Abstract
IMPORTANCE
Anti–N-methyl-D-aspartate receptor (NMDAR) encephalitis is a severe but treatable autoimmune encephalitis affecting mainly young adults and children. The lack of suitable biomarkers of disease activity makes treatment decisions and identification of relapses challenging.
OBJECTIVE
To determine the levels of the B-cell–attracting C-X-C motif chemokine 13 (CXCL13) in serum samples and cerebrospinal fluid (CSF) of patients with anti-NMDAR encephalitis and whether they can be used as biomarkers of treatment response and outcome.
DESIGN, SETTINGS, AND PARTICIPANTS
Retrospective cohort study of 167 patients consecutively diagnosed as having anti-NMDAR encephalitis between May 1, 2008, and January 31, 2013. Concentration of CXCL13 was determined with enzyme-linked immunosorbent assay in all available patients’ samples (272 CSF and 55 serum samples). Samples from 25 patients with noninflammatory neurological disorders and 9 with neuroborreliosis served as controls. Expression of CXCL13 in the brain biopsy of a patient with anti-NMDAR encephalitis was determined by immunohistochemistry.
MAIN OUTCOMES AND MEASURES
Percentage of patients with anti-NMDAR encephalitis and elevated CXCL13 in CSF.
RESULTS
Compared with control individuals, 70% of patients with early-stage anti-NMDAR encephalitis had increased CXCL13 in CSF (>7 pg/mL; P < .001) but none in serum samples (>1047 pg/mL; P > .99). High concentration of CSF CXCL13 was associated with the presence of prodromal fever or headache (P = .01), limited response to therapy (P = .003), clinical relapses (P = .03), and intrathecal NMDAR-antibody synthesis (P < .001). Among patients with monophasic disease assessed 2 to 6 months after starting treatment, 10 of 15 with limited treatment response vs 0 of 13 with favorable response had increased CSF CXCL13 (specificity, 100%; 95% CI, 75–100 and sensitivity, 67%; 95% CI, 38–88; P = .02). Six of 12 patients had elevated CSF CXCL13 at relapse including 3 with previously normal levels. In brain, abundant mononuclear cells in perivascular infiltrates and scattered intraparenchymal microglia expressed CXCL13.
CONCLUSIONS AND RELEVANCE
Seventy percent of patients with early-stage anti-NMDAR encephalitis had increased CSF CXCL13 concentration that correlated with intrathecal NMDAR-antibody synthesis. Prolonged or secondary elevation of CXCL13 was associated with limited response to treatment and relapses. CXCL13 is a potentially useful biomarker of treatment response and outcome in anti-NMDAR encephalitis.
Anti–N-methyl-D-aspartate receptor (NMDAR) encephalitis is an immune-mediated disorder that often affects women and children and associates with promi-effects of patients’ antibodies in cultured neurons or after injection into the hippocampus of rodents and autopsy findings from patients suggest the disorder is mediated by antibodies, causing internalization of the receptors.2 The reversibility of these effects and the recovery of many patients with immunotherapy support a mechanism of neuronal dysfunction rather than irreversible degeneration.3 Yet, patients usually improve over months along with persistent detection of cerebrospinal fluid (CSF) antibodies that has been attributed to their synthesis within the central nervous system (CNS). This is supported by the presence of intrathecal antibody synthesis in most patients and brain-infiltrating plasma cells in pathological studies.1,4 A study showed a correlation between the change of CSF antibody titers (but not serum titers) and clinical relapses; however, in patients with monophasic disease, this correlation was less perfect and the change of titers was slow. Moreover, patients who recovered still had detectable antibodies in serum samples or CSF.5 Therefore, there is a need for biomarkers that may assist in the treatment and follow-up of these patients.
C-X-C motif chemokine 13 (CXCL13) is a B-cell–attracting chemokine that is found mildly elevated in autoimmune disorders such as multiple sclerosis and neuromyelitis optica.6,7 Levels of CSF CXCL13 correlate better with the presence of plasma cells or plasmablasts in CNS than those of any other B-cell–attracting chemokines.8 These findings led us to determine the levels of CXCL13 in patients with anti-NMDAR encephalitis and whether these correlated with clinical outcome and antibody titers. We report here data supporting these hypotheses.
