Abstract
Objective:
To identify reliable predictors of outcome in comatose patients after cardiac arrest using a single routine EEG and standardized interpretation according to the terminology proposed by the American Clinical Neurophysiology Society.
Methods:
In this cohort study, 4 EEG specialists, blinded to outcome, evaluated prospectively recorded EEGs in the Target Temperature Management trial (TTM trial) that randomized patients to 33°C vs 36°C. Routine EEG was performed in patients still comatose after rewarming. EEGs were classified into highly malignant (suppression, suppression with periodic discharges, burst-suppression), malignant (periodic or rhythmic patterns, pathological or nonreactive background), and benign EEG (absence of malignant features). Poor outcome was defined as best Cerebral Performance Category score 3–5 until 180 days.
Results:
Eight TTM sites randomized 202 patients. EEGs were recorded in 103 patients at a median 77 hours after cardiac arrest; 37% had a highly malignant EEG and all had a poor outcome (specificity 100%, sensitivity 50%). Any malignant EEG feature had a low specificity to predict poor prognosis (48%) but if 2 malignant EEG features were present specificity increased to 96% (p < 0.001). Specificity and sensitivity were not significantly affected by targeted temperature or sedation. A benign EEG was found in 1% of the patients with a poor outcome.
Conclusions:
Highly malignant EEG after rewarming reliably predicted poor outcome in half of patients without false predictions. An isolated finding of a single malignant feature did not predict poor outcome whereas a benign EEG was highly predictive of a good outcome.
Out-of-hospital cardiac arrest is common1 and most patients remain unconscious after restoration of spontaneous circulation. Approximately half of these patients will die during the hospital stay, the majority from hypoxic-ischemic brain injury.2
Intensive care is usually continued until a prediction of poor outcome is made, based on clinical neurologic examination, commonly combined with other prognostic tools.3,4 EEG is the most widely used prognostic tool to support a clinical examination and is accessible in most hospitals5; it is recommended for both prognostication and ruling out subclinical seizures.6,7
There is no high-level evidence for predicting poor prognosis using EEG and meta-analysis of available data is confounded by the wide variety of classification systems used.8–10 Other drawbacks of EEG include interrater variability11,12 and the confounding effects of ongoing or residual sedation.
The American Clinical Neurophysiology Society (ACNS) has proposed a standardized critical care EEG terminology13 to facilitate multicenter studies and maximize interrater reliability. We used this recently revised terminology to evaluate prospectively recorded EEGs in the Target Temperature Management (TTM) trial, which randomized 950 patients to 33°C vs 36°C controlled temperature after cardiac arrest and reported no differences in mortality or neurocognitive function.14,15
Using the ACNS terminology, we defined highly malignant EEG patterns16 and found substantial interrater and intrarater agreement.11 The main objective of this study was to evaluate whether the proposed highly malignant EEG patterns reliably predict a poor neurologic outcome for patients who remain in a coma after cardiac arrest. Moreover, we explored the prognostic performance of malignant and benign EEG patterns.16
METHODS
The TTM trial randomized 950 adult comatose patients resuscitated after out-of-hospital cardiac arrest of presumed cardiac cause from November 2010 to January 2013.
Standard protocol approvals, registrations, and patient consents.
The trial protocol17 was approved by the ethics committees in the participating countries and registered at ClinicalTrials.gov (NCT01020916). Consent was obtained from a legal surrogate and from each patient regaining mental capacity.
Sedation was mandatory during temperature management and tapered at normothermia if not needed for intensive care reasons or treatment of status epilepticus.17
A routine EEG was performed during office hours 12–36 hours after rewarming in patients who were still comatose, typically corresponding to 48–72 hours after the cardiac arrest or later if this period coincided with a weekend. The detailed rationale of the present EEG study, including the prespecified hypotheses, was published previously.16 All 36 intensive care units in Europe and Australia participating in the TTM trial performed routine EEGs.
For this EEG study, we included all patients from sites that had an EEG system allowing EEG data export including notations on reactivity testing. Patients who awoke or died before the recommended time point of EEG were excluded. Sedation, antiepileptic medication, and level of consciousness were documented prospectively.14,17
At 72 hours after rewarming, a physician blinded to target temperature, but not to the local EEG report, performed a neurologic evaluation and recommended continuation or withdrawal of life-sustaining therapy (WLST).17–19 Persisting deep coma (Glasgow Coma Scale: motor 1–2) was a criterion of poor prognosis and allowed WLST if combined with either bilateral loss of somatosensory evoked potentials (SSEP) N20 responses or treatment-refractory status epilepticus.17 Treatment refractory was defined as unresponsive to propofol, midazolam, or thiopental in combination with at least 1 IV antiepileptic substance for at least 24 hours. In addition, a clinical finding of early status myoclonus, regardless of EEG correlate, in combination with bilateral loss of N20 potentials after rewarming was a criterion of poor prognosis. In patients not fulfilling these criteria, continued active intensive care was protocolized and patients reevaluated daily. The details and rationale of the prognostication algorithm and how WLST was implemented in the trial was published.14,17–19
EEGs were retrieved as electronic data format files to a central EEG database and analyzed using the ACNS standardized terminology,13 independently by 4 EEG specialists (Sweden: E.W., Denmark: T.W.K., The Netherlands: A.-F.v.R., and Switzerland: A.O.R.). The EEG specialists were blinded to all clinical data including outcome and reported EEG findings through a Web-based electronic case report form structured to assure complete data. The EEGs were full-length (>20 minutes) and at least 16 EEG channels were used. Based on the findings regarding periodic or rhythmic patterns, background pattern, and reactivity, the EEGs were classified according to the prespecified hypotheses into the following categories:
-
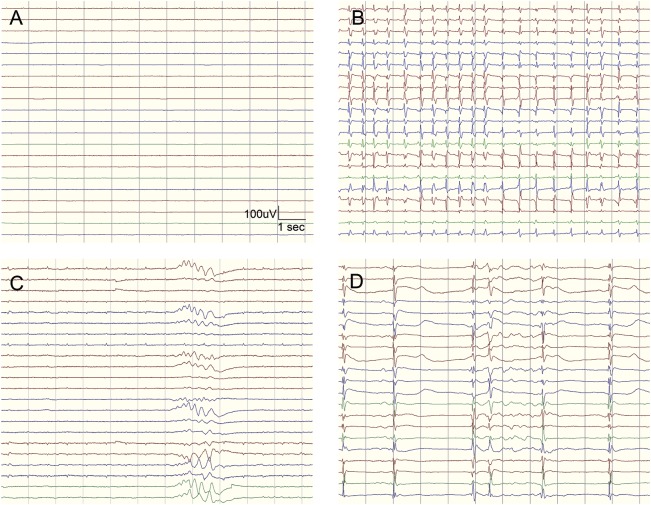
Highly malignant EEG (figure 1)
Suppressed background without discharges
Suppressed background with continuous periodic discharges
Burst-suppression background with or without discharges
-
Malignant EEG
Malignant periodic or rhythmic patterns (abundant periodic discharges; abundant rhythmic polyspike-/spike-/sharp-and-wave; unequivocal electrographic seizure)
Malignant background (discontinuous background; low-voltage background; reversed anterior-posterior gradient)
Unreactive EEG (absence of background reactivity or only stimulus-induced discharges)
Benign EEG (absence of all malignant features stated above)
Figure 1. Highly malignant EEG patterns.
