Abstract
Objective:
To determine whether and how longitudinal rates of change in MRI volumetrics, CSF concentrations of Alzheimer-related proteins, molecular imaging of cerebral fibrillar amyloid with PET using the [11C] benzothiazole tracer, Pittsburgh compound B (PiB), and cognition were associated among asymptomatic middle-aged to older individuals.
Methods:
Multivariate mixed models for repeated measures were used to assess the correlations on the rates of changes across markers.
Results:
Among 209 asymptomatic middle-aged to older individuals longitudinally followed for up to 11 years (mean 6.7 years), a faster intraindividual decrease in CSF Aβ42 was associated with a faster increase in PiB mean cortical standardized uptake value ratio (MCSUVR, p = 0.04), but not others. The rate of change in CSF tau (and Ptau181) was correlated with the rate of change in PiB MCSUVR (p = 0.002), hippocampal volume (p = 0.04), and global cognition (p = 0.008). The rate of change in hippocampal volume was correlated with the rate of change in global cognition (p = 0.04). Only 3 significant correlations were observed at baseline: CSF Aβ42 and PiB MCSUVR (p < 0.001), CSF tau and PiB MCSUVR (p < 0.001), and CSF Aβ42 and global cognition (p = 0.01).
Conclusions:
CSF tau (Ptau181), PiB MCSUVR, and hippocampal volume were all longitudinally correlated with each other, whereas CSF Aβ42 was correlated only with PiB binding. Unlike the baseline values, the longitudinal change in CSF tau (Ptau181) and hippocampal volume were correlated with the longitudinal change in global cognition, validating the role of these biomarkers in Alzheimer disease prevention trials.
Accumulating research suggests that neurodegenerative processes associated with Alzheimer disease (AD) may begin at middle age (∼50 years)1–3 and almost certainly many years prior to symptom onset.4–7 Because by definition there are no clinical symptoms at this preclinical stage of AD, biomarkers can be an effective tool to measure disease progression so that early interventions can be tested and developed. The cross-sectional and longitudinal associations across multiple modalities of AD biomarkers have been well-characterized, especially in elderly individuals 65 years or older with and without clinical symptoms of AD.8–10 It remains unknown, however, how and to what degree the longitudinal changes of AD biomarkers are correlated in asymptomatic middle-aged to older individuals. The objective of this report is to provide a comprehensive assessment of the biomarker relationships on the longitudinal rates of change across major modalities of AD biomarkers and cognition in a cognitively normal middle-aged to older cohort, and compare results to the cross-sectional correlations of baseline values alone.
METHODS
Participants.
Since 2005, the Adult Children Study (ACS) has enrolled a cohort of cognitively normal 43- to 77-year-old individuals in a comprehensive study of biomarkers for AD prior to its symptomatic stages.11 In addition to clinical and cognitive measures, a broad spectrum of biomarkers for AD were longitudinally assessed, including MRI-based regional brain volumes, CSF analytes, and molecular imaging of cerebral fibrillar amyloid with PET using the [11C] benzothiazole tracer, Pittsburgh compound B (PiB). As of October 2014, the ACS enrolled 357 community-living volunteers from the greater St. Louis metropolitan area. Recruitment primarily was through word of mouth and personal inquiries. The design of the ACS was a 2-way stratification by family history (FH) for late-onset AD and 3 age groups at baseline (43–54, 55–64, 65–77 years).12 Eligibility criteria for the ACS were availability of an informant who knew the participant well, cognitive normality at baseline (defined as Clinical Dementia Rating [CDR]13 = 0), and willingness in principle to complete all study procedures at baseline and each follow-up. Individuals with comorbid conditions, including depressive features short of major affective disorder, were allowed in ACS if clinically stable at time of enrollment. Exclusion criteria included conditions that would preclude longitudinal participation or confound cognitive assessment or membership in families with a dominantly inherited pattern of AD or a known causative mutation for AD.
Standard protocol approvals, registrations, and patient consents.
The Washington University Human Research Protection Office approved the study and all participants gave written informed consent.
Clinical and cognitive assessments.
The clinical and cognitive assessments were conducted longitudinally every 3 years except for participants age 65 years or older, who were assessed annually. Details of clinical and cognitive assessments have been described previously.12 In brief, the primary clinical assessment protocol is that of the National Alzheimer Coordinating Center Uniform Data Set,14 which includes standard definitions and diagnostic criteria for detection of dementia and its differential diagnosis.14,15 The presence or absence of dementia and, when present, its severity were operationalized with the CDR.13 The entire clinical assessment takes approximately 90 minutes to complete. Participants completed comprehensive psychometric testing 1–2 weeks after their clinical assessment. The 5 cognitive domains assessed in the 2-hour cognitive battery include episodic memory, working memory, semantic knowledge, executive function and attention, and visuospatial ability. A global cognition score covering all major cognitive domains for early changes was computed by using 7 cognitive tests that were shared by all age groups of the ACS cohort: Logical Memory Delayed and Verbal Paired Associates from the Wechsler Memory Scale (WMS),16 Free and Cued Selective Reminding,17 WMS-III Letter-Number Sequencing,18 Animal Naming,19 and Trailmaking Test A and B.20 The global cognitive score represented the average of the z scores from all 7 tests, each of which was obtained by first subtracting the baseline mean score over the entire ACS cohort from each individual's score and then dividing the difference by the baseline SD.