Methods
We examined 272 CSF and 55 randomly selected serum samples of 167 patients consecutively studied from May 1, 2008, until January 31, 2013, at the University of Pennsylvania, Philadelphia, and Hospital Clinic, University of Barcelona, Barcelona, Spain. The inclusion criteria were based on (1) adequate amounts of CSF and serum samples and (2) clinical follow-up of at least 8 months. Gathering of clinical information, diagnosis, and modified Rankin Scale (mRS) scoring of anti-NMDAR encephalitis and relapses was done as previously reported.1,3,5 Of these 167 patients, 154 had a monophasic course and 13 had clinical relapses. Forty-three of the mono-phasic patients and 11 with relapses had follow-up samples available for testing (median, 3; range, 2–6 samples). Cerebro-spinal fluid and serum samples of 25 randomly selected patients with noninflammatory CNS conditions (normal CSF; brain magnetic resonance imaging without abnormalities suggesting inflammation) served as negative control individuals. Final diagnoses on hospital discharge included primary psychiatric disorders (n = 10), intracranial hypertension (n = 3), epilepsy (n = 3), idiopathic headache disorders (n = 2), systemic viral infection (n = 2), and others (n = 5). Additionally, 9 patients with neuroborreliosis9 (a disorder that typically occurs with very high levels of CSF CXCL13) were included as positive control individuals.
Written consent for studies was obtained from families and patients’ representatives; studies were approved by the institutional review boards of the University of Pennsylvania and the University of Barcelona.
CXCL13 Enzyme-Linked Immunosorbent Assay
All samples were measured in duplicates. Cerebrospinal fluid was measured undiluted except in patients with neuroborreliosis whose CSF was diluted 1:5 and serum samples were diluted 1:2. Sandwich, capture enzyme-linked immunosorbent assay (ELISA) was performed according to the manufacturer’s instructions (Euroimmun). In brief, precoated ELISA plates were serially incubated with patients’ samples for 3 hours, with biotinylated secondary antibody for 30 minutes and with horse-radish peroxidase–avidin conjugate and chromogens for 15 minutes each. All incubations were done at room temperature and between steps, extensive washing was performed with phosphate-buffered saline with 0.05% Tween 20. The optical density was determined on a microplate reader (Biotek, Synergy HT). A cutoff of 7.0 pg/mL was determined as the 97.7 percentile (mean+2SD) of the noninflammatory control group. Using the same approach, the cutoff for serum levels was set at 1047 pg/mL.
All samples were handled similarly, kept frozen at −80°C, and measured in duplicates. The number of freezing-thawing cycles was similar in all samples. To determine whether freezing samples would potentially alter the levels of CXCL13, the CSF samples from 3 patients underwent 10 freezing-thawing cycles. These studies showed a reduction of less than 7% compared with prefreezing levels in all cases (mean, 95.6%; 95% CI, 93.7–97.5; n = 5).
NMDAR Antibody Detection and Titration Experiments
Techniques used for antibody testing (immunohistochemistry with rat brain and cell-based assay), criteria of antibody positivity, and determination of antibody titers using serial dilutions of serum samples or CSF with brain immunohistochemistry have been reported.1,5 For calculation of intrathecal synthesis of NMDAR antibodies, we multiplied the measured ratio of NMDAR antibody titer in CSF/serum samples with the calculated upper limit of IgG ratio between serum samples/CSF.10 In cases with unknown albumin ratio but normal CSF protein levels (<50 mg/dL), the albumin ratio was estimated using age-dependent, calculated albumin ratios.10
Statistics
One-way analysis of variance with Sidak-Holm post hoc test was used to assess differences in CXCL13 concentration between samples of patients with anti-NMDAR encephalitis and control individuals. Two-way analysis of variance with Sidak-Holm post hoc testing was used to compare CSF CXCL13 concentration in initial and follow-up samples between patients with limited and favorable response to treatment in samples obtained at different points after treatment initiation (months 1–2, months 2–6, and >month 6). Univariate general linear modeling was used to identify factors and interaction of factors correlating with initial CSF CXCL13 (eTable in the Supplement).3 We evaluated the correlation of CXCL13 with response to therapy by general linear modeling by including dichotomized functional impairment (independent living with no or mild symptoms [mRS<2] vs severe impairment necessitating help for daily activities [mRS≥3]) at 8 months after symptom onset as a factor representing response to treatment and applying this model to all monophasic patients treated within 90 days after symptom onset (n = 137). Levels of CSF CXCL13 in initial and follow-up samples of relapsing patients were compared using the Kruskal-Wallis test and Dunn post hoc test. The correlation between the initial CSF CXCL13 and CSF NMDAR antibody titer, serum titer, or intrathecal synthesis of antibodies was determined using Pearson correlation. Concentrations of CSF/serum CXCL13 below detection limit of the ELISA (<1 pg/mL) were set to 1 pg/mL. CXCL13 and NMDAR antibody titers were log-transformed because of skewed distribution. Means and 95% CIs were obtained by potentiating log-transformed data. The significance level α was set at .05, 2-tailed. Statistics were done using SPSS IBM version 22 and graphs by GraphPad Prism 6 for MacOS.
