Abstract
Herein, the literature regarding functional imaging of the thalamus during language tasks is reviewed. Fifty studies met criteria for analysis. Two of the most common task paradigms associated with thalamic activation were generative tasks (e.g. word or sentence generation) and naming, though activation was also seen in tasks that involve lexical decision, reading and working memory. Typically, thalamic activation was seen bilaterally, left greater than right, along with activation in frontal and temporal cortical regions. Thalamic activation was seen with perceptually challenging tasks, though few studies rigorously correlated thalamic activation with measures of attention or task difficulty. The peaks of activation loci were seen in virtually all thalamic regions, with a bias towards left-sided and midline activation. These analyses suggest that the thalamus may be involved in processes that involve manipulations of lexical information, but point to the need for more systematic study of the thalamus using language tasks.
Keywords: Thalamus, Pulvinar, Aphasia, Cognitive, Brain, Language, Intralaminar, Thalamocortical, Corticothalamic
1. Introduction
The role of the thalamus in language has been enigmatic for at least half a century. Fisher (1959) was one of the first to describe aphasia in the setting of thalamic damage and Penfield and Roberts (1959) proposed a central integrating role for the thalamus in language. Despite several decades of case reports and series describing patients with thalamic lesions and aphasia, there continues to be controversy regarding the very idea that the thalamus plays any role in language at all. Where physiological models exist, they are quite varied in terms of the sub-nuclei involved and the specific operations taking place in the thalamus. The emergence of functional imaging as a tool to study brain function may permit new insights beyond what has been derived from the clinical–pathological correlative approach. To better understand the potential role of the thalamus in normal language function, the literature associating thalamic lesions with aphasia will briefly be reviewed, followed by an analysis of the literature demonstrating thalamic activation in language tasks by normal subjects.
2. Lesion evidence of thalamic involvement in language
Given the heterogeneity of thalamic nuclei in terms of function and projections to different areas of cortex, it is of interest to understand which thalamic nuclei are most likely involved with language. The thalamus projects to all areas of the neocortex, including those areas in the frontal, temporal, and parietal cortical regions that are commonly associated with language. Assigning individual thalamic nuclei to particular cortical areas is made complicated by convergence of inputs from thalamic nuclei to individual regions of the cortex. For example, restricted tracer injection into the caudal portion of the primate ventral premotor cortex, which bears at least superficial similarity to areas of the human frontal cortex important for language, produces substantial retrograde label in no fewer than 10 thalamic nuclei: ventrolateral nucleus, ventral anterior nucleus, ventral medial nucleus, centrolateral nucleus, centré-median nucleus, medial dorsal nucleus, area X, lateral posterior nucleus, medial pulvinar and ventral posterior nucleus (Morel, Liu, Wannier, Jeanmonod, & Rouiller, 2005). Similarly, injections of tracer into the macaque caudal superior temporal gyrus produce retrogradely-labeled neurons in multiple thalamic nuclei: the medial pulvinar, lateral posterior nucleus, suprageniculate-limitans nucleus and the medial division of the medial geniculate body (Hackett, Stepniewska, & Kaas, 1998). These data suggest that there are a number of thalamic nuclei, based on their projections to the cortex, that have a potential to be involved with language, but place a focus on ventrolateral nuclei, midline nuclei and the pulvinar which most densely project to the ventral premotor cortex and superior temporal gyrus (more extensively reviewed by Barbas et al., Lee and Bartlett, this issue).
Thalamic infarction leading to aphasia has been described in each of the four major vascular distributions of the thalamus: tuberothalamic (Bogousslavsky, Regli, & Assal, 1986; Karussis, Leker, & Abramsky, 2000; Levin, Ben-Hur, Biran, & Wertman, 2005; Raymer, Moberg, Crosson, Nadeau, & Rothi, 1997), paramedian (Bogousslavsky, Miklossy, Deruaz, Regli, & Assal, 1986; Perren, Clarke, & Bogousslavsky, 2005; Radanovic & Scaff, 2003), inferolateral (Karussis et al., 2000; McFarling, Rothi, & Heilman, 1982), and posterior choroidal (Neau & Bogousslavsky, 1996). Although lesions of the anterior and midline group of nuclei (ventral anterior, ventrolateral, anterior thalamic, mediodorsal and intralaminar nuclei) appear more frequently in the literature, reporting biases caused by the vagaries of vasculature have made it difficult for the early aphasiologists to assign a language “center” in the thalamus based on stroke data as they did for the cortex. For example, lesions of the pulvinar, which is extensively connected with the areas of the cortex involved with language, such as the ventrolateral prefrontal cortex and the superior temporal gyrus and sulcus (Romanski, Giguere, Bates, & Goldman-Rakic, 1997), are rarely reported, likely because (1) the pulvinar has a dual blood supply (Morandi et al., 1996; Takahashi et al., 1994); and (2) vascular lesions proximal enough in the posterior circulation to cause pulvinar infarction often cause global cognitive or arousal deficits, making it difficult if not impossible to provide a plausible cognitive decomposition of the deficits in these cases. As a result, approximately 6% of isolated thalamic infarcts are found in the posterior thalamic region (Carrera & Bogousslavsky, 2006). In this regard it should be noted that although the pulvinar is relatively protected from ischemic infarction, there are several reports of focal hemorrhage into the left pulvinar that have caused aphasic deficits (Bruyn, 1989; Crosson et al., 1986; Puel et al., 1992).
The clinical lesion data thus strongly suggest that thalamic lesions impair language function. A recent formal meta-analysis of patients with either ischemic or hemorrhagic thalamic infarction, found that the most common deficit among patients was in naming, with relative preservation of repetition (De Witte, Brouns, Kavadias, Engelborghs, & De Deyn, 2011). Other commonly noted features in patients with thalamic aphasia are a high frequency of semantic paraphasic errors (Demeurisse et al., 1979; Ebert, Vinz, Görtler, Wallesch, & Herrmann, 1999; Karussis et al., 2000; Radanovic & Scaff, 2003; Raymer et al., 1997) and perseverations (Bell, 1968; Bogousslavsky, Regli et al., 1986; Bruyn, 1989; Demeurisse et al., 1979; Graff-Radford, Eslinger, Damasio, & Yamada, 1984; Levin et al., 2005; McFarling et al., 1982; Puel et al., 1992).
While the meta-analysis from De Witte et al. provided a general overview of the thalamic contribution to language, a more detailed examination of individual patients may provide different insights. Note, that that several challenges exist when examining the pooled clinical literature to attempt to gain insights into language function. One difficulty is the variability of exact location and size of the infarctions. This suggests that there may be a role for detailed description of clinical–pathological correlations in small numbers of patients. Another potential difficulty that arises in describing the detailed language performance in aphasic patients is how these deficits are defined. Herein, we will use the term ‘lexical’ to describe the processes that involve processes that involve manipulation of word forms. The term ‘semantic’ will be used to describe processes that involve the manipulation of word meaning.
The language deficits of two thalamic stroke patients were described in great detail by Raymer et al. (1997) and later by Crosson (1999). These patients had strokes in different distributions: one patient with a left tuberothalamic ischemic stroke (damaging the ventrolateral, ventral anterior, centré-median and thalamic reticular nuclei) and a second with a slightly larger left paramedian ischemic stroke (damaging ventral anterior, ventrolateral, mediodorsal, centré-median, parafascicular and thalamic reticular nuclei). In both cases, the authors found that subjects had difficulty with oral picture naming, written picture naming and oral naming to auditory definition. However, tasks that involved direct orthographic output to phonologic input (writing to dictation), or phonologic output from orthographic input (reading aloud) were intact. The subjects also did well on auditory word – picture matching, as well as written word – picture matching. Furthermore, the majority of the naming errors took the form of semantically-related words. These suggested to the authors that the core deficit was one of retrieval of lexical items from semantic input. Further supporting the idea that the thalamus may be involved in the use of semantic information to facilitate lexical retrieval, are the findings of category-specific naming deficits in patients with thalamic infarcts and deficits (Crosson, Moberg, Boone, Gonzalez Rothi, & Raymer, 1997; Levin et al., 2005) and demonstrations that the thalamus may be involved in object recall (Segal, Williams, Kraut, & Hart, 2003; Slotnick, Moo, Kraut, Lesser, & Hart, 2002; Wahl et al., 2008).
Analysis of the effects on language of thalamotomy or thalamic deep brain stimulation can avoid the limitations imparted by the idiosyncrasies of the thalamic vasculature, though the ability to make inferences about thalamic structure-function relationships via this approach is limited by the small range of thalamic targets employed (typically ventrolateral nucleus, pulvinar and intralaminar nuclei). For example, Ojemann, Fedio, and van Buren (1968) and Ojemann and Ward (1971) studied the effects of deep brain stimulation in both the ventrolateral nucleus and pulvinar in separate populations of patients with extrapyramidal movement disorders. In the naming paradigm used in these studies, the subject read aloud a plate on which was printed: “This is a _ “ followed by a line drawing of an object. By requiring motor output by the subject, this paradigm was designed to capture dysnomia that could not be accounted for by motor speech deficits. The investigators observed naming problems after stimulation of the anterior superior pulvinar (5/8 patients on left, 1/7 on right), and in the posterior inferior medial ventrolateral nucleus (6/13 patients on left, 0/12 on right), but not in other areas of the ventrolateral nucleus that were more anterior or superior. The sites producing naming errors were contiguous across the ventrolateral nucleus and pulvinar and there were no qualitative differences in the types of errors produced. More than half of the errors in both series were substitution errors, rather than omissions. This is in contrast to the types of naming errors seen in the same study with stimulation outside of the thalamus, in the subcortical parietal white matter, which produced >80% omission errors (Ojemann et al., 1968). Note that these studies are reviewed in more detail in Hebb and Ojemann (this issue). In addition, Fedio and Van Buren found predominantly substitution error during stimulation of the left pulvinar, but not in adjacent areas outside the pulvinar or in the right pulvinar (Fedio & Van Buren, 1975). Similarly, Vilkki and Laitinen (1976) found decreases in word fluency and token test performance in those undergoing left ventrolateral thalamotomy, and trends towards worsening token test performance and naming for patients undergoing pulvinotomy (Vilkki & Laitinen, 1976). The findings are by bolstered by the known connectivity data, which would suggest that all of the structures described above (ventral lateral thalamus, pulvinar and intralaminar nuclei), project to areas of the cortex important for language (Jones, 2007).