CSF collection and analysis.
Longitudinal CSF was collected in the ACS. The assessment protocol has been described previously.8 Briefly, CSF (20–30 mL) was collected by routine lumbar puncture (LP) in polypropylene tubes at 8:00 am after overnight fasting as previously described.8 The samples were analyzed for total tau, tau phosphorylated at threonine-181 (Ptau181), and Aβ1-42 (Aβ42) by commercial ELISA (Improved INNOTEST, Fujirebio, Ghent, Belgium).21 Longitudinal CSF samples from the same individual were run on the same assay plate (and same lot number) in order to minimize potential interplate and inter-lot methodologic variability. Samples were continuously kept on ice and assays were performed on the same aliquot after a single thaw following initial freezing.
Image acquisition and processing.
MRI scans were obtained on a Sonata 1.5T, Vision 1.5T, or Trio 3.0T scanner (Siemens, Munich, Germany). All participants with longitudinal MRI assessments were included in this report, and all longitudinal scans were obtained on a Trio 3.0T scanner. Structural MRI processing steps have been described in detail previously12,22 and included motion correction, averaging across scans, and atlas transformation. Regional volumes were obtained via the FreeSurfer image analysis suite (Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA). Hippocampal volume was selected as the region of interest (ROI) in this analysis.
PET PiB imaging and analysis procedures have been reported elsewhere.12,23 To summarize, PiB amyloid deposition in specified brain ROI was determined using FreeSurfer,24–26 and a standardized uptake value ratio (SUVR) with and without correction for partial volume effects27,28 was calculated for each ROI, based upon the last 30 minutes acquired as part of a 60-minute dynamic acquisition. Partial volume correction was performed using a regional spread function technique.28 The mean cortical SUVR (MCSUVR) was calculated from FreeSurfer regions within the prefrontal cortex, precuneus, and temporal cortex. The cerebellum was chosen as the reference region.
Genotyping.
Statistical analysis.
All 209 individuals with available longitudinal data from at least 2 modalities (CSF, PET/PiB, MRI, and cognition) were analyzed. For each pair of markers, a bivariate mixed model for repeated measures (BMMRM) was used to simultaneously model the longitudinal courses of both markers.30,31 Specifically, random intercept and slope (i.e., the rate of change) were assumed for each marker across individuals.32 The entire set of random effects from 2 markers including 2 slopes and 2 intercepts from the BMMRM was then assumed to follow a joint 4D multivariate normal distribution with an unstructured covariance matrix across the participants. The unstructured covariance matrix is important because it allows an unbiased assessment on the correlation of the rates of change across biomarkers. Different residual variances were assumed between markers. All fixed effects and variance/covariance components were estimated by the method of maximum likelihood. BMMRM was chosen because of the concern on the convergence in the maximum likelihood estimation with more than 2 markers in the joint analyses. A 95% confidence interval (CI) for the correlation of the bivariate rates of change was estimated through the Delta method after the Fisher z transformation. To adjust for the effect of baseline age, FH, and APOE ε4 allele (APOE4) status, similar BMMRMs were fitted by including the fixed effects of these covariates in both the slopes and the intercepts. These models were flexible in handling unbalanced and unevenly spaced multivariate longitudinal biomarker data as well as missing data in the ACS, and have been previously used in studies of AD.33 Model diagnostics (i.e., residual plots) indicated no major concern on the model goodness-of-fit. All models were implemented in PROC MIXED/SAS.34
RESULTS
Table 1 presents the baseline demographics and biomarkers of 209 individuals in the ACS cohort who were part of the analyses. A total of 145 (69%) individuals were younger than 65 or age 65 years at baseline. The duration and the frequency of longitudinal follow-up for each modality are given in table 2. A total of 207/209 individuals underwent longitudinal clinical and cognitive assessments for up to 11 years. Fifteen progressed to higher CDR after baseline. A total of 169 (81%) had longitudinal LPs to obtain CSF, 152 (73%) completed longitudinal PET PiB scans, and 164 (78%) underwent longitudinal MRI scans.
Table 1.
