Abstract
Study Design Prospective multicenter single-arm interventional clinical trial.
Objective To determine the degree of improvement in sacroiliac (SI) joint pain, disability related to SI joint pain, and quality of life in patients with SI joint dysfunction who undergo minimally invasive SI joint fusion using triangular-shaped titanium implants.
Methods Subjects (n = 172) underwent minimally invasive SI joint fusion between August 2012 and January 2014 and completed structured assessments preoperatively and at 1, 3, 6, and 12 months postoperatively, including a 100-mm SI joint and back pain visual analog scale (VAS), Oswestry Disability Index (ODI), Short Form-36 (SF-36), and EuroQOL-5D. Patient satisfaction with surgery was assessed at 6 and 12 months.
Results Mean SI joint pain improved from 79.8 at baseline to 30.0 and 30.4 at 6 and 12 months, respectively (mean improvements of 49.9 and 49.1 points, p < 0.0001 each). Mean ODI improved from 55.2 at baseline to 32.5 and 31.4 at 6 and 12 months (improvements of 22.7 and 23.9 points, p < 0.0001 each). SF-36 physical component summary improved from 31.7 at baseline to 40.2 and 40.3 at 6 and 12 months (p < 0.0001). At 6 and 12 months, 93 and 87% of subjects, respectively, were somewhat or very satisfied and 92 and 91%, respectively, would have the procedure again.
Conclusions Minimally invasive SI joint fusion resulted in improvement of pain, disability, and quality of life in patients with SI joint dysfunction due to degenerative sacroiliitis and SI joint disruption.
Keywords: minimally invasive surgery, sacroiliac joint, sacroiliac joint dysfunction, sacroiliac joint disruption, degenerative sacroiliitis, sacroiliac joint arthrodesis, spine surgery, prospective clinical trial
Introduction
Pain from the sacroiliac (SI) joint, first described in the early 1900s,1 is very common. It occurs in 14.5 to 22.6% of all patients evaluated for back pain in the outpatient setting.2 3 SI joint pain may be even more prevalent (up to 40%) in patients with prior lumbar fusion.4 5 The SI joint is richly innervated,6 and studies of normal volunteers have shown that local anesthetic injection into the SI joint can eliminate pain provocation.7 8 The impact of SI joint pain on quality of life is substantial and often disabling, similar to that observed with other prominent orthopedic conditions such as lumbar spinal stenosis and degenerative hip arthritis.9
Nonsurgical treatments for SI joint pain include physical therapy, chiropractic manipulations, intra-articular SI joint steroid injections, and radiofrequency (RF) neurotomy (ablation) of the S1–S3 dorsal rami innervating the SI joint. Blinded randomized trials of periarticular injections have shown only short-term pain relief.10 11 12 RF ablation neurotomy trials in patients who respond to dorsal rami blocks have shown modest pain relief13 14; limited evidence for long-term pain relief from this treatment is available.15 Open SI joint arthrodesis was first reported in the 1920s.16 17 18 A small number of retrospective studies suggest that it can be effective in relieving chronic SI joint pain.19 20 21 22 23 24 25 However, open SI joint fusion is rarely performed currently due to relatively large incisions, lengthy hospital stays (3 to 5 days), and long recovery periods (often lasting months), and a relatively high complication rate (13.7% in one systematic review26). The reported nonunion rate is unsatisfactory (9 to 41%),21 27 28 and patient satisfaction with open SI joint fusion surgery has been highly variable (18 to 80%) in reported series.26 Open SI joint fusion is currently used primarily to address acute traumatic, infection- or tumor-related joint instability.
Minimally invasive alternatives to open SI joint fusion have gained popularity in recent years.29 Although other techniques have been reported,30 31 32 most recently published studies describe use of a series of triangular titanium implants coated with a porous titanium plasma spray (TPS) across the SI joint.33 34 35 36 37 38 39 Herein we report 12-month clinical outcomes from a prospective multicenter clinical trial of minimally invasive SI joint fusion using this implant system.
Methods
This study was a prospective, multicenter, postmarket (on-label) single-arm interventional clinical trial of SI joint fusion using an FDA-cleared implant system (iFuse Implant System®, SI-BONE, Inc., San Jose, California, United States). Enrollment took place between August 2012 and December 2013 at 26 sites. The study protocol (registered on clinicaltrials.gov [NCT01640353]) was approved by the institutional review board at each participating clinical site prior to patient enrollment. The study was sponsored by the device's manufacturer (SI-BONE, Inc., San Jose, California, United States). All study sites underwent both remote and on-site data monitoring. All study data were 100% source verified.
Patients were invited to participate if they were between the ages of 21 and 70 and had a diagnosis of SI joint dysfunction due to degenerative sacroiliitis and/or SI joint disruption. Identification of the SI joint as the pain generator was based on a combination of a history of pain at or near the SI joint,57 a positive provocative testing on at least three of five established physical examination tests,40 and at least a 50% decrease in pain after image-guided injection/arthrogram into the SI joint with local anesthetic. SI joint block to diagnose the SI joint as a pain generator is recommended by multiple pain and anesthesia physician societies.41 42 43 44 45 Degenerative sacroiliitis was defined in the study as diagnosed SI joint pain in the context of either radiographic evidence of SI joint degeneration (sclerosis, osteophytes, subchondral cysts, or vacuum phenomenon) on computed tomography (CT) or X-ray or a history of prior lumbar fusion. SI joint disruption was defined in the study as diagnosed SI joint pain in the context of asymmetric widening of SI joints on CT or X-ray or the presence of significant contrast extravasation during a diagnostic SI joint block/arthrogram. Study inclusion also required the patient to have a baseline score of at least 30% on Oswestry Disability Index (ODI) and an SI joint pain score of at least 50 on a 0-to-100-mm visual analog scale (VAS), where 0 represents no pain and 100 represents worst imaginable pain.
Patients were excluded if they met any of the following conditions: severe back pain due to other causes (e.g., lumbar disk degeneration, spinal stenosis, etc.), history of recent (<1 year) major trauma to the pelvis, metabolic bone disease (either induced or idiopathic), or any condition that made treatment with the study devices infeasible or that interfered with the ability to participate in physical therapy. Patients involved in litigation, on disability leave, or receiving workers' compensation related to their back or SI joint pain were also excluded. Patients who agreed to enroll signed a study-specific informed consent form prior to any study-specific procedure.
Baseline (presurgical) assessments included a detailed medical history, physical examination, and several assessments, including SI joint and lower back pain measured using a VAS, disability measured by ODI,46 and quality of life measured by both EuroQoL-5D (EQ-5D) and Short Form-36 (SF-36).47 48 ODI is a validated 10-question survey for disability due to back pain. EQ-5D is a 5-question broad quality-of-life measure that can be combined into a single index and represents the time trade-off utility of current health. EQ-5D also includes a 0- to 100-mm health thermometer. SF-36 is a 36-question, 8-subscale generic quality-of-life measure. The SF-36 physical component summary (PCS) summarizes overall physical health, with population norms with mean 50 and standard deviation of 10. Similarly, the SF-36 mental component summary (MCS) summarizes overall mental health, with similar population norms.
Subjects underwent index-side minimally invasive SI joint fusion as described previously within 30 days of baseline assessment.36 Under general anesthesia, the subject was placed in the prone position on a radiolucent table. A 3- to 5-cm lateral buttocks incision was made, and dissection was carried down to the gluteal fascia to reach the outer table of the ilium. A guide pin was inserted through the ilium across the SI joint into the body of the sacrum, avoiding the sacral neural foramen. Pin placement was confirmed with lateral, inlet, and outlet fluoroscopic views of the pelvis. A soft tissue protector was passed over the pin, followed by use of a drill to create a pathway through the ilium and into the sacrum, and to decorticate the articular surfaces of the joint. A triangular broach was then used to further decorticate the joint and prepare the pathway for placement of the implant (Fig. 1), which was driven into place. Using a parallel drill guide, additional implants (usually a total of three) were placed across the SI joint. Typically, the most cephalad implant was placed within the sacral ala above the S1 foramen, the second implant was positioned above or adjacent to the S1 foramen, and the third implant was positioned between the S1 and S2 foramen. The incision was irrigated and the tissue layers closed in a standard fashion. Subjects requiring treatment of both SI joints could undergo either bilateral same-day surgery or staged surgery.
Fig. 1.