Another commonly reported feature of patients with thalamic lesions, either due to stroke to electrolytic lesion, is a relatively rapid recovery from language deficits. When recovery has been described, most patients recover to a significant degree within 6 months of the ictus (Archer, Ilinsky, Goldfader, & Smith, 1981; Graff-Radford et al., 1984; McFarling et al., 1982; Raymer et al., 1997; Vilkki & Laitinen, 1976), though several patients with persistent aphasic deficits after focal thalamic lesions have been described (Bell, 1968; Demeurisse et al., 1979; Graff-Radford et al., 1984; Karussis et al., 2000; Puel et al., 1992; Radanovic & Scaff, 2003). Recovery of language function after small strokes has also been described in the cortex (Mohr et al., 1978), and the mechanisms for this are not known. It is certainly possible that resolution of edema or microscopic hemorrhage may play a role. It is also possible that there may be previously undescribed redundancy and/or plasticity in thalamocortical networks that are important for language.
To summarize, lesions over multiple areas of the left thalamus have been associated with loss of naming function with relative perseveration of repetition, without a consistent pattern of deficits in comprehension or production of speech. Based on work by Cros-son, Ojemann and others described above, a core deficit in these patients may be one of lexical retrieval, based on semantic information.
3. Imaging of the thalamus in language tasks
The lesion-deficit analysis presented above suggests that the thalamus may play a role in word selection when given information about word meaning. The high prevalence of perseverations and individual variability also suggest that the thalamus may be involved in the maintenance of current, and suppression of previous, word representations. However, the clinical findings represent a “moving target” such that there is apparently rapid re-organization of thalamic and/or cortical networks after thalamic stroke. Therefore, it is not clear what role, if any, the thalamus may play in normal language processing. The clinical data are also confounded by the nature of the lesions: loci of thalamic stroke are dependent on the vasculature and may apparently conjoin multiple structures based on a common blood supply or apparently exclude others based on favorable blood supply. In addition, deep brain stimulation, targeted to ventrolateral, intralaminar nuclei and pulvinar for therapeutic reasons, creates an ascertainment bias and may therefore artificially inflate the role of these specific nuclei in language. For these reasons, an imaging approach might be useful to provide convergent data regarding these issues.
However, data from PET and fMRI demonstrating thalamic activation in language tasks have been fewer than what might have been predicted. There are a number of possible reasons for this. The most probable reason is that most investigators’ a priori hypotheses about language functions do not include subcortical structures, so these are not included in their regions of interest, or a purely cortical analysis is performed. This reason is exacerbated by emerging “surface-based” examinations of cortical activations, which often do not involve examination of subcortical structures. In addition, thalamic nuclei are small (often a few mm in diameter) relative to the voxel size used in fMRI (typically 3 × 3 × 3 mm, with an 8 mm Gaussian filter), and demonstrate high person-to-person variability (Andrew & Watkins, 1969; Rademacher, Bürgel, & Zilles, 2002; Uylings et al., 2008). Furthermore the thalamus demonstrates greater sensitivity to cardiac-induced movement artifacts on MR scanning than does the cortex (Guimaraes et al., 1998). Both of these contribute to potential washout of thalamic signals in across-subjects analysis, particularly when few subjects or when large-kernel spatial averaging is used. In addition, hemodynamic response functions, optimized for the cortex, may not be adequate for the thalamus, which is supported by observations of differences in the time course of activation of thalamus and cortex using metabolic imaging (Llano, Theyel, Mallik, Sherman, & Issa, 2009). Finally, there may be fundamental differences in how the thalamus and cortex process information, leading to different absolute numbers of neurons activated during a task (for example, see small amount of thalamic activation needed to drive large cortical activations in (Llano et al., 2009; Theyel, Llano, & Sherman, 2010)), leading to different metabolic signatures in each structure.
Despite these limitations, a number of previous studies have demonstrated thalamic activation during language tasks. A search of PubMed using “thalamus + language + (SPECT or PET or MRI)” revealed 238 references. Of these, references were removed if they (1) did not involve normal human subjects, (2) only involved tasks that involved overt language production without controls for articulation or (3) did not involve a language task. This yielded 43 publications. In addition, seven publications that demonstrated thalamic activation were pulled from secondary references, producing a total of 50 publications. Studies that required overt responses but did not control for the motor act of speaking were excluded because of the data demonstrating the role of the thalamus in speech motor control (Bohland & Guenther, 2006; Ghosh, Tourville, & Guenther, 2008; Murphy et al., 1997; Riecker, Kassubek, Gröschel, Grodd, & Ackermann, 2006).
Though the tasks and imaging conditions were quite varied, there are common themes among the tasks that tended to cause thalamic activation: (1) generation of words, sentences or concepts (both silent and aloud); (2) lexical or semantic decision tasks; and (3) reading of words or pseudowords (both silent and aloud). To facilitate analysis of the studies, they were classified based on their core experimental paradigm. Five categories were defined:
Generative tasks: Subjects were asked to generate lists of words, to complete the stem of a word, to generate sentences, or to generate new concepts based on semantic information. If overt responses were required, an overt verbal task must have been used as a control.
Object naming: Subjects were asked to generate the name of a photo or line drawing of an object. If overt responses were required, an overt verbal task must have been used as a control.
Listening: Subjects were to make a judgment about, or detect a feature of, a heard stimulus, typically a sentence or a series or phonemes.
Reading: Subjects were asked to silently read a word, pseudoword or sentence vs. a control visual task.
Lexical decision: Subjects were asked to make a judgment about words (e.g. lexical category). If overt responses were required, an overt verbal task must have been used as a control.
Other: Studies not easily classified into one of the above categories.
All studies, their modality (SPECT, PET or fMRI), their thalamic locus of activation and a brief description of the experimental paradigm are listed by category in Tables 1–6. Talairach and Tournoux (TT) coordinates are listed for all studies where these data are provided. For studies reporting MNI coordinates, these were transformed using the nonlinear transformation found in: http://www.bioimagesuite.org/Mni2Tal/index.html. Although other potentially more standardized coordinate systems have been proposed (Devlin & Poldrack, 2007), we have defaulted to TT coordinates. This is because the majority of papers referenced in this analysis used these coordinates, and therefore converting MNI to TT involved the least amount of potential distortions. Occasionally, authors assigned a name to the thalamic subdivision where activation was noted, though most authors simply listed “left thalamus” or “right thalamus.” Where names of thalamic nuclei were assigned by authors, the verbatim names were listed in the tables, along with their coordinates. Two studies had both naming and reading tasks and are thus listed twice (Bookheimer, Zeffiro, Blaxton, Gaillard, & Theodore, 1995; Seghier & Price, 2010).
Table 1.
Generative tasks. List of studies demonstrating thalamic activation in language tasks. Format of 1–6 are identical. Note that smoothing windows are Gaussian unless otherwise specified. For studies where smoothing window in mm is not provided, nothing is listed.
| Study (# of subjects) | Mode (smoothing window) |
Thalamic activation (TT coordinates) | Task with thalamic activation |
|---|---|---|---|
|
Poline et al. (1996) (n = 77) |
rCBF-PET (12 mm) | L thalamus: (8, −12,12) | Silent generation of verbs related to nouns vs. silent rest |
|
Warburton et al. (1996) (n = 4–9) |
rCBF-PET (20 × 20 × 12 mm) |
Experiment 1: (Rate 25/min) R thalamus: (+10, −22, 0) |
Covert noun-verb comparison vs. eyes-closed rest. |
| Experiment 2: (Rate 4/min) | Covert verb generation vs. eyes vs. eyes-closed rest. | ||
| L thalamus:(−16, −10, +12) | |||
| L thalamus: (−4, −12, +4) | Covert verb generation vs. passive listening | ||
| Experiment 3: Bilateral thalamus: (−8, −16, −4), (+8, −16, +12) |
Covert verb generation vs. rest | ||
| Bilateral thalamus: (−16, −18, +8), (+8, −16, +12) |
Covert noun generation vs. rest | ||
| Experiment 4: (10/min) L thalamus: (−18, −16, +12) |
Covert verb generation vs. rest | ||
| R thalamus: (+4, −6, +12) | Covert verb generation vs. repetition | ||
|
Baker et al. (1997) (n = 10) |
rCBF-PET | L thalamus: (2, −8, 0) | Overt verbal fluency in response to visually presented letter vs. Overt repetition |
|
Müller et al. (1998) (n = 5 normals) |
rCBF-PET | L thalamus (Whole thalamus taken as ROI) |
Generating sentences, given a concept and a word vs. repeating sentences |
|
Ojemann et al. (1998) (n = 32) |
PET, fMRI (3.125 mm Hanning) |
L thalamus: (−9, −12, 9) | Word-stem completion task |
|
Gourovitch et al. (2000 (n = 18) |
rCBF-PET (15 mm) | L thalamus: (−14, −18, 8) L ventrolateral nucleus of thalamus: (−4, −10, 4) |
Overt letter fluency vs. recitation of days of week or months of year Overt semantic fluency. vs. recitation of days of week or months of year |
|
Rosen et al. (2000) (n = 5) |
fMRI (3.75 × 3.75 × 8 mm) |
L thalamus: (−9,17, 8) R thalamus: (9, −17, 8) |
Covert word stem completion task vs. visual fixation on crosshair |
|
Kraut, Kremen, Moo, et al. (2002) (n = 11) |
fMRI (6 × 6 × 8 mm) | L thalamus: (−11, −20, 2.1) | Covert generation of new object from non-semantically associated word pairs (event-related design) |
|
Kraut, Kremen, Segal, et al. (2002) (n = 11) |
fMRI (6 × 6 × 8 mm) | Bilateral thalamus (5.5, −16.5,13.6), (−3.8, −9.8, 14.2) |
Covert generation of new object from non-semantically associated word- picture pairs |
|
Kraut et al. (2003) (n = 9) |
fMRI (6 × 6 × 8 mm) | L pulvinar, L dorsomedial nucleus | Covert generation of new object from non-semantically associated word- picture pairs |
|
Assaf et al. (2006) (n = 18) |
fMRI (9 mm) | L thalamus: (−6, −6, 3) | Recall vs. no recall of semantic object |
|
Halari et al. (2006) (n = 19) |
fMRI (7 mm) | R thalamus: (6, −32, 10), (2, −16, 6) | Overt letter fluency vs. Repeat word “rest” |
|
Basho et al. (2007) (n = 12) |
fMRI (6 mm) | L thalamus: (−11, −17,15) | Semantic fluency: overt and covert, paced and unpaced. vs. repetition (overt or covert, paced or unpaced) of the word “nothing.” |
|
Mestres-Misse et al. (2008) (n = 12) |
fMRI (8 mm) | L mediodorsal: (−4, −16, 4) | Sentence reading with novel words where new meanings can be assigned. |
|
Mestres-Misse et al. (2009) (n = 15) |
fMRI (8 mm) | L thalamus: (−8, −20, 16) | Sentence reading and generation of meaning of new word vs. real word (word type effect). |
| R mediodorsal: (8, −20,12) | |||
|
Gauthier et al. (2009) (n = 44) |
fMRI (8 mm) | L thalamus: (0, −9, 10), (−2, −21, 5), (−6, −23, 5), (−2, −10, 2) |
Covert word fluency task vs. covert counting task. |
|
Senhorini et al. (2011) (n = 21) |
fMRI (9 mm) | Experiment 1: L thalamus: (−7, −7, 15) |
Experiment 1: Overt verbal fluency (easy letters) vs. repetition. |
| R thalamus: (4, −30, 4) | |||
| Experiment 2: L thalamus: (−11, −22, 20) |
Experiment 2: Overt verbal fluency (hard letters) vs. repetition. | ||
| R thalamus: (0, −19, 9) |
Table 6.