Demographic and biomarker descriptive statistics at baseline (n = 209)
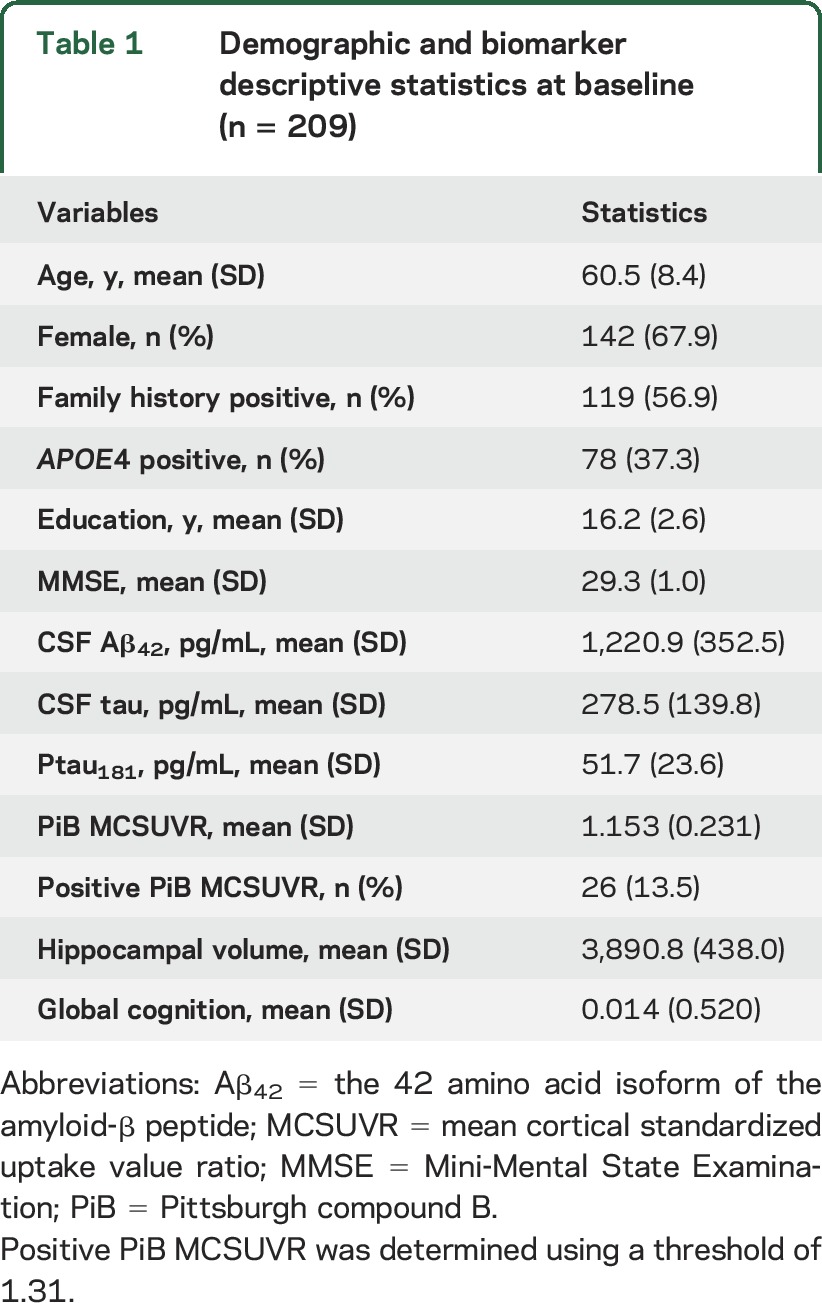
Table 2.
Summary of longitudinal follow-up in the Adult Children Study cohort
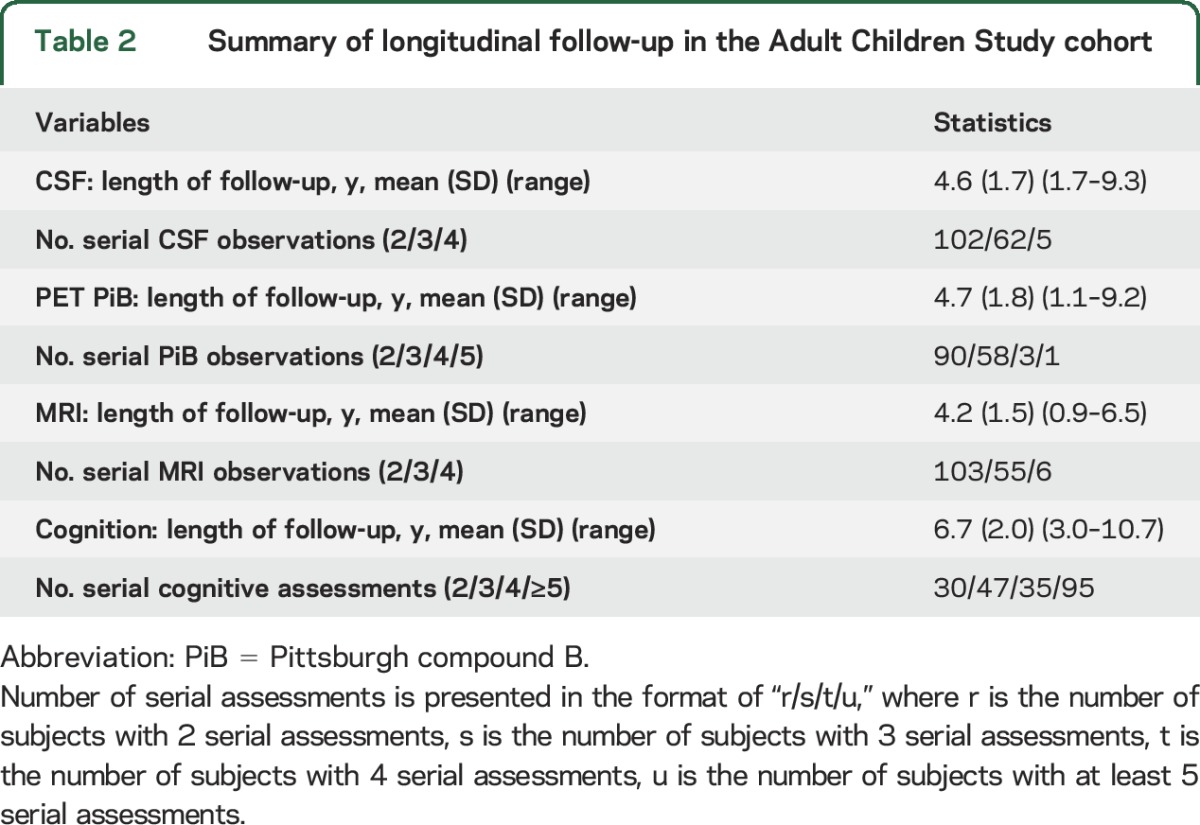
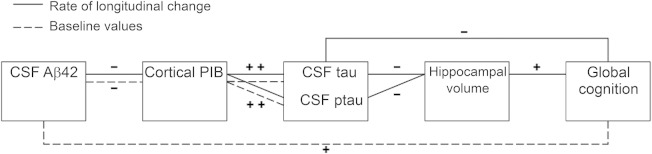
Table 3 presents the associations among CSF Aβ42, CSF tau (and Ptau181), PiB MCSUVR, hippocampal volume, and global cognition at baseline alone. The dashed lines between 2 biomarkers in figure 1 represent statistically significant correlations at baseline (p < 0.05), whereas biomarkers without connecting dashed lines are not significantly correlated at baseline. Specifically, after adjusting for the effects of family