Abstract
IMPORTANCE
Autoantibodies to the γ-aminobutyric acid type B (GABAB) receptor have recently been identified as a cause of autoimmune encephalitis. Most patients with GABAB encephalitis have presented with limbic encephalitis. About half of the cases reported have been paraneoplastic in origin, with the majority of tumors representing small cell lung cancer.
OBSERVATIONS
We describe a 3-year-old boy who presented with a mixed movement disorder (opsoclonus, ataxia, and chorea) as well as seizures refractory to treatment. His seizures required continuous pentobarbital sodium infusion to be controlled. Despite treatment with intravenous corticosteroids and immunoglobulins, the patient ultimately died of overwhelming sepsis.
CONCLUSIONS AND RELEVANCE
To our knowledge, this report represents the first pediatric case of GABAB-associated encephalitis. Our patient presented with encephalopathy, refractory seizures, and a mixed movement disorder rather than limbic encephalitis. γ-Aminobutyric acid type B receptor autoimmunity deserves consideration in pediatric patients presenting with encephalitis.
Immune-mediated encephalitis with autoantibodies directed against synaptic proteins has become an important component of the differential diagnosis of patients with encephalitis. Current estimates suggest that a substantial proportion of patients once suspected to have viral encephalitis in fact have an autoimmune etiology for their symptoms.1 Additional autoantigen targets continue to be identified, and the phenotypic spectrum associated with autoimmune encephalitis continues to expand. We describe a 3-year-old patient who presented with acute-onset confusion, opsoclonus, chorea, and intractable seizures. Neuroimaging disclosed involvement of the brainstem, basal ganglia, and hippocampi. γ-Aminobutyric acid type B (GABAB) receptor autoantibodies were identified in the serum and cerebrospinal fluid (CSF). Despite immuno-modulating therapy, the patient died of overwhelming sepsis. To our knowledge, this is the first description of a pediatric patient with GABAB receptor autoantibodies. The presence of opsoclonus, ataxia, and chorea expands the clinical phenotype and indicates that GABAB receptor auto-immunity should be considered in cases of pediatric encephalitis.
Report of a Case
A previously healthy 3-year-old boy developed confusion and lethargy at home during the course of a single day, prompting his parents to seek medical attention. His initial examination disclosed opsoclonus, dystonic movements of the tongue, ataxia, and chorea affecting the limbs and trunk. Within 24 hours, he developed frequent complex partial seizures and was intubated. His hyperkinetic movements were controlled with midazolam sedation.
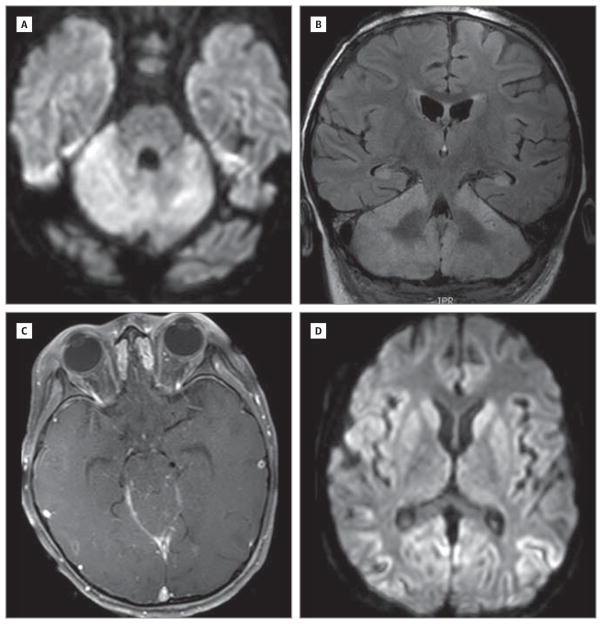
Initial CSF analysis demonstrated a lymphocytic pleocytosis, with a white blood cell count of 154/μL (to convert to ×109 per liter, multiply by 0.001; 94% lymphocytes), a red blood cell count of 228 × 106/μL (to convert to ×1012 per liter, multiply by 1.0), a glucose level of 123 mg/dL (to convert to millimoles per liter, multiply by 0.0555), and a protein level of 59 g/dL (to convert to grams per liter, multiply by 10.0). Extensive evaluation for infectious causes was unrevealing (including herpes simplex virus, varicella-zoster virus, human herpesvirus 6, Epstein-Barr virus, cytomegalovirus, enterovirus, and mycoplasma). A CSF paraneoplastic antibody panel, including antineuronal nuclear antibody 1, Purkinje cell cytoplasmic antibody 1, amphiphysin antibody, antineuronal nuclear antibody 2, Purkinje cell cytoplasmic antibody type Tr, Purkinje cell cytoplasmic antibody 2, antineuronal nuclear antibody 3, collapsin response-mediator protein 5 IgG, anti-glial/neuronal nuclear antibody 1, voltage-gated calcium channel antibody, glutamic acid decarboxylase 65, and N-methyl-D-aspartate receptor antibody, was negative. Neuroimaging demonstrated multifocal diffusion-weighted imaging hyperintensities within the brainstem and cerebellum without corresponding apparent diffusion coefficient restriction, leading to a working diagnosis of rhomboencephalitis (Figure 1). Subsequent imaging showed evolution of these lesions to T2/fluid-attenuated inversion recovery hyperintensity, with involvement of the basal ganglia.
