Abstract
Study Design Combination of narrative and systematic literature reviews.
Objectives Massive perioperative blood loss in complex spinal surgery often requires blood transfusions and can negatively affect patient outcome. Systemic use of the antifibrinolytic agent tranexamic acid (TXA) has become widely used in the management of surgical bleeding. We review the clinical evidence for the use of intravenous TXA as a hemostatic agent in spinal surgery and discuss the emerging role for its complementary use as a topical agent to reduce perioperative blood loss from the surgical site. Through a systematic review of published and ongoing investigations on topical TXA for spinal surgery, we wish to make spine practitioners aware of this option and to suggest opportunities for further investigation in the field.
Methods A narrative review of systemic TXA in spinal surgery and topical TXA in surgery was conducted. Furthermore, a systematic search (using PRISMA guidelines) of PubMed (MEDLINE), EMBASE, and Cochrane CENTRAL databases as well as World Health Organization International Clinical Trials Registry Platform, ClinicalTrials.gov (National Institutes of Health), and International Standard Randomized Controlled Trial Number registries was conducted to identify both published literature and ongoing clinical trials on topical TXA in spinal surgery.
Results Of 1,631 preliminary search results, 2 published studies were included in the systematic review. Out of 285 ongoing clinical trials matching the search criteria, a total of 4 relevant studies were included and reviewed.
Conclusion Intravenous TXA is established as an efficacious hemostatic agent in spinal surgery. Use of topical TXA in surgery suggests similar hemostatic efficacy and potentially improved safety as compared with intravenous TXA. For spinal surgery, the literature on topical TXA is sparse but promising, warranting further clinical investigation and consideration as a clinical option in cases with significant anticipated surgical site blood loss.
Keywords: tranexamic acid (TXA), spinal surgery, topical drug administration, bleeding management, antifibrinolytics
Introduction
Massive blood loss occurs frequently and remains a challenge in complex spinal surgery.1 2 3 4 Significant intra- and postoperative hemorrhage negatively affects patient outcomes by increasing coagulopathy, postoperative hematoma, and anemia.5 The need for allogenic blood transfusions can lead to potential transfusion reactions and infections, in addition to increasing long-term mortality rates.6 There is an economic disadvantage associated with iatrogenic major blood loss relating to the direct costs of the blood products and intraoperative blood salvage technology and indirect costs of prolonged patient hospitalization and complication management.7
Many efforts have focused on achieving better perioperative blood conservation, in particular through prophylactic intravenous administration of antifibrinolytic agents before and during major surgery. Intravenous administration of the inexpensive but highly efficacious lysine analogue tranexamic acid (TXA) reduces perioperative hemorrhage and the need for blood transfusions by one third in major surgery,8 9 including spinal surgery.10 11 12 13 14 There also appears to be an increasingly established role for topical TXA (tTXA) in orthopedic and cardiac surgery to attenuate the perioperative blood loss from the surgical site.15
Here, we first provide a narrative literature review with the rationale to succinctly review the pharmacology of TXA and discuss the current status of its clinical use in spinal surgery. Beyond illustrating the clinical evidence for intravenous TXA as an efficient hemostatic agent in this surgical discipline, we also discuss the increasing use and potential of tTXA in surgery. Furthermore, we have performed a systematic review of existing publications and ongoing clinical trial protocols on tTXA for spinal surgery, to summarize the rationale of this complementary option to reduce postoperative blood loss from the surgical site.
Methods
For the narrative review, we focused on summarizing the mechanisms of action and clinical pharmacology of TXA, illustrating the clinical evidence for its application as a systemic hemostatic agent in spinal surgery as well as discussing an emerging role for its topical use in different kinds of surgery.
For the systematic review, we followed the PRISMA guidelines to identify and review the published literature as well as the currently registered clinical trials on use of tTXA in spinal surgery.16 To identify the relevant published clinical literature (case–control studies, cohort studies, randomized controlled trials [RCTs], meta-analyses), a systematic search of Ovid EMBASE, Ovid MEDLINE, and CENTRAL (Cochrane Library Trials) databases was conducted on March 20, 2015 using the MeSH terms “tranexamic acid (including synonyms: t-amcha, amcha, cyklokapron, transamine)” AND “surgery.” For EMBASE, the available “Search Limits” feature was used to refine the search for “Clinical Trials.” Titles and abstracts of the publications in the search results were manually screened for subject relevance, followed by whole-publication screening of potential candidates. Studies were included if they (1) assessed patient groups undergoing any kind of spinal surgery, (2) investigated tTXA as an independent variable for hemostatic control in spinal procedures, (3) provided any form of outcome measure to evaluate perioperative (i.e., intra- and/or postoperative) blood loss. Those studies using intravenous route of TXA administration were excluded and all duplicates were removed.