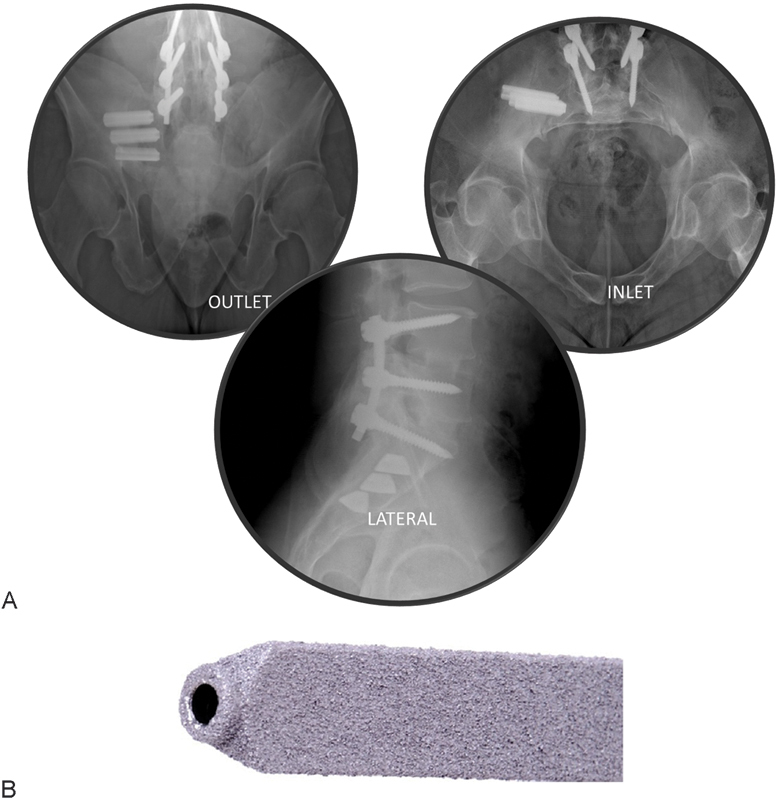
(A) Intraoperative fluoroscopic images showing postplacement outlet (left), inlet (right), and lateral (lower) views. (B) iFuse implant (SI-BONE, Inc., San Jose, California, United States).
Perioperative measures, including estimated blood loss, fluoroscopy time, operating time, number of devices used, and complications, were collected. Three-way postplacement X-ray or fluoroscopic images were obtained. Subjects were discharged home at the surgeon's discretion. Prior to discharge, subjects were re-evaluated for the occurrence of adverse events.
Postoperatively, subjects were asked to remain at heel-toe touchdown-protected weight-bearing using a walker or crutches for 3 weeks followed by progressive increases in weight-bearing until fully ambulatory. Beginning at 1 to 3 weeks postoperatively, subjects were asked to undergo individualized physical therapy twice a week for 6 weeks. Physical therapy involved activity modification to minimize pain recurrence, mobility and stability exercises, as well as adjacent segment joint mobilization for stiffness and pain control. Manipulation of the treated SI joint was discouraged.
Subjects underwent in-clinic follow-up at 1, 3, 6, 12, 18, and 24 months postoperatively. This report describes clinical outcomes up to 12 months (18- and 24-month follow-up is pending). Follow-up assessments consisted of review of adverse changes in health, ambulatory, and work status, medication use for pain, physical examination, and quality-of-life questionnaires. Outcomes of radiographic assessments, including X-ray at 3, 6, and 24 months and high-resolution CT scan at 12 months, will be reported elsewhere.
Adverse events, defined as any negative change in health according to an international clinical trial standard (ISO14155:2011), were monitored continuously and assessed at all study visits. For each event, investigators were asked to rate the severity and relationship to the study device, the device placement procedure and, if present, pre-existing conditions. Relatedness was captured as definitely, probably, possibly, unlikely, and unrelated to the device, procedure, or pre-existing condition, and each event was categorized by body system.
Device Description
The implant system used in this study is cleared by the U.S. Food and Drug Administration (K080398) for SI joint fusion for SI joint dysfunction due to sacroiliac joint disruption and degenerative sacroiliitis. The system consists of a titanium implant that is triangular in shape on cross section and coated with a porous TPS and a delivery system. The implant's triangular shape minimizes rotation, and the procedure incorporates an interference fit between the implant and adjacent osseous walls to reduce micromotion. The porous TPS coating allows for biological fixation in bone, a concept used in several orthopedic devices such as hip, knee, and shoulder implants. Implants are available in configurations ranging from 30 to 70 mm in length and 4 and 7 mm in inscribed diameter. Per the manufacturer, at least two implants should be placed across the SI joint.
Cohorts, Study End Points and Statistical Analysis
The primary analysis cohort consists of subjects who were enrolled, underwent the study procedure, and were available at each follow-up time point. The analysis cohort included those subjects enrolled who were subsequently determined to be ineligible. The primary study end point, evaluated at 6 months after the most recent SI joint fusion (to accommodate subjects who underwent staged bilateral surgery), was a binary success/failure composite end point. A subject was considered a success if all of the following were met: reduction from baseline VAS SI joint pain by at least 20 mm, absence of device-related serious adverse events, absence of neurologic worsening related to the sacral spine, and absence of surgical reintervention (removal, revision, reoperation, or supplemental fixation) for SI joint pain. The 20-mm VAS threshold was selected as the minimum clinically important difference in chronic lower back pain.49 50 Success rates were also evaluated at other time points. The study's final sample size was determined by preplanned interim analysis. The study's success rate goal (Bayesian predicted posterior probability of success > 35%, an estimate of the success rate with continued medical therapy) was met in December 2013 when 60 subjects had 6 months of follow-up, at which point enrollment was terminated. The following subgroup analyses were prespecified: underlying condition (degenerative sacroiliitis versus SI joint disruption), history of prior lumbar fusion, smokers versus nonsmokers, and unilateral versus bilateral SI joint fusion. The study's secondary end points included an analysis of patient success rates at other time points as well as improvement from baseline in VAS, ODI, SF-36 PCS, and EQ-5D scores. For continuous variables, changes from baseline were compared using repeated measure analysis of variance. Confidence intervals for proportions were calculated using standard methods. Analysis of procedure-related variables focused on the index (first side) procedure only. All statistical analyses were performed using R.51
Results
Of 194 subjects who qualified and signed a consent form, 10 withdrew prior to SI joint fusion and data from 12 subjects at a single site were eliminated on the basis of the site's persistent noncompliance with the study protocol. These exclusions left 172 treated subjects at 26 sites. All subjects met eligibility criteria except for the following: 2 subjects were over age 70 at the time of screening, 1 subject had an insufficiently high baseline pain rating, 1 subject had pain for less than 6 months, 1 subject had documented severe back pain from degenerative disk disease, 2 subjects had osteoporosis, 1 subject had rheumatoid arthritis, and 9 subjects were receiving disability payments or were involved in back- or SI joint-related disability claims. (One subject had two reasons.) All enrolled and treated subjects were included in all analyses.
Baseline Characteristics
Patient characteristics are listed in Table 1. Mean subject age was 50.9 years; most (96.5%) subjects were Caucasian and 69.8% were women. At baseline subjects experienced high levels of SI joint pain and had substantial disability, as indicated by high baseline pain ratings (mean 79.8 on the 0-to-100 scale) and ODI scores (mean 55.2). Mean pain duration prior to enrollment was 5.1 years (range 0.43 to 41); 84.3% had pain for >1 year and 64.5% had pain for >2 years. Twenty subjects reported that their pain began in the peripartum period (11.6%). Quality of life was substantially diminished, as indicated by low EQ-5D scores (mean of 0.43 on time trade-off and 57.1 on health thermometer) and low SF-36 scores (mean PCS of 31.7 and MCS of 38.5). These quality-of-life scores represent a significant burden of disease.9 Seventy-six percent were taking opioid medications at baseline, and all reported that multiple activities commonly caused their SI joint pain. Many subjects (44.2%) had a history of prior lumbar fusion, and concomitant spine disease was common. (Note that patients with severe pain from causes other than SI joint dysfunction were excluded from the study.) Pain associated with the SI joint was typically located at the posterior superior iliac spine, but distant and/or radiating pain was frequent anteriorly and posteriorly (Fig. 2). SI joint pain persisted despite prior treatment with physical therapy (64.5% of the subjects), SI joint steroid injections (94.2%), and/or RF ablation of the SI joint (15.7%).
