Abstract
Objective:
To compare the yield of epileptiform abnormalities on 30-minute recordings with those greater than 45 minutes.
Methods:
We performed a prospective observational cross-sectional study of all outpatient routine EEGs comparing the rate of interictal epileptiform discharges (IEDs) and clinical events during the initial 30 minutes (routine) with those occurring in the remaining 30–60 minutes (extended). A relative increase of 10% was considered clinically significant.
Results:
EEGs from 1,803 patients were included; overall EEG duration was 59.4 minutes (SD ±6.5). Of 426 patients with IEDs at any time during the EEG, 81 (19.1%, 95% confidence interval 15.6–23) occurred only after the initial 30 minutes. The rate of late IEDs was not associated with age, indication, IED type, or sleep deprivation. Longer recording times also increased event capture rate by approximately 30%.
Conclusions:
The yield of IED and event detection is increased in extended outpatient EEGs compared to 30-minute studies.
EEG is a cornerstone in diagnosing epilepsy and other paroxysmal events. Routine outpatient EEGs in epilepsy are frequently nondiagnostic. Only 29%–55% of epilepsy patients have epileptiform abnormalities on a single routine outpatient EEG. This yield may be increased by performing serial recordings.1–4 Prolonged ambulatory EEGs are also sometimes used to identify interictal epileptiform discharges (IEDs) in patients with normal or nonepileptiform routine EEGs.5
The duration of standard outpatient EEG varies across practices. The American Clinical Neurophysiology Society recommends a minimum of 20 minutes of artifact-free recording to evaluate baseline wakefulness, but concedes that longer studies may be needed for activation procedures and sleep.6 The International League Against Epilepsy recommends recording for at least 30 minutes.7 Based on these recommendations, many routine outpatient EEGs last 20–30 minutes.
While longer EEGs likely render a higher yield of IEDs, this has not been extensively studied in a comparative manner. This is an important consideration as repeated, prolonged, and take-home ambulatory recordings may be intrusive for the patient, and may impact laboratory efficiency. Additionally, the Centers for Medicare and Medicaid Services (CMS) continue to evaluate the value of neurologic procedures in an effort to control costs. Therefore, it is important to quantify the value of extended outpatient EEG studies with respect to diagnostic yield. In this study, performed by neurophysiology and epilepsy fellows as a quality improvement project, our aim was to prospectively determine the diagnostic yield of extending the duration of outpatient EEG recordings beyond 30 minutes in adults and children.
METHODS
Standard protocol approvals, registrations, and patient consents.
Approval was obtained through the institutional review board for this prospective observational cross-sectional study. As it assessed the quality of a practice already instituted in our EEG laboratory (extended EEG) and did not change the course or interpretation of testing, informed consent was not required.
Patients.
All consecutive adult and pediatric outpatient EEGs performed between October 15, 2013, and May 15, 2014, at the Mayo Clinic in Rochester, Minnesota, were prospectively reviewed and included for analysis. Inpatient EEGs were excluded from analysis since this group often has distinctly different EEG indications and seizure etiologies compared to outpatients. Other exclusionary criteria included studies lasting less than 45 minutes, as these studies may not offer a meaningful extended duration compared to a 30-minute EEG, and incomplete data collection at the time of EEG. If a patient had more than 1 EEG during the study period, only the first EEG was included.
EEG.
All EEGs were performed using the international 10–20 system for electrode placement, including Fpz and Oz electrodes. EEG protocols include photic stimulation, hyperventilation for 3 minutes in patients who are not excluded for health-related reasons, and a period of sleep or attempted sleep. Outpatient EEGs at our institution are typically recorded for 60 minutes. EEG technologists noted the 30-minute mark within the study to trigger the initial review by the electroencephalographer before reviewing the rest of the study.
IEDs and events of interest.
IEDs were defined as spikes, sharp waves, temporal intermittent rhythmic delta activity, and generalized spike and wave discharges, including hypsarrhythmia patterns. IEDs were classified as multifocal if they appeared within both cerebral hemispheres independently over at least 3 head regions.8
Events of interest were defined as any clinical or electrographic event that occurred during the EEG that were potentially of diagnostic value. When identified, the authors retrieved data about the event through the formal EEG report. IEDs or events were considered early if occurring within the first 30 minutes of EEG and late if they occurred only after 30 minutes.