To identify ongoing clinical trials on use of tTXA in spinal surgery, three major clinical trial registries were searched on March 20, 2015: ClinicalTrials.gov (U.S. National Institutes of Health), World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), and the International Standard Randomized Controlled Trial Number (ISRCTN). The search terms included “tranexamic acid” AND “surgery.” All search results were manually screened by title and protocol for subject relevance and then underwent the same selection procedure used for published results (outlined previously).
Data extraction and subsequent analysis of published studies and current registered clinical trials selected for systematic review were performed manually by two independent reviewers (S.F.W. and C.S.). From published studies, data was sought for (1) participants/condition (sample size, type of surgery), (2) interventions (dosing/preparation, intraoperative time point and procedure of tTXA administration), (3) comparisons (control groups, type of placebo used), (4) outcome measures (direct and/or indirect measurements of intra- and/or postoperative blood loss, changes in blood transfusion requirements, systemic exposure following tTXA administration, markers of patient recovery, risk of thromboembolic and other adverse events), and (5) study design (type of study, setup of intervention and comparisons, times of postoperative follow-up). From protocols of current clinical trials, data was sought for (1) participants/condition, (2) interventions, (3) comparisons, (4) outcome measures, (5) study design, (6) inclusion and key exclusion criteria.
There were no language restrictions in our searches. There were no specific date restrictions for searched articles, although articles published before 1962 preceded the discovery of TXA.
Results
Pharmacology of Tranexamic Acid
Okamoto et al discovered trans-4-aminomethyl-cyclohexanecarboxylic acid (tranexamic acid) as a potent antifibrinolytic superior to the previously used antifibrinolytic lysine analogue episilon-aminocaproic acid.17 18 19 TXA has received widespread approval and clinical use as a hemostatic agent, in particular following the 2007 preliminary withdrawal and ongoing controversy associated with aprotinin, a direct inhibitor of free plasmin.20 Since 2011, it is also included on the WHO list of essential medicines.21
TXA is a synthetic lysine analogue that interferes with fibrinolysis through binding reversibly and competitively to lysine-binding domains on plasminogen, plasmin, and tissue plasminogen activator (Fig. 1).22 It chiefly works by attenuating the binding capacity of plasminogen and tissue plasminogen activator to fibrin, thereby decreasing the subsequent conversion of plasminogen to the enzymatically active serine protease plasmin, which leads to dissolution of fibrin clots.23 24
Fig. 1.
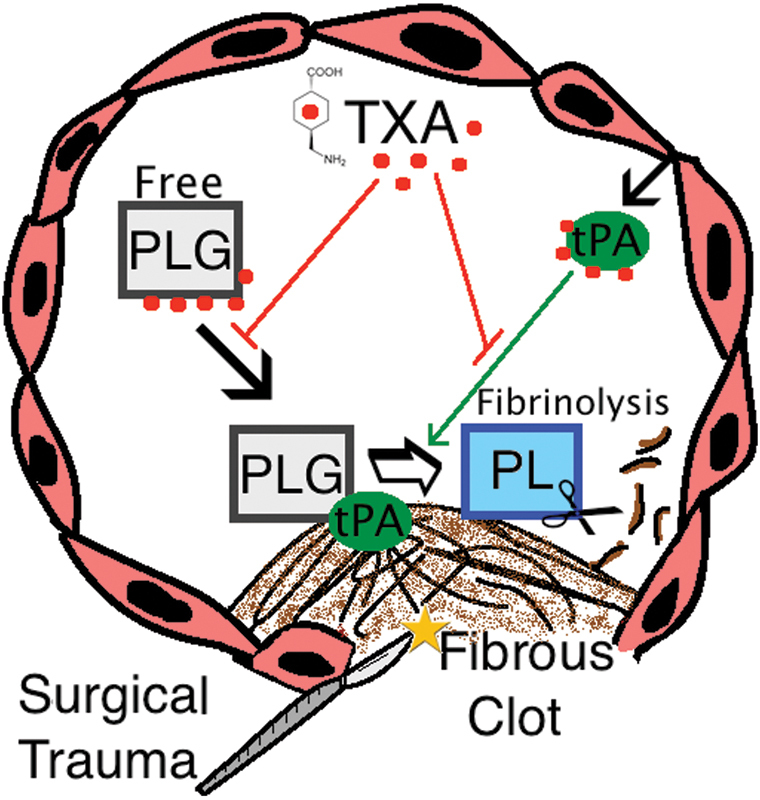
Schematic representation of TXA-mediated antifibrinolytic mechanisms in the intravascular lumen. Abbreviations: PLG, plasminogen; PL, plasmin; tPA, tissue plasminogen activator; TXA, tranexamic acid.
