% CI (1.89, 9.69)]. There was no significant influence of age, PMI, pH or TF on the size of oligodendrocytes in either MDD (age: r=−0.241, p=0.31; PMI: r=−0.166, p=0.48; pH: r=−.0.073, p=0.76; TF: r=0.151, p=0.53) or control (age: r=0.–038, p=0.89; PMI: r=−0.447, p=0.08; pH: r=−0.205, p=0.45; TF: r=0.151, p=0.53) groups. The duration of MDD did not significantly influence soma size of oligodendrocytes (r=−0.029, p=0.91). Suicide did not have a significant effect on oligodendrocyte soma size; MDD subjects dying by suicide had soma sizes similar to MDD subjects dying by other causes (t=0.273, df=18, p=0.79). There was no statistically significant difference in the soma size between MDD subjects with antidepressant drugs present postmortem and MDD subjects with a prescription but no drug present postmortem (t=0.062, df=8, p=0.95).
3.3. Oligodendrocyte morphometry in white matter of vPFC in monkey brain
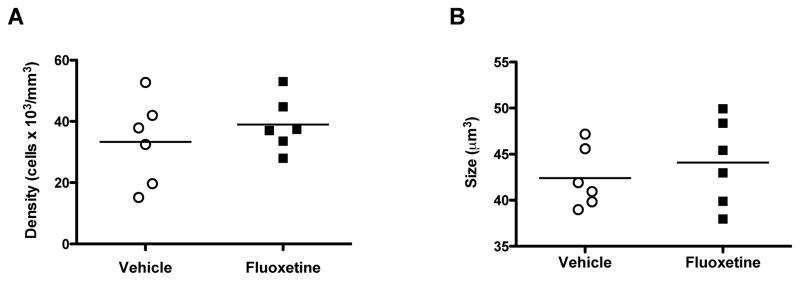
There was no significant difference in the packing density of CNPase-IR oligodendrocytes between fluoxetine-treated and control animals (t=0.8343, df=10, p=0.42) (Fig. 5A). The size of oligodendrocyte cell bodies was also not significantly different between the two cohorts (t=0.7203, df=10, p=0.49) (Fig. 5B).
Figure 5.
Density and soma size of CNPase-immunoreactive oligodendrocytes in the ventral prefrontal white matter in rhesus monkeys treated chronically with fluoxetine or vehicle. Neither cell density (A) nor soma sizes (B) were significantly different between the groups. The horizontal lines represent the mean value of density or size for each treatment group.
3.4. Expression of mRNA for myelin-related proteins in white matter of vPFC in human brain
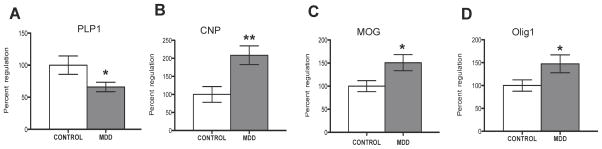
Comparison of the expression of PLP1 mRNA between MDD and control groups revealed a significant, 34 percent down-regulation in MDD (ANCOVA, F(1, 19)=12.370, p=0.002) (Fig. 6A). In contrast, in MDD there was a significant up-regulation in the expression of CNPase mRNA (109 percent, ANCOVA, F(1, 20)=6.958, p=0.016), MOG mRNA (51 percent, ANCOVA, F(1, 15)=9.477, p=0.008) and Olig1 mRNA (47 percent, ANCOVA, F(1, 21)=4.081, p=0.05) (Fig. 6B, C, D). Expression of MBP mRNA (t=0.925, df=23, p=0.36), MOBP mRNA (t=0.825, df=20, p=0.42) and Olig2 mRNA (t=0.328, df=24, p=0.75) was not significantly different between the MDD and control groups. Pearson correlation analyses showed no significant associations between the expression of mRNA for PLP1, CNPase, MOG and Olig1 and age, PMI, pH, TF or duration of MDD (see Tables 2 and 3). Correlational analyses were conducted to test the relationship between expression of mRNA for PLP1, CNPase, MOG and OLIG1 and soma size of oligodendrocytes. We found a significant, positive correlation between PLP1 mRNA and soma size (r=0.464, p=0.03) where the smaller the size, the lower the expression of PLP1. All other correlations were not statistically significant (CNPase vs size: r=−0.149, p=0.68; MOG vs size: r=0.068, p=0.78; Olig1 vs size: r=−0.122, p=0.59).