Other.
| Study (# of subjects) |
Mode (smoothing window) |
Thalamic activation (TT coordinates) |
Task with thalamic activation |
|---|---|---|---|
|
Wallesch et al. (1985) (n = 6) |
rCBF/SPECT | L > R thalamus Spatial resolution of images was poor |
Multiple language tests |
|
Mottaghy et al. (1999) (n = 6) |
fMRI (8 mm) | No coord given. Left dorsomedial thalamus according to authors |
Paired associated learning vs. nonsense words or word pairs. L dorsomedial thalamus activated in PAL vs. nonsense word pairs and with high imagery word pairs |
|
Wiggs et al. (1998) (n = 16) |
rCBF/PET (20 × 20 × 12 mm) |
R thalamus: (8, −18, 4) R thalamus: (6, −14, 4) |
Episodic retrieval vs. naming Episodic retrieval vs. semantic association |
|
Grossman et al. (2002) (n = 30) |
fMRI (12 mm) | L thalamus: (−12, −24, 4) L thalamus: (−12, −24, 4) |
Rule-based categorization of written object task Similar task, with degraded stimulus presentation with brief description. |
| Maguire and Frith (2004) (n = 12) |
fMRI (8 mm) | Left dorsomedial thalamus: (−1, −15,10) |
Semantic knowledge: Hearing sentences that provide new knowledge vs. descriptive sentences |
|
Sach et al. (2004) (n = 12) |
rCBF-PET (20 mm) | R thalamus: (16, −20, −4) | Overt inflection of an infinitive verb vs. overt insertion of the pre-inflected verb |
|
Stringaris et al. (2007) (n = 11) |
fMRI (7.2 mm) | L thalamus: (−7, −15, 4) | Covert decision regarding meaningfulness of sentences with metaphoric meaning vs. sentences with literal meaning or nonmeaningful sentences |
|
Peschke et al. (2009) (n = 20) |
fMRI (10 mm) | R thalamus: (2, −15, 4) | Rapid repetition of sentences spoken by multiple speakers vs. rapid repetition of sentences spoken by single speaker |
|
Ghosh et al. (2010) (n = 10) |
fMRI (8 mm) | Thalamus (L/R and coord not given) |
Semantic association vs. control (lexical decision) |
|
Wittfoth et al. (2010) (n = 20) |
fMRI (8 mm) | L thalamus: (−2, −21,13) | Angrily intoned sentences with positive semantic content (incongruent vs. congruent) |
|
Ye et al. (2011) (n = 21) |
fMRI (8 mm) | Midline thalamus: (−1, −7, 3) | Learning new word meaning and real words from sentences – in same network with left inferior frontal gyrus |
Seventeen studies reported thalamic activation during various generative tasks, all of which either involved covert generation of words or sentences, or, for overt studies, compared overt generation to overt repetition (Table 1). The specific generative tasks were quite varied, involving word stem completion (Ojemann et al., 1998; Rosen, Ojemann, Ollinger, & Petersen, 2000), semantic or phonemic fluency (Baker, Frith, & Dolan, 1997; Basho, Palmer, Rubio, Wulfeck, & Müller, 2007; Bohland & Guenther, 2006; Gourovitch et al., 2000; Halari et al., 2006; Warburton et al., 1996), sentence generation (Brown, Martinez, & Parsons, 2006; Müller et al., 1998), recall of an object from features or images associated with that object (Kraut, Calhoun, Pitcock, Cusick, & Hart, 2003; Kraut, Kremen, Moo, et al., 2002; Kraut, Kremen, Segal, et al., 2002) or novel word meaning (Mestres-Missé, Münte, & Rodriguez-Fornells, 2009). In most cases, there was left greater than right thalamic activation and this was associated with widespread activation of areas of the inferior frontal and superior temporal cortex. Several of these studies showed greater thalamic activation to word generation than with repetition (Baker et al., 1997; Halari et al., 2006; Müller et al., 1998; Warburton et al., 1996), which would be consistent with the clinical literature that shows preserved repetition and impaired naming in thalamic aphasic patients (De Witte et al., 2011). Left greater than right thalamic activation was also seen in five studies involving object naming (Bookheimer et al., 1995; Garn, Allen, & Larsen, 2009; Murtha, Chertkow, Beauregard, & Evans, 1999; Price, Moore, Humphreys, Frackowiak, & Friston, 1996; Seghier & Price, 2010) (Table 2). This is also consistent with the lesion literature, where naming deficits are the primary manifestation of left-lateralized thalamic lesions producing aphasia (De Witte et al., 2011).
Table 2.
Object naming.
| Study (# of subjects) |
Mode (smoothing window) |
Thalamic activation (TT coordinates) | Task with thalamic activation |
|---|---|---|---|
|
Bookheimer et al. (1995)a (n = 16) |
rCBF-PET (6 × 6 × 10 mm) |
L thalamus: (−16, −4,12) L thalamus: (−24, −18, 16) R thalamus: (28, −34, 4) |
Silent naming of objects Silent reading of words |
|
Price et al. (1996) (n = 6) |
rCBF-PET | L thalamus: (−10, −14, 8) | Overt naming of objects and colors vs. saying “Yes” to object and color viewing |
|
Murtha et al. (1999) (n = 10) |
rCBF-PET (20 mm Hanning window) |
L pulvinar: (−17, −23, −3) | Overt picture naming vs. overt “Yes” to visual pattern |
|
Garn et al. (2009) (n = 26) |
fMRI (8 mm) | L thalamus: (−7, −29, 7) | Covert naming vs. visually-matched scrambled control |
|
Seghier and Price (2010)a (n = 28) |
fMRI (6 mm) | Bilateral thalamus. Thalamus was not significant in dynamic causal modeling of activation caused by of word reading. |
Word reading > object naming > saying “1, 2, 3” |
Also listed in Table 4 under “Reading”.
Nine studies found thalamic activation in listening tasks that involved detection of specific acoustic features in speech (Alain et al., 2005; Christensen, Antonucci, Lockwood, Kittleson, & Plante, 2008; Demonet, Price, Wise, & Frackowiak, 1994; Gonzalez-Castillo & Talavage, 2011; Mazziotta, Phelps, & Carson, 1984; Salvi et al., 2002; Seidman et al., 1998; Tervaniemi et al., 2006; von Kriegstein, Patterson, & Griffiths, 2008) (Table 3). Five of these studies reported bilateral activation, while two reported primarily left thalamus and two reported primarily right thalamus activation. For several of these studies, thalamic activation was seen in the most perceptually challenging paradigms used (Alain et al., 2005; Demonet et al., 1994; Seidman et al., 1998), though this was not universal, since thalamic activation was not significant in the most difficult tasks of two studies (Christensen et al., 2008; Salvi et al., 2002). Reaction time was measured by Demonet et al, but not explicitly correlated with neuronal activation function. Regarding localization, with the exception of von Kriegstein et al., none of these studies claimed to find activation in the primary auditory thalamic nucleus, known as the medial geniculate body (MGB). Though the localization of activation functions in the thalamus carries a substantial amount of imprecision (see below), the MGB (and lateral geniculate nucleus) is located several millimeters ventral and lateral to the main body of the thalamus. Therefore it is likely that most the of the activation reported in these studies is not purely driven by acoustic stimulation, which is known to activate the MGB without activating other core thalamic structures (Harms & Melcher, 2002).
Table 3.
Listening tasks.
| Study (# of subjects) | Mode (smoothing window) |
Thalamic activation (TT coordinates) | Task with thalamic activation |
|---|---|---|---|
|
Mazziotta et al. (1984) (n = 21) |
CMRglc-PET | Bilateral thalamus | Auditory verbal stimulation vs. “sensory deprived” |
| Demonet et al. (1994) (n = 9) | rCBF-PET (20 mm) |
L thalamus: (−10, −16, 8) | Detection of a sequence of ambiguous phonemes vs. simple phoneme detection task. |
| R thalamus: (8, −8, 8) | |||
| Seidman et al. (1998) (n = 7–12) | fMRI | Mulitiple loci: (−6, −9, 9), (−21, −9, 16), (−3, −24, 12), (3, −15, 12), (9, −9, 16), (12, −31, 12) |
Demanding vs. simple auditory vigilance task. |
| Salvi et al. (2002) (n = 10) | rCBF-PET (15 mm) |
R thalamus R thalamus near pulvinar |
Noise vs. quiet Speech in noise vs. Quiet |
| Alain et al. (2005) (n = 11) | fMRI (6 mm) | L thalamus: (0.0, −12.0, 5.0) | Correctly identifying two vowels vs. one vowel |
|
Tervaniemi et al. (2006) (n = 17) |
fMRI (5.65 mm) | L thalamus: (−20, −22, 0) R thalamus: (17, −16, 0), (13, −25, 18) |
Duration deviants vs. standard speech Frequency deviants vs. standard speech |
|
Von Kriegstein et al. (2008) (n = 16–17) |
fMRI (4 mm) | Exp 1: MGB, L > R Exp 2: MGB, R > L |
Exp 1: Syllable task > loudness task/ Exp 2: fast-varying signals vs. speaker vocal-tract length |
|
Christensen et al. (2008) (n = 14) |
fMRI (4 mm) | R thalamus: (14, −9,13) L thalamus: (−13, −6, 12) |
Diotic vs. Reverse-speech control |
| R thalamus: (14, −10,15) | Dichotic vs. Reverse-speech control | ||
| R thalamus: (coordinates not given) | Diotic vs. dichotic listening | ||
|
Gonzalez-Castillo and Talavage (2011) (n = 17) |
fMRI (6 mm) | L thalamus (−24, −28, 15)a | CUNY covert sentence comprehension (high attention) vs. fixation |
Assumed AP coordinate was −28, rather than 28, given in the paper.