Results
Patients With Anti-NMDAR Encephalitis Have Increased CXCL13 Concentration in CSF
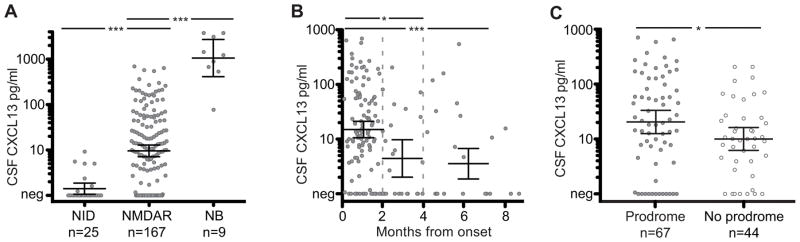
The mean concentration of CSF CXCL13 in patients with anti-NMDAR encephalitis was 9.6 pg/mL (95% CI, 7.2–12.9) compared with 1.4 pg/mL (95% CI, 1.1–1.9) in the control group without neuroinflammatory disorders (P < .001; Figure 1A). All patients with neuroborreliosis (positive control) had very high levels of CSF CXCL13 (mean, 1076 pg/mL; 95% CI, 419–2767; P < .001). There was no significant difference between the concentrations of CXCL13 in serum samples of patients with anti-NMDAR encephalitis and those of the control group without neuroinflammatory disorders (anti-NMDAR encephalitis: mean, 53.1 pg/mL, 95% CI, 33.6–85.7, n = 55 and control: mean, 55.2 pg/mL, 95% CI 32.4–94.4, n = 25; P = .78; eFigure 1 in the Supplement).
Figure 1. Cerebrospinal Fluid (CSF) C-X-C Motif Chemokine (CXCL13) Is Elevated at Early Stages of Anti–N-Methyl-D-Aspartate Receptor (NMDAR) Encephalitis and in Patients With Prodromal Symptoms.
A, Cerebrospinal fluid CXCL13 concentration measured in patients with noninflammatory disorders (NID, control group), anti- NMDA Rencephalitis (NMDAR), and neuroborreliosis (NB). B, Cerebrospinal fluid CXCL13 measured in samples from patients with anti-NMDAR encephalitis, showing disease duration in months. Samples obtained during months 1 to 2 (n = 113), months 3 to 4 (n = 21), or after month 4 (n = 33). C, Cerebrospinal fluid CXCL13 measured in patients with prodromal symptoms (filled circles) or without prodromal symptoms (open circles); samples were obtained during months 1 to 2. Data are presented as logarithmic means and 95% CIs of the mean. Concentrations of CSF CXCL13 below 1 pg/mL are depicted as negative (Neg). For detailed statistics, see the eTable in the Supplement.
aP < .001.
bP < .05.
Clinical Features Related to Increased CSF CXCL13 Concentration
We examined factors that correlated with high concentration of CSF CXCL13 in patients with anti-NMDAR encephalitis using general linear modeling (for detailed statistics, see the eTable in the Supplement). Overall, 96 of 167 patients (57%) with anti-NMDAR encephalitis had elevated CXCL13 (>7 pg/mL) in CSF. This proportion was higher (70%) at early stages of the disease (≤2 months from onset: 78 of 112 [70%]; >2 months: 18 of 55 [33%]; P = .001; Figure 1B). At the early stages of anti-NMDAR encephalitis (months 1–2), CSF CXCL13 concentration was higher in older patients (P = .005; eFigure 2A in the Supplement) and in patients with prodromal symptoms (P = .01; Figure 1C). When prodromal symptoms were present, the levels of CSF CXCL13 were higher in patients with a teratoma than in those without a teratoma (interaction of prodrome × tumor: P = .04; eTable in the Supplement). None of the patients had elevated serum CXCL13 levels (>1047 pg/mL; 0 of 55 [0%]).