Table 1. Characteristics of enrolled subjects.
| Characteristic | Value |
|---|---|
| Age (y), mean (range) | 50.9 (23–72) |
| Women, n (% female) | 120 (69.8%) |
| Race, n (%) White Hawaiian/Pacific Islander Black Other |
166 (96.5%) 1 (0.6%) 2 (1.2%) 3 (1.7%) |
| Ethnicity Hispanic or Latino, n (%) |
7 (4.1%) |
| Body mass index, mean (range) | 29.4 (17–51) |
| Smoking status, n (%) Current smoker Former smoker Never smoker |
44 (25.6%) 49 (28.5%) 79 (45.9%) |
| Ambulatory without assistance, n (%) | 154 (89.5%) |
| Work status, n (%) Working full time Working part time Not working, retired Not working due to back pain Not working, other reason |
64 (37.2%) 9 (5.2%) 36 (20.9%) 59 (34.3%) 4 (2.3%) |
| Prior lumbar fusion, n (%) | 76 (44.2%) |
| Underlying diagnosis Degenerative sacroiliitis Sacroiliac joint disruption |
135 (78.5%) 37 (21.5%) |
| Duration of pain (y), mean (range) | 5.1 (0.43–41) |
| Pain syndrome Pain began peripartum Pain radiates down leg Groin pain Pain worse with sitting Pain worse with rising Pain worse with walking Pain worse with climbing stairs Pain worse descending stairs |
20 (11.6%) 144 (83.7%) 96 (55.8%) 151 (87.8%) 137 (79.9%) 153 (89.0%) 150 (87.2%) 117 (68.0%) |
| Prior treatments Physical therapy Steroid SI joint injection RF ablation |
111 (64.5%) 162 (94.2%) 27 (15.7%) |
| Taking narcotics, n (%) | 131 (76.2%) |
| Proportion with lumbar stenosis, n (%) | 40 (23.3%) |
| Proportion with hip diagnosis, n (%) | 19 (11.0%) |
| Physical exam findings on target side (left/right) FABER/Patrick's Compression Thigh thrust Distraction Gaenslen |
82 (91.1%)/74 (90.2%) 72 (80.0%)/64 (78.0%) 73 (81.1%)/74 (90.2%) 65 (72.2%)/54 (65.9%) 48 (64.4%)/55 (67.1%) |
| VAS pain score, mean (± SD) | 79.8 (12.8) |
| ODI score, mean (± SD) | 55.2 (11.5) |
| SF-36, mean (± SD) PCS MCS |
31.7 (5.6) 38.5 (11.3) |
| EQ-5D TTO index Health thermometer |
0.43 (0.18) 57.1 (23.7) |
Abbreviations: EQ-5D, EuroQOL-5D; MCS, mental component summary; ODI, Oswestry Disability Index; PCS, physical component summary; RF, radiofrequency; SD, standard deviation; SF-36, Short Form-36; SI, sacroiliac; TTO, time trade-off; VAS, visual analog scale; FABER, flexion, abduction and external rotation.
Fig. 2.
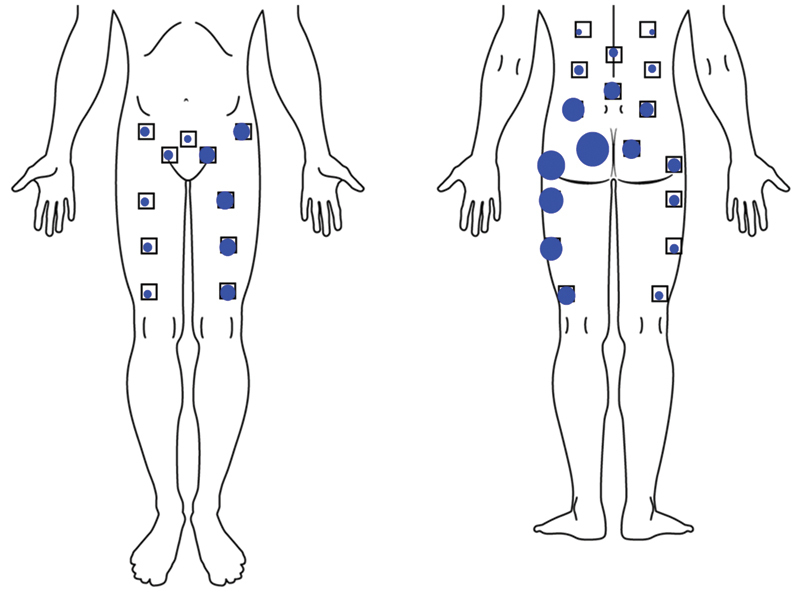
Pain location in subjects reporting primarily left-sided sacroiliac joint pain. Dot size is proportional to the number of subjects reporting pain in that location.
Procedure Characteristics
All study procedures were performed according to the manufacturer's guidelines (instructions for use). Most subjects underwent unilateral treatment; 14 (8.1%) underwent simultaneous (n = 3) or staged (n = 11) bilateral treatment. Mean (standard deviation [SD]) procedure time was 46.6 (± 16.1) minutes, with a range of 13 to 111 minutes (Table 2). Eighty percent of procedures lasted less than 1 hour. Mean (SD) fluoroscopy time was minimal at 2.7 (± 1.8) minutes (range 0.3 to 14). Mean (SD) estimated blood loss was 51.0 mL (± 75.8; range 5 to 800 mL). One subject had 800 mL of blood loss due to injury to the superior gluteal artery. In most cases (144, 83.7%), 3 implants were used; 2 and 4 implants were used in 6 (3.5%) and 22 (12.8%) cases, respectively. Most implants (97.2%) were 7 mm in diameter. Three device-related technical complications occurred (all cases at a single site of excessive pin advancement during drilling), without consequence. Hospital length of stay ranged from 0 to 7 days (median 1 day). Prolonged hospital stays (≥3 days, 8 cases, 4.7%) were related to patient comorbidities, not procedure-related adverse events.
Table 2. Index procedure characteristics (n = 172).
| Characteristic | Value |
|---|---|
| Target joint, n (%) Right Left |
83 (48.3%) 89 (51.7%) |
| Procedure time (min) Mean (SD, range) < 30 30–60 60–120 |
46.6 (16.1, 13–111) 27 (15.7%) 111 (64.5%) 34 (19.8%) |
| Fluoroscopy time (min) Mean (SD, range) 0–1 1–2 2–3 3–4 > 4 |
2.7 (1.8, 0.3–14) 24 (14.0%) 42 (24.4%) 46 (26.7%) 29 (16.9%) 28 (16.3%) |
| Estimated blood loss (mL) Mean (SD, range) 0–50 50–100 > 100 |
51.0 (75.8, 5–800) 130 (75.6%) 22 (12.8%) 13 (7.6%) |
| Number of implants used, n (%) 2 3 4 |
6 (3.5%) 144 (83.7%) 22 (12.8%) |
| Implant length (mm), mean (SD) First implant Second implant Third implant Fourth implant |
49.1 (7.4) 43.4 (5.7) 40.2 (6.2) 39.8 (3.9) |
| Implant diameter (mm), n (%) 4 7 |
15 (2.8%) 517 (97.2%) |
| Hospital length of stay (d) Mean (SD, range) Discharged same day 1 2 3 or more |
0.79 (0.96, 0–7) 69 (40.1%) 86 (50.0%) 9 (5.2%) 8 (4.7%) |
Abbreviation: SD, standard deviation.
Note: Only the index side procedure is reported.
Subject Trial Flow
Trial follow-up was excellent. Of the 172 participants, 169 (98%) had 6-month follow-up and 157 (91%) had 12-month follow-up (Fig. 3). Reasons for study exit prior to the 12-month visit included withdrawal of consent (n = 2) and loss to follow-up (n = 7). Six subjects missed the 12-month study visit but had not exited the study.
Fig. 3.