Highly malignant patterns used in the study defined according to the standardized EEG terminology by the American Clinical Neurophysiology Society. (A) Suppressed background (amplitude <10 μV, 100% of the recording) without discharges. (B) Suppressed background with superimposed continuous periodic discharges. (C) Burst-suppression (periods of suppression with amplitude <10 μV constituting >50% of the recording) without discharges. (D) Burst-suppression with superimposed discharges.
Surviving patients attended a follow-up 180 days after their cardiac arrest by a blinded assessor.14 Outcome was dichotomized according to the Cerebral Performance Category scale (CPC).20 Good neurologic outcome was defined as CPC 1–2 (no or moderate disability) at follow-up or at any time during the hospital stay. Poor outcome was defined as best achieved CPC of 3–5 (severely disabled, comatose, or deceased).
Prognostic ability (specificity, sensitivity) was calculated for the highly malignant patterns (primary hypothesis) and the other prespecified patterns (secondary hypotheses) and is presented as the mode value for the 4 interpreters, i.e., the pattern that 2 or more interpreters have reported, with 95% confidence intervals (CI). If 2 interpreters reported 1 pattern and the other 2 interpreters reported another, the most malignant pattern was assigned.
For comparisons among temperature groups, sedation, and EEG patterns, we applied generalized linear mixed models including interpretations from all 4 EEG specialists.
Statistical analysis was performed using R, version 3.0.2.
RESULTS
Eight study sites randomized 202 patients to targeted temperature management at 33°C (n = 103) or 36°C (n = 99). A routine EEG was recorded at a median 77 hours (interquartile range 53–102) after cardiac arrest in 103 patients still comatose after rewarming.
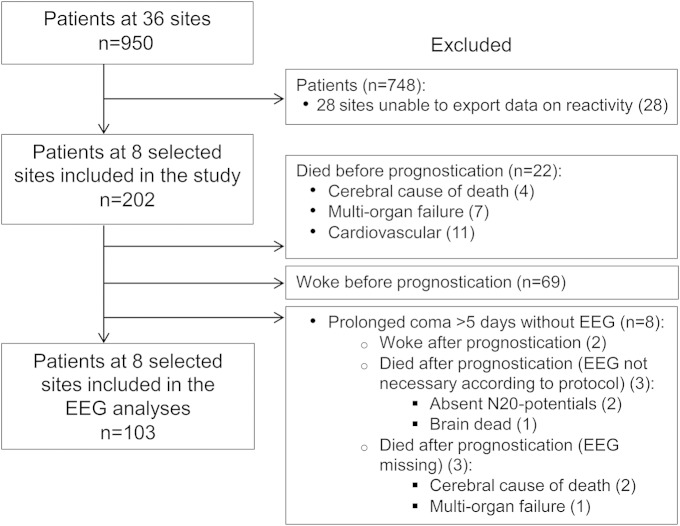
Exclusion and inclusion in the present study is described in figure 2. In 99 of the 202 randomized patients, 69 of whom awoke and 22 died before the recommended time point of prognostication, EEGs were not performed.
Figure 2. Study flow chart of exclusion from and inclusion into the study.
The recommended time point of prognostication was 72 hours after rewarming, corresponding to approximately 108 hours after the cardiac arrest. The most probable cause of death according to the treating physician is reported (cerebral, multiorgan failure, or cardiovascular). Eight study sites were included since they had an EEG system that allowed export of EEG data that included notations regarding testing of reactivity.
Eight patients with prolonged coma (>5 days) did not have an EEG. One of these was brain dead and 2 had absent N20 potentials. In the remaining 5 patients, the reason for missing EEG was unclear. Two of these patients did not have WLST and eventually recovered to CPC 3, 1 died on day 5 of cerebral cause without WLST, 1 died on day 6 due to multiorgan failure without WLST, and 1 died on day 5 of cerebral cause after WLST.