Review process.
All studies were reviewed prospectively by a board-certified electroencephalographer or clinical neurophysiology EEG fellow under the direct supervision of a board-certified electroencephalographer. The first 30 minutes of each study was first reviewed, and the reviewer's impressions recorded. The remaining portion of the study was then reviewed, and the final impression recorded.
Statistical analysis.
Age, sex, previous diagnosis of epilepsy, use of antiepileptic drugs (AEDs), indication for EEG, EEG duration, whether sleep was recorded (and if so, whether this occurred within or after the first 30 minutes of the recording), timing of first detected or new independent IED, and type of IEDs were all collected. If a diagnosis of epilepsy was unknown at the time of the study, use of AEDs was not sufficient to imply a diagnosis as they may be started by non-neurologists based solely on suspicion of a diagnosis even when clinical history is suggestive of other paroxysmal events. When known, the number of hours the patient slept the night before was also recorded to assign sleep deprivation status. Adult patients were considered sleep-deprived if they had 4 hours of sleep or less and children if they had 6 hours or less.
EEG data recorded included IEDs seen within 30 minutes and IEDs seen after 30 minutes. IEDs seen after 30 minutes were considered unique if they were not seen in the first 30 minutes, or if a new IED was seen at a new focality or carried a new syndromic implication (e.g., new generalized discharge with previous fragments that may be interpreted as focal, or the emergence of a hypsarrhythmia pattern).
Data were evaluated for nonequivalence between 30 minutes and extended outpatient studies. In order to calculate the number of EEGs needed for statistical power purposes, extended outpatient EEGs were predicted to be clinically superior to 30-minute EEGs if a relative difference of 10% in IEDs was detected. We estimate that in our own laboratory, this difference would result in approximately 100 new patients with IEDs over a 1-year period. Based on this, 1,644 EEGs (455 children, 1,189 adults) were calculated as necessary to provide 80% power in the detection of a significant difference between 30 minutes and extended EEGs. Variables potentially affecting the timing of IEDs, including sleep deprivation, sleep during the EEG, and type of IED, were evaluated using χ2 analysis. Continuous variables are displayed as means with SDs. Categorical variables are displayed as percentages with 95% confidence interval (CIs). Statistical analysis was performed by D.B.B. using JMP version 9.0.1 statistical software.
RESULTS
Overall, 1,965 patients underwent outpatient EEG during the study timeframe. A total of 162 patients were excluded (43 children, 119 adults), with 130 having incomplete data collection at the time of their EEG, and the remainder due to having studies shorter than 45 minutes. A total of 1,803 patients were included in the analysis, comprising 517 children and 1,286 adults.
Table 1 outlines the group demographics. The most common indication for EEG referral was “spells” in both the child and adult groups, followed by focal and generalized seizures. A seizure-specific indication for EEG comprised 44% of the group. Approximately half of the patients were not taking an AED. The average duration of EEG was approximately 60 minutes in either group.
Table 1.
