Abstract
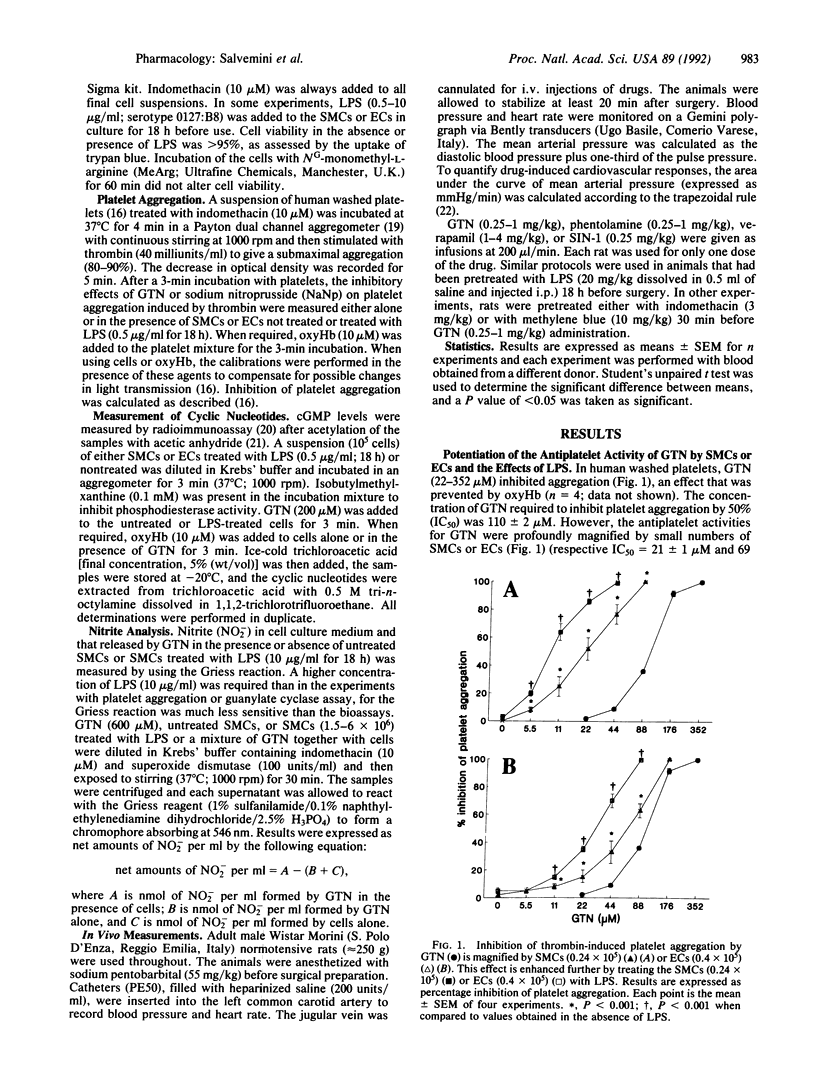
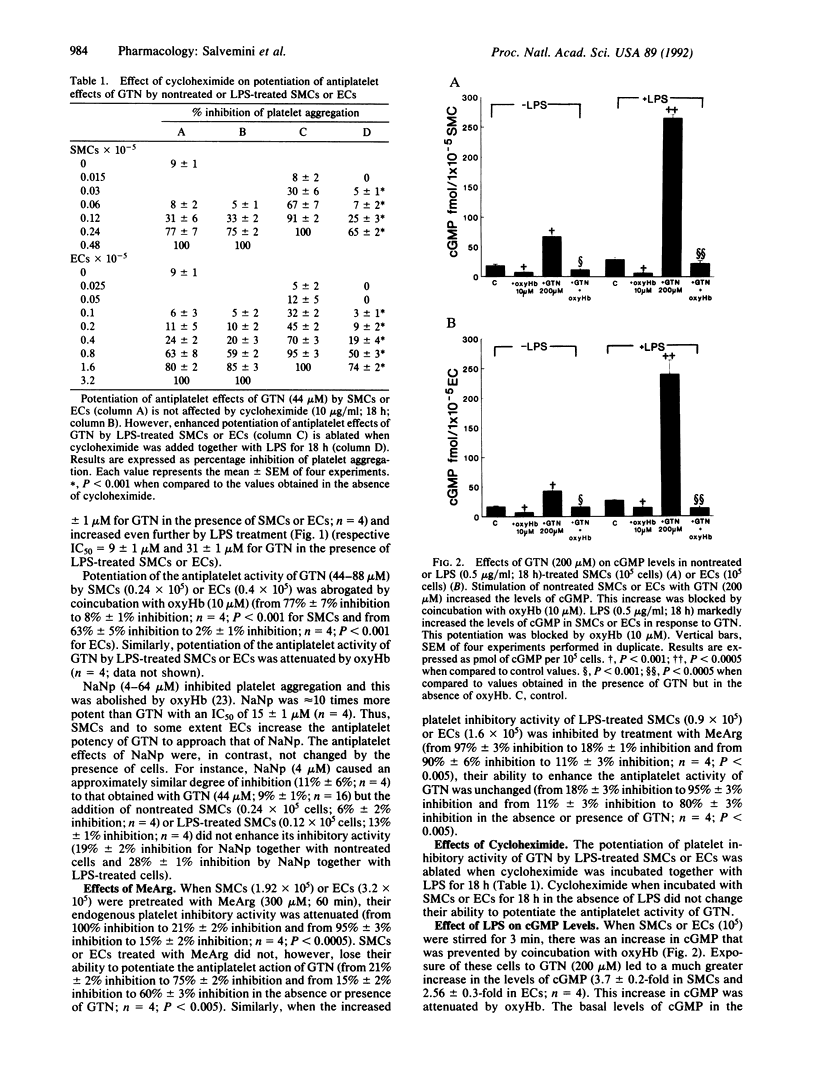
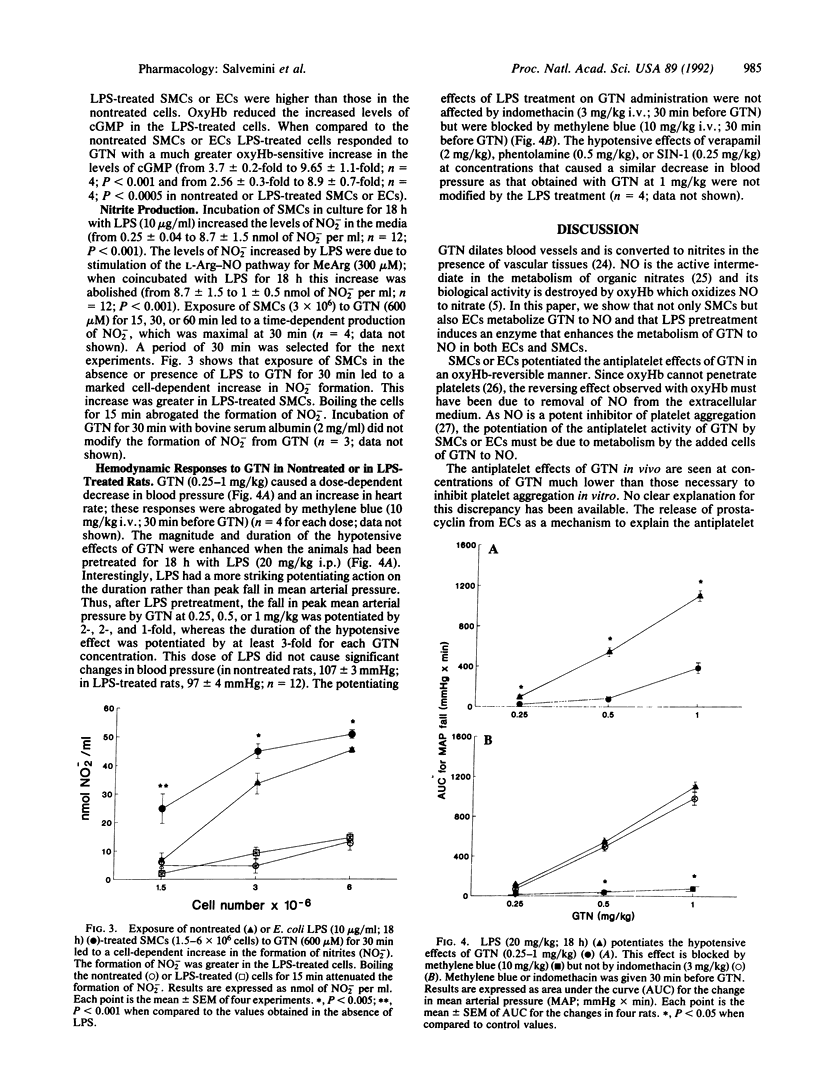
Here, we demonstrate that the metabolism of glyceryl trinitrate (GTN) to nitric oxide (NO) occurs not only in bovine aortic smooth muscle cells (SMCs) but also in endothelial cells (ECs) and that this biotransformation is enhanced by pretreatment with Escherichia coli lipopolysaccharide (LPS). Two bioassay systems were used: inhibition of platelet aggregation and measurement of cGMP after stimulation by NO of guanylate cyclase in SMCs or ECs. In addition, NO produced from GTN by cells was measured as nitrite (NO2-), one of its breakdown products. Indomethacin (10 microM)-treated SMCs or ECs enhanced the platelet inhibitory activity of GTN. This effect was abrogated by coincubation with oxyhemoglobin (oxyHb; 10 microM), indicating release of NO from GTN. LPS (0.5 microgram/ml; 18 h) enhanced at least 2- to 3-fold the capacity of SMCs or ECs to form NO from GTN, and this enhancement was attenuated when cycloheximide (10 micrograms/ml) was incubated together with LPS. Furthermore, when incubated with GTN (200 microM) SMCs or ECs treated with LPS (0.5 microgram/ml; 18 h) released more NO from GTN than nontreated cells as indicated by a much higher (8- to 9-fold) increase in the levels of cGMP. Exposure of SMCs to GTN (600 microM) for 30 min led to an increase in the levels of NO2- dependent on cell numbers, which was enhanced when SMCs were treated with LPS. Incubation of nontreated or LPS-treated cells with NG-monomethyl-L-arginine (300 microM; 60 min) did not influence the metabolism of GTN to NO. SMCs failed to enhance the antiplatelet activity of sodium nitroprusside. Anesthetized rats treated with an intraperitoneal injection of LPS (20 mg/kg) 18 h beforehand showed enhanced hypotensive responses