Figure 6.
Expression of myelin-related mRNA in the human ventral prefrontal white matter. Histograms show significant differences in the expression of A, PLP1; B, CNPase; C, MOG; D, Olig 1 between the subjects with major depressive disorder (MDD) and control subjects. Mean values (with standard error of the means) for subjects with MDD are expressed as percent regulation of the control subjects. *p< 0.05, **p<0.01.
3.5. Expression of CNPase protein in white matter of vPFC in human brain
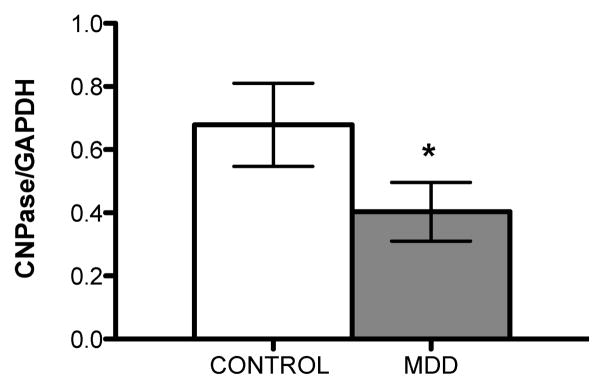
The level of CNPase protein from subjects with MDD was significantly lower (44%) than that of matched controls (paired t-test, t = 2.279, df=13, p = 0.04), Fig. 7). While Pearson correlation analysis revealed a significant negative correlation between the amount of CNPase immunoreactivity and storage time in freezer (TF) (only in MDD; r = −0.7, p = 0.007), levels of CNPase were still significantly less in MDD than in controls (ANCOVA with TF as a covariate, F(1,23) = 4.769, p = 0.039). There was no correlation between the amount of CNPase protein and age at the time of death, PMI or tissue pH in either control or MDD groups, or CNPase expression and the duration of MDD (Table 4). The decrease in CNPase immunoreactivity in MDD subjects was not related to death by suicide since there was no difference in the CNPase level between 8 suicide MDD subjects (0.38 ± 0.13) and 5 MDD non suicide subjects (0.57 ± 0.21; unpaired t-test, t = 0.802, df=11, p = 0.44).
Figure 7.
Expression of CNPase protein in ventral prefrontal white matter from 14 matched pairs of subjects with major depressive disorder (MDD) and control subjects. Mean values (with S.E.M.) of the normalized optical density for each group are presented. *p< 0.05.
Table 4.
Summary of Pearson correlations between control subjects and those with MDD for CNPase protein expression and potentially confounding variables.
| Variable | CNPase protein | |||
|---|---|---|---|---|
| Control | MDD | |||
| r | p | r | p | |
| Age at death | 0.255 | 0.40 | 0.064 | 0.84 |
| PMI | 0.218 | 0.47 | −0.007 | 0.98 |
| Tissue pH | 0.257 | 0.40 | 0.489 | 0.09 |
| TF | −0.416 | 0.16 | −0.703 | 0.007 |
| Duration of depression | - | - | −0.022 | 0.94 |
CNPase - 2,′3′-cyclic nucleotide 3′-phosphodiesterase; MDD – major depressive disorder; PMI - postmortem interval; TF - storage time in freezer.
4. Discussion
Oligodendrocytes were examined in the vPFC as these cells are crucial components in the orbital and medial prefrontal cortical networks and pathology in these glial cells may likely interfere with the role of the vPFC in emotional, motivational and social behaviors, and thereby contribute to the constellation of symptoms comprising MDD (Drevets et al., 2008; Koenigs & Grafman, 2009; Kringelbach, 2005; Kringelbach and Rolls, 2004; Price and Drevets, 2010; Rolls and Grabenhorst, 2008).
A reduction in the size of oligodendrocyte cell bodies immunoreactive for CNPase was noted in gyral but not deep portions of the vPFC white matter in MDD. This reduction in size in the gyral white matter was paralleled by a decrease in the expression of CNPase protein and PLP1 mRNA, and an increase in the expression of mRNA for CNPase, MOG and Olig1 in MDD, as compared to control subjects.