Patient characteristics
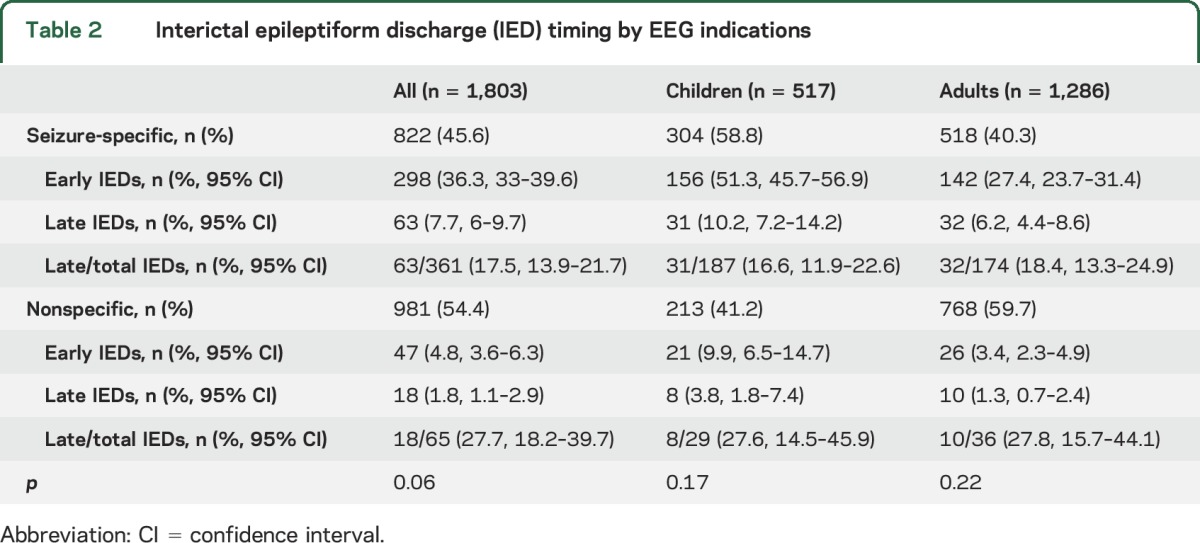
A total of 426 patients (23.6%) had IEDs at any time during their EEG. Eighty-one patients (4.5%) had IEDs occur late. This corresponds to a relative increase of 19% (95% CI 15.6–23) in new IEDs, and remained similar in both children (absolute increase 7.5%, relative 18.1%, 95% CI 13.5–23.8) and adults (absolute increase 3.2%, relative 20%, 15.1–36). The relative increase in IED yield remained true regardless of discharge type (table e-1 on the Neurology® Web site at Neurology.org). Pretest probability was an important factor in the overall likelihood of any IEDs occurring (table 2). Patients with seizure-specific indications were more likely to have IEDs at any time than patients with nonspecific indications (43.9% vs 6.6%, respectively). Despite the absolute difference in IED occurrence between the seizure-specific and nonspecific groups, the relative increase in late IED yield remained high, with 17.5% occurring after 30 minutes in the seizure-specific group and 27.7% in the nonspecific group. This effect held true regardless of age.
Table 2.
Interictal epileptiform discharge (IED) timing by EEG indications
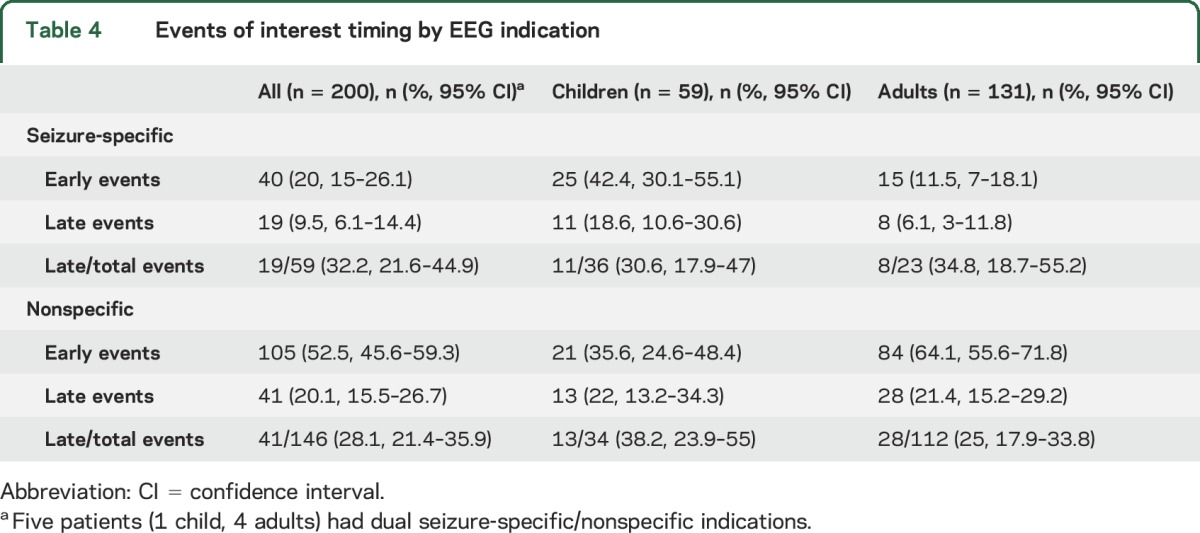
Events of interest were recorded in 214 patients (table 3), of which clinical data were available in 200. Of 54 recorded seizures, 16 (29.6%, 95% CI 19.1–42.9) were detected beyond the initial 30 minutes (late) in the recording. Nonepileptic spells (NES) were the most common event type, with late NES accounting for 20% all events and 29% of all recorded NES. Nonepileptic physiologic events were rare. A similar rate of late events was seen in both seizure-specific and nonspecific EEG indications (table 4). Overall, nonseizure events of any kind were more likely in the nonspecific EEG indication group, while seizures were much more likely in patients with a seizure indication (p ≤ 0.0001) (table e-2).
