ABSTRACT
Dental materials in general are tested in different animal models prior to the clinical use in humans, except for bleaching agents.
Objectives
To evaluate an experimental rat model for comparative studies of bleaching agents, by investigating the influence of different concentrations and application times of H2O2 gel in the pulp tissue during in-office bleaching of rats’ vital teeth.
Material and Methods
The right and left maxillary molars of 50 Wistar rats were bleached with 20% and 35% H2O2 gels, respectively, for 5, 10, 15, 30, or 45 min (n=10 rats/group). Ten animals were untreated (control). The rats were killed after 2 or 30 days, and the maxillae were examined by light microscopy. Inflammation was evaluated through histomorphometric analysis with inflammatory cell count in the coronal and radicular thirds of the pulp. Fibroblasts were also counted. Scores were attributed to odontoblastic layer and vascular changes. Tertiary dentin area and pulp chamber central area were measured histomorphometrically. Data were compared by analysis of variance and Kruskal-Wallis test (p<0.05).
Results
After 2 days, the amount of inflammatory cells increased in the coronal pulp occlusal third up to the 15-min application groups of each bleaching gel. In the groups exposed to each concentration for 30 and 45 min, the number of inflammatory cells decreased along with the appearance of necrotic areas. After 30 days, reduction on the pulp chamber central area and enlargement of the tertiary dentin area were observed, without the detection of inflammation areas.
Conclusion
The rat model of extracoronal bleaching showed to be adequate for studies of bleaching protocols, as it was possible to observe alterations in the pulp tissues and tooth structure caused by different concentrations and application periods of bleaching agents.
Keywords: Bleaching agents, Animal models, Hydrogen peroxide
INTRODUCTION
In-office bleaching with H2O2 gel is considered to be a conservative and affordable aesthetic treatment 18 . Its effectiveness is attributable to the low molecular mass of the main active compound, H2O2, which easily diffuses through enamel and dentin, and releases reactive oxygen species (ROS), thus oxidizing organic structures 2 .
Importantly, H2O2 and its by-products have varying biological effects on human oral tissues 30 . ROS-induced oxidative stress can cause mutation, enzyme inactivation, protein degradation, and fragmentation in pulp cells, which might manifest as pulpitis and tooth sensitivity 3 . The severity of pulp damage depends on the in-office dental bleaching protocol used, and this procedure has been increasingly questioned 2 , 6 , 8 .
An increase in vascular permeability depending on the duration of the bleaching procedures has been observed in rats’ incisors 12 . A 30-min bleaching session using 35% H2O2 gel, with or without heat, caused a severe inflammatory reaction in the dental pulp of dogs, including increased deposition of reparative dentin, thinning of the odontoblastic layer, inflammatory infiltration, and internal root resorption. Some of the changes, such as inflammation and bleeding, reversed after 60 days 25 . In humans, in-office bleaching of mandibular incisors by using the abovementioned protocol caused partial necrosis in the coronal pulp and a mild inflammatory reaction in the radicular pulp 8 . Moreover, 45-min bleaching with 35% H2O2 gel resulted in necrosis near to the pulp horns in rats 6 . On the other hand, the application of 38% H2O2 gel on human premolars did not cause pathological changes in the dental pulp 17 . Therefore, it is evident that anatomical characteristics of the teeth and the in vivo model analyzed, as well as the bleaching protocols employed, determined different results.
Thus, the lesser thickness of enamel and dentin in teeth of rats might allow greater penetration of H2O2, and consequently more damage to pulp tissues 8 . Therefore, it is essential to characterize the experimental model in rats, in order to find an appropriate protocol to be applied in this model and to allow the conduction of further studies on H2O2 damage to pulp tissues. This model will enable the evaluation of new dosages, formulations and concentrations of bleaching agents that arise in the market, in addition to the evaluation of potential therapeutic agents that may be used to minimize the damage caused by H2O2 to the pulp tissue, in different application protocols 6 , 9 .
The choice of rats was due to the ease of standardization and control of these animals, and the possibility of performing other tests 7 , 9 . Thus, it is possible to study different variables in order to, in a second stage, with results already standardized and evaluated in animals, propose the validation of these results in humans, with smaller groups, following ethical principles 9 . Researches involving both dog and human teeth to study bleaching protocols are impractical because of the difficulty in obtaining the required sample as well as ethical principles. Furthermore, Cintra, et al. 6 (2013), when analyzing the influence of the number of bleaching sessions on pulp tissues, indicated the possibility of using teeth of rats for the study of bleaching protocols. Using the rat model for studying bleaching agents is relatively simple and easy to reproduce.
Therefore, the purpose of this study was to characterize an experimental animal model for comparative studies of bleaching agents, by investigating the influence of different concentrations and application times of H2O2 gel during in-office bleaching of rats’ vital teeth. It was hypothesized that: (I) the H2O2 in bleaching gel is capable of penetrating pulp tissue and causing greater damages with increasing time of application and H2O2 concentration; (II) pulp tissue is capable of recuperating from the damages caused by H2O2 after long periods of time.
MATERIAL AND METHODS
Animals
Sixty male Wistar rats (180-200g) were used in this study. The animals were housed in a temperature-controlled environment (22°C±1°C) on a standard light–dark schedule with unrestricted access to food and water. The experimental protocol was approved by the Ethics Committee (CEUA 2013-01253) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD).