Other tasks also activated the thalamus, such as reading (Table 4) and lexical or lexical-semantic decision tasks (Table 5). As in the case of auditory stimulation, it is unlikely that most of the reading-related thalamic activation corresponds to primary activation of visual thalamic structures since the visual thalamus is displaced from the main body of the thalamus, and can be separately activated using visual stimuli (Kastner et al., 2004). Review of the studies in Table 5 shows that activation is seen with the manipulation of both written and heard words, and at least one of these studies reported an a priori hypothesis regarding the role of subcortical structures (Ketteler, Kastrau, Vohn, & Huber, 2008). A host of other paradigms that involve language also have also shown thalamic activation in individual studies, such as paired-associated verbal learning, detection of incongruence of emotional intonation in speech and others (see Table 6). These are not dealt with further since the number of studies for each of these task categories is small.
Table 4.
Reading.
| Study (# of subjects) |
Mode (smoothing window) |
Thalamic activation (TT coordinates) | Task with thalamic activation |
|---|---|---|---|
|
Price et al. (1994) (n = 6 each experiment) |
rCBF-PET (20 mm) |
Bilateral ventromedial thalamus (+6, −6, +4), (−6, −16, +4), (+4, −12, +4) |
Silently viewing real words vs. viewing words with false fonts |
|
Bookheimer et al. (1995)a (n = 16) |
rCBF-PET (6 × 6 × 10 mm) |
L Thalamus: (−16, −4, 12) L thalamus: (−24, −18, 16) R thalamus: (28, −34, 4) |
Silent naming of objects Silent reading of words |
|
Perani et al. (1999) (n = 11) |
rCBF-PET (15 mm) |
L thalamus: (−10, −22,16) | Covert decision if two words referring to living objects were the same despite different font vs. pseudoword discrimination task. |
| Cohen et al. (2002) (n = 7) |
fMRI (8 mm) | L thalamus: (−18, −24, 3) | Right visual field (RVF) word reading vs. left visual field |
|
Fiebach et al. (2002) (n = 12) |
fMRI (5.6 mm) | Bilateral thalamus (coordinates do not distinguished btwn caudate and thalamus, though images clearly show thalamic activation) |
Reading low frequency words vs. high frequency Reading pseudowords vs. high freq |
|
Seghier and Price (2010)a (n = 28) |
fMRI (6 mm) | Bilateral thalamus. Thalamus was not significant in dynamic causal model of word reading. |
Word reading > object naming > saying “1, 2, 3” |
Also listed in Table 2 under “Object naming”.
Table 5.
Lexical Decision.
| Study (# of subjects) | Mode (smoothing window) | Thalamic activation (TT coordinates) | Task with thalamic activation |
|---|---|---|---|
| Rumsey et al. (1997) (n = 14) | rCBF-PET (20 × 20 × 12 mm) |
R thalamus (2, −4, 0) L thalamus (−12, −12, −4) |
Covert phonological lexical decision vs. visual fixation Covert orthographic lexical decision vs. visual fixation |
| Ketteler et al. (2008) (n = 12) | fMRI (7 mm) | L mediodorsal nucleus (−3, −17,12) | 3 Lexical decision tasks with two involving potential ambiguity |
| L pulvinar (−3, −29, 7) | |||
| L ventral anterior nucleus (−12, −8,14) | |||
| Carreiras et al. (2009) (n = 20) | fMRI (8 mm) | L thalamus (−7, −22, 10) | Lexical decision with color incongruency vs. color congruency |
| R thalamus (10, −22,10) | |||
| Vannest et al. (2011) (n = 22) | fMRI (8 mm) | Bilateral thalamus | Lexical decision (low vs. high frequency) |
Two studies explicitly studied the network interactions between subcortical and cortical structures during language tasks. In one study (Seghier & Price, 2010) the authors found that the thalamus was not likely to be the mediator of long-range cortical interactions during a reading task. A second study found that the midline thalamus was part of a larger network (involving several prefrontal cortical regions, inferior parietal lobule and caudate) involved with assigning meaning to new words (Ye, Mestres-Missé, Rodriguez-Fornells, & Münte, 2010).
It can be argued that many of the language tasks used in these studies, such as the generation of lists of words, required a higher level of arousal than control conditions, which often consist of performing a fixation task. Given the known relationship between the thalamus and arousal (McCormick & Bal, 1997), it is possible that thalamic activation may be a byproduct of task difficulty rather than a reflection of language processing per se. However, only a few studies assessed the correlation between thalamic activation and objective measures of task difficulty (e.g. accuracy or reaction time) and found mixed results. For example Assaf et al. (2006) found that thalamic activation was more frequently seen in tasks with a lower accuracy (suggesting that these tasks were more difficult and required more effort). Similarly Fiebach, Friederici, Muller, and Cramon (2002) found that thalamic activation was seen in the more difficult tasks. However, the reaction time data from Kraut, Kremen, Segal et al. (2002), demonstrate similar reaction times across tasks, suggesting similar degrees of effort in this particular study. In addition, five of the studies here compared reaction time (as an index of difficulty), and none of them found a relationship between thalamic activation and reaction time (Kraut, Calhoun, Pitcock, Cusick, & Hart, 2003; Perani, Schnur, Tettamanti, Cappa, & Fazio, 1999; Rumsey et al., 1997; Sach, Seitz, & Indefrey, 2004; Stringaris, Medford, Giampietro, Brammer, & David, 2007). In addition, most studies showed left greater than right activation, even when both thalami were activated, while one would expect symmetric and general activation if the thalamic responses were only reflective of generalized arousal. This does not exclude the more nuanced view of “modality-specific” arousal, to be discussed further below.
4. Anatomic information from functional imaging studies
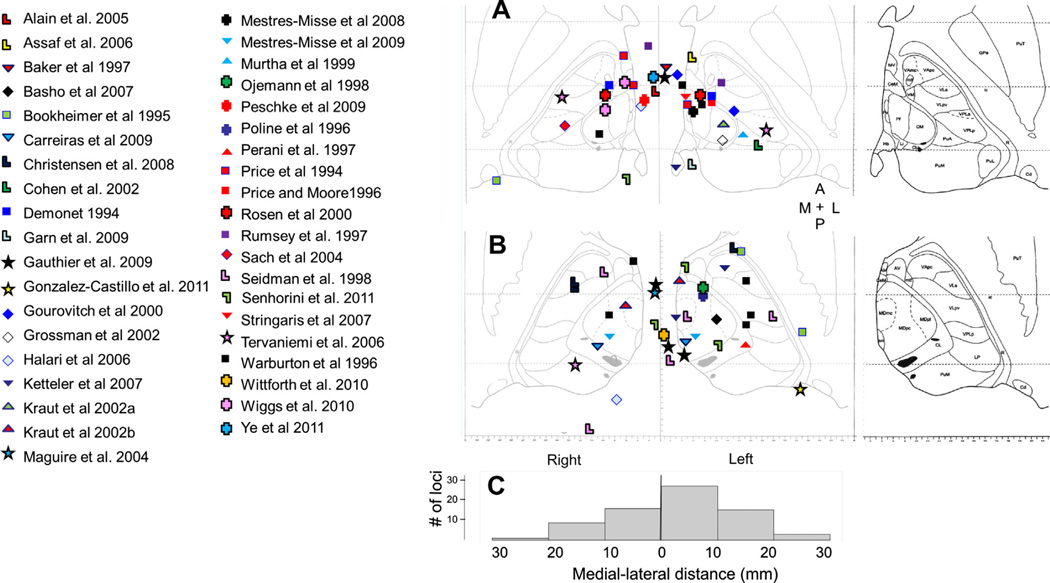
The relatively poor spatial resolution of fMRI and PET relative to the size the thalamic subnuclei limit our ability to make inferences about the roles of specific thalamic subnuclei in language. At a gross level, out of 49 studies where the side of thalamic activation was provided, 25 reported only left thalamic activation, 19 reported bilateral activation (though typically left greater than right), and 5 reported only right activation. This modest degree of unilaterality differs from the lesion data, which very strongly indicate that left thalamic lesions are seen more frequently in aphasia. This difference between the strong unilaterality seen in the aphasia literature, and moderate unilaterality seen in the language imaging literature, is also seen in the cortex, where it has been shown from the earliest functional imaging studies that cortical activation during language behavior is often bilateral, while lesion data point primarily to the left hemisphere (Petersen, Fox, Posner, Mintun, & Raichle, 1988; Poeppel et al., 2004). Beyond lateralization, most authors did not report sublocalization within the thalamus, and as has been noted above, the subnuclei are small relative to the resolution of both PET and BOLD MRI, and their borders tend to be variable, prohibiting precise localization. In all of the studies, we have listed the Talairach and Tournoux coordinates (or converted coordinates, when MNI coordinates were reported) of the voxel of peak activation and have plotted these coordinates in two dimensions (anterior–posterior and medial–lateral) on the same two axial maps of the thalamus shown in Fig. 1. All coordinates with superior–inferior location inferior to 8 mm below the posterior commissure were collapsed to Fig. 1A. Coordinates with location superior to this level were collapsed to Fig. 1B. Collapsing was done for ease of exposition, and because thalamic subnucleus designation did not change when points were collapsed. Review of the distribution of activation foci are suggestive of moderate clustering near the left midline structures (intralaminar nuclei and mediodorsal nucleus, Fig. 1C for histogram); however, foci are scattered throughout the thalamus and across both sides. No statistical analysis was attempted given the heterogeneity of data sources and task types, and because a few studies yielded more than one datapoint if more than one thalamic locus was reported. It is worth pointing out that these foci represent only the peaks of activation, and given limitations on the spatial resolution in imaging deep structures mentioned above, this form of analysis does not have the resolving power to exclude responsible thalamic subnuclei.