Table 3.
Events of interest timing by event type
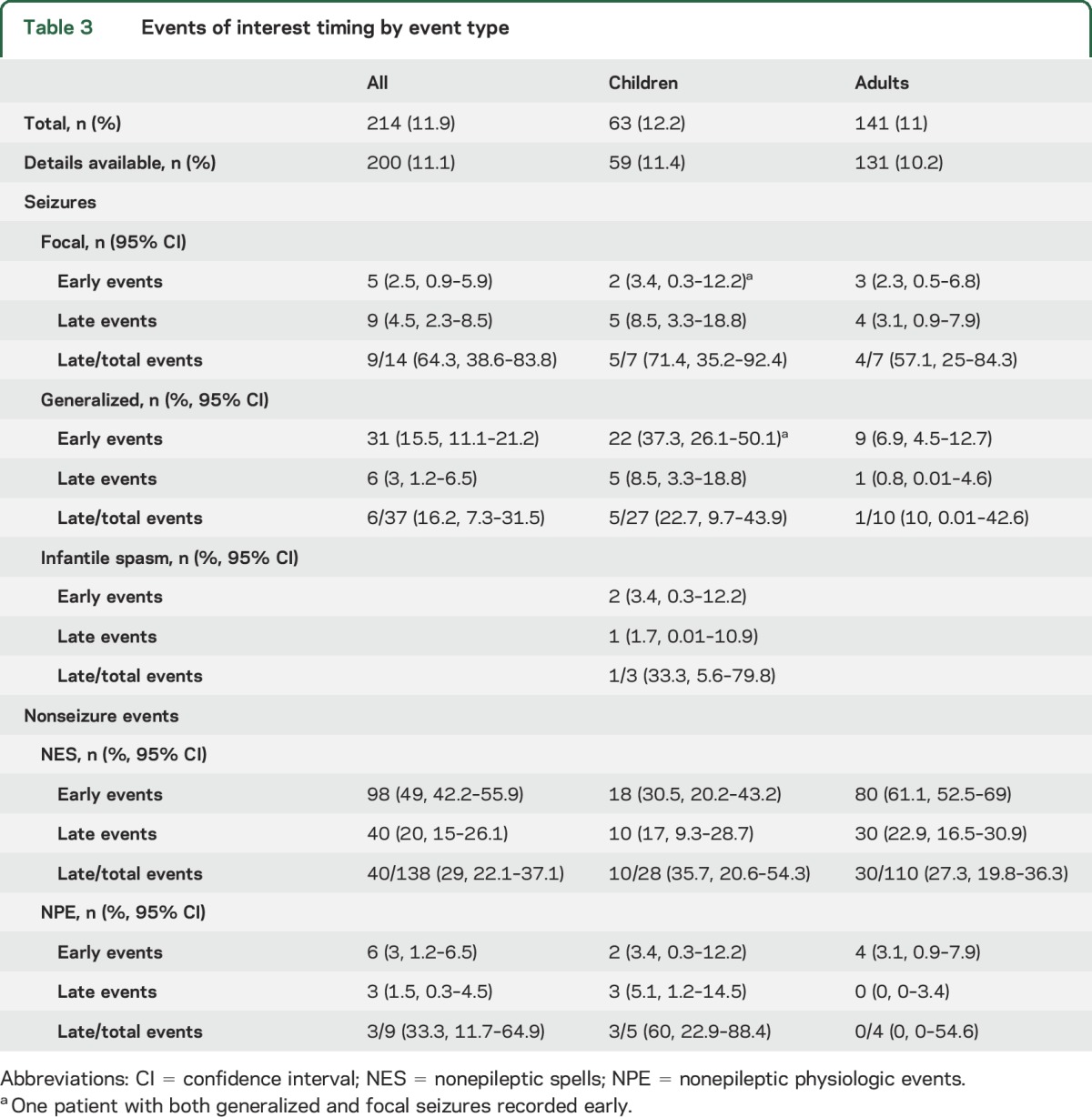
Table 4.