Tooth bleaching
The rats were anesthetized with intramuscular injections of ketamine (87 mg/kg; Francotar, Virbac do Brasil Ind e Com Ltda, Roseira, SP, Brazil) and xylazine (13 mg/kg; Rompum, Bayer SA, São Paulo, SP, Brazil). The right and left molars in every animal were bleached with 20% (Whiteness HP Blue, FGM Dental Products, Joinville, SC, Brazil) and 35% H2O2 (Whiteness HP Maxx, FGM Dental Products, Joinville, SC, Brazil), respectively, for 5, 10, 15, 30, or 45 min (n=10 rats/group). Ten animals (controls) did not receive any treatment.
Histology
Animals were killed with an overdose of the anesthetic solution 2 or 30 days after the bleaching sessions. Their bilateral maxillae were separated, dissected, and fixed in a 10% buffered formalin solution for 24 h. The specimens were decalcified in a 10% ethylenediaminetetraacetic acid (EDTA) solution for three months, and then dehydrated in a graded ethanol series, embedded in paraffin, cut into 6-μm sagittal cross-sections, and stained with hematoxylin and eosin (H&E).
The serial histological sections of each specimen were selected from the point where the mesial root of the first molar was seen in all its longitudinal extension.
The coronal pulp was divided into occlusal, middle, and cervical thirds and the radicular pulp was divided into cervical, middle, and apical thirds 6 . Inflammation was evaluated through histomorphometric analysis with inflammatory cell count in the coronal and radicular thirds of the pulp. Fibroblasts were also counted. The cell count was performed in a 10 μm2 field in each third of the pulp tissue of each specimen, examined under light microscopy (1000× magnification; DM4000 B, Leica Microsystems, Wetzlar, Hesse, Germany).
Scores were attributed to the odontoblastic layer in each third of the pulp tissue, as follows: 1- intact odontoblastic layer; 2- disorganized odontoblastic layer; or 3-disruption of the odontoblastic layer. Scores for vascular changes were also assigned as follows: 1- normality; 2- increase in the number of blood vessels; or 3- necrosis.
The mean central area of the pulp chamber was measured by image processing software (Leica QWin V3, Leica Microsystems, Wetzlar, Hesse, Germany) (Figure 1). With the values obtained, it was possible to calculate the percentage reduction in the central area of the pulp chamber in the treated groups, considering the central area of the control group.
Figure 1. Central area measurement of the pulp chamber in the experimental groups using the Leica QWin V3 Image Processing and Analysis Software (Leica Microsystems, Wetzlar, Hesse, Germany). The values obtained were analyzed by the Kolmogorov-Smirnov normality test and the one-way analysis of variance (p<0.05).
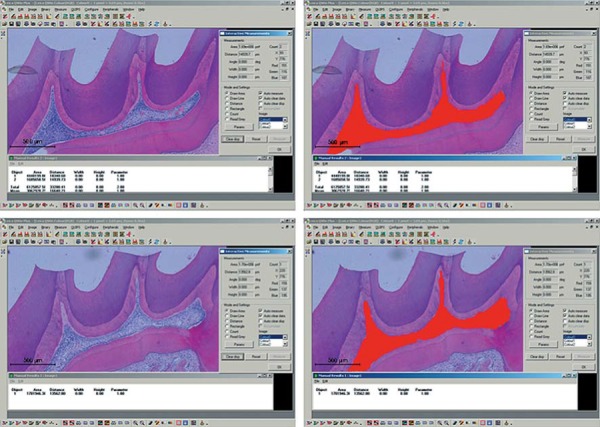
After the application of the Kolmogorov-Smirnov test of normality, the data obtained in counts of inflammatory cells and fibroblasts were submitted to two-way analysis of variance and Tukey’s test for intergroup comparisons at the significance level of 5% (p<0.05). The scores obtained in the analysis of odontoblastic layer and vascular changes were submitted to Kruskal-Wallis and Dunn’s tests (p<0.05). The values obtained in the mean central area of the pulp chamber were submitted to Kolmogorov-Smirnov test of normality and one-way analysis of variance (p<0.05).
RESULTS
Inflammatory response
Control group
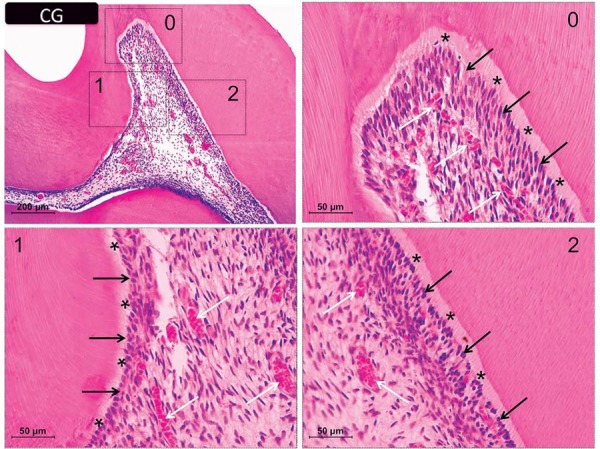
The dental pulp of the control animals exhibited well-defined acellular and cell-rich layers under an intact odontoblastic layer and an even distribution of cells, blood vessels, and extracellular matrix structures (Figure 2).
Figure 2. Representative images of hematoxylin & eosin-stained sections showing the coronal pulp of the controls. Panels 0, 1, and 2 are magnified images (400×) of the respective insets in the upper left panel (100× magnification). The black arrows indicate the odontoblastic layer and the white arrows show the distribution of cells and blood vessels in the subjacent tissue. Asterisks indicate the predentin layer.