The smaller size of oligodendrocyte cell bodies in MDD does not appear due to antidepressant therapy. The size of cell bodies was not significantly different between MDD subjects treated or not with antidepressant medications. Moreover, monkeys treated for nine months with the antidepressant fluoxetine showed no significant change in oligodendrocyte size in vPFC white matter, as compared to vehicle-treated controls. In contrast to size, the density of oligodendrocytes was not significantly different between MDD and control subjects in either gyral or deep white matter of the vPFC. However, there was a significant positive correlation between the age of MDD subjects and the density of oligodendrocyte in gyral white matter. The increased density in older MDD subjects may be a result of degeneration of some axons, which in turn leaves oligodendrocyte cell bodies more densely packed.
This is the first study in MDD to estimate the density and size of oligodendrocytes visualized with an antibody specific to this type of glial cell. Published studies estimated the packing density of oligodendrocytes in MDD used Nissl staining, where it can be difficult to distinguish oligodendrocytes from other glial cells (Mosebach et al., 2013; Sibille et al, 2009; Uranova et al., 2004, Williams et al., 2013). Nonetheless, the present study noted no change in oligodendrocytes density in white matter of vPFC in MDD and is consistent with similar reports using Nissl staining in the dorsolateral prefrontal and subgenual cingulate white matter in MDD (Uranova et al., 2004; Williams et al., 2013).
Morphometry of CNPase-IR oligodendrocytes was analyzed in the white matter of vPFC. Since it is difficult to differentiate axon bundles with nearby vs. distant projections in histological sections, oligodendrocytes here were analyzed in two parts of the vPFC white matter selected by their proximity to the gray matter: the deep white matter, consisting of the ventral portions of medial and lateral prefrontal white matter, and the gyral white matter, located within the medial orbitofrontal gyrus (BA 47) (Malykhin et al., 2011; Uylings et al., 2010). Significant changes noted in oligodendrocyte morphometry in gyral but not deep white matter may be due to the differences in fiber bundle composition in these regions. Gyral white matter lies immediately beneath the gray matter of cortical gyri and is predominantly composed of short “U” shaped fibers connecting adjacent gyri, and in addition, longer intra-lobar fibers connecting areas within the same lobe, and finally, deep white matter fiber extensions, with all of them having origin or destination in a restricted cortical area (Catani et al., 2012, Schmahmann and Pandya, 2006; Yeterian et al., 2012). By contrast, deep white matter contains long association, commissural and projection fibers connecting various areas of the vPFC with limbic structures and other cortical and subcortical regions (Carpenter, 1991; Filley, 2001; Jbabdi et al., 2013; Schmahmann and Pandya, 2006). Reduced size of oligodendrocyte cell bodies, as detected in the gyral white matter of the vPFC, suggests that myelinated axons of local origin or destination in the overlying gray matter are involved in the pathology of white matter in MDD.
The lack of significant changes in oligodendrocyte density or size in the deep white matter in MDD may be due to the variety of destinations and origins of axonal groups traversing this region of the white matter. Some axonal bundles may not be affected in MDD to the same extent as others and a statistically significant difference, in aggregate, might be masked. In neuroimaging studies, this aggregation of deep and gyral locations of white matter might obscure pathology such that it would only be noted in some but not other studies in MDD (Abe et al., 2010; Choi et al., 2014; Du and Ongur, 2013; Murphy and Frodl, 2011; Sexton et al., 2009; Tham et al., 2011). Neuroimaging studies at higher resolution may yet note bundle-specific changes in MDD.
While DTI studies have not yet examined gyral cortical white matter in MDD, such a study in schizophrenia revealed alterations in the superficial (gyral) frontal white matter which were related to disturbances in cognitive performance (Nazeri et al, 2013). Interestingly, this study also noted that U-shaped and intraregional fibers, rather than extensions of the deep white matter fibers, showed pathological changes in superficial frontal white matter. This pathology may be related to data from postmortem tissue showing changes in neuron density and distribution in gyral white matter in the prefrontal cortex in schizophrenia (Akbarian et al., 1996; Eastwood et al., 2005; Joshi et al., 2012; Yang et al., 2011). Whether the same is true for MDD remains to be determined by DTI and postmortem studies.