to GTN (0.25-1 mg/kg). These effects were blocked by methylene blue (10 mg/kg) but not by indomethacin (3 mg/kg). LPS did not alter the hypotensive responses induced by phentolamine, verapamil, or SIN-1. Thus, both in vitro and in vivo, LPS induces the enzyme(s) metabolizing GTN to NO.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin N., Dutton J. A., Ritter J. M. Human vascular smooth muscle cells inhibit platelet aggregation when incubated with glyceryl trinitrate: evidence for generation of nitric oxide. Br J Pharmacol. 1991 Apr;102(4):847–850. doi: 10.1111/j.1476-5381.1991.tb12264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenger F. P., Cano J. P., Rolland P. H. Antithrombogenic endothelial cell defense. Basal characteristics in cultured endothelial cells and modulation by short-term and long-term exposure to isosorbide nitrates. Circ Res. 1987 Apr;60(4):612–620. doi: 10.1161/01.res.60.4.612. [DOI] [PubMed] [Google Scholar]
- Chung S. J., Fung H. L. Identification of the subcellular site for nitroglycerin metabolism to nitric oxide in bovine coronary smooth muscle cells. J Pharmacol Exp Ther. 1990 May;253(2):614–619. [PubMed] [Google Scholar]
- De Caterina R., Dorso C. R., Tack-Goldman K., Weksler B. B. Nitrates and endothelial prostacyclin production: studies in vitro. Circulation. 1985 Jan;71(1):176–182. doi: 10.1161/01.cir.71.1.176. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Noack E. A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987 Jul 2;139(1):19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- Frank L., Summerville J., Massaro D. Potection from oxygen toxicity with endotoxin. Role of the endogenous antioxidant enzymes of the lung. J Clin Invest. 