history, baseline age, and APOE4 status, only 3 significant correlations were observed at baseline: CSF Aβ42 and PiB MCSUVR (r = −0.49, 95% CI −0.63 to 0.32), CSF tau and PiB MCSUVR (r = 0.52, 95% CI 0.36–0.65), and CSF Aβ42 and global cognition (r = 0.22, 95% CI 0.05–0.39).
Table 3.
Biomarker correlations (95% confidence interval) and p value on the baseline values, without (the first 4 numbers of each cell) and with (the last 4 numbers of each cell) adjusting for the effects of APOE4, family history, and baseline age
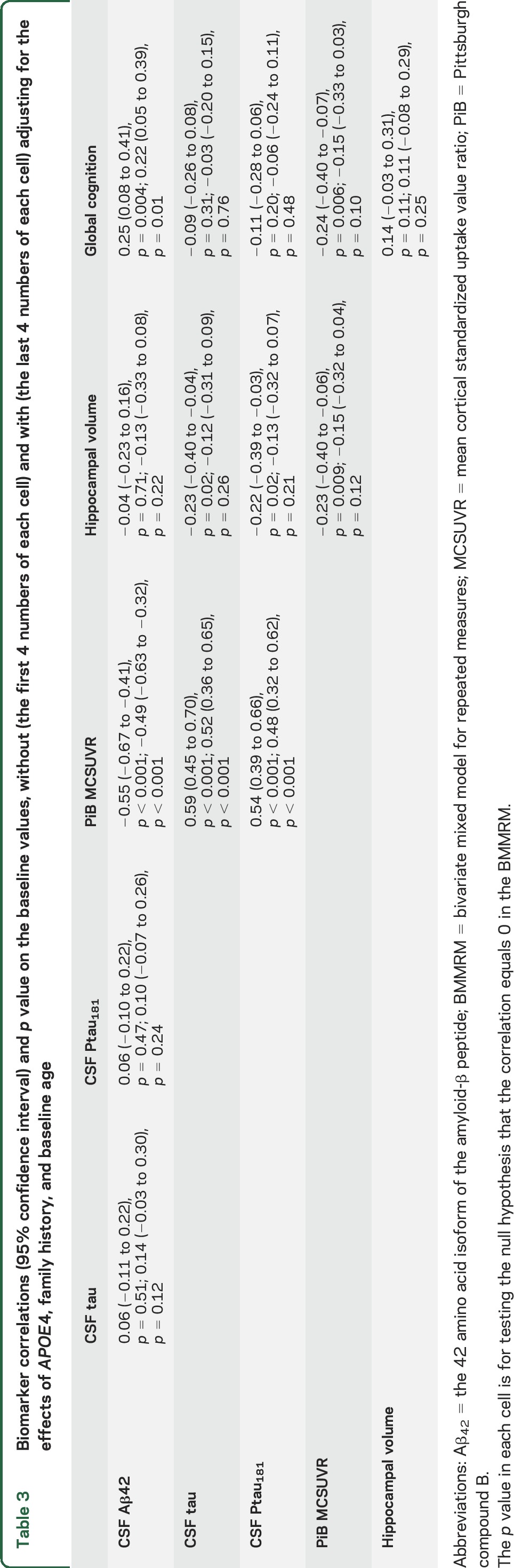
Figure 1. Graphical presentation of longitudinal and cross-sectional (i.e., at baseline) biomarkers correlations across multiple modalities.