Clinical Outcome and Follow-up of CSF CXCL13 Concentration
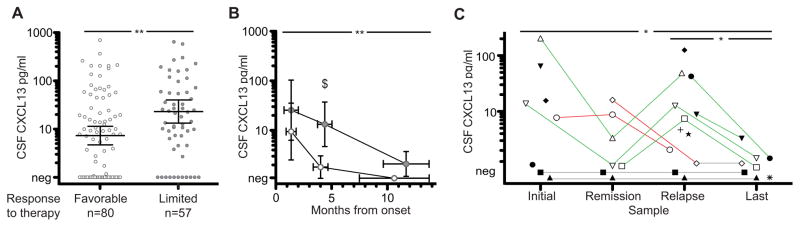
To determine the prognostic implications of CSF CXCL13 levels, we focused the study on a homogeneous subgroup of patients who received early treatment (within 90 days of symptom onset) and were treated similarly with first-line immunotherapies (steroids, intravenous immunoglobulin, plasma exchange, or tumor removal, if applicable). Among 137 patients fulfilling these criteria, those with higher CSF CXCL13 at the initial evaluation were more likely to have limited improvement (mRS score ≥3) at 8 months follow-up (P = .003; Figure 2A; eTable in the Supplement). These findings were independent of whether the sample examined for CXCL13 was acquired before or after initiation of first-line immunotherapy (only 9 patients [6.6%] had received second-line therapy at the time of sample acquisition; eTable in the Supplement). However, after determination of CXCL13 levels, 54 of 137 patients received second-line therapy. At the 8-month follow-up, 28 of 80 (35%) with favorable outcomes and 35 of 57 (61%) with limited improvement had received second-line immunotherapy (P = .003).
Figure 2. Cerebrospinal Fluid (CSF) C-X-C Motif Chemokine (CXCL13) Is Elevated in Patients With Limited Response to Therapy and in Patients With Relapses.
A, Cerebrospinal fluid CXCL13 measured in the first sample of patients with a favorable response to treatment (open circles; modified Rankin Scale score ≤2) or limited response (filled circles; modified Rankin Scale score ≥s3) to therapy; modified Rankin Scale score assessed 8 months after symptom onset. Samples obtained from patients with immunosuppressive treatment initiated within 90 days of symptom onset. Significance by general linear modeling corrected for covariates. B, Concentration of CSF CXCL13 measured in initial and follow-up samples of 35 patients with monophasic anti–N-methyl-D-aspartate receptor encephalitis with favorable (open circles) or limited response (filled circles) to treatment. Immunosuppressive treatment was initiated within 90 days of symptom onset. Samples acquired during months 1 to 2, 3 to 6, or more than 6 months after treatment initiation. Time from onset to sample acquisition is shown on the x-axis. Two-way analysis of variance showed a significant effect of treatment response (P = .003); the result of post hoc testing is indicated. The time from onset of disease to sample acquisition between response groups was not significantly different (horizontal error bars represent mean and 95% CI of the mean). C, Concentrations of CSF CXCL13 in patients with clinical relapses (n = 13) are shown. Patients are represented by individual symbols and consecutive samples are connected by a line. Black lines indicate expected changes (eg, increase of CXCL13 on relapse); orange lines, unexpected changes; and gray lines, persistently negative CSF CXCL13. Data are presented as logarithmic mean and 95% CI of the mean. Concentrations of CXCL13 below 1 pg/mL are depicted as negative (Neg). For detailed statistics, see the eTable in the Supplement.
aP < .01.
bP < .05.
cP = .02.
Although these findings were statistically significant, we noted a substantial overlap in the initial levels of CSF CXCL13 between patients with favorable and limited outcome. Therefore, we next investigated the period in which the detection of an increased CSF CXCL13 (>7 pg/mL) offered the best sensitivity and specificity for response to treatment. For these studies, follow-up CSF samples of 35 patients with monophasic illness and early treatment were available, demonstrating that CXCL13 in samples obtained between 2 and 6 months after initiation of immunotherapy offered the best sensitivity and specificity for outcome (P = .02; Figure 2B; eTable in the Supplement). In this analysis, the number of patients who received second-line immunotherapy was similar in both groups (patients with favorable response: 9 of 16 [56%] and patients with limited response: 14 of 19 [74%]; P = .31). Within this time frame, the CSF of 28 of 35 patients was available: 10 of 15 with limited response and 0 of 13 with complete response had elevated CSF CXCL13, indicating a specificity of 100% (95% CI, 75–100) and sensitivity of 67% (95% CI, 38–88).