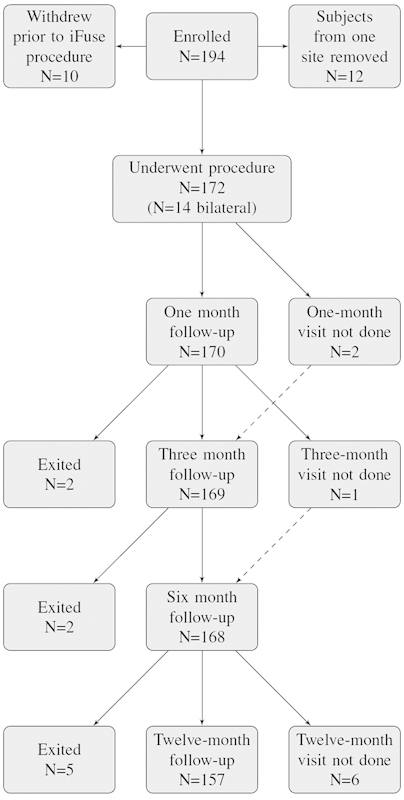
Patient flow. Some subjects who missed the 1- or 3-month visit returned for subsequent visits (dashed lines).
Primary End Point
By month 6, 136 of 169 subjects (80.5%, 95% posterior credible interval 74.0 to 85.9%) met the study's primary success end point. The observed success rate exceeded the threshold for study success (Bayesian posterior probability of study success > 0.999). The 12-month success rate was similar (125/157 or 79.6%). Prespecified subgroup analyses (Table 3) showed no statistically significant differences in success rates by underlying diagnosis, history of prior lumbar fusion, smoking status, or unilateral versus bilateral SI joint fusion surgery.
Table 3. Success rates at 6 mo.
| Subgroup | Rate | p Valuea |
|---|---|---|
| Underlying condition Degenerative sacroiliitis Sacroiliac joint disruption |
104/132 (78.8%) 32/37 (86.5%) |
0.4183 |
| Prior lumbar fusion No Yes |
75/95 (78.9%) 61/74 (82.4%) |
0.7103 |
| Smoking Current smoker Former smoker Never smoker |
31/42 (73.8%) 38/49 (77.6%) 67/78 (85.9%) |
0.2330 |
| Bilateral procedure No Yes |
123/155 (79.4%) 13/14 (92.9%) |
0.3851 |
| Overall | 136/169 (80.5%) |
Chi-square test.
Pain and Quality-of-Life Outcomes
During follow-up, mean (SD) SI joint pain improved from a baseline of 79.8 points to 30.0 points at 6 months and 30.4 at 12 months (Table 4 and Fig. 4). Changes from baseline in VAS SI joint pain (49 to 50 points at each time point 3 months or thereafter) were statistically significant (p < 0.0001) and clinically substantial. At 3, 6, and 12 months, the proportion with pain improvements of ≥20 points was 88, 82, and 82%, respectively. The proportion with improvements of ≥40 points at 3, 6, and 12 months was 64, 68, and 66%, respectively. ODI score, a measurement of disability due to back pain, improved from a mean baseline score of 55.2 to 32.5 at 6 months and 31.4 at 12 months. Improvements from baseline in ODI at 3, 6, and 12 months (−21.3, −22.7, and −23.9 points, respectively) were statistically significant and clinically substantial. At 6 and 12 months, 129 (76%) and 122 (77%) subjects had an ODI improvement of 10 or more points and 111 (66%) and 106 (67.0%) had an improvement of 15 or more points. No important difference in pain improvement or ODI were observed across prespecified subgroups except that current smokers had a somewhat diminished ODI response compared with nonsmokers (∼6 points less, p = 0.0710).
Table 4. Change in pain, Oswestry Disability Index, SF-36, and EQ-5D.
| Measure | Baseline | 1 mo | 3 mo | 6 mo | 12 mo | p Valuea |
|---|---|---|---|---|---|---|
| VAS SI joint pain (SD) VAS SI joint pain change (SD) |
79.8 (12.8) | 36.9 (26.3) −42.7 (28.5) |
30.7 (25.9) −49.2 (25.6) |
30.0 (26.5) −49.9 (28.3) |
30.4 (27.6) −49.1 (29.5) |
<0.0001 |
| ODI ODI change (SD) |
55.2 (11.5) | 42.6 (17.4) −12.5 (19.2) |
33.8 (18.8) −21.3 (19.2) |
32.5 (19.7) −22.7 (20.6) |
31.4 (19.2) −23.9 (20.4) |
<0.0001 |
| EQ-5D TTO index TTO change Health thermometer Health thermometer change |
0.43 (0.18) 57.1 (23.7) |
– – |
– – |
0.69 (0.21) 0.25 (0.24) 69.1 (20.2) 12.1 (27.1) |
0.71 (0.20) 0.27 (0.24) 68.8 (20.7) 11.4 (27.9) |
<0.0001 <0.0001 |
| SF-36 PCS PCS change MCS MCS change |
31.7 (5.6) 38.5 (11.3) |
– – |
– – |
40.2 (9.7) 8.4 (9.7) 47.8 (11.5) 9.4 (12.6) |
40.3 (9.5) 8.7 (9.9) 48.0 (12.4) 9.2 (11.7) |
<0.0001 <0.0001 |
| Satisfaction (%) Somewhat or very satisfied Might or definitely would have implant again |
– – |
– – |
– – |
157 (93.5%) 155 (92.3%) |
137 (87.3%) 143 (91.1%) |
– – |
Abbreviations: EQ-5D, EuroQOL-5D; MCS, mental component summary; ODI, Oswestry Disability Index; PCS, physical component summary; RF, radiofrequency; SD, standard deviation; SF-36, Short Form-36; SI, sacroiliac; TTO, time trade-off; VAS, visual analog scale.
Note: The top row shows group mean and the bottom row shows change from baseline.
Repeated measures analysis of variance. A dash indicates assessment not required per protocol.
Fig. 4.
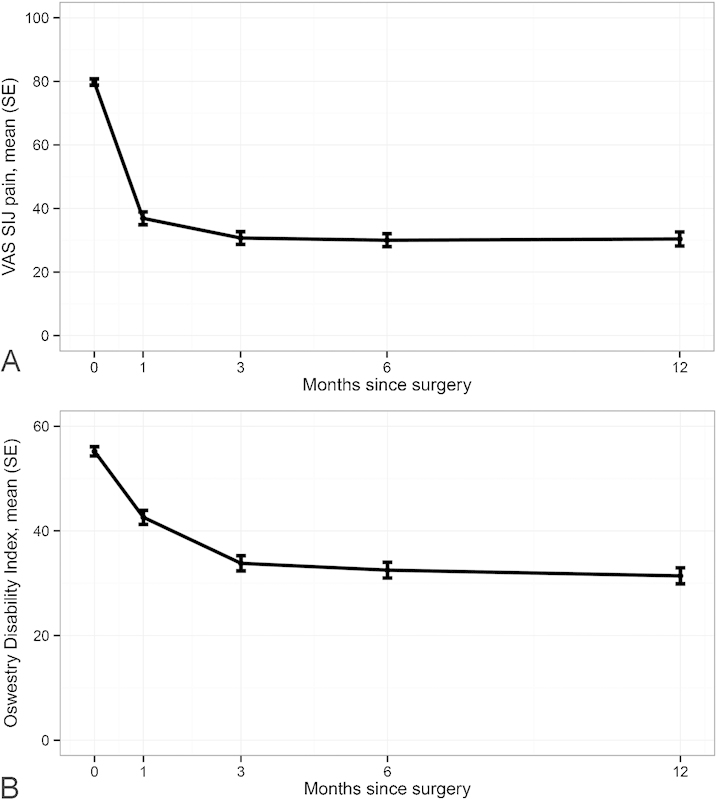
Improvement in VAS SI joint pain (A) and Oswestry Disability Index (B). Abbreviations: SE, standard error; SIJ, sacroiliac joint; VAS, visual analog scale.
Quality of life was measured using two generic assessments, EQ-5D and SF-36. Mean EQ-5D time trade-off index improved from 0.43 at baseline to 0.69 at 6 months and 0.71 at 12 months, increases of 0.25 and 0.27 points, respectively (Table 4, p < 0.0001). Mean EQ-5D global health thermometer rating improved from 57.1 at baseline to 69.1 at 6 months and 68.8 at 12 months (improvements of 12.1 and 11.4 points, p < 0.0001). All SF-36 individual domains showed statistically significant improvements (p = 0.006 and 0.003 for general health at 6 and 12 months, respectively, and p < 0.0001 for all other domains and time points, Fig. 5). SF-36 PCS and MCS were depressed at baseline (mean 31.7 and 38.5, respectively); by 6 and 12 months after SI joint fusion, respectively, these values improved by 8.4 and 8.7 points for PCS and 9.4 and 9.2 points for MCS (p < 0.0001 each). Satisfaction rates were high: at 6 and 12 months, most (93.5 and 87.3%) were somewhat or very satisfied. Similarly, 92.3 and 91.1% of subjects stated at 6 and 12 months that they might or would definitely have the procedure again. Opioid use decreased somewhat from 76% at baseline to 60% at 6-month follow-up and 57% at 12 months.