20%–5 min group
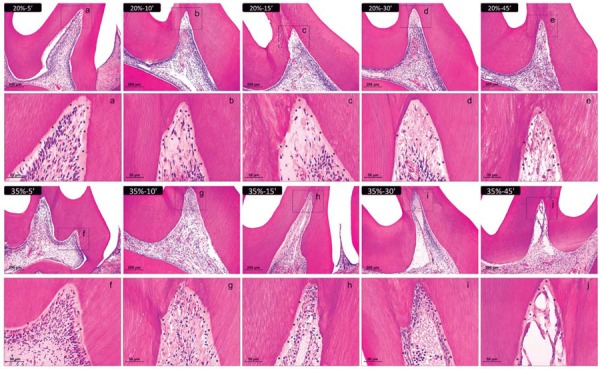
This group exhibited no inflammatory infiltrate. The dental pulp appeared similar to that of the control group. The odontoblastic layer was intact and the blood vessels showed normal characteristics. The cementum, periodontal ligament, alveolar bone, and other supporting structures also seemed normal (Figure 3A).
Figure 3. Representative images of hematoxylin & eosin-stained sections showing the coronal pulp 2 days after bleaching. Panels A, B, C, D, and E represent the groups treated with 20% H2O2 gel and panels F, G, H, I, and J represent those treated with 35% H2O2 gel for 5, 10, 15, 30, and 45 min, respectively (100× magnification). Panels a–j are magnified images (400×) of the insets in panels A–J, respectively. Asterisks indicate the predentin layer. The number of inflammatory cells and fibroblasts was obtained in each third of the pulp tissue (at 1000× magnification) and subjected to the Kolmogorov-Smirnov normality test, two-way analysis of variance, and Tukey’s test (p<0.05); the scores of odontoblastic layer and vascular changes underwent Kruskal-Wallis and Dunn’s tests (p<0.05).
20%–10 min group
This group did not exhibit a considerable amount of inflammatory cells. There was a reduction in the amount of fibroblasts in the occlusal and middle thirds of the coronal pulp. The odontoblastic layer was partially disorganized in the occlusal third, and there was an increase in the number of blood vessels in the occlusal and middle thirds of the coronal pulp (Figure 3B).
20%–15 min group
There was an increased amount of inflammatory cells in the occlusal and middle thirds of the coronal pulp, the amount of fibroblasts was reduced, and there was an increased amount of blood vessels. The odontoblastic layer was partially disorganized in the occlusal third (Figure 3C).
20%–30 min group
The highest number of inflammatory cells was found in the middle third of the coronal pulp in this group. There was a large reduction in the amount of fibroblasts in the occlusal third, where there was disruption of the odontoblastic layer. The amount of blood vessels increased in the occlusal and middle thirds of the coronal pulp (Figure 3D). The radicular pulp seemed normal in all cases.
20%–45 min group
This group showed an increased number of inflammatory cells in the cervical and middle thirds of the coronal pulp. The occlusal third showed necrotic areas. A reduction in the number of fibroblasts was observed in the cervical third of the crown. The odontoblastic layer was absent in the occlusal third and partly disorganized in the middle third of the coronal pulp. There was an insignificant amount of inflammatory cells in the cervical third of the radicular pulp (Figure 3E).
35%–5 min group
The amount of inflammatory cells in this group was not significant. The amount of fibroblasts reduced in the occlusal third of the coronal pulp, where the odontoblastic layer was partially disorganized. An increase in the number of blood vessels was observed in all areas of the coronal pulp (Figure 3F).
35%–10 min group
There was an increase in the number of inflammatory cells in the occlusal and middle thirds of the coronal pulp in this group. The amount of fibroblasts reduced in the occlusal third. The odontoblastic layer was absent in the occlusal third, and partly disorganized in the middle third of the coronal pulp. There was an increase in the number of blood vessels throughout the coronal pulp (Figure 3G).
35%–15 min group
There was an increase in the number of inflammatory cells in the occlusal and middle thirds of the coronal pulp, while the number of fibroblasts reduced. The odontoblastic layer was absent in the occlusal third, and partly disorganized in the middle third of the coronary pulp. There was an increase in the number of blood vessels throughout the coronal pulp (Figure 3H).
35%–30 min group
The number of inflammatory cells and fibroblasts reduced in the occlusal third, where necrotic areas were present. There was an increase in the number of inflammatory cells in the middle and cervical thirds of the coronary pulp. The amount of fibroblasts was still low in the middle third of the coronary pulp. The odontoblastic layer was absent in the occlusal and middle thirds of the crown. There was an increase in the number of blood vessels in the middle and cervical thirds of the coronal pulp. The occlusal third was characterized as necrotic. A small amount of inflammatory cells was found in the cervical third of the radicular pulp (Figure 3I).
35%–45 min group
There was necrosis in the occlusal third of this group, with absence of inflammatory cells and fibroblasts. The number of inflammatory cells increased in the cervical and middle thirds of the coronal pulp, and in the cervical third of the radicular pulp. The number of fibroblasts reduced in these thirds. The odontoblastic layer was absent in the occlusal and middle thirds of the crown, and partially disorganized in the cervical third. The number of blood vessels increased in the cervical third of the coronary and radicular pulp. The remaining thirds seemed normal (Figure 3J).