Events of interest timing by EEG indication
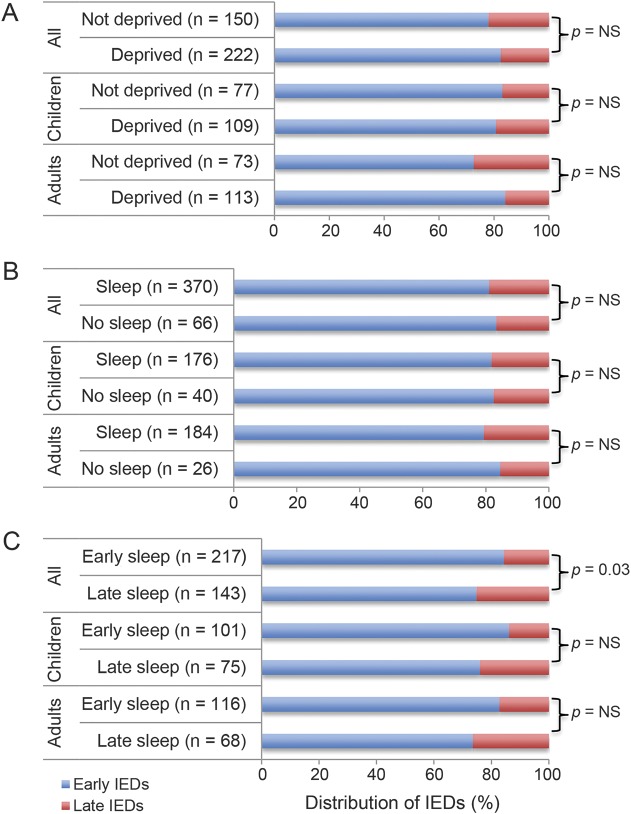
Sleep deprivation and sleep during EEG also did not affect IED occurrence. Sleep timing, however, was important. Late IEDs were significantly more likely when patients slept only after the first 30 minutes of the study in the entire group (p = 0.03), but not in either the adult or child groups individually (figure).
Figure. Early- and late-occurring interictal epileptiform discharges (IEDs) by sleep status.
Early and late IEDs by (A) sleep deprivation at time of EEG, (B) sleep during EEG, and (C) early or late sleep during EEG.
Among the entire group, 530 (30.4%) previously had an EEG at our institution, with 391 demonstrating IEDs in at least one prior recording. Similar to patients with a seizure-specific indication, 193 (49.3%) patients with a previous IED had an IED on their current EEG, with 36 (18.7%, 95% CI 13.8–24.8) occurring late. Of 139 patients with previously normal EEGs, 14 (10.1%) showed an IED on their current EEG, with only 1 (7.1%, 95% CI 0–33.5) occurring late.
DISCUSSION
This large, prospective study shows that increasing the duration of outpatient EEG from 30 to 45 minutes or longer increases the relative yield of new IEDs by approximately 20%, exceeding our a priori cutoff for clinical significance. This equates to a 4.5% absolute increase in IEDs across all patients. This relative increase was irrespective of age, EEG indication, or IED type. This is particularly relevant for patients with seizure-specific indications, where the relative increase was 17.5% with an absolute increase of 7.7%. Put in more practical terms, extended EEGs result in a newly seen IED approximately every 22 studies regardless of indication, and once in every 13 studies when the pretest probability is high.