TXA is most commonly administered systemically and at 10 mg/kg intravenous administration has a half-life of around 80 minutes, reaching peak plasma concentrations within 1 hour postinjection.25 As TXA is eliminated renally, appropriate dose adjustments have to be made with patients suffering from renal impairment.26 Optimal dosing for intravenous TXA (ivTXA) is unknown; however, there is general consensus that ivTXA is clinically efficacious at doses of 10 to 15 mg/kg (or 1 g), with higher doses providing little additional hemostatic benefit.8 12 27 This observation is reflected by in vitro studies showing that ivTXA has high tissue penetrance and absorption, with doses of 10 mg/kg reaching 80% inhibition of fibrinolysis in tissue removed from patients who received TXA systemically during surgery.28 For most kinds of surgery, including spinal surgery, ivTXA is administered as a bolus dose (10 to 20 mg/kg) preoperatively and/or as a maintenance infusion (1 to 10 mg/kg/h) during the operation, most commonly at doses of 10 mg/kg followed by infusion of 1 mg/kg/h.22 24
Systemically administered TXA can penetrate the blood–brain barrier and distribute throughout the central nervous system (CNS) and eye, where it reaches the cerebrospinal fluid and aqueous humor concentrations at around 10% of TXA plasma concentrations.22 ivTXA has been used at higher doses (≥100 mg/kg) in cardiopulmonary bypass (CPB) surgery, where it was found to produce epileptogenic side effects leading to postoperative convulsive seizures in a subgroup of susceptible patients.29 Analogously, experimental studies with rodents revealed TXA may be neurotoxic when applied topically to CNS tissue,30 31 likely by interfering with central GABAA receptors and glycine receptors.32 Some cases of other rare serious side effects of TXA such as visual disturbances and acute renal cortical necrosis have been reported.33 34 35
Despite a lack of evidence in the literature, there remains considerable uncertainty regarding a theoretical thrombogenicity of TXA.36 Most meta-analyses assessing association of TXA with thromboembolic events (TEs) have reported inconclusive findings, because RCTs are underpowered due to small sample sizes and because reporting of thromboembolic complications is often biased and poorly communicated.9 The potential risk of TEs would likely vary across different surgical disciplines and specific patient groups, with a higher expected cumulative risk in cardiovascular procedures owing to the type of surgery, patient factors, and more frequent use of high-dose ivTXA. Importantly, the CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Hemorrhage) trial assessing the hemostatic effects of TXA in over 20,000 adult trauma patients found no increase in thromboembolic risk but did find a decrease in postoperative myocardial infarction in TXA-treated patients.37 Similarly, a large retrospective cohort study (510 U.S. hospitals, n = 872,416 patients) on perioperative ivTXA use in arthroplasty surgery revealed no increase in complications, including TE and renal failure, in patients treated with the drug.38 In addition, Neilipovitz has pointed out a need to raise awareness about the pharmacodynamics of antifibrinolytics in hemostasis to curtail a common misconception in the medical community that lysine analogues work like procoagulant drugs and increase blood clotting.3
Use of Intravenous Tranexamic Acid in Spinal Surgery
Intravenous use of the antifibrinolytic agent TXA in the management of bleeding is well established.39 40 The results from the CRASH-2 trial have confirmed its efficacy in significantly reducing mortality from trauma-related bleeding. Specifically, mortality is reduced to 5.3 from 7.7% in the placebo group when TXA is administered within 1 hour of injury occurrence. The potential clinical impact of providing routine ivTXA administration to all patients with or at risk of trauma-related hemorrhage has been estimated at a reduction of the global number of deaths by 120,000 annually.39 Beyond its use for trauma-related hemorrhage,41 ivTXA has found major applications in other hemorrhagic conditions, including postpartum hemorrhage,42 menorrhagia,43 and many kinds of surgery.24 Ker et al have conducted several meta-analyses to identify efficacy of ivTXA as a hemostatic agent in surgery, reporting a one-third reduction in blood loss from surgery (104 trials, n = 8,030 patients) and similarly a 38% reduction in the probability of receiving a blood transfusion (95 trials, n = 7,838 patients) in patients receiving treatment.8 9