Fig. 1.
Compilation of center of activation foci taken from Talairach and Tournoux coordinates (either given in original publications or transformed from MNI coordinates). Two axial sections are shown. (A) Taken from 2.7 mm superior–inferior (base image reproduced from Morel (2007)). (B) Taken from 8.1 mm superior–inferior. Each study is represented by a different symbol. (C) Histogram of number of activation loci within each 10 mm of midline in the medio-lateral dimension. Where activation foci fell on the border, half a locus was placed into each of the neighboring bins. Abbreviations: A = anterior, P = posterior, M = medial, L = lateral, AM = anteromedial nucleus, AV = anteroventral nucleus, Cd = caudate nucleus, CeM = central medial nucleus, CL = centrolateral nucleus, CM = center median nucleus, GPe = globus pallidus externa, Hb = habenular nucleus, ic = internal capsule, Li = limitans nucleus, LP = lateral posterior nucleus, MDmc = mediodorsal nucleus, magnocellular division, MDpc = mediodorsal nucleus, parvocellular division, MDpl = mediodorsal nucleus, paralamellar division, mtt = mammillothalamic tract, Pf = parafascicular nucleus, PuA = anterior pulvinar, PuL = lateral pulvinar, PuM = medial pulvinar, PuT = putamen, Pv = paraventricular, R = thalamic reticular nucleus, sm = stria medullaris, VAmc = Ventral anterior nucleus, magnocellular, VApc = Ventral anterior nucleus, parvocellular, VLa = ventrolateral anterior, VLpd = ventrolateral posterior nucleus, dorsal division, VLpv = ventrolateral posterior nucleus, ventral division, VM = ventromedial, VPLa = ventral posterior nucleus, anterior division, VPLp = ventral posterior nucleus, posterior division. Anatomical image reproduced with permission from Informa Healthcare.
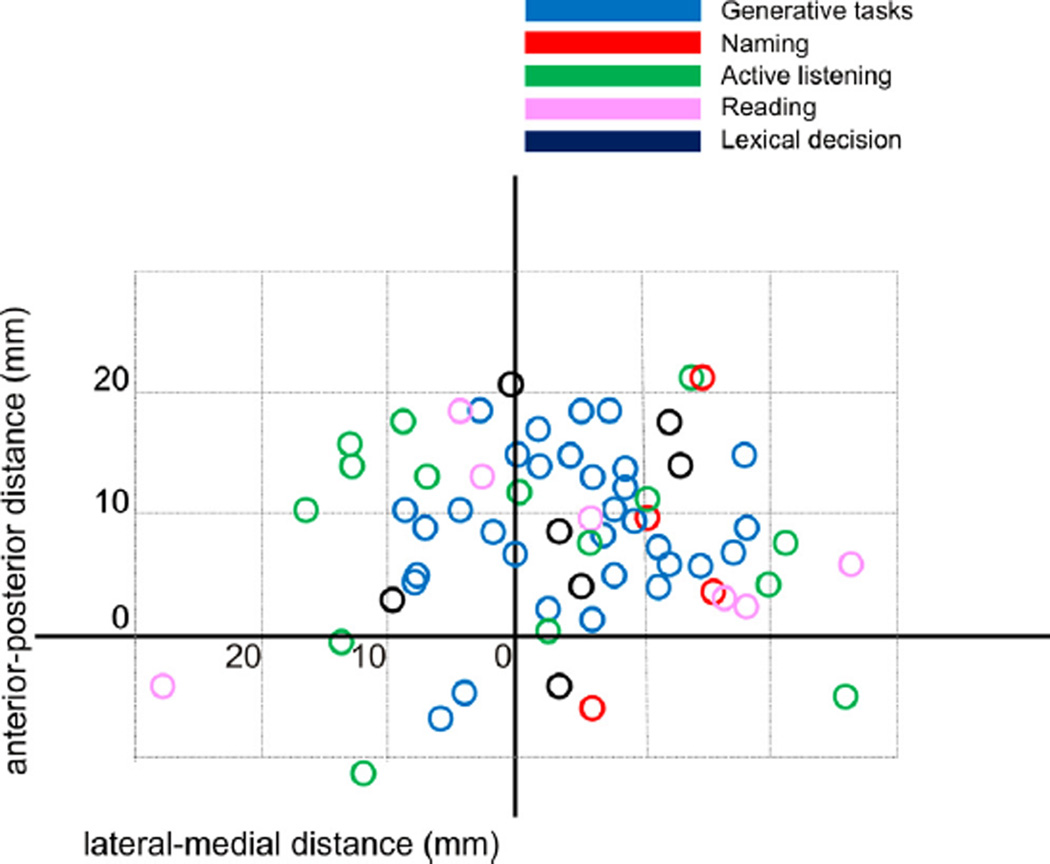
In addition, foci of activation were separated based on the task types denoted in Tables 1–6. Given the small numbers of studies in each task type, the maps of the thalamus were collapsed in the superior–inferior plane to yield two-dimensional maps (anterior– posterior, medial–lateral). Studies from the ‘other’ category (Table 6) are not plotted. Where single studies have more than one task type (Bookheimer et al., 1995; Seghier & Price, 2010), the separate tasks are shown in different colors. As shown, there are trends for the generative tasks (blue circles) to be found near the left midline, while all naming tasks activated foci left laterally (red circles), and active speech listening tasks tended to activate the thalamus bilaterally (green circles). For the reasons denoted above, no statistical analysis was attempted.
5. Weaknesses of the current analysis
Based on the search criteria described above, we obtained 49 reports of thalamic activation in language tasks that involve brain imaging. A quick search of PubMed shows that there have been 1213 publications in 2011 alone that are found with the search terms “fMRI” and “language.” Clearly, what is not known is whether the thalamic activations reported herein represent the outliers of the imaging literature or whether the thalamus is a core part of a network involved with language. One approach to this problem would be to begin with a language task and survey the literature to assess for the frequency of thalamic activation during this task. This was done by Indefrey and Levelt, who showed in their meta-analysis of 82 PET and fMRI naming or word generation experiments, that the most commonly activated structures were the inferior frontal gyrus, superior temporal gyrus as well as the left thalamus (Indefrey & Levelt, 2004). This is consistent with the current analysis which shows that naming and word generation tasks are among the most commonly encountered tasks in studies showing thalamic activation with language.
In addition, it is recognized that the anatomical meta-analyses in Figs. 1 and 2 are limited in their interpretability since the datapoints were obtained with different modalities (rCBF-PET, 1.5 T fMRI, 3T fMRI), using different statistical thresholds, in some cases using transformed coordinate systems, etc. However, treated as a hypothesis-generating exercise, these analyses may help to shape further studies into this issue.
Fig. 2.
Foci taken from Fig. 1, color coded by nature of the task. All foci were collapsed onto a single superior–inferior plane. Units are in millimeters.
6. Summary and conclusions
The current analysis of the literature suggests that the left thalamus may play a role in processes that involve the manipulation of lexical information and that thalamic activation may be modulated by the difficulty of task demands. These data are consistent with predictions made by previously-proposed models of the thalamus and language. Most of these models have ascribed an attentional role to the thalamus (Johnson & Ojemann, 2000; McFarling et al., 1982; Riklan & Cooper, 1975). For example, George Ojemann and colleagues have attributed a “specific alerting response” to the thalamus, generated by ventrolateral thalamus and pulvinar, which gates the activation of language-related areas of the cortex. In addition, Bruce Crosson and colleagues have proposed several potential models of the role of the thalamus in language (Crosson, 1985, 1992, 1999; Nadeau & Crosson, 1997), and these have been derived primarily by the detailed study of patients with thalamic damage and aphasia. In the most recent instantiation, Crosson and colleagues hypothesized that the language deficits in their patients were caused by a failure to activate cortical circuits needed to retrieve names of objects based on their descriptions or drawings. Further, they proposed that the failure was due to a lesion in the fronto-inferior thalamic peduncle-nucleus reticularis-centré-median thalamus system.
The notion that the thalamus plays a role in attention has been explored in great depth in the literature (Frith & Friston, 1996). A particularly interesting idea is that the thalamus may play a role in activating particular cortical circuits during specific cognitive functions, providing specific activation functions, rather than nonspecific arousal. Multiple mechanisms for this have been proposed. For example, thalamic intralaminar nuclei project to layer 1 of cortex and may provide a gating signal to particular cortical areas during cognitive tasks (Llinas, Leznik, & Urbano, 2002; Purpura & Schiff, 1997), which may be potentially be expanded to involve language. In addition, the thalamic reticular nucleus, potentially via its communication between thalamic nuclei, has been proposed to be involved in the selection of particular thalamocortical circuits to direct the focus of attention (Crabtree & Isaac, 2002; Crick, 1984; Mc Alonan & Brown, 2002). Also, direct projections from the pulvinar have been proposed to be involved in visual attention (Olshausen, Anderson, & Van Essen, 1993). Given the pulvinar’s projection to multiple language-related cortical areas and the effects of pulvinar deep brain stimulation on naming (Ojemann et al., 1968), this might be another candidate region for language.
Since all of these thalamic nuclei project to areas of cortex relevant for language (Jones, 2007), they are all reasonable candidates to be involved in language, and activation foci were seen either in or near all of these regions (Fig. 1). However, given the heterogeneity of sources of imaging data and generally poor spatial resolution of these imaging techniques relative to thalamic subnuclei, the anatomical data presented in Figs. 1 and 2 should not be used to as strong evidence that any particular thalamic subnucleus may or may not be involved in language.
Ultimately, to better understand the role of the thalamus in language, it will be important to design imaging studies that specifically assess thalamic activation in particular language tasks. This may involve optimizing the spatial resolution and noise minimization around subcortical structures and assessing whether or not a generic hemodynamic response function adequately captures thalamic activation. In addition, it will be important that future studies assess the impact of potential confounders, such as arousal and task difficulty, on the activation of the thalamus. It will also be necessary to ensure that other subcortical structures that are strongly connected to the thalamus and implicated in language, such as the basal ganglia (Crosson et al., 2003; Radanovic & Scaff, 2003; Riecker et al., 2005; Robles, Gatignol, Capelle, Mitchell, & Duffau, 2005), are optimally imaged during these tasks. Finally, it will be critical to perform both language testing and functional imaging of cortical and subcortical activation in the acute and resolution phase of thalamic aphasia to better understand mechanisms of recovery after thalamic stroke. These approaches will allow us to more precisely determine what role the thalamus plays in language, and ultimately to better understand how large-scale brain networks interact to support language function.