Among 12 patients with assessable clinical relapses, 6 (50%) had increased CSF CXCL13 concentration in samples obtained during relapse. In contrast, only 2 of 7 (29%; no samples available for the other 5 patients) had increased CSF CXCL13 levels in samples obtained during symptom remission or improvement (P = .03; Figure 2C; eTable in the Supplement). In 1 of these 2 cases, the prerelapse sample was obtained during clinical improvement but within the first 2 months of the disease while most patients still have increased CXCL13 levels (Figure 2C, open diamond). All 3 patients examined who had normal CXCL13 levels at remission and later developed a clinical relapse had elevated CSF CXCL13 levels at relapse.
Correlation of CSF CXCL13 With NMDAR Antibody Titers
The correlation between CSF CXCL13 concentration and serum or CSF NMDAR antibody titers and intrathecal synthesis of antibodies was determined in 31 of 35 patients with monophasic disease and sufficient sample volume for studies. Of these, CSF titers could be evaluated in 30 patients, serum titers in 25, and intrathecal synthesis of antibodies in 24. These studies showed that the level of CSF CXCL13 correlated significantly with the level of intrathecal synthesis of NMDAR antibodies (P < .001; Figure 3) but not with CSF titers (eFigure 2B in the Supplement) or serum titers (not shown).
Figure 3. Cerebrospinal Fluid (CSF) C-X-C Motif Chemokine (CXCL13) Concentration Correlates With the Magnitude of Intrathecal Synthesis of N-Methyl-D-Aspartate Receptor (NMDAR) Antibodies.
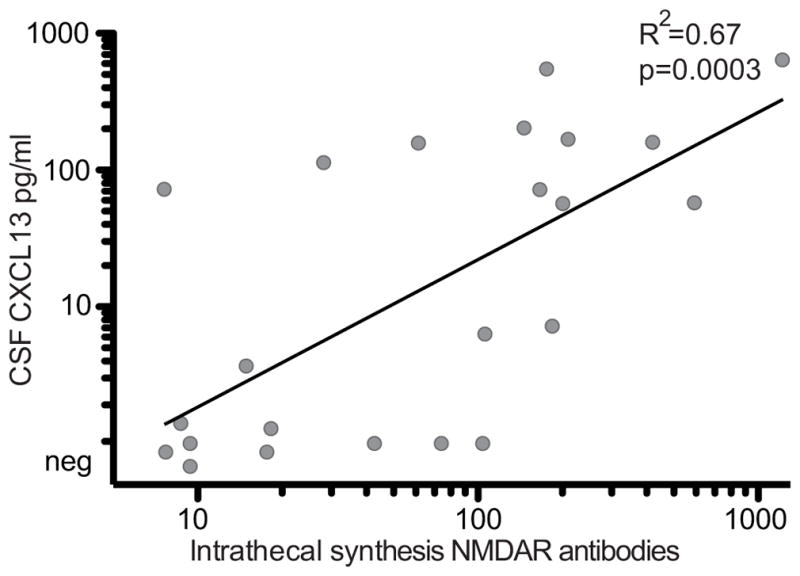
Concentration of CSF CXCL13 is plotted against the intrathecal NMDAR-antibody synthesis in the first available CSF sample (onset of neurological symptoms to sample: median, 1.6 months [interquartile range, 0.5–3.6 months]) of patients with monophasic anti-NMDAR encephalitis (n = 24). Pearson R2 and significance P value indicated. For graphical reasons, CSF CXCL13 concentrations below 1 pg/mL are depicted as negative (Neg).
Brain Inflammatory Infiltrates Express CXCL13
Immunostaining of human brain biopsy samples (eAppendix in the Supplement) obtained from a patient with anti-NMDAR encephalitis 22 days after symptom onset (case 5)4 revealed abundant CXCL13 expression in perivascular infiltrates that were predominantly made of activated monocytes/ macrophages, as well as in scattered activated microglia in the brain parenchyma (eFigure 3 in the Supplement).