Fig. 5.
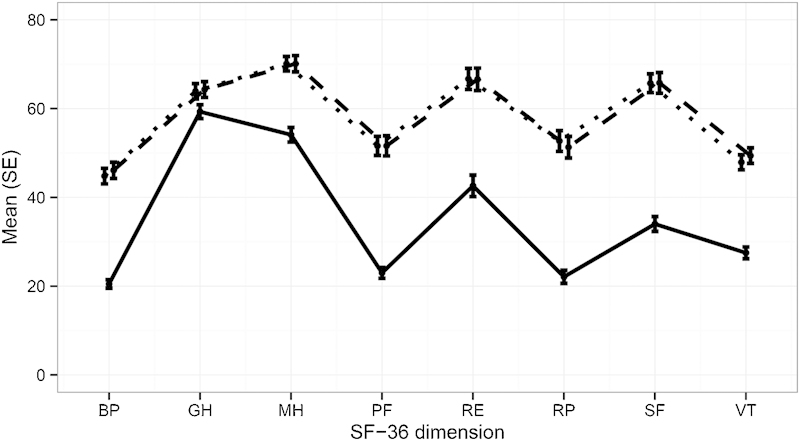
Improvement in SF-36 domains at 6 months compared with baseline. All p < 0.0001 versus baseline except for GH (p = 0.0063 for 6 months versus baseline and p = 0.0031 for 12 months versus baseline). Solid line = baseline; dotted line = month 6; dashed line = month 12. Abbreviations: SF-36, Short Form-36; SE, standard error; BP, bodily pain; GH, general health; MH, mental health; PF, physical function; RE, role emotional; RP, role physical; SF, social function; VT, vitality.
Device-Related Events
As per the study protocol and ISO 14155:2011 compliance, all negative changes in health were collected as adverse events. Adverse events occurring up to 12 months are listed in Table 5. Five adverse events (2.9% of subjects) were rated as probably or definitely related to the study implant (Table 6). Two subjects experienced implant-related L5–S1 nerve root irritation postoperatively, which resolved in both cases with repositioning of the involved implant. (One additional patient had a similar event after revision of a contralateral SI joint fusion initially performed prior to study entry.) One subject had ipsilateral buttocks pain attributed to iliac bone cortex periosteal bone growth around the proximal end of the implants. One subject had persistent SI joint pain after a fall associated with a misstep. A CT scan of this patient's treated SI joint showed that the second and third implants were not fully across the SI joint, and this subject eventually underwent revision surgery, which resulted in substantial pain improvement. In the fifth case, mild (2 out of 10) buttock pain starting at postoperative day 182 was attributed to the device.
Table 5. Adverse events by body system.
| Category | n (%)a |
|---|---|
| Pelvis | 55 (32) |
| Back | 36 (20.9) |
| Leg | 35 (20.3) |
| Fall | 30 (17.4) |
| Hip | 19 (11) |
| Gastroenterologic | 13 (7.6) |
| Neck | 10 (5.8) |
| Surgical wound | 10 (5.8) |
| Cardiovascular | 9 (5.2) |
| Infection | 9 (5.2) |
| Miscellaneous | 7 (4.1) |
| Foot | 6 (3.5) |
| Genitourinary | 6 (3.5) |
| Allergic | 5 (2.9) |
| Arm/hand | 5 (2.9) |
| Pulmonary | 4 (2.3) |
| Shoulder | 4 (2.3) |
| Trauma | 4 (2.3) |
| Endocrinologic | 2 (1.2) |
| Gynecologic | 2 (1.2) |
| Psychological | 2 (1.2) |
| Thoracic | 2 (1.2) |
| Hematologic | 1 (0.6) |
| Ophthalmologic | 1 (0.6) |
| Rheumatologic | 1 (0.6) |
Number of events and rate (events divided by total number of trial subjects).
Table 6. Device- or procedure-related events.
| Event category | n (%)a |
|---|---|
| Related to deviceb
Neuropathy related to malposition SI joint pain after fall associated with inadequate device placement Hip pain related to periosteal bone growth around implant Mild SI joint pain |
5 (2.9) 2 (1.2) 1 (0.6) 1 (0.6) 1 (0.6) |
| Related to procedureb
Wound infection or drainage Buttocks or SI joint pain Postoperative nausea/vomiting Neuropathy related to malpositionc Staple irritation Numbness around surgical wound Gluteal artery bleeding Urinary retention Fall causing SI joint paind |
21 (12.2) 5 (2.9) 5 (2.9) 3 (1.7) 2 (1.1) 1 (0.6) 1 (0.6) 1 (0.6) 1 (0.6) 1 (0.6) |
Abbreviation: SI, sacroiliac.
Number (rate) of events divided by number undergoing surgical procedure.
Events rated as probably or definitely related to the device/procedure by the study site investigator. One additional subject had neuropathy related to iFuse implant placement in a nonindex side.
Same two events as those noted to be device-related. One additional similar event related to contralateral SI joint fusion not part of study.
Fall possibly related to inadequate placement of second and third implants not fully across SI joint.
Procedure-Related Events
Twenty-one events (12.2% rate) were rated as probably or definitely related to the placement procedure (Table 6). Notable events include 5 cases of wound infection or drainage (all of which resolved with antibiotic treatment or surgical debridement, which was necessary in one case), 2 cases of radiculopathy related to implant malposition (described previously), 1 case of hemorrhage due to an injured gluteal artery, and 1 case of pain resulting from both a fall and inadequate device placement (case described above). All remaining events were related to anesthesia or postoperative recovery only.
Severe Events
Twenty-nine events were noted to be severe. Of the 29, 1 event was both severe and device-related: L5–S1 nerve irritation due to implant malposition, described previously (Table 7). Four events were severe and probably or definitely procedure-related: 1 case of implant radiculopathy (same case as above), 1 case of postoperative surgical pain requiring brief hospitalization, 1 case of postoperative nausea/vomiting requiring prolonged hospitalization, and 1 case of deep wound infection requiring surgical wound debridement. All remaining events were unassociated with the SI joint surgery.
Table 7. Adverse events related to device or procedure rated as severe.
| Event | n (%)a | Pre-existing conditionb |
|---|---|---|
| Related to device Neuropathy due to malpositioned implant |
1 (0.6%) 1 (0.6%) |
NA |
| Related to procedure Neuropathy due to malpositioned implantc Postoperative nausea/vomiting Postoperative pain requiring hospitalization Wound infection |
4 (2.3%) 1 (0.6%) 1 (0.6%) 1 (0.6%) 1 (0.6%) |
NA NA Chronic pain disorder, opiate dependence NA |
Abbreviation: NA, not applicable.
Note: One additional site was terminated from the study due to poor study compliance. All data from this site was excluded in this report and the site reported no serious adverse events.
Number of events and rate.
Pre-existing condition, if present; NA if no known condition.
Event also rated as device-related.
Revisions
Revision surgery occurred in 4 cases (2.3%). In 3 cases, subjects awoke with new-onset leg pain (1 case was not related to a study procedure). Leg pain resolved when implants were repositioned slightly. In 2 additional cases, SI joint pain relief was either minor or pain recurred, probably due in both cases to poor implant positioning, with 1 or more implants barely engaging the sacrum. Both subjects underwent placement of 1 or more additional implants with subsequent improvement in SI joint pain. One of these subjects had also undergone interval anterior lumbar interbody fusion of L5–S1 for lower back pain. With a mean of 18.5 months of follow-up to date (total 3,152 person-months), the cumulative revision rate is 2.8% (95% confidence interval 0 to 5.5%).
One further subject, a 44-year-old man, underwent staged bilateral SI joint fusion. He had initial pain relief at the index side after implant placement, but developed recurrent pain 6 months later. Extensive workup showed bilateral labral tears and evidence of possible femoral acetabular impingement. Repeat bilateral SI joint block provided 50% temporary pain relief and intra-articular hip injections provided no diagnostic benefit; he therefore underwent traditional open SI joint arthrodesis followed by placement of 1 additional implant in each SI joint. He reported excellent SI joint pain relief at month 12 but was still troubled by lower back pain of unknown cause. One subject, who had no improvement in SI joint pain at 6 months, underwent placement of a spinal cord stimulator ∼13 months after SI joint fusion, which provided substantial pain relief and suggested a preoperative neuropathic cause of pain.