In addition to oligodendrocyte morphometry, the expression of mRNA transcripts for 7 proteins associated with myelin and expressed by oligodendrocytes, including CNPase, MBP, PLP1, MOG, MOBP, Olig1 and Olig2, were also evaluated. The mRNA for CNPase, PLP1, MOG, MOBP and MBP encode structural components of myelin and are related to myelination (Berger and Reindl, 2007; Diehl et al., 1986; Myllykoski et al., 2012; Nave, 2010; Scherer et al., 1994; Trapp et al., 1988; Yu et al., 1994). Olig1 and Olig2 are transcription factors found in human white matter and expressed by oligodendrocytes during maturation and regeneration (Fu et al., 2002; Marie et al., 2001). A significant decrease in the expression of PLP1 mRNA was noted here in the vPFC white matter in MDD vs. controls. In contrast, the expression of mRNA for CNPase, OLIG1 and MOG was increased in the same region of the prefrontal white matter in MDD. Reductions in PLP1 mRNA suggest a degeneration of cortical axons since both mice lacking PLP1 and humans with a naturally-occurring PLP1 point mutation exhibit degeneration of cortical axons in the absence of demyelination and inflammation (Garbern et al., 2002). However, it cannot be ruled out that an excess of PLP1 protein may down-regulate PLP1 mRNA synthesis. This PLP1 pathology in MDD may also be related to the smaller soma sizes of oligodendrocytes as there was a positive correlation between size and expression of PLP1 mRNA.
The levels of CNPase protein and mRNA were also determined. CNPase protein levels were significantly decreased in MDD. CNPase is involved in the formation of myelin membranes and cytoskeletal microtubules in oligodendrocytes (Bifulco et al., 2002; Yin et al., 1997). A deficit in CNPase in MDD, potentially affecting membranes and microtubule formation, may result in altered morphology and smaller oligodendrocyte soma size. In contrast, levels of CNPase mRNA were significantly increased in MDD. This discordance between CNPase mRNA and protein may reflect a compensatory increase in mRNA in response to a decrease in levels of the protein. In addition, the increase in expression of CNPase mRNA in MDD may be related to smaller oligodendrocyte soma size and altered expression of myelin-related mRNA since overexpression of this transcript in transgenic mice alters oligodendrocyte development and induces aberrant myelination (Gravel et al., 1996). Likewise, overexpression of MOG in MDD may be related to aberrant production of myelin sheaths as this gene is considered important in the completion and/or maintenance of myelin sheaths (Roth et al., 1995). Finally, increased expression of Olig1 in MDD may point to dysfunctional differentiation of oligodendrocytes. Overexpression of Olig 1 in cell culture prevents oligodendrocytes from reaching a mature, fully functional phenotype (Copray et al., 2006). In summary, changes in gene expression for four myelin proteins (i.e., PLP1, CNPase, MOG and OLIG1) in the white matter of vPFC in MDD suggest dysfunctional maturation of oligodendrocytes with accompanying alterations in axonal integrity.
A few postmortem studies have examined myelin-related and oligodendrocyte-specific gene expression in MDD (Aston et al., 2005; Kim and Webster, 2010; Klempan et al., 2009). Reductions in mRNA expression for PLP1, CNPase, MOG, MOBP and Olig2 (among other genes) were found in a mixture of gray and white matter of the temporal cortex in MDD (Aston et al., 2005). Similar to our observation, Aston et al. (2005) did not note any alteration in myelin basic protein (MBP) in the vPFC white matter. While some data in MDD presented here (increase in mRNA for CNPase and MOG) are opposite those noted by Aston et al. (2005), this may be due to a different cortical region examined (prefrontal vs. temporal), white matter vs. a mixture of gray and white matter, or differences in clinical characteristics of the subjects. However, in the present study, the decrease in mRNA expression for PLP1 is consistent with Aston et al. (2005). The increase in the expression of Olig1 mRNA in the present study is consistent with an increase in Olig1 immunoreactivity in white matter of anterior cingulate cortex in MDD (Mosebach et al, 2013).
Decreased expression of mRNA for PLP1 in prefrontal white matter in MDD may be regulated by the expression of other genes, e.g. CNPase, MOG and OLIG1. PLP1 composes about 50 percent of myelin in the CNS (Baumann and Pham-Dinh, 2001). Other myelin-related genes may increase their expression to compensate for reductions in PLP1. Animal and cell culture studies report mutual interactions between PLP1, CNPase, OLIG1 and MOG. For example, OLIG1 directly binds to PLP1 and CNPase promoter regions and regulates transcription of the major myelin-specific genes, such as PLP1 (Xin et al., 2005) and MOG (Lu et al., 2002; Xin et al., 2005).