Although used extensively in orthopedic and cardiac surgery,44 45 46 47 ivTXA is also becoming a standard of care for bleeding management in spinal surgery. The prevalent clinical use and hemostatic benefits of ivTXA in spinal surgery are reflected by multiple meta-analyses of relevant RCTs that consistently reported its efficacy in reducing blood loss from surgery and a concurrent reduction in the need for allogenic blood products.8 10 11 12 13 14 48 The RCTs were performed for patients undergoing posterior instrumented spinal surgery for thoracic/lumbar instability or scoliosis.49 50 51 52 53 54 55 56 57 58 59 60 61 62
In one RCT conducted in our institution, Wong et al demonstrated the hemostatic benefits of ivTXA (10 mg/kg initial bolus and 1 mg/kg/h maintenance infusion until skin closure) on patients (n = 147) undergoing elective posterior thoracic/lumbar instrumented spinal fusions.49 Total perioperative blood loss was reduced by 25 to 30% in ivTXA-treated patients corresponding to higher postoperative hemoglobin levels as well as reduced frequency and amount of transfused cell saver blood in the treatment group.
ivTXA has also been used for hemostasis in cervical spinal procedures and spinal tumor surgery63 64; several RCTs have demonstrated its beneficial effects in overall complex spinal surgery procedures.65 66 An RCT conducted by Elwatidy et al involving patients (n = 64) undergoing different kinds of major, mostly multilevel, spinal surgery used a high-dose protocol (2 g loading dose for adults or 30 mg/kg for children, followed by a maintenance dose of 100 mg/h for adults or 1 mg/kg/h for children) of ivTXA, which was continuously administered up to 5 hours after the surgery.65 Total blood loss in the treatment group was reduced by almost half, with a 48% reduction intraoperatively and 55% reduction in postoperative drain output. In summary, the established hemostatic role of ivTXA in surgery extends to the field of spinal surgery, where the current literature confirms the efficacy and suggests the safety of ivTXA in consistently reducing perioperative blood loss and blood transfusion requirements associated with major spinal surgery. Just like in other hemorrhage-related conditions, there is no optimal dosing regimen for TXA in spinal surgery. However, its known efficacy at relatively low doses was also demonstrated for spinal surgery.8 12 27 49 Future RCTs should investigate whether prolonged ivTXA administration beyond the perioperative period is safe and provides additional hemostatic benefit following skin closure.65
Use of Topical Tranexamic Acid in Surgery
The unresolved discussion about potential thrombogenic risks as well as rare but known epileptogenic side effects and restrictions of using TXA systemically in patients with clotting disorders or renal impairment have prompted investigations on the use of tTXA for bleeding management. Moreover, the fact that excessive fibrinolysis as a result of acute consumptive coagulopathy from surgical trauma is most severe in the surgical wound itself and often persists throughout the postoperative period has sparked interest in exploring whether topically applied antifibrinolytics might improve the current standard of care for hemostasis in surgery.15
tTXA has been used to control hemorrhage in dental extraction and epistaxis,67 68 69 and applications in many varieties of surgery are now increasingly identified. tTXA application is widely established in major orthopedic surgery, particularly for hip and knee arthroplasty,70 71 72 73 74 75 76 77 where it is applied via intra-articular injection or irrigated into the wound before skin closure.