Perhaps just as useful, extending the EEG beyond 45-minute events increased the capture rate of clinical events. Events of any kind were recorded relatively frequently, with approximately 30% occurring only after the first 30 minutes of the study regardless of indication. This equates to a new captured event every 31 EEGs in extended recordings compared to shorter studies. Additionally, pretest probability again was important as NES were much more common in patients with nonspecific EEG indications, and seizures more likely with seizure-specific indications. Capturing events on EEG is the gold standard of diagnosis when there is a question of epilepsy, making it invaluable in decision-making.
These results have many potential implications. Clinically, an increased yield in IEDs and recorded events with extended recordings provide improved diagnostic support for epilepsy (or nonepileptic entities), which may result in a reduction in the need for other tests such as prolonged ambulatory or inpatient EEGs. Along similar lines, this also allows for more timely appropriate management. Improvements in diagnostic yield may impact laboratory efficiency, and to that end further evaluation of the potential effects of extended routine EEG recordings on health care delivery and use of prolonged EEG monitoring and ambulatory EEG evaluations merit evaluation.
Past studies have demonstrated that serial EEGs are useful in increasing the diagnostic yield of epileptiform abnormalities. Single EEGs are positive in 29%–55% of patients with seizures. Serial routine EEGs may increase that yield up to 59%–82%.1–4 Looking at individual serial EEG studies, a single repeat recording may increase the rate of overall IED-positive EEGs by 8%–17%, reflecting a relative increase of IEDs identified of approximately 18%–26%.2,3 The IED increase on successive EEGs is concordant with the increase seen in a single extended recording in our study. This is also corroborated by one study evaluating IED latency in 171 extended EEG recordings lasting up to 6 hours. In that study, 71% of IED-containing EEGs had the first discharge within 30 minutes, and another 22% of patients between 30 and 90 minutes.9 Performing a single study of longer duration may prevent the need for repeated studies, thereby potentially reducing the expense of performing multiple EEGs for individual patients in some cases.
Previous studies have evaluated the effect of reduced recording times on EEG diagnostic yield in an effort to increase laboratory efficiency.10,11 These have involved taking studies of 20–25 minutes, and reviewing truncated protocols lasting from 10 to 15 minutes in comparison, which in turn often compare favorably. This may not be surprising as patients who display IEDs within 20–25 minutes are more likely to have a shorter IED latency; however, our data suggest that a meaningful proportion of patients with longer IED latencies would likely be missed by this approach.
Sleep timing was an important factor in the occurrence of IEDs, and the duration of the EEG may be a significant factor in this regard. Patients who did not sleep until 30 minutes or later into the study were more likely to have late IEDs. The duration of EEG may be important in achieving sleep. Only about half of the total group slept during the first 30 minutes of the study, while another 25% of the group was able to sleep after 30 minutes.
It is known that sleep modulates activation of IEDs.12 A shorter 20- to 30-minute study is often insufficient to allow for a complete set of activating procedures, such as photic stimulation and 3–5 minutes of hyperventilation, as well as an adequate sampling of sleep. One study looking at the maximum efficiency and shortest duration in which an adequate EEG could be performed supported that all appropriate states could be seen within either 15 or 25 minutes; however, this only included time built in for resting EEG and did not specifically allow for sleep.10 Another study comparing various EEG protocols showed that in patients who had 2 or more generalized tonic-clonic seizures, IEDs were more likely to be seen using a sleep deprived protocol (SD-EEG) compared to routine (r-EEG) or temazepam protocols (DI-EEG).13 In that study, the median duration of both SD-EEG and DI-EEG protocols was more than 40 minutes, while r-EEG was about 21 minutes. The DI-EEG protocol called for administering temazepam 10 mg orally 30 minutes prior to recording the EEG. Patients were much more likely to achieve sleep in either of the longer protocols (89%–95% in SD or DI-EEG vs 46% in r-EEG). It may be that the increased sleep and IED yield in that study was a function of time as much as any sleep-inducing procedure, and was actually hindered by the application of a sedative medication. Our study supports the concept that sampling an adequate amount of sleep can result in an increased yield of IEDs, and that longer outpatient EEG times allow for a higher chance of recording natural sleep.