Reparative dentin area
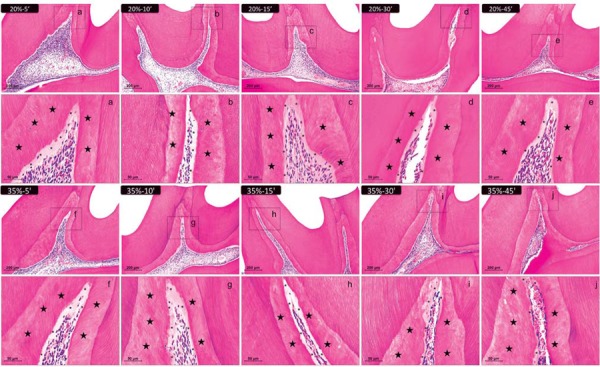
Thirty days after the bleaching sessions, all the specimens showed normal dental pulp. However, the central area of the pulp chamber reduced, and the tertiary dentin area increased (Figure 4).
Figure 4. Representative images of hematoxylin & eosin-stained sections showing the coronal pulp 30 days after bleaching. Panels A, B, C, D, and E represent the groups treated with 20% H2O2 gel and panels F, G, H, I, and J represent those treated with 35% H2O2 gel for 5, 10, 15, 30, and 45 min, respectively (100× magnification). Panels a–j are magnified images (400×) of the insets in panels A–J, respectively. Stars indicate the reparative dentin layer; asterisks indicate the predentin layer. The values of the pulp chamber area were obtained as shown in Figure 1 to carry out the statistical analysis.
Intergroup comparisons
Table 1 shows the amount of inflammatory cells of the experimental groups. The most predominant inflammatory cells found were mononuclear cells, such as lymphocytes, macrophages and plasmocytes, characterizing a chronic inflammatory infiltrate. The amount of inflammatory cells gradually increased with increasing concentrations and application time of the bleaching gel, up to the 15-min application groups of each bleaching gel, in the occlusal third of the coronal pulp. The groups that received the application of 30 and 45-min of each bleaching agent showed areas of necrosis in the occlusal third with a decrease in the amount of inflammatory cells. Significant differences were observed between the bleached groups and the control group in the occlusal third (p<0.05), except for the 35%–45 min group, which presented absence of cells. Significant differences in the middle third of the coronal pulp were noted between the control group and the 20%–10 to 45 min and 35%–5 to 45 min groups (p<0.05). In the cervical third, the difference from the control group was also present in the 20%–15 to 45 min and 35%–10 to 45 min groups (p<0.05). In the cervical third of the radicular pulp, significant differences were noted between the 20%–45 min and 35%–30 min groups from the other groups, and between the 35%–45 min group and all groups. Significant differences were not observed in the other radicular thirds (p>0.05).
Table 1. Inflammatory cell count (per 10 μm2) in the pulp thirds of each group (mean ±standard deviation).
| Group | Coronal | Radicular | |||||
|---|---|---|---|---|---|---|---|
| Occlusal | Middle | Cervical | Cervical | Middle | Apical | ||
| Control | 0.0 ±0.0a | 0.0 ±0.0a | 0.0 ±0.0a | 0.0 ±0.0a | 0.0 ±0.0a | 0.0 ±0.0a | |
| 20% H2O2 gel | 5 min | 3.6 ±0.5b | 2.2 ±0.4ab | 1.0 ±0.4ab | 0.0 ±0.0a | 0.0 ±0.0a | 0.0 ±0.0a |
| 10 min | 5.6 ±1.1bc | 4.6 ±1.1bcd | 2.2 ±0.5abc | 0.0 ±0.0a | 0.0 ±0.0a | 0.0 ±0.0a | |
| 15 min | 7.6 ±1.5c | 6.0 ±1.4ce | 3.4 ±0.9bd | 0.0 ±0.0a | 0.0 ±0.0a | 0.0 ±0.0a | |
| 30 min | 5.2 ±0.8bc | 8.2 ±1.3e | 5.2 ±1.1d | 0.0 ±0.0a | 0.0 ±0.0a | 0.0 ±0.0a | |
| 45 min | 3.2 ±0.8b | 13.0 ±2.9fg | 9.0 ±2.3e | 5.6 ±0.9b | 0.0 ±0.0a | 0.0 ±0.0a | |
| 35% H2O2 gel | 5 min | 4.0 ±0.7b | 4 ±1.0bc | 2.2 ±0.5abc | 0.0 ±0.0a | 0.0 ±0.0a | 0.0 ±0.0a |
| 10 min | 10.6 ±2.3d | 7.4 ±1.1de | 4.4 ±1.1cd | 0.0 ±0.0a | 0.0 ±0.0a | 0.0 ±0.0a | |
| 15 min | 14.6 ±3.6e | 11.8 ±2.3f | 5.2 ±1.3d | 0.0 ±0.0a | 0.0 ±0.0a | 0.0 ±0.0a | |
| 30 min | 6.0 ±1.4bc | 15.6 ±1.3g | 9.8 ±2.6e | 5.8 ±0.8b | 0.0 ±0.0a | 0.0 ±0.0a | |
| 45 min | 0.0 ±0.0a | 12.0 ±2.9f | 11.0 ±2.6e | 7.6 ±1.5c | 0.0 ±0.0a | 0.0 ±0.0a | |
*Different letters in the columns indicate significant difference between the groups (Kolmogorov-Smirnov normality test and the Two Way ANOVA and Tukey test - P<0.05)
Table 2 shows the amount of fibroblasts of the experimental groups. The 20%–10 to 45 min and 35% groups showed a significant decrease in the number of fibroblasts in the occlusal third, compared with the control group (p<0.05). This decrease was also present in the middle third of the coronal pulp in the 20%–15 to 45 min and 35%–15 to 45 min groups (p<0.05). In the cervical third, only the groups that received the bleaching gels for 45 min showed a significant difference from the control group (p<0.05). Significant differences were not observed in the radicular thirds of any group (p>0.05).