1980 May;65(5):1104–1110. doi: 10.1172/JCI109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter C. A., Barry B. K., McNamara D. B., Gruetter D. Y., Kadowitz P. J., Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5(3):211–224. [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Kuhn M., Otten A., Frölich J. C., Förstermann U. Endothelial cyclic GMP and cyclic AMP do not regulate the release of endothelium-derived relaxing factor/nitric oxide from bovine aortic endothelial cells. J Pharmacol Exp Ther. 1991 Feb;256(2):677–682. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Mollace V., Salvemini D., Anggard E., Vane J. Nitric oxide from vascular smooth muscle cells: regulation of platelet reactivity and smooth muscle cell guanylate cyclase. Br J Pharmacol. 1991 Nov;104(3):633–638. doi: 10.1111/j.1476-5381.1991.tb12481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Rees D. D., Schulz R., Palmer R. M. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M., Kaplan K. L., Nossel H. L. Prostacyclin production by perturbed bovine aortic endothelial cells in culture. Blood. 1984 Oct;64(4):801–806. [PubMed] [Google Scholar]
- Needleman P., Johnson E. M., Jr Mechanism of tolerance development to organic nitrates. J Pharmacol Exp Ther. 1973 Mar;184(3):709–715. [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland P. H., Bory M., Leca F., Sainsous J., Gueydon E., Juhan I., Serradimigni A., Cano J. P. Evidence for isosorbide dinitrate (ISDN) promoting effect on prostacyclin release by the lung and prostacyclin implication in ISDN-induced inhibition of platelet aggregation in humans. Prostaglandins Leukot Med. 1984 Dec;16(3):333–346. doi: 10.1016/0262-1746(84)90190-2. [DOI] [PubMed] [Google Scholar]
- Salvemini D., Masini E., Anggard E., Mannaioni P. F., Vane J. Synthesis of a nitric oxide-like factor from L-arginine by rat serosal mast cells: stimulation of guanylate cyclase and inhibition of platelet aggregation. Biochem Biophys Res Commun. 1990 Jun 15;169(2):596–601. doi: 10.1016/0006-291x(90)90372-t. [DOI] [PubMed] [Google Scholar]