The review process was limiting as both the first 30 minutes and the complete extended EEG were reviewed during a single session rather than blinded and as separate records. Alternatively, the first 30 minutes and post-30-minutes recordings could have been presented as separate studies in a blinded fashion to reduce bias. However, the need to re-review 1,800 EEGs would have proven impractical for laboratory throughput. Instead, the 30-minute mark was indicated by EEG technologists as the study was recorded, and reviewers were asked to record their impressions on the first 30 minutes prior to reviewing the remainder of the recording to help prevent bias. Reviewers were also not blinded to the clinical circumstances of the patients, and were able to review previous EEG results in the medical record. This was not likely a significant factor as patients with first-time EEGs displayed late IEDs at the same rate as the total group, indicating no evidence that reviewers were significantly biased by these factors. All studies included periods of sleep or attempted sleep and standard activating procedures (hyperventilation and photic stimulation), but these were not performed at the same time for every patient and either may have been attempted only after the first 30 minutes. Theoretically, this could create instances where IEDs were seen only after 30 minutes by using a provoking maneuver that could have otherwise been performed earlier.
Differing Current Procedural Terminology codes are utilized for routine EEG studies, which are based in large part on recording duration. Codes 95816 and 95819 are used for recordings lasting 20–40 minutes, 95812 for 41–60 minutes, and 95813 for recordings lasting longer than 60 minutes. Historically, these codes have been reimbursed at a differential rate, with codes for longer studies generally reimbursed at rates greater than shorter studies. CMS recently decreased the relative value units for 95812 and 95813, making them nearly equal to 20- to 40-minute recordings. Our data would support a higher reimbursement rate for longer duration studies based on higher diagnostic yield.
Performing extended outpatient EEG of 45 minutes or more results in a relative increase in the detection of IEDs and capture of clinical events. This appears most relevant for patients with a high pretest probability of having epilepsy as this group may have a new IED identified in as few as 1 in every 13 studies. This difference may be less in patients with a low pretest probability in terms of the detection of IEDs due to low overall rates of IEDs at baseline; however, the increased capture of clinical events supports the use of longer recordings in this population as well. The potential impact on health care economy and delivery as related to EEG duration warrants further study.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the EEG technologists at the Mayo Clinic.
GLOSSARY
- AED
antiepileptic drug
- CI
confidence interval
- CMS
Centers for Medicare and Medicaid Services
- DI-EEG
temazepam EEG protocol
- IED
interictal epileptiform discharge
- NES
nonepileptic spell
- r-EEG
routine EEG protocol
- SD-EEG
sleep-deprived EEG protocol
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Burkholder was involved in study concept and design, data acquisition, analysis and interpretation of data, statistical analysis, and drafting and revising the manuscript. Dr. Britton was involved in study concept and design, data acquisition, analysis and interpretation of data, and drafting and revising the manuscript. Dr. Rajasekaran was involved in data acquisition and drafting and revising the manuscript. Dr. Fabris was involved in data acquisition and drafting and revising the manuscript. Dr. Perumpillichira was involved in data acquisition and drafting and revising the manuscript. Dr. Kelly-Williams was involved in data acquisition and drafting and revising the manuscript. Dr. So was involved in data acquisition and drafting and revising the manuscript. Dr. Nickels was involved in data acquisition and drafting and revising the manuscript. Dr. Wong-Kisiel was involved in data acquisition and drafting and revising the manuscript. Dr. Lagerlund was involved in data acquisition and drafting and revising the manuscript. Dr. Cascino was involved in data acquisition and drafting and revising the manuscript. Dr. Worrell was involved in data acquisition and drafting and revising the manuscript. Dr. Wirrell was involved in study concept and design, data acquisition, analysis and interpretation of data, and drafting and revising the manuscript.
STUDY FUNDING
No targeted funding is reported.