Table 2. Fibroblast count (per 10 μm2) in the pulp thirds of each group (mean ±standard deviation).
| Group | Coronal | Radicular | |||||
|---|---|---|---|---|---|---|---|
| Occlusal | Middle | Cervical | Cervical | Middle | Apical | ||
| Control | 65.2 ±8.7a | 67.0 ±7.8ab | 44.0 ±4.6abc | 38.0 ±3.7a | 33.8 ±2.9a | 37.2 ±3.3a | |
| 20% H2O2 gel | 5 min | 51.8 ±6.6ab | 72.2 ±11.9a | 52.8 ±8.5bc | 39.4 ±4.2a | 35.8 ±3.0a | 37.0 ±2.3a |
| 10 min | 45.2 ±9.2bc | 54.0 ±4.2bc | 55.6 ±11.2c | 36.8 ±2.9a | 37.6 ±3.1a | 35.0 ±3.2a | |
| 15 min | 36.2 ±3.6c | 46.4 ±2.4cd | 43.2 ±8.4ac | 41.2 ±0.8a | 38.2 ±3.6a | 35.2 ±3.3a | |
| 30 min | 5.8 ±1.6de | 32.4±7.5de | 34.6 ±5.5ad | 39.6 ±1.9a | 36.2 ±3.8a | 37.2 ±2.2a | |
| 45 min | 0.0 ±0.0d | 26.0 ±1.6efg | 29.0 ±8.2def | 36.0 ±4.5a | 37.6 ±4.2a | 38.2 ±2.6a | |
| 35% H2O2 gel | 5 min | 15.2 ±2.8e | 64.8 ±11.3ab | 40.8 ±8.0abe | 35.2 ±2.9a | 34.8 ±3.9a | 39.0 ±2.3a |
| 10 min | 13.2±3.0de | 54.6±12.4abc | 35.2 ±3.6ae | 38.6 ±1.5a | 36.2 ±4.3a | 37.6 ±3.6a | |
| 15 min | 10.8±2.9de | 41.6±9.7cdf | 34.2 ±3.0ae | 37.6 ±2.5a | 38.8 ±1.6a | 37.6 ±4.3a | |
| 30 min | 5.8±0.8de | 23.2±5.0eg | 30.4 ±4.0aef | 36.0 ±4.2a | 37.8 ±2.2a | 39.6 ±4.5a | |
| 45 min | 0.0 ±0.0d | 10.2±2.7g | 19.6±1.8f | 38.0±2.7a | 36.8 ±2.6a | 40.2 ±4.2a | |
*Different letters in the columns indicate significant difference between the groups (Kolmogorov-Smirnov normality test and the Two Way ANOVA and Tukey test - P<0.05)
Table 3 shows the scores assigned to the odontoblast layer of each experimental group. In the occlusal third, the 20%–45 min and 35%–30 and 45 min groups differed significantly from the control and 20%–5 min groups (p<0.05). In the middle third of the coronal pulp, the 35%–45 min group also differed significantly from the control and 20%–5 to 30 min groups (p<0.05). There were no significant differences in the cervical third and radicular thirds (p>0.05).
Table 3. Comparison of odontoblastic layer scores (median).
| Group | Coronal | Radicular | |||||
|---|---|---|---|---|---|---|---|
| Occlusal | Middle | Cervical | Cervical | Middle | Apical | ||
| Control | 1a | 1a | 1a | 1a | 1a | 1a | |
| 20% H2O2 gel | 5 min | 1a | 1a | 1a | 1a | 1a | 1a |
| 10 min | 2ab | 1a | 1a | 1a | 1a | 1a | |
| 15 min | 2ab | 1a | 1a | 1a | 1a | 1a | |
| 30 min | 3ab | 1a | 1a | 1a | 1a | 1a | |
| 45 min | 3b | 2ab | 1a | 1a | 1a | 1a | |
| 35% H2O2 gel | 5 min | 2ab | 1ab | 1a | 1a | 1a | 1a |
| 10 min | 3ab | 2ab | 1a | 1a | 1a | 1a | |
| 15 min | 3ab | 2ab | 1a | 1a | 1a | 1a | |
| 30 min | 3b | 3ab | 1a | 1a | 1a | 1a | |
| 45 min | 3b | 3b | 2a | 1a | 1a | 1a | |
*Different letters in the columns indicate significant difference between the groups (Kruskal-Wallis and Dunn tests - P<0.05)
Table 4 shows the scores assigned to the vascular changes in each experimental group. The 20%–45 min and 35%–45 min groups differed significantly from the control and 20%–5 min groups in the occlusal third (p<0.05). In the middle third of the coronal pulp, the 35%–45 min group also differed significantly from the control and 20%–5 groups (p<0.05). There was no significant difference in the cervical third and radicular thirds (p>0.05).