Nonsevere Adverse Events Unrelated to the Procedure or Implant
In all, 257 additional events were not severe and not related to either the device or procedure. Of these events, 83 (32.3%) were probably or definitely related to pre-existing conditions.
Discussion
The SI joint has been recognized as a pain generator since the early 1900s,1 and it has more recently garnered attention as a significant contributor to low back pain.3 In the setting of a prior lumbar fusion, degeneration of the SI joint is frequent and may be the most common cause of post-fusion lower back pain,52 possibly as a result of adjacent segment degeneration or increased energy transfer causing SI joint instability.4 5 In patients undergoing lumbar laminectomy alone, increased scintigraphic uptake has been demonstrated within the SI joint, suggesting altered spinal mechanics.53
Diagnosing the SI joint as a source of pain involves a history suggestive of pain from the SI joint, physical examination that includes at least three positive physical examination maneuvers predictive of SI joint pain, and confirmatory diagnostic SI joint block. X-ray and cross-sectional imaging is important to rule out neural compressive or other lesions that could cause referred pain to the affected buttocks or groin. In our cohort, pain was reported primarily near the posterior superior iliac spine but radiation into the hip, leg, upper back, or groin was common (Fig. 2). All study subjects had physical examination signs common in patients with SI joint pain.40 Finally, all subjects had marked acute reduction in pain on fluoroscopy-guided injection of local anesthetic into the SI joint, a test with substantial support from pain and anesthesia societies.41 42 43 44 45
Our study is the first to provide strong prospective, multicenter evidence of the effectiveness of minimally invasive SI joint fusion. Over 80% of subjects reported a clinically significant improvement (≥20 point decrease) in SI joint pain at 6 and 12 months after surgery as rated using a VAS. Study subjects reported improvement in pain as early as 1 month; pain improvement was maintained between 3 and 12 months. Improvements in pain were reflected by statistically significant and clinically important improvements in disability as measured by ODI. Approximately three quarters of study subjects had a decrease in ODI of 10 or more points.
Quality of life is substantially depressed in patients with SI joint pain; the degree of depression is similar to that reported for other major orthopedic conditions commonly treated surgically, such as lumbar spinal stenosis and degenerative hip arthritis.9 In our cohort, quality of life, measured using two quality-of-life instruments (EQ-5D and SF-36), showed marked improvements at 6 and 12 months. Improvements in pain, disability, and quality of life were remarkable given the long duration of SI joint pain (on average 5 years) and the high rate of failure of prior therapies (64.5% had received physical therapy, 94.2% had received SI joint steroid injections, and 15.7% had received RF ablation). Moreover, the enrolled patient population was complex, with many participants having a history of prior spine surgery (lumbar fusion, 44%) and concomitant spine and hip disease (lumbar stenosis, 23%; hip disease, 11%). The occurrence of concomitant degenerative spine and hip disease in our cohort is not surprising given that osteoarthritic degeneration of the SI joint explained SI joint pain in the majority of trial subjects.
Improvements in pain, disability, and quality of life observed in our study were similar to results reported in retrospective cohorts using the same device,33 34 35 36 37 38 39 as well as a recently published randomized clinical trial with identical eligibility criteria.54 Quality-of-life score changes were also similar to results observed in a retrospective case series of patients undergoing SI joint fusion using hollow modular anchorage screws plus demineralized bone matrix.31 Prespecified subgroup analyses showed that smokers responded similarly to nonsmokers and that subjects with a history of prior lumbar fusion responded similarly to those without a history of fusion. Fusion of the lumbar spine may be a risk factor for SI joint degeneration,52 and prior lumbar fusion is common in patients with SI join pain.4 It is also possible (though our trial provides no direct evidence to support this point) that some participants may have previously undergone lumbar fusion when SI joint pain was the true underlying diagnosis.
The etiology of SI joint dysfunction in our cohort included osteoarthritic degeneration of the SI joint and/or joint hypermobility due to joint disruptions, neither of which are reliably diagnosed radiographically. Rather, diagnosis relies on history, physical examination, and diagnostic SI joint block. The mechanism of pain relief with iFuse is two-fold: early pain relief occurs as a result of immediate surgical joint stabilization, and late pain relief occurs as a result of both surgical stabilization and long-term fusion, as demonstrated in a 5-year clinical and radiographic follow-up study of patients treated with the same device.55 Proper implant positioning is probably important in achieving pain relief. In two cases, subjects with persistent pain and inadequate device placement evident on CT underwent placement of additional iFuse implants; both subjects experienced pain relief after this revision surgery. Additional potential explanations for inadequate pain relief after iFuse placement include loosening of the implants after placement (one possible case observed to date in this study), inaccurate diagnosis, and the presence of concomitant disease (e.g., hip osteoarthritis, lumbar facet arthropathy) that can present in a manner similar to SI joint pain, or new-onset piriformis syndrome.
All adverse events, defined in the study protocol according to an international clinical trial standard (ISO14155:2011), were carefully collected in this prospective study. Five events were deemed related to the study implant. Two subjects required early revision due to the placement of study implants too close to sacral nerves with resulting radiculopathy. Three additional subjects had recurrent SI joint pain deemed by the investigators to be related to the study implant; in one case, pain was associated with a fall. Twenty-one events were deemed related to the study procedure; these events were not unexpected and all resolved. Twenty-nine events were severe, but most were unrelated to the study device or procedure.
Taken together with the modest rate of device- and procedure-related adverse events, the degree of pain relief observed in our study, combined with improvements in disability and quality of life, provide strong evidence that the benefits of the procedure outweigh the risks for most patients, at least in the 12-month time frame. Existing longer-term studies using the same device showed similar 1-year findings and suggested sustained pain relief at 2,34 36 4.5,56 and 5 years.55 Long-term radiographic fusion of the treated SI joint was observed in 87% of patients at 5-year follow-up.55 Combined with the results from previously published studies,33 34 35 36 37 38 39 54 the results from our prospective trial demonstrate that for patients with SI joint pain unresponsive to nonsurgical treatments, minimally invasive SI joint fusion is an acceptable option.
This study has several advantages. All participants were carefully screened against predetermined eligibility criteria, and the results represent the prospective experience of multiple surgeons. The trial was executed according to an international clinical trial standard (ISO14155:2011). Study data were collected on electronic case report forms at predetermined postoperative times, and all data were rigorously monitored and source verified. The 12-month follow-up rate was high. The primary study limitations were lack of a concurrent control group treated nonsurgically and the subjective nature of the patient-reported outcomes used. These concerns may be mitigated by the following observations. First, all study participants had at least 6 months of pain prior to study enrollment and treatment, mean pain duration was >5 years, and many commonly provided interventions for SI joint pain, such as SI joint steroid injections and physical therapy, had failed to provide relief prior to enrollment. The significant regression of pain, disability, and quality of life observed in this study would be highly unusual with continued nonsurgical care. Second, in a recently published randomized controlled trial with identical enrollment criteria, subjects who were assigned to nonsurgical management showed only modest 6-month improvements in these parameters compared with those who underwent surgical fusion.54 The patient-reported outcomes used in our study are commonly used in orthopedic clinical trials. Commonly accepted and validated physical function end points are not available for SI joint pain.
The observed improvement in pain, disability, and quality of life in our study is considerable given our complex patient population, in which many subjects had pre-existing concomitant spinal disease and close to half had undergone prior lumbar fusion, a suspected risk factor for degenerative sacroiliitis.52 Twenty-four month follow-up from this study will be helpful to determine the long-term maintenance of relief of SI joint pain and its associated effects on disability and quality of life and to evaluate long-term radiographic outcomes.
Conclusions
This study provided strong evidence that minimally invasive SI joint fusion using triangular porous TPS coated implants placed across the SI joint improved pain, disability, and quality of life at 12 months in patients with SI joint dysfunction due to degenerative sacroiliitis and SI joint disruption.