Acknowledgments
Thank you to Drs. Steven Small, Anthony Dick, Brad Sutton, Uri Hasson, Joe Beatty and Iraklis Petrof on their comments on earlier versions of this manuscript.
References
- Alain C, Reinke K, McDonald KL, Chau W, Tam F, Pacurar A, et al. Left thalamo-cortical network implicated in successful speech separation and identification. NeuroImage. 2005;26(2):592–599. doi: 10.1016/j.neuroimage.2005.02.006. http://dx.doi.org/10.1016/j.neuroimage.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Andrew J, Watkins E. A variability study. Williams and Wilkins; 1969. A stereotactic atlas of the human thalamus and adjacent structures. [Google Scholar]
- Archer C, Ilinsky I, Goldfader P, Smith K. Case report. Aphasia in thalamic stroke: CT stereotactic localization. Journal of Computer Assisted Tomography. 1981;5(3):427–432. doi: 10.1097/00004728-198106000-00024. [DOI] [PubMed] [Google Scholar]
- Assaf M, Calhoun VD, Kuzu CH, Kraut MA, Rivkin PR, Hart J, Jr, et al. Neural correlates of the object-recall process in semantic memory. Psychiatry Research: Neuroimaging. 2006;147(2–3):115–126. doi: 10.1016/j.pscychresns.2006.01.002. http://dx.doi.org/10.1016/j.pscychresns.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Baker S, Frith C, Dolan R. The interaction between mood and cognitive function studied with PET. Psychological Medicine. 1997;27:565–578. doi: 10.1017/s0033291797004856. [DOI] [PubMed] [Google Scholar]
- Basho S, Palmer ED, Rubio MA, Wulfeck B, Müller R-A. Effects of generation mode in fMRI adaptations of semantic fluency: Paced production and overt speech. Neuropsychologia. 2007;45(8):1697–1706. doi: 10.1016/j.neuropsychologia.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DS. Speech functions of the thalamus inferred from the effects of thalamotomy. Brain. 1968;91(4):619–638. doi: 10.1093/brain/91.4.619. http://dx.doi.org/10.1093/brain/91.4.619. [DOI] [PubMed] [Google Scholar]
- Bogousslavsky J, Miklossy J, Deruaz J, Regli F, Assal G. Unilateral left paramedian infarction of thalamus and midbrain: A clinico-pathological study. Journal of Neurology, Neurosurgery and Psychiatry. 1986;49(6):686–694. doi: 10.1136/jnnp.49.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogousslavsky J, Regli F, Assal G. The syndrome of unilateral tuberothalamic artery territory infarction. Stroke. 1986;17(3):434–441. doi: 10.1161/01.str.17.3.434. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. NeuroImage. 2006;32(2):821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Gaillard W, Theodore W. Regional cerebral blood flow during object naming and word reading. Human Brain Mapping. 1995;3(2):93–106. [Google Scholar]
- Brown S, Martinez M, Parsons L. Music and language side by side in the brain: A PET study of the generation of melodies and sentences. European Journal of Neuroscience. 2006;23(10):2791–2803. doi: 10.1111/j.1460-9568.2006.04785.x. [DOI] [PubMed] [Google Scholar]
- Bruyn R. Thalamic aphasia. A conceptional critique. Journal of Neurology. 1989;236:21–25. doi: 10.1007/BF00314212. [DOI] [PubMed] [Google Scholar]
- Carrera E, Bogousslavsky J. The thalamus and behavior: Effects of anatomically distinct strokes. Neurology. 2006;66(12):1817–1823. doi: 10.1212/01.wnl.0000219679.95223.4c. http://dx.doi.org/10.1212/01.wnl.0000219679.95223.4c. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Riba J, et al. Syllable congruency and word frequency effects on brain activation. Human Brain Mapping. 2009;30(9):3079–3088. doi: 10.1002/hbm.20730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen TA, Antonucci SM, Lockwood JL, Kittleson M, Plante E. Cortical and subcortical contributions to the attentive processing of speech. NeuroReport. 2008;19(11):1101–1105. doi: 10.1097/WNR.0b013e3283060a9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, et al. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125(5):1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Crabtree JW, Isaac JTR. New intrathalamic pathways allowing modality-related and cross-modality switching in the dorsal thalamus. The Journal of Neuroscience. 2002;22(19):8754–8761. doi: 10.1523/JNEUROSCI.22-19-08754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: The searchlight hypothesis. Proceedings of the National Academy of Sciences. 1984;81(14):4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B. Subcortical functions in language: A working model. Brain and Language. 1985;25:257–292. doi: 10.1016/0093-934x(85)90085-9. [DOI] [PubMed] [Google Scholar]
- Crosson B. Subcortical mechanisms in language: Lexical-semantic mechanisms and the thalamus. Brain and Cognition. 1999;40(2):414–438. doi: 10.1006/brcg.1999.1088. [DOI] [PubMed] [Google Scholar]
- Crosson B, Benefield H, Cato M, Sadek J, Moore A, Wierenga C, et al. Left and right basal ganglia and frontal activity during language generation: Contributions to lexical, semantic, and phonological processes. Journal of the International Neuropsychological Society. 2003;9(7):1061–1077. doi: 10.1017/S135561770397010X. [DOI] [PubMed] [Google Scholar]
- Crosson B, Moberg PJ, Boone JR, Gonzalez Rothi LJ, Raymer A. Category-specific naming deficit for medical terms after dominant thalamic/ capsular hemorrhage. Brain and Language. 1997;60(3):407–442. doi: 10.1006/brln.1997.1899. http://dx.doi.org/10.1006/brln.1997.1899. [DOI] [PubMed] [Google Scholar]
- Crosson B, Parker J, Kim A, Warren R, Kepes J, Tully R. A case of thalamic aphasia with postmortem verification. Brain and Language. 1986;29:301–314. doi: 10.1016/0093-934x(86)90050-7. [DOI] [PubMed] [Google Scholar]
- Crosson B. Subcortical functions in language and memory. Guilford Press. 1992 [Google Scholar]
- De Witte L, Brouns R, Kavadias D, Engelborghs S, De Deyn P. Cognitive, affective and behavioural disturbances following vascular thalamic lesions: A review. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior. 2011;47(3):273–319. doi: 10.1016/j.cortex.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Demeurisse G, Derouck M, Coekaerts M, Deltenre P, Van Nechel C, Demol O, et al. Study of two cases of aphasia by infarction of the left thalamus, without cortical lesion. Acta Neurologica Belgica. 1979;79:450–459. [PubMed] [Google Scholar]
- Demonet JF, Price C, Wise R, Frackowiak RSJ. A PET study of cognitive strategies in normal subjects during language tasks: Influence of phonetic ambiguity and sequence processing on phoneme monitoring. Brain. 1994;117(4):671–682. doi: 10.1093/brain/117.4.671. http://dx.doi.org/10.1093/brain/117.4.671. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Poldrack RA. In praise of tedious anatomy. NeuroImage. 2007;37(4):1033–1041. doi: 10.1016/j.neuroimage.2006.09.055. http://dx.doi.org/10.1016/j.neuroimage.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert A, Vinz B, Görtler M, Wallesch C, Herrmann M. Is there a syndrome of tuberothalamic artery infarction? A case report and critical review. Journal of Clinical and Experimental Neuropsychology. 1999;21(3):397–411. doi: 10.1076/jcen.21.3.397.915. [DOI] [PubMed] [Google Scholar]
- Fedio P, Van Buren JM. Memory and perceptual deficits during electrical stimulation in the left and right thalamus and parietal subcortex. Brain and Language. 1975;2:78–100. doi: 10.1016/s0093-934x(75)80056-3. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, Cramon DYv. FMRI evidence for dual routes to the mental lexicon in visual word recognition. Journal of Cognitive Neuroscience. 2002;14(1):11–23. doi: 10.1162/089892902317205285. http://dx.doi.org/10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fisher C. The pathologic and clinical aspects of thalamic hemorrhage. Transactions of the American Neurological Association. 1959;84:56–59. [PubMed] [Google Scholar]
- Frith CD, Friston KJ. The role of the thalamus in “top down” modulation of attention to sound. Neuroimage. 1996;4(3):210–215. doi: 10.1006/nimg.1996.0072. http://dx.doi.org/10.1006/nimg.1996.0072. [DOI] [PubMed] [Google Scholar]
- Garn CL, Allen MD, Larsen JD. An fMRI study of sex differences in brain activation during object naming. Cortex. 2009;45(5):610–618. doi: 10.1016/j.cortex.2008.02.004. http://dx.doi.org/10.1016/j.cortex.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Gauthier CT, Duyme M, et al. Sex and performance level effects on brain activation during a verbal fluency task: A functional magnetic resonance imaging study. Cortex. 2009;45(2):164–176. doi: 10.1016/j.cortex.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Tourville JA, Guenther FH. A neuroimaging study of premotor lateralization and cerebellar involvement in the production of phonemes and syllables. Journal of Speech and Hearing Research. 2008;51(5):1183–1202. doi: 10.1044/1092-4388(2008/07-0119). http://dx.doi.org/10.1044/1092-4388(2008/07-0119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Basu A, et al. Functional mapping of language networks in the normal brain using a word-association task. Indian J Radiol Imaging. 2010;20(3):182–187. doi: 10.4103/0971-3026.69352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Talavage TM. Reproducibility of fMRI activations associated with auditory sentence comprehension. NeuroImage. 2011;54(3):2138–2155. doi: 10.1016/j.neuroimage.2010.09.082. http://dx.doi.org/10.1016/j.neuroimage.2010.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, et al. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology. 2000;14(3):353–360. doi: 10.1037//0894-4105.14.3.353. [DOI] [PubMed] [Google Scholar]
- Graff-Radford N, Eslinger P, Damasio A, Yamada T. Nonhemorrhagic infarction of the thalamus: Behavioral, anatomic, and physiologic correlates. Neurology. 1984;34:14–23. doi: 10.1212/wnl.34.1.14. [DOI] [PubMed] [Google Scholar]