- Salvemini D., Radziszewski W., Korbut R., Vane J. The use of oxyhaemoglobin to explore the events underlying inhibition of platelet aggregation induced by NO or NO-donors. Br J Pharmacol. 1990 Dec;101(4):991–995. doi: 10.1111/j.1476-5381.1990.tb14194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D., de Nucci G., Gryglewski R. J., Vane J. R. Human neutrophils and mononuclear cells inhibit platelet aggregation by releasing a nitric oxide-like factor. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6328–6332. doi: 10.1073/pnas.86.16.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorer A. E., Kaplan M. E., Rao G. H., Moldow C. F. Interleukin 1 stimulates endothelial cell tissue factor production and expression by a prostaglandin-independent mechanism. Thromb Haemost. 1986 Dec 15;56(3):256–259. [PubMed] [Google Scholar]
- Schröder H., Leitman D. C., Bennett B. M., Waldman S. A., Murad F. Glyceryl trinitrate-induced desensitization of guanylate cyclase in cultured rat lung fibroblasts. J Pharmacol Exp Ther. 1988 May;245(2):413–418. [PubMed] [Google Scholar]
- Sneddon J. M., Vane J. R. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2800–2804. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Wood K. S., Buga G. M., Byrns R. E., Ignarro L. J. Vascular smooth muscle-derived relaxing factor (MDRF) and its close similarity to nitric oxide. Biochem Biophys Res Commun. 1990 Jul 16;170(1):80–88. doi: 10.1016/0006-291x(90)91243-l. [DOI] [PubMed] [Google Scholar]
- Yeates R. A., Schmid M., Leitold M. Antagonism of glycerol trinitrate activity by an inhibitor of glutathione S-transferase. Biochem Pharmacol. 1989 Jun 1;38(11):1749–1753. doi: 10.1016/0006-2952(89)90408-5. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]