Acknowledgments
The authors acknowledge the 59 investigators and coordinators in the SIFI Study Group: Harry Lockstadt, MD, Elaine Wilhite, MS, James Farris, PA-C (Bluegrass Orthopaedics and Hand Care, Lexington, Kentucky); Don Kovalsky, MD, Cristy Newman, Laura Pestka, RN (Orthopaedic Center of Southern Illinois, Mount Vernon, Illinois); Cheng Tao, MD, Jackie Makowski, Toni Kelly (Spine and Neuro Center, Huntsville, Alabama); S. Craig Meyer, MD, Vicki Jones (Columbia Orthopaedic Group, Columbia, Missouri); Scott Kutz, MD, Linda Thompson, RN, BSN, FNP (Mercy Medical Research Center, Springfield, Missouri); Dimitriy Kondrashov, MD, Irina Kondrashov (SF Spine Group, San Francisco, California); Andy J. Redmond, MD, Jennifer Piazza, MS, Laurie Doredant (Precision Spine Care, Tyler, Texas); CL Soo, MD, Julie White, MBA, Kallena Haynes (Medical Research International, Oklahoma City, Oklahoma); Bradley Duhon, MD, Amber Pfister (Neurosurgical and Spine Specialists, Parker, Colorado); Ali Mesiwala, MD, Stephanie Bose, RN (Southern California Center for Neuroscience and Spine, Pomona, California); Leonard Rudolf, MD, John Thibodeau Jr RN (Alice Peck Day Memorial Hospital, Lebanon, New Hampshire); Kevin Stevenson, MD, Logan Honeycutt, LPN (Piedmont Orthopaedic Complex, Macon, Georgia); Fabien Bitan, MD, Stephanie Gomez (Manhattan Orthopaedics, New York City, New York); John Stevenson, MD, Ana Marichal (The Orthopaedic Institute, Gainesville, Florida); Donald Sachs, MD, Robin Cambron, MSN, MBA, RN (Center for Spinal Stenosis and Neurological Care, Lakeland, Florida); Abhineet Chowdhary, MD, Tina Fortney, RN, BSN (Overlake Hospital Medical Center, Bellevue, Washington); Gowriharan Thaiyananthan, MD, Tungie Williams (BASIC Spine, Orange, California); Michael Oh, MD, Gary Schmidt, MD, Matthew Yeager (Allegheny General Hospital, Pittsburgh, Pennsylvania); David Wiles, MD, Susan Maye, RN, MS (East Tennessee Brain & Spine, Johnson City, Tennessee); Michael Hasz, MD, Carrie Califano (Virginia Spine Institute, Reston, Virginia); William Rosenberg, MD, Pamela McCann, RN, BSN (Midwest Division-RMC, LLC,-Research Medical Center, Kansas City, Missouri); Jeffrey D. Coe, MD, Julia Coe (Silicon Valley Spine, Campbell, California); Jed Vanichkachorn, MD, Jessica Lynch (Tuckahoe Orthopaedics Associates, Richmond, Virginia); Mark C. Gillespy, MD, Sherri Zicker, RN (Orthopaedic Clinic of Daytona Beach, Daytona Beach, Florida); Ralph Rashbaum, MD, Shannon Rusch, BA, CCRC (Texas Back Institute, Plano, Texas); Emily A. Darr, MD, John A. Glaser, MD, Laura Fields (Medical University of South Carolina, Charleston, South Carolina).
Disclosures Bradley S. Duhon, Paid consultant: SI-BONE; Research grant: SI-BONE Daniel J. Cher, Employee: SI-BONE Kathryn D. Wine, Employee: SI-BONE Don A. Kovalsky, Research grant: SI-BONE Harry Lockstadt, Paid consultant: SI-BONE; Research grant: SI-BONE
Financial/Material Support
The study reported herein was sponsored by SI-BONE, Inc. (San Jose, California, United States). Portions of this work were previously presented at the NASS 2014 meeting (San Francisco, CA). An early version of this work was published at: http://dx.doi.org/10.2147/MDER.S55197.
References
- 1.Goldthwait J E, Osgood R B. A consideration of the pelvic articulations from an anatomical, pathological and clinical standpoint. Boston Med Surg J. 1905;152(21):593–601. [Google Scholar]
- 2.Bernard T N Jr, Kirkaldy-Willis W H. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res. 1987;(217):266–280. [PubMed] [Google Scholar]
- 3.Sembrano J N, Polly D W Jr. How often is low back pain not coming from the back? Spine (Phila Pa 1976) 2009;34(1):E27–E32. doi: 10.1097/BRS.0b013e31818b8882. [DOI] [PubMed] [Google Scholar]
- 4.Liliang P-C, Lu K, Liang C-L, Tsai Y-D, Wang K-W, Chen H-J. Sacroiliac joint pain after lumbar and lumbosacral fusion: findings using dual sacroiliac joint blocks. Pain Med. 2011;12(4):565–570. doi: 10.1111/j.1526-4637.2011.01087.x. [DOI] [PubMed] [Google Scholar]
- 5.DePalma M J, Ketchum J M, Saullo T R. Etiology of chronic low back pain in patients having undergone lumbar fusion. Pain Med. 2011;12(5):732–739. doi: 10.1111/j.1526-4637.2011.01098.x. [DOI] [PubMed] [Google Scholar]
- 6.Forst S L, Wheeler M T, Fortin J D, Vilensky J A. The sacroiliac joint: anatomy, physiology and clinical significance. Pain Physician. 2006;9(1):61–67. [PubMed] [Google Scholar]
- 7.Dreyfuss P, Henning T, Malladi N, Goldstein B, Bogduk N. The ability of multi-site, multi-depth sacral lateral branch blocks to anesthetize the sacroiliac joint complex. Pain Med. 2009;10(4):679–688. doi: 10.1111/j.1526-4637.2009.00631.x. [DOI] [PubMed] [Google Scholar]
- 8.Fortin J D, Dwyer A P, West S, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: Asymptomatic volunteers. Spine (Phila Pa 1976) 1994;19(13):1475–1482. [PubMed] [Google Scholar]
- 9.Cher D, Polly D, Berven S. Sacroiliac joint pain: burden of disease. Med Devices (Auckl) 2014;7:73–81. doi: 10.2147/MDER.S59437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luukkainen R K, Wennerstrand P V, Kautiainen H H, Sanila M T, Asikainen E L. Efficacy of periarticular corticosteroid treatment of the sacroiliac joint in non-spondylarthropathic patients with chronic low back pain in the region of the sacroiliac joint. Clin Exp Rheumatol. 2002;20(1):52–54. [PubMed] [Google Scholar]
- 11.Luukkainen R, Nissilä M, Asikainen E. et al. Periarticular corticosteroid treatment of the sacroiliac joint in patients with seronegative spondylarthropathy. Clin Exp Rheumatol. 1999;17(1):88–90. [PubMed] [Google Scholar]
- 12.Maugars Y, Mathis C, Berthelot J M, Charlier C, Prost A. Assessment of the efficacy of sacroiliac corticosteroid injections in spondylarthropathies: a double-blind study. Br J Rheumatol. 1996;35(8):767–770. doi: 10.1093/rheumatology/35.8.767. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S P, Hurley R W, Buckenmaier C C III, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279–288. doi: 10.1097/ALN.0b013e31817f4c7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel N, Gross A, Brown L, Gekht G. A randomized, placebo-controlled study to assess the efficacy of lateral branch neurotomy for chronic sacroiliac joint pain. Pain Med. 2012;13(3):383–398. doi: 10.1111/j.1526-4637.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- 15.Patel N Twelve-month follow-up of a randomized trial assessing cooled radiofrequency denervation as a treatment for sacroiliac region pain Pain Practice 2015; doi: 10.1111/papr.12269 [DOI] [PubMed] [Google Scholar]
- 16.Smith-Petersen M N. Arthrodesis of the sacroiliac joint. A new method of approach. J Bone Joint Surg. 1921;3:400–405. [Google Scholar]
- 17.Smith-Petersen M N, Rogers W A. Arthrodesis for tuberculosis of the sacro-iliac joint. J Am Med Assoc. 1926;86(1):26–30. [Google Scholar]
- 18.Smith-Petersen M N, Rogers W A. End-result study of arthrodesis of the sacro-iliac joint for artritis—traumatic and non-traumatic. J Bone Joint Surg. 1926;8:118–136. [Google Scholar]
- 19.Belanger T A, Dall B E. Sacroiliac arthrodesis using a posterior midline fascial splitting approach and pedicle screw instrumentation: a new technique. J Spinal Disord. 2001;14(2):118–124. doi: 10.1097/00002517-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Berthelot J M, Gouin F, Glemarec J, Maugars Y, Prost A. Possible use of arthrodesis for intractable sacroiliitis in spondylarthropathy: report of two cases. Spine (Phila Pa 1976) 2001;26(20):2297–2299. doi: 10.1097/00007632-200110150-00028. [DOI] [PubMed] [Google Scholar]