Oligodendrocytes play a crucial role in preserving the integrity and survival of axons (Edgar and Sibille, 2012; Nave, 2010). Axons in Plp1-null mice undergo Wallerian degeneration (Griffiths et al., 1998). Thus, significant reductions in the expression of PLP1 mRNA observed here in MDD, even if not dramatic, may compromise axonal integrity and result in reduced fractional anisotropy noted in some DTI studies in MDD.
In conclusion, altered mRNA expression of PLP1, CNPase, Olig1 and MOG and levels of CNPase protein in the vPFC white matter in MDD suggests myelin pathology and is tied to a decrease in size of oligodendrocyte cell bodies, with no change in cell density. In contrast, changes in expression of these genes in schizophrenia are linked to a reduction in the density of oligodendrocytes (Davis and Haroutunian, 2003; Farkas et al., 2010; Hakak et al., 2001; Hof et al., 2003; Segal et al., 2007; Tkachev et al., 2003; Uranova et al., 2001, 2004). Future studies of all myelin- and oligodendrocyte-related genes, as well as higher resolution neuroimaging, should more clearly reveal the cellular and molecular basis of aberrant connections of the prefrontal white matter in MDD.
Size and density of oligodendrocytes was examined in prefrontal white matter in depression.
Sizes were smaller in subjects with depression as compared to controls.
Expression of mRNA for myelin and oligodendrocyte markers was altered in depression.
These changes may be related to white matter pathology reported by neuroimaging.
Acknowledgments
We gratefully acknowledge the assistance of Drs. James C. Overholser, George Jurjus and Lisa Konick in establishing the psychiatric diagnoses and in collecting the human tissues. We thank the Cuyahoga County Medical Examiner’s Office, Cleveland, Ohio, and the next-of-kin of our subjects for their participation and support. We also acknowledge Gillian O’Dwyer, Yilianys Pride and Warren May, Ph.D., for their excellent technical assistance. This study was supported by the Imaging and Postmortem Brain Cores of the Center for Psychiatric Neuroscience, funded by COBRE P30 GM103328.
We gratefully acknowledge the assistance of Drs. James C. Overholser, George Jurjus and Lisa Konick in establishing the psychiatric diagnoses and in collecting the human tissues. We thank the Cuyahoga County Medical Examiner’s Office, Cleveland, Ohio, and the next-of-kin of our subjects for their participation and support. We also acknowledge Gillian O’Dwyer, Yilianys Pride and Dr. Warren May for their excellent technical assistance. This study was supported by the Imaging and Postmortem Brain Cores of the Center for Psychiatric Neuroscience, funded by COBRE P30 GM103328.
Role of the Funding Source: This study was supported by the Imaging and Postmortem Brain Cores of the Center for Psychiatric Neuroscience, funded by COBRE P30 GM103328. Funding source had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors: Dr. Rajkowska designed the morphometric part of the study, performed statistical analyses of morphometric and PCR data, interpreted the results, constructed all figures and tables and wrote the first draft of this manuscript. She also delineated regions of interest on every slide which was analyzed for oligodendrocyte density and soma size.
Dr. Mahajan performed morphometric analyses (cell counting and soma size measurements) in human postmortem tissue and in monkey brains.
Dr. Maciag performed, analzyed and described experiment on defining levels of CNPase protein by using western blotting technique.
Dr. M. Sathyanesan isolated RNA, performed quality control analysis, designed primers and performed quantitative PCR runs on the expression of myelin related mRNAs for PLP1, MBP, MOG, MOBP, Olig1 and Olig2.
Dr. Iyo designed the primer and performed PCR experiments on mRNA expression for CNPase.
Dr. Moulana helped Dr. Mahajan with counting oligodendrocyte cells and measuring their soma size in human postmortem brain tissue.
Dr. Kyle measured levels of fluoxetine in monkey’s blood during chronic treatment with fluoxetine to verify that the levels were physiologically relevant.