Figure 1. Magnetic Resonance Imaging Findings.
Evidence of brainstem, cerebellar, and basal ganglia involvement on diffusion-weighted (A and D), T1 contrast-enhanced (B), and T2 fluid-attenuated inversion recovery (C) images.
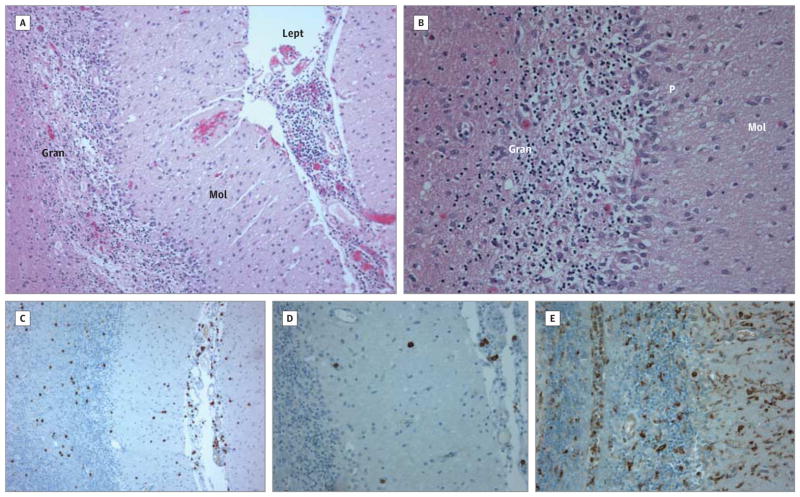
Electroencephalography demonstrated diffuse delta range slowing and bioccipital spike-wave discharges with rapid secondary generalization. The patient’s electrographic seizures persisted despite administration of multiple anticonvulsants. Seizures were eventually controlled with a continuous infusion of pentobarbital sodium, but status epilepticus recurred each time a wean was attempted. The patient developed worsening cerebral edema, and a decompressive posterior craniectomy was performed. At this time, a cerebellar biopsy specimen was also obtained. This demonstrated widespread astrogliosis and microglial activation with Purkinje and granule cell loss (Figure 2). A CD3+ T-cell infiltrate was also observed. Bacterial and viral culture of biopsy tissue as well as 16S ribosomal RNA polymerase chain reaction for bacteria, acid-fast bacilli, fungus, and mold did not detect any pathogens.
Figure 2. Brain Biopsy Findings.
A and B, Biopsy demonstrates profound loss of cerebellar granule neurons and Purkinje cells and prominent leptomeningeal inflammatory infiltrate (hematoxylin-eosin, original magnification ×10 [A] and ×20 [B]). Gran indicates granule cell layer; Lept, leptomeninges; Mol, molecular layer; and P, Purkinje cell layer. C, Prominent CD3+ T cells are seen (3,3′-diaminobenzidine, original magnification ×10). D, Occasional CD20+ B cells are evident (3,3′-diaminobenzidine, original magnification ×20). E, Marked CD68+ microglial activation is present (3,3′-diaminobenzidine, original magnification ×20).
The patient was treated with high-dose intravenous corticosteroids and intravenous immunoglobulin without clear benefit. A search for an occult tumor was not performed. Despite intensive supportive care and aggressive seizure management, the patient developed overwhelming sepsis and died 4 weeks after his initial presentation. The parents declined an autopsy.
Two years after the patient’s initial presentation, testing of archived serum and CSF identified diffuse reactivity to rat neuropil with prominent reactivity in the cerebellum, thalamus, and hippocampus. Follow-up studies using HEK293 cells transfected with GABAB1 and GABAB2 were performed. Transfected cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton-X100, and incubated overnight at 4°C with the patient’s CSF (neat) and guinea pig GABAB1 or GABAB2 polyclonal antibody. AlexaFluor secondary antibodies were used for visualization, and colocalization was performed using an ImageJ-based macro. These studies indicated high-titer anti-GABAB1 receptor autoantibodies.