- Grossman M, Smith EE, et al. The Neural Basis for Categorization in Semantic Memory. NeuroImage. 2002;17(3):1549–1561. doi: 10.1006/nimg.2002.1273. [DOI] [PubMed] [Google Scholar]
- Guimaraes A, Melcher J, Talavage T, Baker J, Ledden P, Rosen B, et al. Imaging subcortical auditory activity in humans. Human Brain Mapping. 1998;6(1):33–41. doi: 10.1002/(SICI)1097-0193(1998)6:1<33::AID-HBM3>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett T, Stepniewska I, Kaas J. Thalamocortical connections of the parabelt auditory cortex in macaque monkeys. The Journal of Comparative Neurology. 1998;400(2):271–286. doi: 10.1002/(sici)1096-9861(19981019)400:2<271::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Halari R, Sharma T, Hines M, Andrew C, Simmons A, Kumari V. Comparable fMRI activity with differential behavioural performance on mental rotation and overt verbal fluency tasks in healthy men and women. Experimental Brain Research. 2006;169(1):1–14. doi: 10.1007/s00221-005-0118-7. [DOI] [PubMed] [Google Scholar]
- Harms MP, Melcher JR. Sound repetition rate in the human auditory pathway: Representations in the waveshape and amplitude of fMRI activation. Journal of Neurophysiology. 2002;88(3):1433–1450. doi: 10.1152/jn.2002.88.3.1433. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92(1–2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Ojemann GA. The role of the human thalamus in language and memory: Evidence from electrophysiological studies. Brain and Cognition. 2000;42(2):218–230. doi: 10.1006/brcg.1999.1101. [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamus. Cambridge University Press. 2007 [Google Scholar]
- Karussis D, Leker RR, Abramsky O. Cognitive dysfunction following thalamic stroke: A study of 16 cases and review of the literature. Journal of the Neurological Sciences. 2000;172(1):25–29. doi: 10.1016/s0022-510x(99)00267-1. [DOI] [PubMed] [Google Scholar]
- Kastner S, O’Connor DH, Fukui MM, Fehd HM, Herwig U, Pinsk MA. Functional imaging of the human lateral geniculate nucleus and pulvinar. Journal of Neurophysiology. 2004;91(1):438–448. doi: 10.1152/jn.00553.2003. http://dx.doi.org/10.1152/jn.00553.2003. [DOI] [PubMed] [Google Scholar]
- Ketteler D, Kastrau F, Vohn R, Huber W. The subcortical role of language processing. High level linguistic features such as ambiguity-resolution and the human brain; an fMRI study. NeuroImage. 2008;39(4):2002–2009. doi: 10.1016/j.neuroimage.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Calhoun V, Pitcock JA, Cusick C, Hart J. Neural hybrid model of semantic object memory: Implications from event-related timing using fMRI. Journal of the International Neuropsychological Society. 2003;9:1031–1040. doi: 10.1017/S135561770397007X. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Kremen S, Moo LR, Segal JB, Calhoun V, Hart J. Object activation in semantic memory from visual multimodal feature input. Journal of Cognitive Neuroscience. 2002;14(1):37–47. doi: 10.1162/089892902317205302. http://dx.doi.org/10.1162/089892902317205302. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Kremen S, Segal JB, Calhoun V, Moo LR, Hart J. Object activation from features in the semantic system. Journal of Cognitive Neuroscience. 2002;14(1):24–36. doi: 10.1162/089892902317205294. http://dx.doi.org/10.1162/089892902317205294. [DOI] [PubMed] [Google Scholar]
- Levin N, Ben-Hur T, Biran I, Wertman E. Category specific dysnomia after thalamic infarction: A case-control study. Neuropsychologia. 2005;43:1385–1390. doi: 10.1016/j.neuropsychologia.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Llano DA, Theyel BB, Mallik AK, Sherman SM, Issa NP. Rapid and sensitive mapping of long-range connections in vitro using flavoprotein autofluorescence imaging combined with laser photostimulation. Journal of Neurophysiology. 2009;101(6):3325–3340. doi: 10.1152/jn.91291.2008. http://dx.doi.org/10.1152/jn.91291.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: A voltage-dependent dye-imaging study in mouse brain slices. Proceedings of the National Academy of Sciences. 2002;99(1):449–454. doi: 10.1073/pnas.012604899. http://dx.doi.org/10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta JC, Phelps ME, Carson RE. Tomographic mapping of human cerebral metabolism: Subcortical responses to auditory and visual stimulation. Neurology. 1984;34:825–828. doi: 10.1212/wnl.34.6.825. [DOI] [PubMed] [Google Scholar]
- Mc Alonan K, Brown VJ. The thalamic reticular nucleus: More than a sensory nucleus? The Neuroscientist. 2002;8(4):302–305. doi: 10.1177/107385840200800405. http://dx.doi.org/10.1177/107385840200800405. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: Thalamocortical mechanisms. Annual Review of Neuroscience. 1997;20(1):185–215. doi: 10.1146/annurev.neuro.20.1.185. http://dx.doi.org/10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McFarling D, Rothi L, Heilman K. Transcortical aphasia from ischaemic infarcts of the thalamus: A report of two cases. Journal of Neurology, Neurosurgery and Psychiatry. 1982;45:107–112. doi: 10.1136/jnnp.45.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestres-Missé A, Càmara E, et al. Functional Neuroanatomy of Meaning Acquisition from Context. Journal of Cognitive Neuroscience. 2008;20(12):2153–2166. doi: 10.1162/jocn.2008.20150. [DOI] [PubMed] [Google Scholar]
- Mestres-Missé A, Münte TF, Rodriguez-Fornells A. Functional neuroanatomy of contextual acquisition of concrete and abstract words. Journal of Cognitive Neuroscience. 2009;21(11):2154–2171. doi: 10.1162/jocn.2008.21171. http://dx.doi.org/10.1162/jocn.2008.21171. [DOI] [PubMed] [Google Scholar]
- Mohr J, Pessin M, Finkelstein S, Funkenstein H, Duncan G, Davis K. Broca aphasia: Pathologic and clinical. Neurology. 1978;28(4):311–324. doi: 10.1212/wnl.28.4.311. [DOI] [PubMed] [Google Scholar]
- Morandi X, Brassier G, Darnault P, Mercier P, Scarabin J, Duval J. Microsurgical anatomy of the anterior choroidal artery. Surgical and Radiologic Anatomy. 1996;18:275–280. doi: 10.1007/BF01627605. [DOI] [PubMed] [Google Scholar]
- Morel A, Liu J, Wannier T, Jeanmonod D, Rouiller E. Divergence and convergence of thalamocortical projections to premotor and supplementary motor cortex: A multiple tracing study in the macaque monkey. European Journal of Neuroscience. 2005;21(4):1007–1029. doi: 10.1111/j.1460-9568.2005.03921.x. [DOI] [PubMed] [Google Scholar]
- Morel A. Stereotactic atlas of the human thalamus and basal ganglia. Informa Health Care. 2007 [Google Scholar]
- Mottaghy FM, Shah NJ, et al. Neuronal correlates of encoding and retrieval in episodic memory during a paired-word association learning task: a functional magnetic resonance imaging study. Experimental Brain Research. 1999;128(3):332–342. doi: 10.1007/s002210050853. [DOI] [PubMed] [Google Scholar]
- Müller R-A, Chugani DC, Behen ME, Rothermel RD, Muzik O, Chakraborty PK, et al. Impairment of dentato-thalamo-cortical pathway in autistic men: Language activation data from positron emission tomography. Neuroscience Letters. 1998;245(1):1–4. doi: 10.1016/s0304-3940(98)00151-7. [DOI] [PubMed] [Google Scholar]
- Murphy K, Corfield DR, Guz A, Fink GR, Wise RJS, Harrison J, et al. Cerebral areas associated with motor control of speech in humans. Journal of Applied Physiology. 1997;83(5):1438–1447. doi: 10.1152/jappl.1997.83.5.1438. [DOI] [PubMed] [Google Scholar]
- Murtha S, Chertkow H, Beauregard M, Evans A. The neural substrate of picture naming. Journal of Cognitive Neuroscience. 1999;11(4):399–423. doi: 10.1162/089892999563508. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Crosson B. Subcortical aphasia. Brain and Language. 1997;58(3):355–402. doi: 10.1006/brln.1997.1707. [DOI] [PubMed] [Google Scholar]
- Neau JP, Bogousslavsky J. The syndrome of posterior choroidal artery territory infarction. Annals of Neurology. 1996;39:779–788. doi: 10.1002/ana.410390614. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Buckner RL, Akbudak E, Snyder AZ, Ollinger JM, Mckinstry R, et al. Functional MRI studies of word-stem completion: Reliability across laboratories and comparison to blood flow imaging with PET. Human Brain Mapping. 1998;6(4):203–215. doi: 10.1002/(SICI)1097-0193(1998)6:4<203::AID-HBM2>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA, Fedio P, van Buren JM. Anomia from pulvinar and subcortical parietal stimulation. Brain. 1968;91(1):99–116. doi: 10.1093/brain/91.1.99. http://dx.doi.org/10.1093/brain/91.1.99. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Ward AAJ. Speech represenation in the ventrolateral thalamus. Brain. 1971;94(4):669–680. doi: 10.1093/brain/94.4.669. http://dx.doi.org/10.1093/brain/94.4.669. [DOI] [PubMed] [Google Scholar]
- Olshausen BA, Anderson CH, Van Essen DC. A neurobiological model of visual attention and invariant pattern recognition based on dynamic routing of information. Journal of Neuro Science. 1993;13(11):4700–4719. doi: 10.1523/JNEUROSCI.13-11-04700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Roberts L. Speech and brain-mechanisms. Princeton University Press. 1959 [Google Scholar]
- Perani D, Schnur T, Tettamanti M, Cappa SF, Fazio F. Word and picture matching: A PET study of semantic category effects. Neuropsychologia. 1999;37(3):293–306. doi: 10.1016/s0028-3932(98)00073-6. [DOI] [PubMed] [Google Scholar]
- Perren F, Clarke S, Bogousslavsky J. The syndrome of combined polar and paramedian thalamic infarction. Archives of Neurology. 2005;62(8):1212–1216. doi: 10.1001/archneur.62.8.1212. http://dx.doi.org/10.1001/archneur.62.8.1212. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331(6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Peschke C, Ziegler W, et al. Auditory-motor integration during fast repetition: The neuronal correlates of shadowing. NeuroImage. 2009;47(1):392–402. doi: 10.1016/j.neuroimage.2009.03.061. [DOI] [PubMed] [Google Scholar]