Dr. Woolverton designed experiment on monkeys chronically treated with fluoxetine and vehicle animals and was responsible for daily delivery of the drug. He also sacrificed all the experimental animals and harvested their brains.
Dr. Miguel-Hidalgo dissected and froze blocks from monkey brains. He also provided essential input to the manuscript.
Dr. Stockmeier established and maintains the brain collection from which the tissues were selected, he participated in the study design and co-wrote the manuscript.
Dr. Newton designed the QPCR experiments, prepared the PCR data for statistical analyses and wrote the part of manuscript related to PCR experiments.
All authors (except William Woolverton, deceased) contributed to the drafting of this manuscript and have approved its final form.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Grazyna Rajkowska, Email: grajkowska@umc.edu.
Gouri Mahajan, Email: gmahajan@umc.edu.
Dorota Maciag, Email: dorotamaciag@yahoo.com.
Monica Sathyanesan, Email: Monica.Sathyanesan@usd.edu.
Abiye H. Iyo, Email: alawarik@yahoo.ca.
Mohadetheh Moulana, Email: mmoulana@umc.edu.
Patrick B. Kyle, Email: pkyle@umc.edu.
Jose Javier Miguel-Hidalgo, Email: jmiguel-hidalgo@umc.edu.
Craig A. Stockmeier, Email: cstockmeier@umc.edu.
Samuel S. Newton, Email: Samuel.Sathyanesan@usd.edu.
References
- Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Inoue H, et al. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Res. 2010;181:64–70. doi: 10.1016/j.pscychresns.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE, Jr, Jones EG. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry. 1996;53:425–36. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: American Psychiatric Press; 2013. [Google Scholar]
- Aston C, Jiang L, Sokolov BP. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry. 2005;10:309–22. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Berger T, Reindl M. Multiple sclerosis: disease biomarkers as indicated by pathophysiology. J Neurol Sci. 2007;259:21–6. doi: 10.1016/j.jns.2006.05.070. [DOI] [PubMed] [Google Scholar]
- Bifulco M, Laezza C, Stingo S, Wolff J. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase: a membrane-bound, microtubule-associated protein and membrane anchor for tubulin. Proc Natl Acad Sci U S A. 2002;99:1807–12. doi: 10.1073/pnas.042678799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M. Core text of neuroanatomy. 4. Baltimore: Williams & Wilkins; 1991. [Google Scholar]
- Catani M, Dell’Acqua F, Vergani F, Malik F, Hodge H, Roy P, et al. Short frontal lobe connections of the human brain. Cortex. 2012;48:273–91. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Choi KS, Holtzheimer PE, Franco AR, Kelley ME, Dunlop BW, Hu XP, et al. Reconciling Variable Findings of White Matter Integrity in Major Depressive Disorder. Neuropsychopharmacology. 2014;39:1332–9. doi: 10.1038/npp.2013.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copray S, Balasubramaniyan V, Levenga J, de Bruijn J, Liem R, Boddeke E. Olig2 overexpression induces the in vitro differentiation of neural stem cells into mature oligodendrocytes. Stem Cells. 2006;24:1001–10. doi: 10.1634/stemcells.2005-0239. [DOI] [PubMed] [Google Scholar]
- Davis KL, Haroutunian V. Global expression-profiling studies and oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:758. doi: 10.1016/S0140-6736(03)14297-3. [DOI] [PubMed] [Google Scholar]
- Diehl HJ, Schaich M, Budzinski RM, Stoffel W. Individual exons encode the integral membrane domains of human myelin proteolipid protein. Proc Nat Acad Sci USA. 1986;83:9807–11. doi: 10.1073/pnas.83.24.9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Ongür D. Probing myelin and axon abnormalities separately in psychiatric disorders using MRI techniques. Front Integr Neurosci. 2013;7:24. doi: 10.3389/fnint.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial white matter neuron density in the dorsolateral prefrontal cortex and parahippocampal gyrus in schizophrenia. Schizophr Res. 2005;79:181–8. doi: 10.1016/j.schres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Edgar N, Sibille E. A putative functional role for oligodendrocytes in mood regulation. Transl Psychiatry. 2012;2:e109. doi: 10.1038/tp.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas N, Lendeckel U, Dobrowolny H, Funke S, Steiner J, Keilhoff G, et al. Reduced density of ADAM 12-immunoreactive oligodendrocytes in the anterior cingulate white matter of patients with schizophrenia. World J Biol Psychiatry. 2010;11:556–66. doi: 10.3109/15622970903497936. [DOI] [PubMed] [Google Scholar]