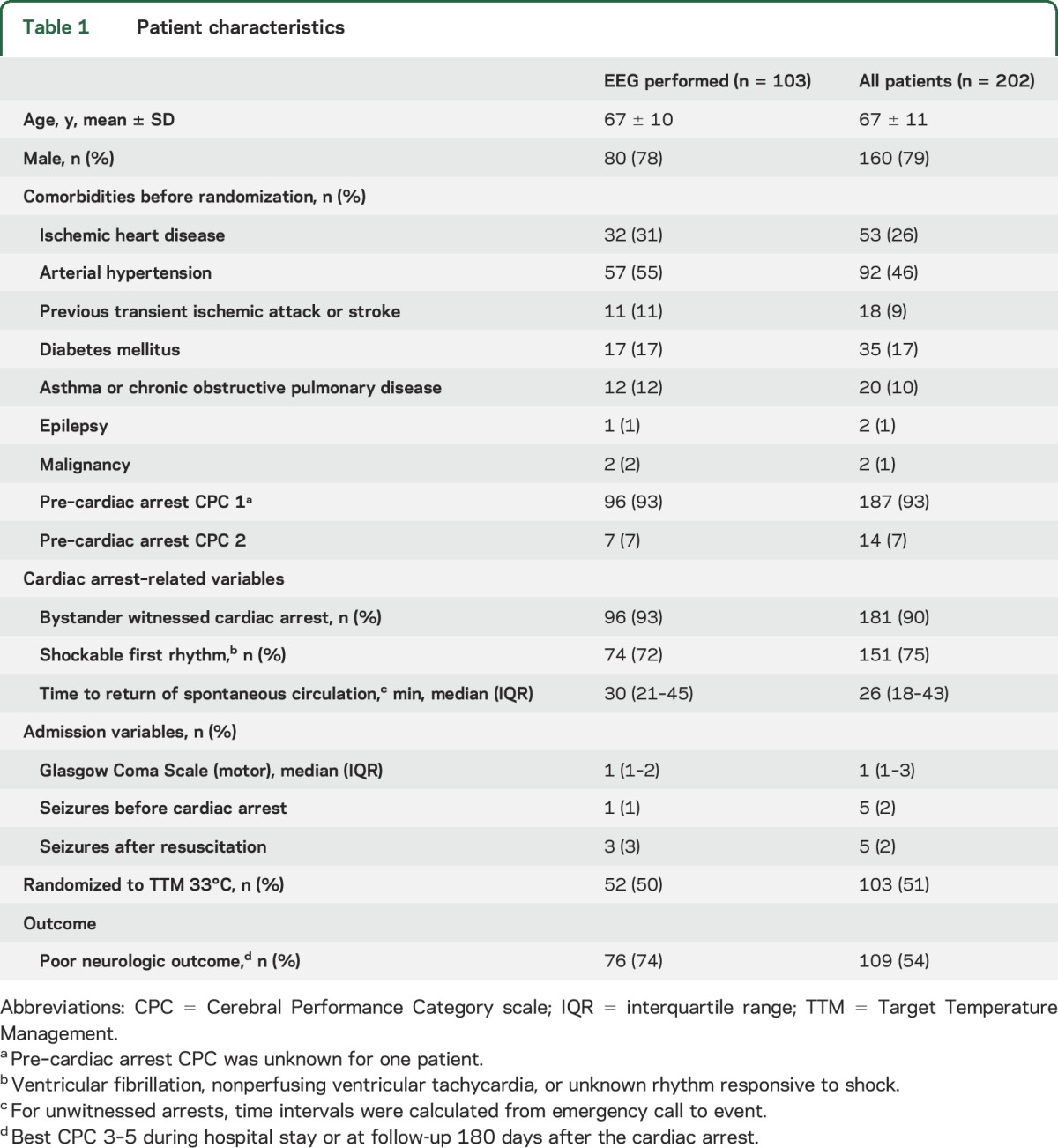
The patients' characteristics are presented in table 1. Patients with EEG were more likely to have a poor outcome.
Table 1.
Patient characteristics
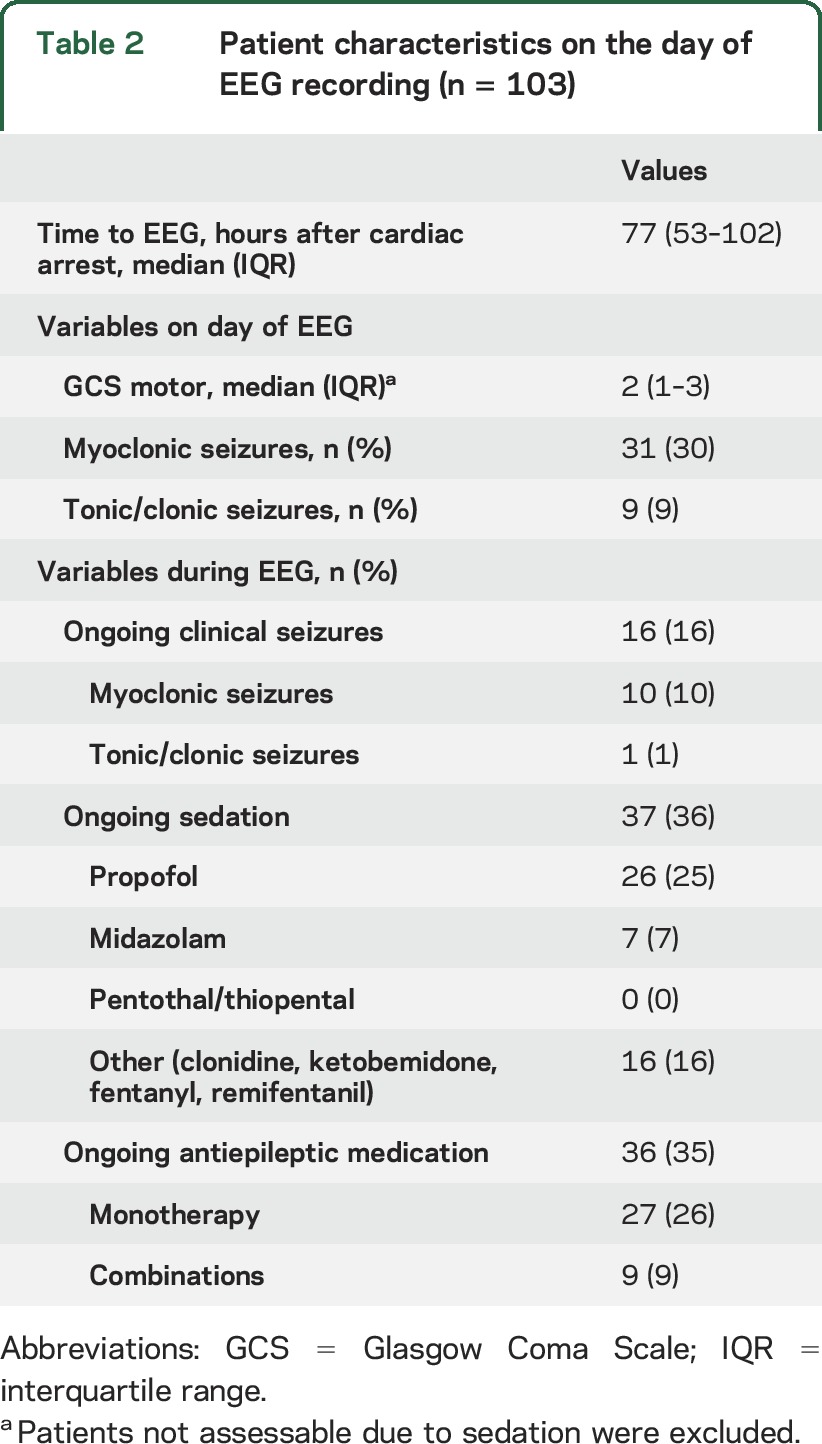
Characteristics of the patients on the day of EEG registration are presented in table 2. At this time point the majority were deeply comatose. Clinical seizures occurred during the EEG recording in 16%, mostly myoclonus, and 35% of patients had antiepileptic treatment.
Table 2.
Patient characteristics on the day of EEG recording (n = 103)
Highly malignant EEG.