Discussion
This study provides several novel findings regarding CXCL13 as an adjuvant biomarker during anti-NMDAR encephalitis: (1) at the early stage of the disease, 70% of the patients had increased concentration of CXCL13 in CSF but not in serum, (2) CSF CXCL13 concentration was higher in patients with prodromal symptoms and in older patients, (3) patients with limited response to therapy had higher and persistent elevation of CSF CXCL13 concentration compared with patients with favorable response to therapy, (4) a secondary increase of CSF CXCL13 concentration was observed in some patients with relapsing symptoms, (5) CSF CXCL13 levels correlated with intrathecal synthesis of NMDAR antibodies, and (6) expression of CXCL13 was identified in perivascular infiltrates of macrophages and microglia in the brain biopsy of a patient with this disorder.
One predictor of increased CSF CXCL13 in samples obtained at early stages of anti-NMDAR encephalitis was the presence of prodromal symptoms (headache and fever), and this association was more robust in patients with ovarian teratomas. CXCL13 is not only produced in response to activation of toll-like receptor 2 by membrane components of Borrelia burgdorferi but also by activation of toll-like receptor 7 by single-stranded DNA in viral infections.11–14 It is well known that anti-NMDAR encephalitis is frequently preceded or accompanied at the early stage of the disease by fever, headache, and sometimes meningeal signs. These features, often considered prodromal symptoms, and the current findings suggest that a potential inflammatory process (eg, meningeal inflammation, viral, or yet unknown) could trigger a CXCL13-mediated B-cell attraction and contribute to the development of anti-NMDAR encephalitis. Our findings and previous studies examining the levels of antibodies in patients with or without a teratoma suggest that this mechanism would be more intense in patients with strong or persistent priming of B-cells due to NMDAR-expressing teratomas. We also observed an association between young age and lower CSF CXCL13 level at the early stage of anti-NMDAR encephalitis; this age dependency is likely unrelated to the type of underlying disease given that a similar correlation has been noted in neuroborreliosis.15
As far as the possible correlation between levels of CXCL13 and outcome is concerned, the clinical significance of CSF CXCL13 became evident with prolonged follow-up. Indeed, within the first 2 months of first-line immunotherapy and tumor removal, if applicable, there was a substantial overlap of CSF CXCL13 levels between patients who eventually had favorable clinical recovery and those with limited recovery. However, at later follow-up (third to sixth month after treatment initiation), patients with limited improvement had CSF CXCL13 levels that were significantly higher than those who had substantial clinical recovery, and there was also much less overlap between groups. Of note, the use of second-line immunotherapy was similar between both groups (favorable response to immunotherapy: 9 of 16 [56%] and limited response: 14 of 19 [74%]; P = .31). These findings suggest that persistently elevated CSF CXCL13 levels are associated with limited recovery, probably reflecting an active autoimmune CNS process.
CXCL13 is the major chemoattractant of short-lived antibody-producing plasmablasts.8 Hypothetically, their attraction to the CNSs of patients with acute anti-NMDAR encephalitis drives the initial disease manifestation. If patients respond to therapy, the level of CSF CXCL13 would rapidly decline and plasmablasts undergo apoptosis, leading to a slow decrease of antibody titers and recovery. This is supported by a previous study showing a beneficial effect of rituximab eliminating plasmablasts.16 In limited responders, increased CSF CXCL13 levels persist and might attract circulating follicular T-helper cells expressing high amounts of CXCR5, the receptor of CXCL13.17,18 These cells would be able to orchestrate B-cells and antigen-presenting cells into ectopic lymphoid follicles, resulting in long-standing antibody production, a model similar to that proposed for multiple sclerosis.19–21 This could provide a plausible explanation for the observed delayed or limited response to therapy in patients with prolonged elevation of CSF CXCL13. This hypothesis is supported by 3 findings: the observation of brain and meningeal plasma cells in pathological studies of patients with anti-NMDAR encephalitis,4 the correlation between the course of the disease and CSF NMDAR antibody titers,5 and the association between intrathecal synthesis of NMDAR antibodies and levels of CSF CXCL13. The presence of abundant CXCL13-expressing cells and meningeal inflammatory infiltrates, but absence of secondary lymphoid structures in the biopsy of our patient, might well be due to the relatively short disease course of only 22 days.