- Filley CM. The behavioral neurology of white matter. New York: Oxford University Press; 2001. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for the DSMIV axis I disorders (SCID patient edition), version 2.0. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Fu H, Qi Y, Tan M, Cai J, Takebayashi H, Nakafuku M, et al. Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2. 2 in the control of oligodendrocyte differentiation. Development. 2002;129:681–93. doi: 10.1242/dev.129.3.681. [DOI] [PubMed] [Google Scholar]
- Garbern JY, Yool DA, Moore GJ, Wilds IB, Faulk MW, Klugmann M, et al. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain. 2002;125:551–61. doi: 10.1093/brain/awf043. [DOI] [PubMed] [Google Scholar]
- Gravel MJ, Peterson J, Yong VW, Kottis V, Trapp B, Braun PE. Overexpression of 2′,3′-cyclic nucleotide 3′-phosphodiesterase in transgenic mice alters oligodendrocyte development and produces aberrant myelination. Mol Cell Neurosci. 1996;7:453–66. doi: 10.1006/mcne.1996.0033. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–3. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–51. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, Jr, Byne W, Buitron C, Perl DP, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–85. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- Jbabdi S, Lehman JF, Haber SN, Behrens TE. Human and monkey ventral prefrontal fibers use the same organizational principles to reach their targets: tracing versus tractography. J Neurosci. 2013;33:3190–201. doi: 10.1523/JNEUROSCI.2457-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D, Fung SJ, Rothwell A, Weickert CS. Higher gamma-aminobutyric acid neuron density in the white matter of orbital frontal cortex in schizophrenia. Biol Psychiatry. 2012;72:725–33. doi: 10.1016/j.biopsych.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol. 2010;13:411–20. doi: 10.1017/S1461145709990587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Webster MJ. Correlation analysis between genome-wide expression profiles and cytoarchitectural abnormalities in the prefrontal cortex of psychiatric disorders. Mol Psychiatry. 2010;15:326–36. doi: 10.1038/mp.2008.99. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, et al. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. 2009;14:175–89. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–43. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Mackey S, Petrides M. Quantitative demonstration of comparable architectonic areas within the ventromedial and lateral orbital frontal cortex in the human and the macaque monkey brains. European J Neurosci. 2010;32:1940–50. doi: 10.1111/j.1460-9568.2010.07465.x. [DOI] [PubMed] [Google Scholar]
- Malykhin N, Vahidy S, Michielse S, Coupland N, Camicioli R, Seres P, et al. Structural organization of the prefrontal white matter pathways in the adult and aging brain measured by diffusion tensor imaging. Brain Struct Funct. 2011;216:417–31. doi: 10.1007/s00429-011-0321-1. [DOI] [PubMed] [Google Scholar]
- Marie Y, Sanson M, Mokhtari K, Leuraud P, Kujas M, Delattre JY, et al. OLIG2 as a specific marker of oligodendroglial tumour cells. Lancet. 2001;358:298–300. doi: 10.1016/S0140-6736(01)05499-X. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord. 2010;127:230–40. doi: 10.1016/j.jad.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosebach J, Keilhoff G, Gos T, Schiltz K, Schoeneck L, Dobrowolny H, et al. Increased nuclear Olig1-expression in the pregenual anterior cingulate white matter of patients with major depression: a regenerative attempt to compensate oligodendrocyte loss? J Psychiatr Res. 2013;47:1069–79. doi: 10.1016/j.jpsychires.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Murphy ML, Frodl T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol Mood Anxiety Disord. 2011;1:3–12. doi: 10.1186/2045-5380-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012;17:132–41. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllykoski M, Raasakka A, Han H, Kursula P. Myelin 2′,3′-cyclic nucleotide 3′-phosphodiesterase: active-site ligand binding and molecular conformation. PLoS One. 2012;7:e32336. doi: 10.1371/journal.pone.0032336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–52. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Nazeri A, Chakravarty MM, Felsky D, Lobaugh NJ, Rajji TK, Mulsant BH, et al. Alterations of superficial white matter in schizophrenia and relationship to cognitive performance. Neuropsychopharmacology. 2013;38:1954–62. doi: 10.1038/npp.