The prognostic ability of the highly malignant patterns is presented in table 3 (primary hypotheses). A highly malignant pattern was reported by the majority of the 4 interpreters (mode value) in 38 patients (37%) and all had a poor neurologic outcome. The sensitivity for a highly malignant pattern to predict a poor outcome was 50% (mode value). Sensitivity varied among the 4 interpreters but specificity was 100% for all individual interpreters (table e-1 on the Neurology® Web site at Neurology.org).
Table 3.
Ability of highly malignant and malignant patterns to predict poor outcome
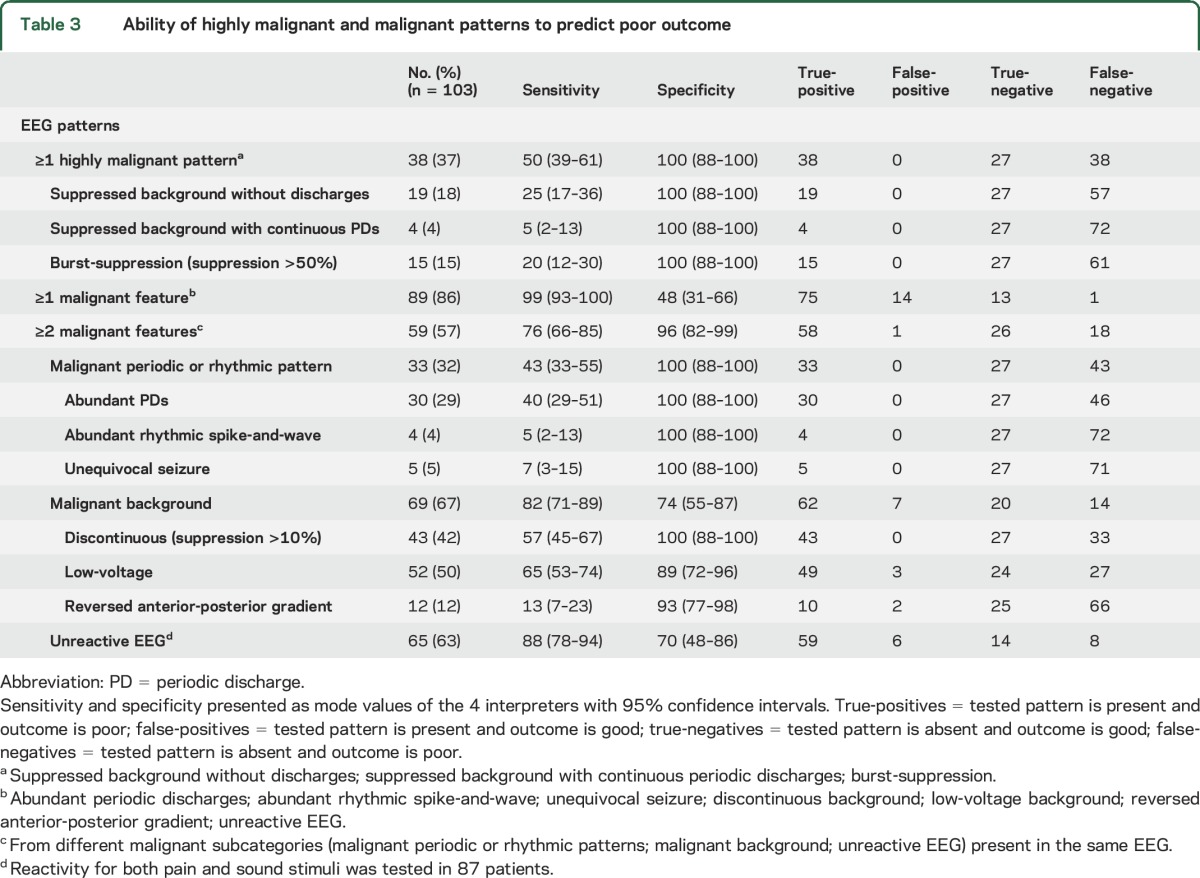
Malignant EEG.
The prognostic ability of the malignant features is presented in table 3. A malignant EEG, defined as presence of at least one malignant feature and thus also including the highly malignant patterns, was reported in 89 patients (86%). The sensitivity to predict a poor outcome was 99% with a specificity of 48% (mode values). Sensitivity and specificity varied considerably among the 4 interpreters (table e-1). Regarding the subcategories of malignant features, any malignant periodic or rhythmic pattern showed a higher specificity (100%) to predict a poor outcome compared to any malignant background pattern (74%) or a nonreactive EEG (70%). An isolated finding of a single malignant feature occurred in 30 patients, 17 of whom had a good outcome.
If at least 2 of the 3 malignant subcategories were present in the same EEG, the specificity to predict a poor outcome increased significantly to 96% (p < 0.001), while sensitivity decreased to 76% (p < 0.001).
Benign EEG.
Fourteen patients (14%) had a benign EEG, defined as a lack of all malignant features. Of these, 13 (93%) had a good outcome. A benign EEG was found in 1% (95% CI 0%–7%) of the 76 patients with a poor outcome and in 48% (95% CI 31%–66%) of the 27 patients with a good outcome.
Eight patients (8%) had a benign and reactive EEG, defined as absence of all malignant features and presence of reactivity to either sound or pain stimuli, and none of these patients had a poor outcome. A benign and reactive EEG was found in none (95% CI 0%–5%) of the patients with a poor outcome and in 30% (95% CI 16%–49%) of the patients with a good outcome.
The ability for a benign EEG to predict good outcome did not significantly differ compared to that of a benign and reactive EEG (p = 0.08) in this small sample size.
Level of target temperature management.
There were no significant differences between patients treated with 33°C compared to 36°C regarding prevalence or prognostic ability of highly malignant EEG (prevalence p = 0.58; sensitivity p = 0.58; specificity p = not applicable) or malignant EEG (prevalence p = 0.17; sensitivity p = 0.64; specificity p = 0.81).
Sedation.
Ongoing or residual sedation affected the level of consciousness in 56% of the patients on the day of the routine EEG, according to the treating physician. In 36%, sedation was ongoing during recording of the EEG. There were no significant differences regarding prognostic ability of highly malignant EEG (sensitivity p = 0.81; specificity p = not applicable) or malignant EEG (sensitivity p = 0.44; specificity p = 0.21) between patients with ongoing sedation and those without ongoing sedation.