Table 4. Comparison of scores of vascular changes (median).
| Group | Coronal | Radicular | |||||
|---|---|---|---|---|---|---|---|
| Occlusal | Middle | Cervical | Cervical | Middle | Apical | ||
| Control | 1a | 1a | 1a | 1a | 1a | 1a | |
| 20% H2O2 gel | 5 min | 1a | 1a | 1a | 1a | 1a | 1a |
| 10 min | 2ab | 2ab | 1a | 1a | 1a | 1a | |
| 15 min | 2ab | 2ab | 1a | 1a | 1a | 1a | |
| 30 min | 2ab | 2ab | 1a | 1a | 1a | 1a | |
| 45 min | 3b | 2ab | 1a | 1a | 1a | 1a | |
| 35% H2O2 gel | 5 min | 2ab | 2ab | 2a | 1a | 1a | 1a |
| 10 min | 2ab | 2ab | 2a | 1a | 1a | 1a | |
| 15 min | 2ab | 2ab | 2a | 1a | 1a | 1a | |
| 30 min | 3ab | 2ab | 2a | 1a | 1a | 1a | |
| 45 min | 3b | 3b | 2a | 1a | 1a | 1a | |
*Different letters in the columns indicate significant difference between the groups (Kruskal-Wallis and Dunn tests - P<0.05)
At 30 days, the specimens showed a gradual increase in the tertiary dentin area. Significant differences were observed between the 35%–45 min group and the other groups, except for the 35%–30 min group (p<0.05). The 20%–5 min, 20%–10 min, 20%–15 min, and 35%–5 min groups did not differ significantly from the control group (p>0.05) (Table 5).
Table 5. Change in the pulp chamber central area (μm2).
| Mean (105)* | SD (105) | % reduction | ||
|---|---|---|---|---|
| Control | 18.46a | 2.38 | 0.00 | |
| 20% H2O2 gel | 5 min | 17.57a | 2.30 | 4.82 |
| 10 min | 17.18a | 1.85 | 6.93 | |
| 15 min | 15.65ab | 1.78 | 15.22 | |
| 30 min | 13.47bc | 1.43 | 27.03 | |
| 45 min | 11.23cd | 1.17 | 39.16 | |
| 35% H2O2 gel | 5 min | 14.61ab | 0.46 | 20.85 |
| 10 min | 13.41bc | 0.72 | 27.36 | |
| 15 min | 12.56bcd | 0.71 | 32.33 | |
| 30 min | 10.01de | 0.62 | 45.77 | |
| 45 min | 6.98e | 0.51 | 62.18 |
*Different letters in the column indicate significant difference (Kolmogorov-Smirnov normality test and the One Way ANOVA test - P<0.05)
DISCUSSION
Tooth bleaching is an aesthetic alternative for discolored teeth, but it has potential adverse effects that are not yet completely understood 28 . A single bleaching session can produce significant aesthetic results, but longer application time and multiple sessions may be required for optimal outcomes, increasing the risk of tooth sensitivity 20 and pulp damage 6 .
A large number of in vitro studies have shown that ROS generated by the H2O2 of bleaching gels are capable of causing histochemical and morphological changes in enamel and dentin 4 , 5 .
In vivo studies showed cellular damage, classified as mild to severe. These include studies performed in dog teeth 25 , 26 , human mandibular incisors 8 , 19 , rat incisors 12 , 13 , and rat molars 6 . Cell culture studies also demonstrated cellular damage as apoptosis 14 , inflammation 3 , cytotoxicity 30 , damage to the DNA 23 , cell viability reduction 27 , or ageing of the dental pulp 1 , 28 . The cytotoxicity of bleaching gel to pulp tissue was also observed in this study.
Studies predominantly with ex vivo manipulated cells have significant importance in preliminary studies of bleaching agents. However, in those studies, the pulp cells are not examined as organized tissues. Teeth have vital pulp components that can prevent or hinder the H2O2 effects in pulp tissues, such as dentinal fluid, cytoplasmatic extensions 17 , 30 , and antioxidant enzymes as superoxide dismutase and catalase, which promote an enzymatic degradation of H2O2 11 , 17 . Therefore, in vivo experiments are the ones that best represent the reality of bleaching effects.
Application of 38% H2O2 gel on human premolars does not cause pathological changes in the dental pulp 17 . However, application of the same concentration on human mandibular incisors causes necrosis in the coronal pulp, similar to what was observed in rat molars 6 , possibly because of the thinner enamel and dentin 8 . These findings indicate that morphological characteristics of different tooth structures influence pulp damage directly. Ideally, upper anterior human teeth should be used to exactly determine pulp changes. Even under these conditions, other factors would influence the results, such as age, presence of restorations, previous trauma, among others.
Even though variations of the pulp response have been shown in human teeth, our study aimed to characterize an experimental animal model of easy reproduction and standardization for the study of new bleaching agents, posology, concentrations, and application time.
In dog teeth, dental bleaching using 35% H2O2 showed greater changes immediately beneath the region where the gel was applied 25 , similar to the findings in this study with 35% H2O2 gel applied for 30 or 45 min and 20% H2O2 gel for 45 min. Severe pulp damage may occur when bleaching agents are applied on the buccal surface of teeth with thin enamel and dentin 8 , 25 , 26 . Dog teeth present difficulties in standardization and insufficient number of similar teeth for new studies. Furthermore, studies in dogs have been avoided nowadays for ethical reasons.
The use of rats as the experimental model presents advantages such as ease of handling, reproduction, control, predictability 22 , and standardization 6 . Moreover, this model further presents better acceptance regarding ethical and economic concerns 9 .