Correlations are after adjusting for the effects of APOE4, family history, and baseline age. Solid lines identify significant longitudinal correlations on the rates of change, and dashed lines significant cross-sectional correlations at baseline. Markers that do not significantly correlate are not connected by lines; + and − represent positive and negative correlations, respectively. PiB = Pittsburgh compound B.
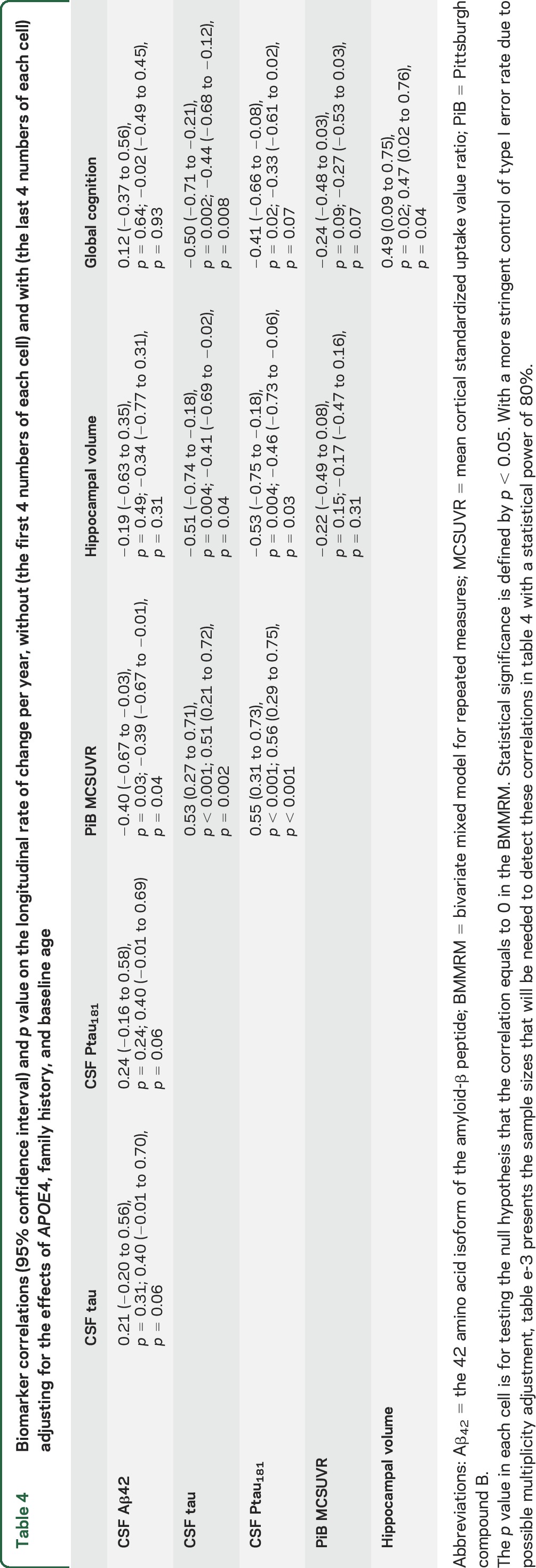
Table 4 presents the estimated correlations on the longitudinal rates of change for the same biomarker and cognitive measures (see tables e-1 through e-4 on the Neurology® Web site at Neurology.org for the estimated rates of changes and figures e-1 and e-2 for plots). Results for MCSUVR with and without correction for partial volume effects28 were very similar, so only those without correction are presented. The solid lines between 2 biomarkers in figure 1 represent statistically significant correlations on the rates of change (p < 0.05), whereas biomarkers without solid line connections are not significantly correlated in their longitudinal changes. The rate of change in CSF Aβ42 was correlated with the rate of change in PiB MCSUVR (r = −0.40, 95% CI −0.67 to −0.03), indicating that among asymptomatic middle-aged to older individuals, a faster decrease of CSF Aβ42 over time is associated with a faster increase of PiB MCSUVR. The rate of change in CSF Aβ42 was not significantly correlated with that of CSF tau (or Ptau181), hippocampal volume, or global cognition. Longitudinal rate of change in CSF tau, however, was correlated with longitudinal rate of change in PiB MCSUVR (r = 0.53, 95% CI 0.27–0.71), hippocampal volume (r = −0.51, 95% CI −0.74 to −0.18), and global cognition (r = −0.50, 95% CI −0.71 to −0.21). Similar significant correlations were also observed between the longitudinal rate of change in CSF Ptau181 and that in PiB MCSUVR, hippocampal volume, and global cognition. Further, the rate of change in hippocampal volume was not significantly correlated with that in PiB MCSUVR (r = −0.22, 95% CI −0.49 to 0.08), but was correlated with the rate of change in global cognition (r = 0.49, 95% CI 0.09–0.75). In contrast, the rates of change in PiB MCSUVR and global cognition were not significantly correlated (r = −0.24, 95% CI −0.48 to 0.03). The correlations of biomarkers on the rate of change after adjusting for the effects of FH, baseline age, and APOE4 were consistent with those from the unadjusted analyses (table 4). Additional sensitivity analyses by further adjusting for the effect of sex, education, hypertension, diabetes, medications, and the length of follow-up as well as by excluding subjects who were biomarker positive at baseline or progressed to higher CDR resulted in largely consistent estimates of the biomarker correlations on the rate of longitudinal change. Finally, baseline values of CSF Aβ42, CSF tau, Ptau181, PiB MCSUVR, and hippocampal volume were all significantly associated with the longitudinal rate of global cognition (table e-2).