DISCUSSION
EEG is a standard investigation to assess prognosis after cardiac arrest, but its reliability may be limited by lack of consensus on definitions of malignant patterns8–10 and by interrater variability.11,12 We found that the proposed highly malignant EEG patterns reliably predicted a poor outcome in our cohort of comatose post–cardiac arrest patients. These patterns were previously found to have substantial interrater and intrarater reliability11 and the sensitivity to detect patients with poor prognosis was 50%, exceeding that of SSEP and absent ocular reflexes.19 Since routine EEG is a generally available, noninvasive examination, these findings, if verified in a different cohort, may enhance the safe use of EEG as a key component in decisions on continuation or withdrawal of life-sustaining therapy. Nevertheless, it is strongly advisable to combine the EEG findings with other tests to perform a multimodal prognostication.7
Standardization of EEG interpretation is critical for reproducibility of clinical studies, meta-analyses, and application of these results into everyday clinical practice. Therefore, the use of an international well-defined terminology, such as the ACNS EEG terminology, is strongly recommended.
The highly malignant patterns used in this study were strictly defined according to the ACNS EEG terminology and adhere closely to previously described malignant patterns.8–10
Our secondary hypothesis that a malignant EEG should nearly always be associated with a poor outcome16 was discarded due to lack of specificity and considerable variability between interpreters. Our results therefore discourage the use of isolated findings of a malignant feature in decisions on WLST. Combination of at least 2 malignant features from diverse malignant subcategories significantly increased the specificity to 96%, indicating that such combinations, if confirmed in another cohort, may be of use to predict a poor prognosis.
Low-voltage background (most background activity <20 μV) was a common pattern with several false-positive predictions, in contrast to the highly malignant pattern of suppressed background (all background activity <10 μV), which had a specificity of 100% for predicting poor outcome. This is of considerable importance since 20 μV has been used as a limit of the background voltage for predicting a poor outcome.8–10,21,22
Our results highlight that the degree of discontinuity is an important predictor of prognosis. A discontinuous background (suppression periods constituting 10%–49%) resulted in some false-positives (for 2 individual interpreters) while a burst-suppression background (suppression periods constituting 50%–99%) was a reliable predictor of poor prognosis for all 4 interpreters. This is in concordance with a recent retrospective single-center study using the same standardized ACNS terminology that found a zero false-positive rate for burst-suppression to predict a poor outcome.22 However, that study also reported no false-positives for a low-voltage background 24 hours after cardiac arrest or later, which was not the case in our study using 4 interpreters from different centers.
A proposed malignant pattern is the alpha-theta coma pattern,23–25 which has a nonreactive background with a reversed anterior-posterior gradient (pathologic distribution of the background activity). A reversed anterior-posterior gradient could per se not reliably predict a poor outcome in our study.
There is no consensus on the definition of postanoxic electrographic seizure activity, but several investigators using different definitions have shown a strong correlation to poor outcome.26–30 All 5 patients who had unequivocal electrographic seizure activity during the limited time period of the routine EEG had a poor outcome in this study. In contrast, abundant periodic discharges were not inevitably associated with a poor outcome for the individual interpreters (table e-1), but no patients with periodic discharges on a suppressed background (<10 μV) according to any interpreter had a good outcome.
The treatment of epileptiform activity was not defined in the protocol and not included in the intervention of this trial. Whether patients with electrographic status epilepticus may benefit from antiepileptic medication is unclear and currently addressed in a separate randomized trial.31 Since continuous EEG monitoring was not available in most centers of this study, it is likely that some patients with intermittent electrographic seizures were missed.
It is well-known that EEG activity is affected by sedation32 and in sufficient doses it may cause burst-suppression, but whether sedation in clinically used doses affects the prognostic value of malignant EEG patterns is unknown. Lacking detailed data on the amount of sedation used, we found that the majority of patients were still considered affected by sedation by the reporting physician at the time of EEG, which is close to the recommended time point for neurologic prognostication in recent guidelines.7 However, the predictive ability of our malignant patterns was not significantly affected by ongoing sedation.
Background reactivity was previously found to have strong prognostic implications in several single-center studies.23,33–35 We found only fair interrater agreement for this variable among our 4 interpreters.11 It is therefore not surprising that the specificity for an unreactive EEG varied considerably among our interpreters. False predictions of a poor outcome ranged from none to the majority of patients among the interpreters. This might reflect different traditions in assessing reactivity in different centers, since the interpreter who had the highest specificity also had the lowest sensitivity and vice versa. A more conservative approach when stating absence of reactivity by the former interpreter compared to the latter may partly explain the differences. More strict definitions, standardized stimuli, video recordings, bedside assessments, use of muscle relaxants, or quantitative measurements36 could possibly improve agreement and specificity. Awareness of the limitations of reactivity as a prognostic tool is important.
We termed EEGs that lacked any malignant features as benign, according to our prespecified definitions. The proportion of patients with a poor outcome who had a benign EEG was very low (1%), which is an important finding since few markers indicating a good prognosis are available. However, it is important to recognize that many patients with good outcome lacked a benign EEG due to presence of some malignant EEG features and that the definition of good outcome includes a significant proportion of patients with mild cognitive impairment.37
An important limitation of our study is that the local EEG report was available at the neurologic evaluation. Although treatment-refractory postanoxic status epilepticus was the only EEG criterion that justified withdrawal of treatment according to the TTM trial protocol,17 other EEG features in the local EEG report may have had influence, causing a self-fulfilling prophecy. Importantly, all 4 EEG readers in this study were blinded to outcome and all clinical data.
Another limitation is that our EEG investigations are limited to patients who failed to wake up when the active temperature control phase was over. Thus we lack data on spontaneously awakening patients, but we hypothesize that the occurrence of malignant EEG patterns would be highly unlikely in awake patients. Exclusion of early awakening patients with favorable prognosis38 explains the worse prognosis among patients with EEG recordings in our study. Yet this cohort is likely to be representative of comatose patients eligible for an EEG for prognostication after cardiac arrest.
The power of the study is limited by the exclusion of sites that could not export EEG data of reactivity. This selection of centers allows for homogenous high-quality data and the possibility to confirm findings, apart from reactivity, in the remaining cohort. We recruited our EEG specialists from different countries and centers without prior collaboration, which would increase the generalizability of our results. We recognize that all 4 EEG readers are specialists in clinical neurophysiology with 10–14 years of experience in reading EEGs and that we cannot conclude on the transferability of our results to less experienced readers.