Despite the difference in enamel and dentin thickness between humans and rat teeth (2.5 mm vs. 100 µm, respectively), they both show the same proportion of these structures 6 , 9 . In addition, rat molars have anatomical, histological, biological and physiological features similar to human molars 9 , 24 . Also, rat molars exhibit the same structural characteristics of the pulp chamber and pulp tissues, where the essential biological reactions and the wound healing of rat molar teeth are comparable to that of other mammals 9 . Conversely, rat incisors are typical of rodents, of permanent growth, with a wide-open apex, and cannot be compared to human teeth 9 .
In the present study, 35% H2O2 gel applied for 30 or 45 min caused necrosis or a severe inflammatory response in the dental pulp, especially in the upper coronal two-thirds. Clinically, in-office bleaching with high H2O2 concentrations for 30 to 45 min in a single session is frequently associated with a high incidence of tooth sensitivity 8 . Considering the similarity of the results found in this study to the results of Costa, et al. 8 (2010), we suggested that rat molars can be targeted and improved as an experimental model to predict the results of procedures performed in human mandibular incisors in this concentration and application time 6 .
The amount of H2O2 detected in the pulp chamber is related to the concentration and application time of the gel 2 . The use of 35% H2O2 gel applied for 30 min, as well as 20% and 35% H2O2 gel for 45 min, was related to changes in vascular permeability 12 . Therefore, our study was conducted with several application times and two concentrations, one of which more commonly employed in clinical dentistry (35% H2O2) 16 , 29 , and a lower one (20% H2O2). Our results allow choosing a concentration and time of application for comparative analysis in the initial inflammatory process (at two days) as well as in the subsequent reparative process (at 30 days).
In our evaluation at 30 days after bleaching, we observed that all the groups showed signs of repair. Tertiary dentin was formed to protect the dental pulp, reducing the pulp chamber central area, and inflammatory cells were absent. The groups of low concentration/application time showed significant differences from those of high concentration/application time. Studies of the effects of high concentrations of bleaching gels on pulp cell cultures have shown that products released by 35% H2O2 gel can diffuse through enamel and dentin and cause significant cell damage 10 , 30 .
H2O2 can penetrate the cell membrane, increase alkaline phosphatase activity, and induce apoptosis in the periodontal ligament and dental pulp 15 as well as stimulate mineralization 21 . Increased alkaline phosphatase activity and extracellular matrix mineralization reveal the dentin production 28 . The model in rats can also be used in long-term analysis to determine clinical protocols of application that produce less pulp damages over time.
The characterization of this experimental model does not replace human trials, but allows the knowledge of new bleaching agents mechanism of action; the comparison between protocols of bleaching; and the study of desensitizing and remineralizing agents used before and after bleaching to minimize effects on pulp tissues.
CONCLUSION
In conclusion, the rat model of extracoronal bleaching showed to be adequate for studies of bleaching protocols, as it was possible to observe alterations in pulp tissues and tooth structure caused by different concentrations and application times of bleaching agents. In-office bleaching with H2O2 gel caused immediate inflammation and accelerated aging of the dental pulp by inducing deposition of tertiary dentin, and the degree of damage increased with increasing concentration and application time of the bleaching agent.
ACKNOWLEDGEMENTS
We thank the financial support from FAPESP projects 2011/13709-2 and 2013/25429-0.
REFERENCES
- 1.Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59:27–33. doi: 10.1080/15216540601156188. [DOI] [PubMed] [Google Scholar]
- 2.Benetti AR, Valera MC, Mancini MN, Miranda CB, Balducci I. In vitro penetration of bleaching agents into the pulp chamber. Int Endod J. 2004;37:120–124. doi: 10.1111/j.0143-2885.2004.00761.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya S, Dudeja PK, Tobacman JK. Carrageenan-induced NFkappaB activation depends on distinct pathways mediated by reactive oxygen species and Hsp27 or by Bcl10. Biochim Biophys Acta. 2008;1780:973–982. doi: 10.1016/j.bbagen.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borges AB, Torres CR, Souza PA, Caneppele TM, Santos LF, Magalhães AC. Bleaching gels containing calcium and fluoride: effect on enamel erosion susceptibility. Int J Dent. 2012;2012:1–6. doi: 10.1155/2012/347848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen HP, Chang CH, Liu JK, Chuang SF, Yang JY. Effect of fluoride containing bleaching agents on enamel surface properties. J Dent. 2008;36(9):718–725. doi: 10.1016/j.jdent.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Cintra LT, Benetti F, Silva Facundo AC, Ferreira LL, Gomes JE, Filho, Ervolino E, et al. The number of bleaching sessions influences pulp tissue damage in rat teeth. J Endod. 2013;39(12):1576–1580. doi: 10.1016/j.joen.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Costa CA, Oliveira MF, Giro EM, Hebling J. Biocompatibility of resin-based materials used as pulp-capping agents. Int Endod J. 2003;36:831–839. doi: 10.1111/j.1365-2591.2003.00702.x. [DOI] [PubMed] [Google Scholar]