In our study, 30% of patients with acute anti-NMDAR encephalitis did not have increased CSF CXCL13 concentration. There are several possible explanations for this finding. Levels of CXCL13 can rapidly normalize within 1 week in neuroborreliosis22 and rapid changes of CSF CXCL13 levels were also evident in some of our anti-NMDAR patients. Thus, nonstandardized sampling in a cohort of heterogeneously treated patients, as occurred in this retrospective study, might have missed transient changes in CXCL13 concentration. Furthermore, although CXCL13 appears to be a major contributor of B-cell attraction and the only one consistently elevated in multiple sclerosis8 and neuromyelitis optica, additional chemokines might potentially be involved (eg, B-cell–attracting factor [BAFF], a proliferation inducing ligand [APRIL], CXCL12, CCL19, and CCL21). In contrast to CXCL13, testing for these other chemokines is less available in routine diagnostics. In addition, patients with normal or low levels of CSF CXCL13 and limited response to treatment could have altered compensatory mechanisms of synaptic homeostasis, which was reported in a previous study.23 Future studies examining in a prospective manner the CSF levels of CXCL13 and other chemokines should provide answers to some of these questions.
Conclusions
Findings from this study have several practical implications. The assessment of CSF CXCL13 concentration between 2 and 6 months after starting therapy seems to be potentially useful for prognostic and treatment decisions. Within this time frame, the data reported here suggest that detection of elevated CXCL13 levels (>7 pg/mL) after first-line immunotherapy argue for the use of second-line therapy. Whether elevated CSF CXCL13 levels detected within 2 months of treatment initiation might also predict limited response needs to be evaluated in a prospective, homogeneously treated cohort. Moreover, in patients with a history of anti-NMDAR encephalitis, the development of atypical or partial symptoms accompanied by elevation of CSF CXCL13 suggests a relapse. This is important considering that NMDAR-antibody titers can remain detectable for a long time5,24 and their change along the course of the disease might be slower than that of CXCL13 levels.
Supplementary Material
Acknowledgments
Funding/Support: Dr Leypoldt was supported by Forschungsförderungsfonds Hamburg-Eppendorf. Dr Höftberger was partially funded by the Fonds zur Förderung der wissenschaftlichen Forschung, Austria, project J3230. Dr Titulaer was supported by an Erasmus MC fellowship and has received funding from the Netherlands Organisation for Scientific Research (NWO, Veni incentive). Dr Armangue has received a personal grant from the Instituto Carlos III (FI12/00366). Dr Gresa-Arribas has received funding from the Fundació la Marató de TV3. Dr Dalmau has received funding from the National Institutes of Health (grant RO1NS077851), Fundació la Marató TV3, and Fondo de Investigaciones Sanitarias (FIS, PI11/01780).
Footnotes
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions: Dr Leypoldt had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Leypoldt, Höftberger, and Titulaer contributed equally to the manuscript.
Study concept and design: Leypoldt, Titulaer, Rosenfeld, Dalmau.
Acquisition, analysis, or interpretation of data: Leypoldt, Höftberger, Titulaer, Armangue, Gresa-Arribas, Jahn, Rostasy, Schlumberger, Meyer, Wandinger, Graus, Dalmau.
Drafting of the manuscript: Leypoldt, Höftberger, Titulaer, Gresa-Arribas, Jahn, Schlumberger, Rosenfeld, Graus, Dalmau.
Critical revision of the manuscript for important intellectual content: Leypoldt, Titulaer, Armangue, Rostasy, Meyer, Wandinger, Rosenfeld, Dalmau.
Statistical analysis: Leypoldt, Titulaer, Dalmau.
Obtained funding: Leypoldt, Dalmau.
Administrative, technical, or material support: Armangue, Jahn, Meyer, Wandinger, Rosenfeld, Dalmau.
Study supervision: Graus, Dalmau.
Conflict of Interest Disclosures: Dr Leypoldt has received speakers honoraria and travel support from Grifols. Dr Titulaer has received travel support from Sun Pharma. Dr Schlumberger is an executive board member and shareholder of Euroimmun AG. Dr Meyer is a consultant to Spirig Pharma and has received speaker honoraria from Becton-Dickinson and Gilead. Dr Rosenfeld holds a patent for the use of N-methyl-D-aspartate receptor as an autoantibody test and receives royalties for antibody testing. Dr Dalmau received a research grant from Euroimmun and holds a patent application for the use of N-methyl-D-aspartate receptor as an autoantibody test; he receives royalties for this antibody testing. No other disclosures were reported.