All EEGs were performed after rewarming and the majority within 2–4 days after the cardiac arrest and we stress that our results are representative for this time period only. It has previously been shown that some of our malignant patterns can occur during the early phase after resuscitation and ongoing target temperature management among survivors with good outcome.26,39
A highly malignant EEG pattern, defined according to the standardized ACNS EEG terminology, predicted poor outcome in half of patients who remained in a coma after rewarming without false-positives. If replicated in a larger cohort, ideally with blinding of the EEGs to the treating physician, these patterns are promising candidates to be included in a multimodal prognostication algorithm. On the other hand, an isolated finding of a single malignant feature could not be used to predict poor outcome. For these patients, prognostication must rely on other methods. A benign EEG was conversely highly predictive of a good functional outcome. Neither the level of temperature management nor ongoing sedation in clinically used doses significantly affected the prognostic ability of these patterns.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the EEG laboratories of the 8 sites that contributed EEG data: Academic Medical Center, the Netherlands; Haukeland University Hospital Bergen (Harald Aurlien), Norway; Skane University Hospital Lund, Skane University Hospital Malmö, Helsingborg Hospital, and Örebro University Hospital (Gunilla Ahlsén), Sweden; and St. Gallen (Dominique Flügel) and La Chaux de Fonds (Philippe Olivier), Switzerland.
GLOSSARY
- ACNS
American Clinical Neurophysiology Society
- CPC
Cerebral Performance Category scale
- CI
confidence intervals
- SSEP
somatosensory evoked potentials
- TTM
Target Temperature Management
- WLST
withdrawal of life-sustaining therapy
Footnotes
Supplemental data at Neurology.org
Editorial, page 1470
Contributor Information
Collaborators: TTM-trial investigators, Manoj Saxena, Jennene Miller, Deborah Inskip, Lewis Macken, Simon Finfer, Noel Eatough, Naomi Hammond, Frances Bass, Elizabeth Yarad, Anne O'Connor, Simon Bird, Timothy Jewell, Gareth Davies, Karl Ng, Sharon Coward, Anders Åneman, Antony Stewart, Sharon Micallef, Sharyn Parker, Dennis Cortado, Sharon Micallef, Ann Gould, Meg Harward, Kelly Thompson, Naomi Hammond, Parisa Glass, John Myburgh, Ondrej Smid, Jan Belholavek, Marketa Kreckova, Ales Kral, Jan Horak, Michal Otahal, Jan Rulisek, Jan Malik, Martin Prettl, Jesper Kjaergaard, Christian Hassager, Michael Wascher, Soeren Boesgaard, Jacob E Moller, John Bro-Jeppesen, Ane Loof Johansen, Vincenzo Campanile, Alberto Peratoner, Francesca Verginella, Daniele Leone, Thomas Pellis, Andrea Roncarati, Eliana Franceschino, Anna Sanzani, Alice Martini, Micol Perlin, Paolo Pelosi, Iole Brunetti, Angelo Insorsi, Stefano Pezzato, Giorgio De Luca, Emanuela Gazzano, Gian Andrea Ottonello, Andrea Furgani, Rosanna Telani, Simona Maiani, Pascal Stammet, Christophe Werer, Jaqueline Kieffer, Janneke Horn, Annelou L vd Veen, Tineke Winters, Nicole P Juffermans, Michael Kuiper, PH Egbers, EC Boerma, RT Gerritsen, H Buter, C de Jager, F de Lange, M Loos, PM Koetsier, WP Kingma, N Bruins, L de Kock, M Koopmans, Frank Bosch, Monique AM Raaijmakers, SWL Metz-Hermans, Henrik Endeman, Saskia Rijkenberg, Addy Bianchi, Jan Hovdenes, Jan Frederik Bugge, Hilde Norum, Andreas Espinoza, Viesturs Kerans, Helene Brevik, Morten Svalebjørg, Guro Grindheim, Arne Jan Petersen, Andreas Baratt-Due, Jon Henrik Laake, Ulrik Spreng, Marte Marie Wallander Karlsen, Jørund Langøren, Rune Fanebust, Marianne Sætrang Holm, Stine Iren Flinterud, Carsten Wickman, Jesper Johnsson, Florian Ebner, Nerida Gustavsson, Heléne Petersson, Jörgen Petersson, Faezheh Nasiri, Frida Stafilidou, Kristine Edqvist, Sven Uhlig, Gunilla Sköld, Johan Sanner, Jesper Wallskog, Nicholas Wyon, Martin Golster, Anders Samuelsson, Carl Hildebrand, Taichi Kadowaki, Jessica Larsson-Viksten, Lina De Geer, Patrik Hansson, Henrik Appelberg, Anders Hellsten, Susanne Lind, Malin Rundgren, Thomas Kander, Johan Persson, Martin Annborn, Anne Adolfsson, Ingrid Corrigan, Tobias Cronberg, Hans Friberg, Irina Dragancea, Johan Undén, Marina Larsson, Michelle Chew, Mårten Unnerbäck, Per Petersen, Anna Svedung-Rudebou, Robert Svensson, Hilde Elvenes, Carl Bäckman, Christian Rylander, Patrik Martner, Louise Martinell, Björn Biber, Marita Ahlqvist, Caisa Jacobson, Marie-Louise Forsberg, Roman Desta Lindgren, Fatma Bergquist, Anders Thorén, Martin Fredholm, Johan Sellgren, Lisa Hård af Segerstad, Mikael Löfgren, Ingvor Gustavsson, Christina Henström, Marita Ahlqvist, Bertil Andersson, Karin Thiringer, Nadja Rydholm, Stefan Persson, Jawad Jawad, Ingela Östman, Ida Berglind, Eric Bergström, Annika Andersson, Cathrine Törnqvist, Yvan Gasche, Nubia Lafayete Marques de Mello, Valérie Gardaz, Gian-Reto Kleger, Claudia Schrag, Edith Fässler, Hervé Zender, Matthew Wise, Nicki Palmer, Jen Fouweather, Jade M Cole, Eve Cocks, Paul J Frost, Anton G Saayman, Tom Holmes, Christopher D Hingston, Gareth M Scholey, Helen Watkins, Stephen Fernandez, Andrew Walden, Jane Atkinson, Nicola Jacques, Abby Brown, Julius Cranshaw, Peter Berridge, Robert McCormick, Martin Schuster-Bruce, Michelle Scott, Nigel White, Emma Vickers, Guy Glover, Marlies Ostermann, Paul Holmes, Michael Koutroumanidis, Katie Lei, Barnaby Sanderson, John Smith, Nawaf Al-Subaie, Matthew Moore, Paul Randall, Johannes Mellinghoff, Azul Forti Buratti, Chris Ryan, Jonathan Ball, and Gaynor Francis
AUTHOR AFFILIATIONS
Divisions of Clinical Neurophysiology (E.W., I.R.), Intensive and Perioperative Care (H.F.), and Neurology (T.C.), Department of Clinical Sciences, Lund University, Sweden; Department of Neurology (A.O.R.), CHUV and University of Lausanne, Switzerland; Departments of Neurology/Clinical Neurophysiology (A.-F.v.R.) and Intensive Care Medicine (J. Horn), Academic Medical Center, University of Amsterdam, the Netherlands; Neurophysiology Center (T.W.K.), Roskilde University Hospital, Roskilde, Denmark; R&D Centre Skane (S.U.), Skane University Hospital, Lund; Department of Anaesthesia and Intensive Care (N.N.), Intensive Care Unit, Helsingborg Hospital, Sweden; Department of Intensive Care (A.Å.), Liverpool Hospital, Sydney, New South Wales, Australia; Department of Clinical Sciences (D.E.), Lund University, Sweden; Department of Anesthesiology, Pharmacology and Intensive Care (Y.G.), Geneva University Hospital, Switzerland; Departments of Cardiology (C.H., J.K.) and Cardiothoracic Anesthesiology (M.W.), The Heart Centre, and Copenhagen Trial Unit, Centre of Clinical Intervention Research (J.W.), Copenhagen University Hospital Rigshospitalet, Denmark; Department of Anesthesiology (J. Hovdenes), Oslo University Hospital, Rikshospitalet, Norway; Department of Intensive Care, Medical Center Leeuwarden (M.K.), the Netherlands; Intensive Care Unit (T.P.), Santa Maria degli Angeli, Pordenone, Italy; Department of Anesthesiology and Intensive Care (P.S.), Centre Hospitalier de Luxembourg; and Adult Critical Care (M.P.W.), University Hospital of Wales, Cardiff, UK.