- 8.Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J. Human pulp responses to in-office tooth bleaching treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e59–e64. doi: 10.1016/j.tripleo.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Dammaschke T. Rat molar teeth as a study model for direct pulp capping research in dentistry. Lab Anim. 2010;44(1):1–6. doi: 10.1258/la.2009.008120. [DOI] [PubMed] [Google Scholar]
- 10.Duncan HF, Smith AJ, Fleming GJ, Cooper PR. Histone deacetylase inhibitors induced differentiation and accelerated mineralization of pulp-derived cells. J Endod. 2012;38(3):339–345. doi: 10.1016/j.joen.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Esposito P, Varvara G, Murmura G, Terlizzi A, Caputi S. Ability of healthy and inflamed human dental pulp to reduce hydrogen peroxide. Eur J Oral Sci. 2003;111:454–456. doi: 10.1034/j.1600-0722.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira VG, Nabeshima CK, Marques MM, Paris AF, Gioso MA, Reis RS, et al. Tooth bleaching induces changes in the vascular permeability of rat incisor pulps. Am J Dent. 2013;26(5):298–300. [PubMed] [Google Scholar]
- 13.Frigo L, Pallota RC, Meneguzzo D, Marcos RL, Penna SC, Lopes-Martins RAB. Evaluation of the photo-activated dental bleaching effect on dental pulp in an in vivo rat experimental model. Rev Dental Press Estét. 2009;6(1):102–114. [Google Scholar]
- 14.Han YH, Kim SZ, Kim SH, Park WH. Pyrogallol as a glutathione depletor induces apoptosis in HeLa cells. Int J Mol Med. 2008;21:721–730. [PubMed] [Google Scholar]
- 15.Hanks CT, Fat JC, Wataha JC, Corcoran JF. Cytotoxicity and dentin permeability of carbamide peroxide and hydrogen-peroxide vital bleaching materials, in vitro. J Dent Res. 1993;72(5):931–938. doi: 10.1177/00220345930720051501. [DOI] [PubMed] [Google Scholar]
- 16.Joiner A. The bleaching of teeth: a review of the literature. J Dent. 2006;34:412–419. doi: 10.1016/j.jdent.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Kina JF, Huck C, Riehl H, Martinez TC, Sacono NT, Ribeiro AP, et al. Response of human pulps after professionally applied vital tooth bleaching. Int Endod J. 2010;43(7):572–580. doi: 10.1111/j.1365-2591.2010.01713.x. [DOI] [PubMed] [Google Scholar]
- 18.Marson FC, Gonçalves RS, Silva CO, Cintra LT, Pascotto RC, Santos PH, et al. Penetration of hydrogen peroxide and degradation rate of different bleaching products. Oper Dent. 2015;40(1):72–79. doi: 10.2341/13-270-L. [DOI] [PubMed] [Google Scholar]
- 19.Marson FC, Guedes AA, Camargo WR, Progiante PS, Oliveira e Silva C. The gel cytotoxicity in relation to the dental pulp. J Surg Clin Dent. 2014;1:10–13. [Google Scholar]
- 20.Marson FC, Sensi LG, Vieira LC, Araújo E. Clinical evaluation of in-office dental bleaching treatments with and without the use of light-activation sources. Oper Dent. 2008;33:15–22. doi: 10.2341/07-57. [DOI] [PubMed] [Google Scholar]
- 21.Matsui S, Takahashi C, Tsujimoto Y, Matsushima K. Stimulatory effects of low-concentration reactive oxygen species on calcification ability of human dental pulp cells. J Endod. 2009;35:67–72. doi: 10.1016/j.joen.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 22.Penna LA, Rode SM. Morphological study of the pulp of Wistar rats molars under experimental occlusal interference. Pesqui Odontol Bras. 2000;14(2):159–164. [Google Scholar]
- 23.Sanz A, Gómez J, Caro P, Barja G. Carbohydrate restriction does not change mitochondrial free radical generation and oxidative DNA damage. J Bioenerg Biomembr. 2006;38:327–333. doi: 10.1007/s10863-006-9051-0. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki T, Kawamata-Kido H. Providing an environment for reparative dentine induction in amputated rat molar pulp by high molecular-weight hyaluronic acid. Arch Oral Biol. 1995;40:209–219. doi: 10.1016/0003-9969(95)98810-l. [DOI] [PubMed] [Google Scholar]
- 25.Seale NS, McIntosh JE, Taylor AN. Pulpal reaction to bleaching of teeth in dogs. J Dent Res. 1981;60:948–953. doi: 10.1177/00220345810600051701. [DOI] [PubMed] [Google Scholar]
- 26.Seale NS, Wilson CF. Pulpal response of bleaching of teeth in dogs. Ped Dent. 1985;7:209–214. [PubMed] [Google Scholar]
- 27.Soares DG, Pontes EC, Ribeiro AP, Basso FG, Hebling J, Costa CA. Low toxic effects of a whitening strip to cultured pulp cells. Am J Dent. 2013;26:283–285. [PubMed] [Google Scholar]
- 28.Soares DG, Ribeiro AP, Sacono NT, Coldebella CR, Hebling J, Costa CA. Transenamel and transdentinal cytotoxicity of carbamide peroxide bleaching gels on odontoblast-like MDPC-23 cells. Int Endod J. 2011;44(2):116–125. doi: 10.1111/j.1365-2591.2010.01810.x. [DOI] [PubMed] [Google Scholar]
- 29.Sulieman M, Addy M, Macdonald E, Rees JS. The bleaching depth of a 35% hydrogen peroxide based in-office product: a study in vitro. J Dent. 2005;33(1):33–40. doi: 10.1016/j.jdent.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Trindade FZ, Ribeiro AP, Sacono NT, Oliveira CF, Lessa FC, Hebling J, et al. Trans-enamel and trans-dentinal cytotoxic effects of a 35% H2O2 bleaching gel on cultured odontoblast cell lines after consecutive applications. Int Endod J. 2009;42:516–524. doi: 10.1111/j.1365-2591.2009.01544.x. [DOI] [PubMed] [Google Scholar]