Additional Contributions: We thank Mercè Alba, Eva Caballero, and Esther Aguilar (August Pi i Sunyer Biomedical Research Institute [IDIBAPS], Service of Neurology, Hospital Clínic, University of Barcelona, Barcelona, Spain) for excellent technical support. They did not receive compensation from a funding sponsor for their contributions. We also thank the patients and their families for volunteering to participate in this study.
References
- 1.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30(17):5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Hernandez E, Horvath J, Shiloh-Malawsky Y, Sangha N, Martinez-Lage M, Dalmau J. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. 2011;77(6):589–593. doi: 10.1212/WNL.0b013e318228c136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167–177. doi: 10.1016/S1474-4422(13)70282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong X, Wang H, Dai Y, et al. Cerebrospinal fluid levels of CXCL13 are elevated in neuromyelitis optica. J Neuroimmunol. 2011;240–241:104–108. doi: 10.1016/j.jneuroim.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Khademi M, Kockum I, Andersson ML, et al. Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult Scler. 2011;17(3):335–343. doi: 10.1177/1352458510389102. [DOI] [PubMed] [Google Scholar]
- 8.Kowarik MC, Cepok S, Sellner J, et al. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J Neuroinflammation. 2012;9:93. doi: 10.1186/1742-2094-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mygland A, Ljøstad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I European Federation of Neurological Societies. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol. 2010;17(1):8–16. e1–4. doi: 10.1111/j.1468-1331.2009.02862.x. [DOI] [PubMed] [Google Scholar]
- 10.Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci. 2001;184(2):101–122. doi: 10.1016/s0022-510x(00)00501-3. [DOI] [PubMed] [Google Scholar]
- 11.Krumbholz M, Derfuss T, Hohlfeld R, Meinl E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol. 2012;8(11):613–623. doi: 10.1038/nrneurol.2012.203. [DOI] [PubMed] [Google Scholar]
- 12.Baker DG, Woods TA, Butchi NB, et al. Toll-like receptor 7 suppresses virus replication in neurons but does not affect viral pathogenesis in a mouse model of Langat virus infection. J Gen Virol. 2013;94(pt 2):336–347. doi: 10.1099/vir.0.043984-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rupprecht TA, Kirschning CJ, Popp B, et al. Borrelia garinii induces CXCL13 production in human monocytes through toll-like receptor 2. Infect Immun. 2007;75(9):4351–4356. doi: 10.1128/IAI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayan K, Dail D, Li L, et al. The nervous system as ectopic germinal center: CXCL13 and IgG in lyme neuroborreliosis. Ann Neurol. 2005;57(6):813–823. doi: 10.1002/ana.20486. [DOI] [PubMed] [Google Scholar]
- 15.Wutte N, Berghold A, Löffler S, et al. CXCL13 chemokine in pediatric and adult neuroborreliosis. Acta Neurol Scand. 2011;124(5):321–328. doi: 10.1111/j.1600-0404.2010.01477.x. [DOI] [PubMed] [Google Scholar]
- 16.Hachiya Y, Uruha A, Kasai-Yoshida E, et al. Rituximab ameliorates anti-N-methyl-D-aspartate receptor encephalitis by removal of short-lived plasmablasts. J Neuroimmunol. 2013;265(1–2):128–130. doi: 10.1016/j.jneuroim.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Krumbholz M, Theil D, Cepok S, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129(pt 1):200–211. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- 18.Kivisäkk P, Imitola J, Rasmussen S, et al. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65(4):457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134(Pt 9):2755–2771. doi: 10.1093/brain/awr182. [DOI] [PubMed] [Google Scholar]
- 20.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14(2):164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chevalier N, Jarrossay D, Ho E, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186(10):5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 22.Senel M, Rupprecht TA, Tumani H, Pfister HW, Ludolph AC, Brettschneider J. The chemokine CXCL13 in acute neuroborreliosis. J Neurol Neurosurg Psychiatry. 2010;81(8):929–933. doi: 10.1136/jnnp.2009.195438. [DOI] [PubMed] [Google Scholar]
- 23.Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice-Gordon RJ. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;76(1):108–119. doi: 10.1002/ana.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen HC, Klingbeil C, Dalmau J, Li W, Weissbrich B, Wandinger KP. Persistent intrathecal antibody synthesis 15 years after recovering from anti-N-methyl-D-aspartate receptor encephalitis. JAMA Neurol. 2013;70(1):117–119. doi: 10.1001/jamaneurol.2013.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.