DISCLOSURE
D. Burkholder reports no disclosures relevant to the manuscript. J. Britton receives funding as a coinvestigator from GW Pharma. V. Rajasekaran, R. Fabris, J. Perumpillichira, K. Kelly-Williams, E. So, K. Nickels, L. Wong-Kisiel, and T. Lagerlund report no disclosures relevant to the manuscript. G. Cascino serves as associate editor for Neurology®, is an investigator in the Human Epilepsy Project, receives technology royalties from Mayo Medical Ventures (high-frequency nerve stimulation to treat lower back pain, Nervo), receives honoraria from the American Academy of Neurology and American Epilepsy Society, and receives funding as a coinvestigator from GW Pharma. G. Worrell reports no disclosures relevant to the manuscript. E. Wirrell receives funding as PI from GW Pharma. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Marsan CA, Zivin LS. Factors related to the occurrence of typical paroxysmal abnormalities in the EEG records of epileptic patients. Epilepsia 1970;11:361–381. [DOI] [PubMed] [Google Scholar]
- 2.Salinsky M, Kanter R, Dasheiff RM. Effectiveness of multiple EEGs in supporting the diagnosis of epilepsy: an operational curve. Epilepsia 1987;28:331–334. [DOI] [PubMed] [Google Scholar]
- 3.Doppelbauer A, Zeitlhofer J, Zifko U, Baumgartner C, Mayr N, Deecke L. Occurrence of epileptiform activity in the routine EEG of epileptic patients. Acta Neurol Scand 1993;87:345–352. [DOI] [PubMed] [Google Scholar]
- 4.Baldin E, Hauser WA, Buchhalter JR, Hesdorffer DC, Ottman R. Yield of epileptiform electroencephalogram abnormalities in incident unprovoked seizures: a population-based study. Epilepsia 2014;55:1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faulkner HJ, Arima H, Mohamed A. Latency to first interictal epileptiform discharge in epilepsy with outpatient ambulatory EEG. Clin Neurophysiol 2012;123:1732–1735. [DOI] [PubMed] [Google Scholar]
- 6.American Clinical Neurophysiology Society. Guideline 1: minimum technical requirements for performing clinical electroencephalography. J Clin Neurophysiol 2006;23:86–91. [DOI] [PubMed] [Google Scholar]
- 7.Fink R, Pedersen B, Guekht AB, et al. Guidelines for the use of EEG methodology in the diagnosis of epilepsy: International League Against Epilepsy: Commission report: Commission on European Affairs: Sub-commission on European Guidelines. Acta Neurol Scand 2002;106:1–7. [DOI] [PubMed] [Google Scholar]
- 8.Ebersole JS, Husain AM, Nordli DR, eds. Current Practice of Clinical Electroencephalography, 4th ed Philadelphia: Wolters Kluwer; 2014. [Google Scholar]
- 9.Losey TE, Uber-Zak L. Time to first interictal epileptiform discharge in extended recording EEGs. J Clin Neurophysiol 2008;25:357–360. [DOI] [PubMed] [Google Scholar]
- 10.Reardon KA, Scheffer IE, Smith LJ, Jolley D, Horne MK. How long should a routine EEG be? J Clin Neurosci 1999;6:492–493. [DOI] [PubMed] [Google Scholar]
- 11.Agbenu J, Newton RW, Martland T, Ismayl O, Hargreaves S. Effect of reducing the recording time of standard EEGs on the detection of EEG-abnormalities in the management of the epilepsies of childhood. Seizure 2012;21:422–425. [DOI] [PubMed] [Google Scholar]
- 12.Ferrillo F, Beelke M, Nobili L. Sleep EEG synchronization mechanisms and activation of interictal epileptic spikes. Clin Neurophysiol 2000;111:S65–S73. [DOI] [PubMed] [Google Scholar]
- 13.Leach JP, Stephen LJ, Salveta C, Brodie MJ. Which electroencephalography (EEG) for epilepsy? The relative usefulness of different EEG protocols in patients with possible epilepsy. J Neurol Neurosurg Psychiatry 2006;77:1040–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.