Discussion
Autoimmune encephalitis associated with anti-GABAB auto-antibodies is a recently described entity.2–4 Thus far, affected patients have been adults with limbic encephalitis and seizures, and about half of the cases have been paraneoplastic in origin, with small cell lung cancer being the most commonly identified neoplasm. To our knowledge, our patient is the first pediatric patient described with this new form of autoimmune encephalitis. Our patient’s case was not associated with an identified tumor (although an exhaustive search for an occult neoplasm was not performed) or a recognized preceding infection. In addition, although mesial temporal lobe involvement was demonstrable on the patient’s neuroimaging, he did not present with classic signs and symptoms of limbic encephalitis (amnesia, psychosis, or agitation), although his young age complicated assessment.
Severe, refractory seizures dominated the clinical course in our patient, similar to previously described adult patients. Although the patient’s seizures did not respond to immuno-modulating treatments, it is not known whether more aggressive immunosuppressive therapy may have led to a treatment response.5,6 The opsoclonus, chorea, and lingual dystonia in our patient are novel phenotypic features (1 adult patient presented with orobuccolingual dyskinesias without limb involvement2). Such abnormal hyperkinetic movements are consistent with the role of GABAB receptors in modulating dopaminergic neurotransmission7 and suggest that this entity should be considered in the differential diagnosis of autoimmune movement disorders8 and perhaps even in cases of atypical opsoclonus-myoclonus-ataxia syndrome.
Acknowledgments
Funding/Support: Dr Kruer is supported by grant K08NS083739 from the National Institute of Neurological Disorders and Stroke, the Sanford Health Foundation, the Sanford-Mayo Collaborative Fund, the Dystonia Medical Research Foundation, the Child Neurology Society, and the American Academy of Cerebral Palsy and Developmental Medicine. Dr Woltjer is supported by grant P30 AG008017 23 from the National Institute on Aging. Dr Dalmau is supported by Euroimmun, grants R01CA107192 and R01CA89054-06A2 from the National Institutes of Health, and the McKnight Foundation.
Footnotes
Conflict of Interest Disclosures: Dr Dalmau serves on the editorial board of Neurology; has a patent issued for the Ma2 autoantibody test (US 6,387,639; issued May 14, 2002); has a patent for the N-methyl-D-aspartate receptor autoantibody test (US 7,972,796 B2; issued July 5, 2011); has filed for patents for the GABAB receptor and LGI1 (leucine-rich, glioma inactivated 1) autoantibody tests; receives license fee payments for the N-methyl-D-aspartate receptor autoantibody test from Euroimmun; receives royalties from the patented Ma2 autoantibody test and the patented N-methyl-D-aspartate receptor autoantibody test; and has a patent application pending for the use of GABAB receptor and LGI1 as an autoantibody test. No other disclosures were reported.
Author Contributions: Dr Kruer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Kruer and Hoeftberger are joint first authors.
Study concept and design: Kruer.
Acquisition of data: All authors.
Analysis and interpretation of data: Kruer, Dalmau.
Drafting of the manuscript: Kruer.
Critical revision of the manuscript for important intellectual content: All authors.
Obtained funding: Woltjer, Dalmau.
Administrative, technical, and material support: Hoeftberger, Svoboda, Woltjer, Dalmau.
Role of the Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54(7):899–904. doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9(1):67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABA(B) receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. 2011;76(9):795–800. doi: 10.1212/WNL.0b013e31820e7b8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frisullo G, Della Marca G, Mirabella M, et al. A human anti-neuronal autoantibody against GABA B receptor induces experimental autoimmune agrypnia. Exp Neurol. 2007;204(2):808–818. doi: 10.1016/j.expneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Kruer MC, Koch TK, Bourdette DN, et al. NMDA receptor encephalitis mimicking seronegative neuromyelitis optica. Neurology. 2010;74(18):1473–1475. doi: 10.1212/WNL.0b013e3181dc1a7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vacher CM, Gassmann M, Desrayaud S, et al. Hyperdopaminergia and altered locomotor activity in GABAB1-deficient mice. J Neurochem. 2006;97(4):979–991. doi: 10.1111/j.1471-4159.2006.03806.x. [DOI] [PubMed] [Google Scholar]
- 8.Panzer J, Dalmau J. Movement disorders in paraneoplastic and autoimmune disease. Curr Opin Neurol. 2011;24(4):346–353. doi: 10.1097/WCO.0b013e328347b307. [DOI] [PMC free article] [PubMed] [Google Scholar]