- Poeppel D, Guillemin A, Thompson J, Fritz J, Bavelier D, Braun AR. Auditory lexical decision, categorical perception, and FM direction discrimination differentially engage left and right auditory cortex. Neuropsychologia. 2004;42(2):183–200. doi: 10.1016/j.neuropsychologia.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Poline JB, Vandenberghe R, et al. Reproducibility of PET Activation Studies: Lessons from a Multi-Center European Experiment: EU Concerted Action on Functional Imaging. NeuroImage. 1996;4(1):34–54. doi: 10.1006/nimg.1996.0027. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, et al. Brain activity during reading The effects of exposure duration and task. Brain. 1994;117(6):1255–1269. doi: 10.1093/brain/117.6.1255. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Frackowiak RSJ, Friston KJ. The neural regions sustaining object recognition and naming. Proceedings of the Royal Society B: Biological Sciences. 1996;263(1376):1501–1507. doi: 10.1098/rspb.1996.0219. [DOI] [PubMed] [Google Scholar]
- Puel M, Demonet JF, Cardebat D, Berry I, Celsis P, Marc-Vergnes JP, et al. Three topographical types of thalamic aphasia: a neurolingusitic, MRI, and SPECT study. In: Valla G, Cappa SF, Wallesch CW, editors. Neuropsychological disorders associated with subcortical lesions. Oxford University Press; 1992. [Google Scholar]
- Purpura KP, Schiff ND. The thalamic intralaminar nuclei: A role in visual awareness. The Neuroscientist. 1997;3:8–15. [Google Scholar]
- Radanovic M, Scaff M. Speech and language disturbances due to subcortical lesions. Brain and Language. 2003;84(3):337–352. doi: 10.1016/s0093-934x(02)00554-0. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Bürgel U, Zilles K. Stereotaxic localization, intersubject variability, and interhemispheric differences of the human auditory thalamocortical system. NeuroImage. 2002;17(1):142–160. doi: 10.1006/nimg.2002.1178. [DOI] [PubMed] [Google Scholar]
- Raymer A, Moberg P, Crosson B, Nadeau S, Rothi L. Lexical-semantic deficits in two patients with dominant thalamic infarction. Neuropsychologia. 1997;35(2):211–219. doi: 10.1016/s0028-3932(96)00069-3. [DOI] [PubMed] [Google Scholar]
- Riecker A, Kassubek J, Gröschel K, Grodd W, Ackermann H. The cerebral control of speech tempo: Opposite relationship between speaking rate and BOLD signal changes at striatal and cerebellar structures. Neurology. 2006;29(1):46–53. doi: 10.1016/j.neuroimage.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, et al. FMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64(4):700–706. doi: 10.1212/01.WNL.0000152156.90779.89. http://dx.doi.org/10.1212/01.wnl.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Riklan M, Cooper IS. Psychometric studies of verbal functions following thalamic lesions in humans. Brain and Language. 1975;2:45–64. doi: 10.1016/s0093-934x(75)80053-8. [DOI] [PubMed] [Google Scholar]
- Robles SG, Gatignol P, Capelle L, Mitchell MC, Duffau H. The role of dominant striatum in language: A study using intraoperative electrical stimulations. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76(7):940–946. doi: 10.1136/jnnp.2004.045948. http://dx.doi.org/10.1136/jnnp.2004.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. The Journal of Comparative Neurology. 1997;379(3):313–332. [PubMed] [Google Scholar]
- Rosen HJ, Ojemann JG, Ollinger JM, Petersen SE. Comparison of brain activation during word retrieval done silently and aloud using fMRI. Brain and Cognition. 2000;42(2):201–217. doi: 10.1006/brcg.1999.1100. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace K, Maisog JM, Andreason P. Phonological and orthographic components of word recognition. A PET-rCBF study. Brain. 1997;120(5):739–759. doi: 10.1093/brain/120.5.739. http://dx.doi.org/10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Sach M, Seitz RJ, Indefrey P. Unified inflectional processing of regular and irregular verbs: A PET study. NeuroReport. 2004;15(3):533–537. doi: 10.1097/00001756-200403010-00030. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Lockwood AH, Frisina RD, Coad ML, Wack DS, Frisina DR. PET imaging of the normal human auditory system: Responses to speech in quiet and in background noise. Hearing Research. 2002;170(1–2):96–106. doi: 10.1016/s0378-5955(02)00386-6. http://dx.doi.org/10.1016/s0378-5955(02)00386-6. [DOI] [PubMed] [Google Scholar]
- Segal JB, Williams R, Kraut MA, Hart J., Jr Semantic memory deficit with a left thalamic infarct. Neurology. 2003;61(2):252–254. doi: 10.1212/01.wnl.0000073145.08816.e2. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Price CJ. Reading aloud boosts connectivity through the putamen. Cerebral Cortex. 2010;20(3):570–582. doi: 10.1093/cercor/bhp123. http://dx.doi.org/10.1093/cercor/bhp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Breiter HC, Goodman JM, Goldstein JM, Woodruff PWR, O’Craven K, et al. A functional magnetic resonance imaging study of auditory vigilance with low and high information processing demands. Neuropsychology. 1998;12(4):505–518. doi: 10.1037//0894-4105.12.4.505. http://dx.doi.org/10.1037/0894-4105.12.4.505. [DOI] [PubMed] [Google Scholar]
- Senhorini MCT, Cerqueira CT, et al. Brain activity patterns during phonological verbal fluency performance with varying levels of difficulty: A functional magnetic resonance imaging study in Portuguese-speaking healthy individuals. Journal of Clinical and Experimental Neuropsychology. 2011;33(8):864–873. doi: 10.1080/13803395.2011.561299. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Kraut MA, Lesser RP, Hart J. Interactions between thalamic and cortical rhythms during semantic memory recall in human. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(9):6440–6443. doi: 10.1073/pnas.092514899. http://dx.doi.org/10.1073/pnas.092514899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris AK, Medford NC, Giampietro V, Brammer MJ, David AS. Deriving meaning: Distinct neural mechanisms for metaphoric, literal, and non-meaningful sentences. Brain and Language. 2007;100(2):150–162. doi: 10.1016/j.bandl.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Suzuki M, Matsumoto K, Ishii K, Higano S, Fukasawa H, et al. Extent and location of cerebral infarcts on multiplanar MR images: Correlation with distribution of perforating arteries on cerebral angiograms and on cadaveric microangiograms. American Journal of Roentgenology. 1994;163(5):1215–1222. doi: 10.2214/ajr.163.5.7976904. [DOI] [PubMed] [Google Scholar]
- Tervaniemi M, Szameitat AJ, Kruck S, Schröger E, Alter K, De Baene W, et al. From air oscillations to music and speech: Functional magnetic resonance imaging evidence for fine-tuned neural networks in audition. The Journal of Neuroscience. 2006;26(34):8647–8652. doi: 10.1523/JNEUROSCI.0995-06.2006. http://dx.doi.org/10.1523/jneurosci.0995-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nature Neuroscience. 2010;13(1):84–88. doi: 10.1038/nn.2449. < http://www.nature.com/neuro/journal/v13/n1/suppinfo/nn.2449_S1.htm> (10.1038/nn.2449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings H, Amunts K, Mohlberg H, Pool C, Zilles K, Smeets W. 3D Probabilistic map of cytoarchitectonically defined human thalamic nuclei in standard MRI. Frontiers in Neuroinformatics (Conference Abstract. Neuroinformatics) 2008 [Google Scholar]
- Vannest J, Newport EL, et al. Interplay between morphology and frequency in lexical access: The case of the base frequency effect. Brain Research. 2011;1373:144–159. doi: 10.1016/j.brainres.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilkki J, Laitinen L. Effects of pulvinotomy and ventrolateral thalamotomy on some cognitive functions. Neuropsychologia. 1976;14(1):67–78. doi: 10.1016/0028-3932(76)90008-7. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Patterson RD, Griffiths TD. Task-dependent modulation of medial geniculate body is behaviorally relevant for speech recognition. Current Biology. 2008;18(23):1855–1859. doi: 10.1016/j.cub.2008.10.052. http://dx.doi.org/10.1016/j.cub.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Marzinzik F, Friederici AD, Hahne A, Kupsch A, Schneider G-H, et al. The human thalamus processes syntactic and semantic language violations. Neuron. 2008;59(5):695–707. doi: 10.1016/j.neuron.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Wallesch C-W, Henriksen L, et al. Observations on regional cerebral blood flow in cortical and subcortical structures during language production in normal man. Brain and Language. 1985;25(2):224–233. doi: 10.1016/0093-934x(85)90082-3. [DOI] [PubMed] [Google Scholar]
- Warburton E, Wise RJS, Price CJ, Weiller C, Hadar U, Ramsay S, et al. Noun and verb retrieval by normal subjects studies with PET. Brain. 1996;119(1):159–179. doi: 10.1093/brain/119.1.159. http://dx.doi.org/10.1093/brain/119.1.159. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Weisberg J, et al. Neural correlates of semantic and episodic memory retrieval. Neuropsychologia. 1998;37(1):103–118. doi: 10.1016/s0028-3932(98)00044-x. [DOI] [PubMed] [Google Scholar]
- Wittfoth M, Schröder C, et al. On Emotional Conflict: Interference Resolution of Happy and Angry Prosody Reveals Valence-Specific Effects. Cerebral Cortex. 2010;20(2):383–392. doi: 10.1093/cercor/bhp106. [DOI] [PubMed] [Google Scholar]
- Ye Z, Mestres-Missé A, Rodriguez-Fornells A, Münte TF. Two distinct neural networks support the mapping of meaning to a novel word. Human Brain Mapping. 2010 doi: 10.1002/hbm.21093. http://dx.doi.org/10.1002/hbm.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Mestres-Missé A, et al. Two distinct neural networks support the mapping of meaning to a novel word. Human Brain Mapping. 2011;32(7):1081–1090. doi: 10.1002/hbm.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]