AUTHOR CONTRIBUTIONS
E.W., I.R., A.O.R., A.-F.v.R., T.W.K., J.Horn, N.N., and T.C. are members of the EEG group of the TTM trial. E.W., I.R., A.O.R., A.-F.v.R., T.W.K., and T.C. designed the EEG analyses. S.U. is the statistician of the EEG analyses. E.W., A.O.R., A.-F.v.R., and T.W.K. performed the EEG interpretations. In the TTM trial: N.N. is principal investigator; H.F. is senior investigator; N.N., H.F., T.C., J.Horn, A.Å., D.E., Y.G., C.H., J.Hovdenes, J.K., M.K., T.P., P.S., M.W., J.W. and M.P.W. are members of the steering group. E.W. and T.C. drafted the manuscript, which was critically revised for intellectual content and finally approved by all coauthors.
STUDY FUNDING
Supported by The Swedish Heart and Lung Association, the Skåne University Hospital Foundations, the Gyllenstierna-Krapperup Foundation, the Segerfalk Foundation, the Swedish National Health System (ALF), the County Council of Skåne, the Swedish Society of Medicine, the Koch Foundation, The Swedish Heart-Lung Foundation, AFA Insurance, The Swedish Research Council, and the Hans-Gabriel and Alice Trolle-Wachtmeister Foundation, Sweden; The Tryg Foundation, Denmark; EU programme Interreg IV A; and the Swiss National Research Foundation (CR32I3_143780) (A.O.R.).
DISCLOSURE
E. Westhall receives academic support from the County Council of Skåne. A. Rossetti receives support by the Swiss National Research Foundation (CR32I3_143780) and received research support from UCB Pharma and SAGE Pharmaceuticals. A. van Rootselaar reports no disclosures. T. Wesenberg Kjaer is principal investigator in trials with Widex A/S and has received honoraria from Eisai and UCB Pharma for lectures. J. Horn receives government research support (ZonMw no. 92003586) and support from the Dutch Heart Foundation (no. 2007B039) and serves on the scientific advisory board of Bard Medical. S. Ullén reports no disclosures. H. Friberg receives support from the Swedish National Health System (ALF), serves as European editor of Therapeutic Hypothermia and Temperature Management, is a member of the editorial board of Resuscitation, and has received honoraria for lectures from Bard Medical and Natus Inc. N. Nielsen receives support from the Swedish National Health System (ALF). I. Rosén and A. Åneman report no disclosures. D. Erlinge has received minor speaker fees or advisory board compensation from ZOLL, AstraZeneca, and Lilly. Y. Gasche reports no disclosures. C. Hassager receives grant support from the Interreg IVA ØKS program; honorarium for lectures from AstraZeneca, Teva, Novartis, and ViCare Medical; and travel support to congresses from Novartis. J. Hovdenes, J. Kjaergaard, and M. Kuiper report no disclosures. T. Pellis has received speakers honorarium from Bard Medical. P. Stammet and M. Wanscher report no disclosures. J. Wetterslev's institution, Copenhagen Trial Unit (CTU), received an unrestricted grant from TRYGFONDEN in Denmark for involvement in the TTM Trial. M. Wise was funded 40% WTE during the study by a National Institute for Social Care and Health Research (NISCHR) Academic Health Science Collaboration (AHSC) Clinical Research Fellowship; received travel costs from the British Thoracic Society, Intensive Care Society, Scottish Intensive Care Society, and Orion Ltd.; received royalties from Wiley Publishing; received honorarium for lecturing at educational meetings from Fisher & Paykel and Merck; serves on the advisory board for Kalobius Pharmaceuticals, Bard; and is Editor of BMJ Open Respiratory Research. T. Cronberg receives academic support from the County Council of Skåne. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Atwood C, Eisenberg MS, Herlitz J, Rea TD. Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation 2005;67:75–80. [DOI] [PubMed] [Google Scholar]
- 2.Dragancea I, Rundgren M, Englund E, Friberg H, Cronberg T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation 2013;84:337–342. [DOI] [PubMed] [Google Scholar]
- 3.Samaniego EA, Persoon S, Wijman CA. Prognosis after cardiac arrest and hypothermia: a new paradigm. Curr Neurol Neurosci Rep 2011;11:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oddo M, Rossetti AO. Predicting neurological outcome after cardiac arrest. Curr Opin Crit Care 2011;17:254–259. [DOI] [PubMed] [Google Scholar]
- 5.Friberg H, Cronberg T, Dunser MW, Duranteau J, Horn J, Oddo M. Survey on current practices for neurological prognostication after cardiac arrest. Resuscitation 2015;90:158–162. [DOI] [PubMed] [Google Scholar]
- 6.Deakin CD, Nolan JP, Soar J, et al. European Resuscitation Council guidelines for resuscitation 2010 Section 4: adult advanced life support. Resuscitation 2010;81:1305–1352. [DOI] [PubMed] [Google Scholar]
- 7.Sandroni C, Cariou A, Cavallaro F, et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1816–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;67:203–210. [DOI] [PubMed] [Google Scholar]
- 9.Sandroni C, Cavallaro F, Callaway CW, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis: part 2: patients treated with therapeutic hypothermia. Resuscitation 2013;84:1324–1338. [DOI] [PubMed] [Google Scholar]
- 10.Sandroni C, Cavallaro F, Callaway CW, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis: part 1: patients not treated with therapeutic hypothermia. Resuscitation 2013;84:1310–1323. [DOI] [PubMed] [Google Scholar]
- 11.Westhall E, Rosen I, Rossetti AO, et al. Interrater variability of EEG interpretation in comatose cardiac arrest patients. Clin Neurophysiol 2015;126:2397–2404. [DOI] [PubMed] [Google Scholar]