Table 4.
Biomarker correlations (95% confidence interval) and p value on the longitudinal rate of change per year, without (the first 4 numbers of each cell) and with (the last 4 numbers of each cell) adjusting for the effects of APOE4, family history, and baseline age
DISCUSSION
Clinicopathologic studies demonstrate that asymptomatic elderly individuals can manifest the neuropathologic changes of AD, notably senile plaques and neurofibrillary tangles (NFT).2,4,35 Such neuropathologic changes may begin in middle age.1–3 In the absence of clinical and cognitive symptoms, biomarkers can be an effective tool to measure disease progression so that early interventions can be tested and developed. Ongoing secondary prevention trials including the Anti-Amyloid Treatment in Asymptomatic Alzheimer's (A4) trial, the Dominantly Inherited Alzheimer Network Trials Unit (DIAN TU) trials, and the Alzheimer's Prevention Initiative trial all depend on the ability to recruit asymptomatic individuals with the highest probability of manifesting measurable, reliable cognitive changes over a given study period. Understanding the longitudinal relationship among biomarkers and cognitive measures is of paramount importance.
The cross-sectional associations across established AD biomarkers have been well-characterized, especially in elderly individuals 65 years or older with and without clinical symptoms of AD.8–10 It remains unknown, however, how and to what degree the longitudinal changes of these biomarkers are correlated in asymptomatic middle-aged to older individuals. To our knowledge, our report represents the first attempt to address this question with all major AD biomarker modalities. Our findings reveal that the longitudinal rates of change in different modalities of markers and cognition are already correlated among asymptomatic middle-aged to older individuals. Specifically, the rates of change in CSF biomarkers (Aβ42, tau, and Ptau181) were all correlated with that of PET PiB MCSUVR, which was then correlated with the rate of change in hippocampal volume. Furthermore, the rates of change in CSF tau, Ptau181, and MRI hippocampal volume were also correlated with the rate of cognitive decline. These correlations establish the longitudinal validity of CSF tau and Ptau181 and MRI volumetrics as prognostic biomarkers for cognitive decline in preclinical AD, and also imply the validity of PET PiB MCSUVR and CSF Aβ42 in tracking early disease progression years prior to symptomatic onset. Thus, these findings support the current design of ongoing secondary prevention trials of AD in which biomarkers are either the primary inclusion and exclusion criteria (the A4 trial) or the primary efficacy outcomes (DIAN TU) prior to a critical phase III trial that will utilize a cognitive endpoint.
Perhaps some of the most important findings from our study are the nonsignificant correlations from a relatively large cohort of asymptomatic middle-aged to older individuals. It has been well-established that senile plaques and NFT are the hallmark neuropathologies of AD. Essentially all brains from individuals who die with late-onset AD exhibit both pathologies at autopsy,36 and significant pathologic overlap also exists among individuals who died with absence of clinical symptoms.5 Yet we found no significant correlations, either cross-sectionally or longitudinally, between CSF Aβ42 and CSF tau (or Ptau181) in the asymptomatic middle-aged to older individuals, suggesting statistically independent early Aβ- and tau-related pathologic processes during early disease stages, consistent with our prior models.37 One possible explanation for this lack of relationship is differential time windows within individuals during which one neuropathologic process has started whereas the other process remains either latent or only fluctuated in a random fashion independent of the former process, especially after adjusting for the effect of major covariates (FH, baseline age, and APOE4). It is also important to note that the observed correlation on the adjusted rate of change between CSF Aβ42 and CSF tau (or Ptau181) is fairly high (0.4). Hence the lack of relationship may be due to limited statistical power. Although the rate of change in CSF Aβ42 was not correlated with the rate of cognitive decline, the baseline values of CSF Aβ42 (table e-2), along with the rates of changes in CSF tau and Ptau181, were already correlated with that of cognition in the middle-aged to older asymptomatic individuals, suggesting that the changes in CSF tau and Ptau181 as well as the baseline levels of CSF Aβ42 all predict the changes in global cognition. Further, the rate of change in PiB MCSUVR is associated with the rate of change in all major CSF biomarkers including CSF Aβ42, tau, and Ptau181, but not in global cognition, supporting the argument that longitudinal changes in amyloid may precede that of cognition for a relatively long duration. Figure 1 demonstrates a pattern of longitudinally correlated biomarkers that is consistent with the hypothesized temporal orderings,38 i.e., the adjacent biomarkers from the hypothesized biomarker temporal orderings were correlated on their rates of changes, whereas biomarkers farther distant and not adjacent to each other were not significantly correlated. Although our findings support the recently proposed diagnostic criteria of preclinical AD,39 it is important to note that correlations on the rates of changes in biomarkers do not themselves indicate a temporal ordering of these markers.