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, et al. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2006;77:120–2. doi: 10.1136/jnnp.2004.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press; 2000. [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Hughes J, Stockmeier CA, Miguel-Hidalgo J, Maciag D. Coverage of blood vessels by astrocytic endfeet is reduced in major depressive disorder. Biol Psychiatry. 2013;73:613–21. doi: 10.1016/j.biopsych.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Pittman SD, Dilley G, Overholser J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–98. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008;86:216–44. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Roth MP, Malfroy L, Offer C, Sevin J, Enault G, Borot N, et al. The human myelin oligodendrocyte glycoprotein (MOG) gene: complete nucleotide sequence and structural characterization. Genomics. 1995;28:241–50. doi: 10.1006/geno.1995.1137. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Braun PE, Grinspan J, Collarini E, Wang DY, Kamholz J. Differential regulation of the 2′,3′-cyclic nucleotide 3′-phosphodiesterase gene during oligodendrocyte development. Neuron. 1994;6:1363–75. doi: 10.1016/0896-6273(94)90451-0. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Segal D, Koschnick JR, Slegers LH, Hof PR. Oligodendrocyte pathophysiology: a new view of schizophrenia. Int J Neuropsychopharmacol. 2007;10:503–11. doi: 10.1017/S146114570600722X. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry. 2009;66:814–23. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Shimony JS, Sheline YI, D’Angelo G, Epstein AA, Benzinger TL, Mintun MA, et al. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66:245–52. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, Belzung C, et al. A molecular signature of depression in the amygdala. Am J Psychiatry. 2009;166:1011–24. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–22. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Rogaeva A, Fitzgibbon H, May WL, Rajkowska G, et al. Decreased expression of Freud-1/CC2D1A, a transcriptional repressor of the 5-HT1A receptor, in the prefrontal cortex of subjects with major depression. Int J Neuropsychopharmacol. 2010;13:1089–101. doi: 10.1017/S1461145710000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham MW, Woon PS, Sum MY, Lee TS, Sim K. White matter abnormalities in major depression: evidence from post-mortem, neuroimaging and genetic studies. J Affect Disord. 2011;132:26–36. doi: 10.1016/j.jad.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Bernier L, Andrews SB, Colman DR. Cellular and subcellular distribution of 2′,3′-cyclic nucleotide 3′-phosphodiesterase and its mRNA in the rat central nervous system. J Neurochem. 1988;51:859–68. doi: 10.1111/j.1471-4159.1988.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–75. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Sanz-Arigita EJ, de Vos K, Pool CW, Evers P, Rajkowska G. 3-D cytoarchitectonic parcellation of human orbitofrontal cortex correlation with postmortem MRI. Psychiatry Res. 2010;183:1–20. doi: 10.1016/j.pscychresns.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Sakurai Y, Ichinose T, Aikawa Y, Kotani M, Itoh K. Monoclonal antibody Rip specifically recognizes 2′,3′-cyclic nucleotide 3′-phosphodiesterase in oligodendrocytes. J Neurosci Res. 2006;84:525–33. doi: 10.1002/jnr.20950. [DOI] [PubMed] [Google Scholar]
- Williams MR, Hampton T, Pearce RK, Hirsch SR, Ansorge O, Thom M, et al. Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2013;263:41–52. doi: 10.1007/s00406-012-0328-5. [DOI] [PubMed] [Google Scholar]
- Xin M, Yue T, Ma Z, Wu FF, Gow A, Lu QR. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci. 2005;25:1354–65. doi: 10.1523/JNEUROSCI.3034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M. The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex. 2012;48:156–65. doi: 10.1016/j.cortex.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Peterson J, Gravel M, Braun PE, Trapp BD. CNP overexpression induces aberrant oligodendrocyte membranes and inhibits MBP accumulation and myelin compaction. J Neurosci Res. 1997;50:238–47. doi: 10.1002/(SICI)1097-4547(19971015)50:2<238::AID-JNR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Yu WP, Collarini EJ, Pringle NP, Richardson WD. Embryonic expression of myelin genes: evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron. 1994;6:1353–62. doi: 10.1016/0896-6273(94)90450-2. [DOI] [PubMed] [Google Scholar]