- 12.Gaspard N, Hirsch LJ, LaRoche SM, Hahn CD, Westover MB, Critical care EEGMRC: interrater agreement for critical care EEG terminology. Epilepsia 2014;55:1366–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch LJ, LaRoche SM, Gaspard N, et al. American clinical neurophysiology Society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med 2013;369:2197–2206. [DOI] [PubMed] [Google Scholar]
- 15.Cronberg T, Lilja G, Horn J, et al. Neurologic function and health-related quality of life in patients following targeted temperature management at 33°C vs 36°C after out-of-hospital cardiac arrest: a randomized clinical trial. JAMA Neurol 2015;72:634–641. [DOI] [PubMed] [Google Scholar]
- 16.Westhall E, Rosen I, Rossetti AO, et al. Electroencephalography (EEG) for neurological prognostication after cardiac arrest and targeted temperature management: rationale and study design. BMC Neurol 2014;14:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen N, Wetterslev J, Al-Subaie N, et al. Target temperature management after out-of-hospital cardiac arrest: a randomized, parallel-group, assessor-blinded clinical trial: rationale and design. Am Heart J 2012;163:541–548. [DOI] [PubMed] [Google Scholar]
- 18.Cronberg T, Horn J, Kuiper MA, Friberg H, Nielsen N. A structured approach to neurologic prognostication in clinical cardiac arrest trials. Scand J Trauma Resusc Emerg Med 2013;21:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dragancea I, Horn J, Kuiper M, et al. Neurological prognostication after cardiac arrest and targeted temperature management 33 degrees C versus 36 degrees C: results from a randomised controlled clinical trial. Resuscitation 2015;93:164–170. [DOI] [PubMed] [Google Scholar]
- 20.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;1:480–484. [DOI] [PubMed] [Google Scholar]
- 21.Hofmeijer J, Beernink TM, Bosch FH, Beishuizen A, Tjepkema-Cloostermans MC, van Putten MJ. Early EEG contributes to multimodal outcome prediction of postanoxic coma. Neurology 2015;85:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivaraju A, Gilmore EJ, Wira CR, et al. Prognostication of post-cardiac arrest coma: early clinical and electroencephalographic predictors of outcome. Intensive Care Med 2015;41:1264–1272. [DOI] [PubMed] [Google Scholar]
- 23.Crepeau AZ, Rabinstein AA, Fugate JE, et al. Continuous EEG in therapeutic hypothermia after cardiac arrest: prognostic and clinical value. Neurology 2013;80:339–344. [DOI] [PubMed] [Google Scholar]
- 24.Grindal AB, Suter C, Martinez AJ. Alpha-pattern coma: 24 cases with 9 survivors. Ann Neurol 1977;1:371–377. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan PW, Genoud D, Ho TW, Jallon P. Etiology, neurologic correlations, and prognosis in alpha coma. Clin Neurophysiol 1999;110:205–213. [DOI] [PubMed] [Google Scholar]
- 26.Rundgren M, Westhall E, Cronberg T, Rosen I, Friberg H. Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med 2010;38:1838–1844. [DOI] [PubMed] [Google Scholar]
- 27.Rundgren M, Rosen I, Friberg H. Amplitude-integrated EEG (aEEG) predicts outcome after cardiac arrest and induced hypothermia. Intensive Care Med 2006;32:836–842. [DOI] [PubMed] [Google Scholar]
- 28.Rossetti AO, Logroscino G, Liaudet L, et al. Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology 2007;69:255–260. [DOI] [PubMed] [Google Scholar]
- 29.Rossetti AO, Oddo M, Liaudet L, Kaplan PW. Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology 2009;72:744–749. [DOI] [PubMed] [Google Scholar]
- 30.Legriel S, Bruneel F, Sediri H, et al. Early EEG monitoring for detecting postanoxic status epilepticus during therapeutic hypothermia: a pilot study. Neurocrit Care 2009;11:338–344. [DOI] [PubMed] [Google Scholar]
- 31.Ruijter BJ, van Putten MJ, Horn J, et al. Treatment of electroencephalographic status epilepticus after cardiopulmonary resuscitation (TELSTAR): study protocol for a randomized controlled trial. Trials 2014;15:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sessler CN, Grap MJ, Ramsay MA. Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care 2008;12(suppl 3):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol 2010;67:301–307. [DOI] [PubMed] [Google Scholar]
- 34.Rossetti AO, Carrera E, Oddo M. Early EEG correlates of neuronal injury after brain anoxia. Neurology 2012;78:796–802. [DOI] [PubMed] [Google Scholar]
- 35.Oddo M, Rossetti AO. Early multimodal outcome prediction after cardiac arrest in patients treated with hypothermia. Crit Care Med 2014;42:1340–1347. [DOI] [PubMed] [Google Scholar]
- 36.Noirhomme Q, Lehembre R, Lugo Zdel R, et al. Automated analysis of background EEG and reactivity during therapeutic hypothermia in comatose patients after cardiac arrest. Clin EEG Neurosci 2014;45:6–13. [DOI] [PubMed] [Google Scholar]
- 37.Lilja G, Nielsen N, Friberg H, et al. Cognitive function in survivors of out-of-hospital cardiac arrest after target temperature management at 33°C versus 36°C. Circulation 2015;131:1340–1349. [DOI] [PubMed] [Google Scholar]
- 38.Fugate JE, Wijdicks EF, White RD, Rabinstein AA. Does therapeutic hypothermia affect time to awakening in cardiac arrest survivors? Neurology 2011;77:1346–1350. [DOI] [PubMed] [Google Scholar]
- 39.Tjepkema-Cloostermans MC, Hofmeijer J, Trof RJ, Blans MJ, Beishuizen A, van Putten MJ. Electroencephalogram predicts outcome in patients with postanoxic coma during mild therapeutic hypothermia. Crit Care Med 2015;43:159–167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.