In comparison, at baseline, the only significant correlations were between CSF Aβ42 and PiB MCSUVR, CSF tau (or Ptau181) and PiB MCSUVR, and CSF Aβ42 and global cognition. Although baseline CSF tau, Ptau181, and hippocampal volume were not correlated with baseline global cognition, their longitudinal rates of change were all significantly correlated with the rate of cognitive decline, long before the symptomatic onset of AD. Recently revised guidelines from the Food and Drug Administration for clinical trials in early-stage AD still mandate that treatments only be approved if they demonstrate cognitive and functional benefits. Our results suggest that, in order to adequately power future prevention trials of AD using cognitive outcomes, these trials may need to focus on individuals in the time window when their biomarker values in CSF tau, Ptau181, and hippocampal volume are starting to change, because these changes predict changes in cognition. Intriguingly, at baseline, CSF Aβ42 and global cognition were correlated, but their rates of longitudinal change were not. These observations suggest that, whereas CSF Aβ42 may be associated with cognition across middle-aged to older asymptomatic individuals at a single time point, the intraindividual change of CSF Aβ42 from baseline does not predict the intraindividual cognitive decline. Because these results are not entirely consistent with some of the previous reports,40 future studies are needed to fully understand the biological and behavioral mechanisms behind this observation.
The major strengths of the study include the wide baseline age starting from ∼43 years and large sample size of carefully characterized cognitively normal individuals for whom all major AD biomarkers were obtained longitudinally. The study also has limitations. First, the ACS is an observational study on a convenience sample. Unobserved factors could contribute to and confound the findings. Second, although the longitudinal follow-up was relatively long, it was not long enough to cover the entire preclinical disease course, thus preventing us from evaluating the cascade of early AD pathogenesis events in its entirety. It is also possible that there is a temporal lag in biomarker associations, which would require longitudinal changepoints or inflection points models over a relatively large number of longitudinal follow-ups. The ongoing longitudinal follow-up in the ACS cohort will provide much more insight to comprehensively understand the preclinical progression of AD.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the Genetics Core (Alison Goate, DPhil, Core Leader) of the Knight ADRC (P50 AG05681) for the APOE data.
GLOSSARY
- A4
Anti-Amyloid Treatment in Asymptomatic Alzheimer's
- Aβ42
the 42 amino acid isoform of the amyloid-β peptide
- ACS
Adult Children Study
- AD
Alzheimer disease
- BMMRM
bivariate mixed model for repeated measures
- CDR
Clinical Dementia Rating
- CI
confidence interval
- DIAN TU
Dominantly Inherited Alzheimer Network Trials Unit
- FH
family history
- LP
lumbar puncture
- MCSUVR
mean cortical standardized uptake value ratio
- NFT
neurofibrillary tangle
- PiB
Pittsburgh compound B
- ROI
region of interest
- SUVR
standardized uptake value ratio
- WMS
Wechsler Memory Scale
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
C.X., A.M.F., T.L.S.B., D.H., J.H., V.B., K.L.M., and J.C.M. all contributed to the design of the study. D.H. and J.H. contributed to the cognitive data collection and analyses. A.M.F. and C.L.S. contributed to the collection of CSF samples and CSF data analyses. T.L.S.B. contributed to imaging data processing and analyses. C.X., M.S.J., and E.G. contributed to statistical analyses and manuscript drafting. E.G. contributed to database analyses. All authors contributed to the critical review of the manuscript.
STUDY FUNDING
Supported by National Institute on Aging (NIA) grant P01 AG026276 (Dr. Morris) and the support of Fred Simmons and Olga Mohan. Additional support was provided by NIA R01 AG034119 (Dr. Xiong) and by the McDonnell Center for Systems Neuroscience (22 3922 26239N), NIH 5P30NS048056, NIH 2UL1TR000448.