- 21.Buchowski J M Kebaish K M Sinkov V Cohen D B Sieber A N Kostuik J P Functional and radiographic outcome of sacroiliac arthrodesis for the disorders of the sacroiliac joint Spine J 200555520–528., discussion 529 [DOI] [PubMed] [Google Scholar]
- 22.Giannikas K A, Khan A M, Karski M T, Maxwell H A. Sacroiliac joint fusion for chronic pain: a simple technique avoiding the use of metalwork. Eur Spine J. 2004;13(3):253–256. doi: 10.1007/s00586-003-0620-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kibsgård T J, Røise O, Sudmann E, Stuge B. Pelvic joint fusions in patients with chronic pelvic girdle pain: a 23-year follow-up. Eur Spine J. 2013;22(4):871–877. doi: 10.1007/s00586-012-2512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuire R A, Chen Z, Donahoe K. Dual fibular allograft dowel technique for sacroiliac joint arthrodesis. Evid Based Spine Care J. 2012;3(3):21–28. doi: 10.1055/s-0032-1327806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore M R. New York, NY: Churchill Livingstone; 1997. Surgical treatment of chronic painful sacroiliac joint dysfunction; pp. 563–572. [Google Scholar]
- 26.Ashman B, Norvell D C, Hermsmeyer J T. Chronic sacroiliac joint pain: fusion versus denervation as treatment options. Evid Based Spine Care J. 2010;1(3):35–44. doi: 10.1055/s-0030-1267066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schütz U, Grob D. Poor outcome following bilateral sacroiliac joint fusion for degenerative sacroiliac joint syndrome. Acta Orthop Belg. 2006;72(3):296–308. [PubMed] [Google Scholar]
- 28.Waisbrod H, Krainick J U, Gerbershagen H U. Sacroiliac joint arthrodesis for chronic lower back pain. Arch Orthop Trauma Surg. 1987;106(4):238–240. doi: 10.1007/BF00450461. [DOI] [PubMed] [Google Scholar]
- 29.Lorio M P, Polly D W Jr, Ninkovic I, Ledonio C GT, Hallas K, Andersson G. Utilization of minimally invasive surgical approach for sacroiliac joint fusion in surgeon population of ISASS and SMISS membership. Open Orthop J. 2014;8:1–6. doi: 10.2174/1874325001408010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Khayer A, Hegarty J, Hahn D, Grevitt M P. Percutaneous sacroiliac joint arthrodesis: a novel technique. J Spinal Disord Tech. 2008;21(5):359–363. doi: 10.1097/BSD.0b013e318145ab96. [DOI] [PubMed] [Google Scholar]
- 31.Khurana A, Guha A R, Mohanty K, Ahuja S. Percutaneous fusion of the sacroiliac joint with hollow modular anchorage screws: clinical and radiological outcome. J Bone Joint Surg Br. 2009;91(5):627–631. doi: 10.1302/0301-620X.91B5.21519. [DOI] [PubMed] [Google Scholar]
- 32.Wise C L, Dall B E. Minimally invasive sacroiliac arthrodesis: outcomes of a new technique. J Spinal Disord Tech. 2008;21(8):579–584. doi: 10.1097/BSD.0b013e31815ecc4b. [DOI] [PubMed] [Google Scholar]
- 33.Cummings J Jr, Capobianco R A. Minimally invasive sacroiliac joint fusion: one-year outcomes in 18 patients. Ann Surg Innov Res. 2013;7(1):12. doi: 10.1186/1750-1164-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith A G, Capobianco R, Cher D. et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14. doi: 10.1186/1750-1164-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudolf L. MIS fusion of the SI joint: does prior lumbar spinal fusion affect patient outcomes? Open Orthop J. 2013;7:163–168. doi: 10.2174/1874325001307010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudolf L. Sacroiliac joint arthrodesis-MIS technique with titanium implants: report of the first 50 patients and outcomes. Open Orthop J. 2012;6:495–502. doi: 10.2174/1874325001206010495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sachs D, Capobianco R. Minimally invasive sacroiliac joint fusion: one-year outcomes in 40 patients. Adv Orthop. 2013;2013:536128. doi: 10.1155/2013/536128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachs D, Capobianco R. One year successful outcomes for novel sacroiliac joint arthrodesis system. Ann Surg Innov Res. 2012;6(1):13. doi: 10.1186/1750-1164-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroeder J E, Cunningham M E, Ross T, Boachie-Adjei O. Early results of sacro-iliac joint fixation following long fusion to the sacrum in adult spine deformity. HSS J. 2014;10(1):30–35. doi: 10.1007/s11420-013-9374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szadek K M, van der Wurff P, van Tulder M W, Zuurmond W W, Perez R SGM. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354–368. doi: 10.1016/j.jpain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 41.American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine . Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810–833. doi: 10.1097/ALN.0b013e3181c43103. [DOI] [PubMed] [Google Scholar]
- 42.Bogduk N. San Rafael, CA: International Spine Intervention Society; 2004. Practice Guidelines for Spinal Diagnostic and Treatment Procedures. 2nd ed. [Google Scholar]
- 43.Manchikanti L Abdi S Atluri S et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations Pain Physician 201316(2, Suppl):S49–S283. [PubMed] [Google Scholar]
- 44.Manchikanti L, Boswell M V, Singh V. et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009;12(4):699–802. [PubMed] [Google Scholar]
- 45.Pauza K Educational Guidelines for Interventional Spinal Procedures. American Academy of Physical Medicine and Rehabilitation Available at: http://www.aapmr.org/practice/guidelines/documents/edguidelines.pdf. Accessed October 1, 2014
- 46.Fairbank J C Pynsent P B The Oswestry Disability Index Spine (Phila Pa 1976) 200025222940–2952., discussion 2952 [DOI] [PubMed] [Google Scholar]
- 47.EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 48.Ware J E Jr, Sherbourne C D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 49.Childs J D, Piva S R, Fritz J M. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila Pa 1976) 2005;30(11):1331–1334. doi: 10.1097/01.brs.0000164099.92112.29. [DOI] [PubMed] [Google Scholar]
- 50.Copay A G, Glassman S D, Subach B R, Berven S, Schuler T C, Carreon L Y. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008;8(6):968–974. doi: 10.1016/j.spinee.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 51.R Core Team R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing; 2013 Available at: http://www.R-project.org/. Accessed September 1, 2013
- 52.Ha K-Y, Lee J-S, Kim K-W. Degeneration of sacroiliac joint after instrumented lumbar or lumbosacral fusion: a prospective cohort study over five-year follow-up. Spine (Phila Pa 1976) 2008;33(11):1192–1198. doi: 10.1097/BRS.0b013e318170fd35. [DOI] [PubMed] [Google Scholar]
- 53.Onsel C, Collier B D, Kir K M. et al. Increased sacroiliac joint uptake after lumbar fusion and/or laminectomy. Clin Nucl Med. 1992;17(4):283–287. doi: 10.1097/00003072-199204000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Whang P, Cher D, Polly D. et al. Sacroiliac joint fusion using triangular titanium implants vs. non-surgical management: six-month outcomes from a prospective randomized controlled trial. Int J Spine Surg. 2015;9:6. doi: 10.14444/2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rudolf L, Capobianco R. Five-year clinical and radiographic outcomes after minimally invasive sacroiliac joint fusion using triangular implants. Open Orthop J. 2014;8:375–383. doi: 10.2174/1874325001408010375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanaclocha-Vanaclocha V, Verdu-Lopez F, Sanchez-Pardo M, Gonzalbes-Esterelles L. Minimally invasive sacroiliac joint arthrodesis: experience in a prospective series with 24 patients. J Spine. 2014;3:185. [Google Scholar]
- 57.Fortin J D, Falco F J. The Fortin finger test: an indicator of sacroiliac pain. Am J Orthop Belle Mead NJ. 1997;26:477–480. [PubMed] [Google Scholar]