DISCLOSURE
C. Xiong reports grants from the NIH during the conduct of the study. M. Jasielec and H. Weng report no disclosures relevant to the manuscript. A. Fagan reports personal fees from IBL International and personal fees from Roche, outside the submitted work. T. Benzinger reports grants and other from Avid Radiopharmaceuticals, other from Eli Lilly, and other from Hoffman La Roche, outside the submitted work. D. Head, J. Hassenstab, E. Grant, C. Sutphen, V. Buckles, and K. Moulder report no disclosures relevant to the manuscript. J. Morris reports grants from NIH P50AG005681, P01AG003991, P01AG026276, and U19AG032438 during the conduct of the study. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 1997;18:351–357. [DOI] [PubMed] [Google Scholar]
- 2.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol 1999;45:358–368. [DOI] [PubMed] [Google Scholar]
- 3.Kok E, Haikonen S, Luoto T, et al. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol 2009;65:650–657. [DOI] [PubMed] [Google Scholar]
- 4.Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early stage Alzheimer's disease. J Mol Neurosci 2001;17:101–118. [DOI] [PubMed] [Google Scholar]
- 5.Price JL, McKeel DW, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 2009;30:1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman RJ, Xiong C, Benzinger TL, et al. ; Dominantly Inherited Alzheimer Network. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med 2012;367:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katzman R. Editorial: the prevalence and malignancy of Alzheimer disease: a major killer. Arch Neurol 1976;33:217–218. [DOI] [PubMed] [Google Scholar]
- 8.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol 2006;59:512–519. [DOI] [PubMed] [Google Scholar]
- 9.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol 2009;65:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo RY, Hubbard AE, Shaw LM, et al. For the Alzheimer's disease neuroimaging initiative. Longitudinal change of biomarkers in cognitive decline. Arch Neurol 2011;68:1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coats M, Morris JC. Antecedent biomarkers of Alzheimer's disease: the adult children study. J Geriatr Psychiatry Neurol 2005;18:242–244. [DOI] [PubMed] [Google Scholar]
- 12.Xiong C, Roe CM, Buckles V, et al. Role of family history for Alzheimer biomarker abnormalities in the Adult Children Study. Arch Neurol 2011;68:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006;20:210–216. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed Washington, DC: American Psychiatric Association; 1997. [Google Scholar]
- 16.Wechsler D. Wechsler Memory Scale (3rd ed): Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 1977. [Google Scholar]
- 17.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology 1988;3:900–903. [DOI] [PubMed] [Google Scholar]
- 18.Peterson L, Peterson MJ. Short-term retention of individual verbal items. J Exp Psychol 1959;58:193–198. [DOI] [PubMed] [Google Scholar]
- 19.Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination Booklet, III, Oral Expression, J. Animal Naming (Fluency in Controlled Association). Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 20.Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psychological Monographs 1945;60:1–48. [Google Scholar]
- 21.Fagan AM, Xiong C, Jasielec MS, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer's disease. Sci Transl Med 2014;6:226ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage 2004;23:724–738. [DOI] [PubMed] [Google Scholar]
- 23.Mintun MA, LaRossa GN, Sheline YI, et al. [11C] PiB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006;67:446–452. [DOI] [PubMed] [Google Scholar]
- 24.Su Y, D'Angelo GM, Vlassenko AG, et al. Quantitative analysis of PiB-PET with FreeSurfer ROIs. PLoS One 2013;8:e73377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 26.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 27.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med 1998;39:904–911. [PubMed] [Google Scholar]
- 28.Su Y, Blazey TM, Snyder AZ, et al. Partial volume correction in quantitative amyloid imaging. NeuroImage 2015;107:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talbot C, Lendon C, Craddock N, Shears S, Morris JC, Goate A. Protection against Alzheimer's disease with apoE ε2 [letter]. Lancet 1994;343:1432–1433. [DOI] [PubMed] [Google Scholar]
- 30.Shah A, Laird NM, Schoenfeld DA. A random-effects model for multiple characteristics with possibly missing data. J Am Statist Assn 1997;92:775–779. [Google Scholar]
- 31.Fieuws S, Verbeke G. Joint modelling of multivariate longitudinal profiles: pitfalls of the random-effects approach. Stat Med 2004;23:3093–3104. [DOI] [PubMed] [Google Scholar]
- 32.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963–974. [PubMed] [Google Scholar]
- 33.Beckett LA, Tancredi DJ, Wilson RS. Multivariate longitudinal models for complex change processes. Stat Med 2004;23:231–239. [DOI] [PubMed] [Google Scholar]
- 34.Gao F, Miller JP, Xiong C, Beiser JA, Gordon M; The Ocular Hypertension Treatment Study (OHTS) Group. A joint-modeling approach to assess the impact of biomarker variability on the risk of developing clinical outcome. Stat Methods Appt 2011;20:83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006;66:1837–1844. [DOI] [PubMed] [Google Scholar]
- 36.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 2012;71:362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storandt M, Head D, Fagan AM, Holtzman DM, Morris JC. Toward a multifactorial model of Alzheimer disease. Neurobiol Aging 2012;33:2262–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vemuri P, Wiste HJ, Weigand SD, et al. , on behalf of the Alzheimer's Disease Neuroimaging Initiative. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology 2009;73:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.