Ker et al performed a large systematic review (29 RCTs, n = 2,612 patients) to investigate the hemostatic effects of tTXA in surgery.78 Out of 28 surgical trials, tTXA was either applied as irrigation directly into the wound before skin closure in 24 RCTs or by means of intra-articular injection in several RCTs involving arthroplasty surgery. The effects of tTXA on blood loss were reported in 18 RCTs, the majority of which involved either cardiac (7 RCTs, n = 511 patients) or knee arthroplasty (5 RCTs, n = 427 patients) surgery. tTXA-related reduction in blood loss was highly significant in both types of surgery as well as in otolaryngologic surgery (2 RCTs, n = 456 patients) and spinal surgery (2 RCTs, n = 130 patients), resulting in an overall reduction of blood loss by 29% in tTXA-treated patients. This percentage is comparable to what has been reported for intravenously administered TXA.8 Furthermore, tTXA reduced the risk of receiving a blood transfusion by 45% (14 RCTs, n = 1,523 patients), exceeding the average risk reduction documented for ivTXA.9
The Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists now recommend both ivTXA and tTXA for surgical bleeding management,15 and it is expected that this rationale will be adopted in other surgical disciplines as tTXA becomes increasingly established.
For knee arthroplasty, several RCTs have directly compared tTXA to ivTXA performance,71 79 demonstrating noninferiority of tTXA for blood loss reduction, blood transfusion requirement, and thromboembolic risk compared with systemic administration of the drug. Similar findings were also reported in a recent meta-analysis comparing the two routes of TXA administration in arthroplasty.80 An RCT conducted at our institution first demonstrated the beneficial hemostatic effects of using tTXA in arthroplasty,70 which is now becoming a popular blood conservation strategy. Wong et al observed a 20 to 25% reduction in postoperative blood loss following a 5-minute application of 1.5 g or 3 g tTXA via irrigation before wound closure.70 TXA plasma levels at 1 hour postoperatively were minimal and ∼70% smaller than the average levels present when TXA is used systemically. Several other studies also report minimal postoperative systemic absorption following tTXA application,81 82 83 estimated at only one-tenth of the concentration observed with ivTXA treatment.78
Furthermore, substantial economic benefits were demonstrated in a cost analysis, where tTXA reduced blood transfusion-related cost by two thirds. TXA itself is relatively inexpensive and deemed a highly cost-efficient drug.84 It has been pointed out that other commonly used topical hemostatic agents such as fibrin sealants or spray have several disadvantages, as they are much more expensive than TXA and are derived from human plasma, bearing a small but relevant risk for infection.70 The blood conservation-related cost-saving aspects of tTXA in arthroplasty are evident and would suggest similar economic benefits in other surgical fields.85 86 87 88 89
Analogous to ivTXA, no definite dosing protocol for tTXA has yet been established. However, unlike systemically administered TXA, dosing of tTXA will presumably vary more across different types of surgery as it can be tailored toward the extent of hemorrhage. Ker et al found that doses of tTXA administered in the RCTs assessing blood loss (18 RCTs, n = 1,651 patients) were highly heterogeneous (ranging from 0.7 mg to 100 mg/mL of saline solution); however, the observed hemostatic effect was not dose-dependent.78 This result likely suggests effective tissue absorption and distribution of locally applied TXA and dose–response properties comparable to systemic TXA, which is highly efficacious already at relatively low doses (i.e., 10 mg/kg [or 1 g] initial bolus with 1 mg/kg/h maintenance dose).27
In summary, the current literature indicates that TXA has been used topically in several surgical disciplines and may have comparable hemostatic efficacy to systemic administration of the drug, albeit optimal dosing protocols remain to be established. Noninferiority of topical use has been demonstrated in arthroplasty and CPB surgery and further RCTs are needed to determine benefits across other surgical procedures. The clinical rationale for using a local and more targeted treatment strategy to manage surgical bleeding is evident, and the resultant minimization of systemic exposure suggests that patients at higher risk for TE, renal impairment, or epilepsy—where ivTXA use would be relatively contraindicated—might safely benefit from its topical use. In addition, a combinatorial treatment protocol with ivTXA and tTXA has been described and is a promising strategy that might augment hemostasis and perhaps reduce the current dose of ivTXA needed to achieve good hemostatic results, thus further diminishing potential side effects.90
Use of Topical Tranexamic Acid in Spinal Surgery
Existing medical literature on ivTXA for spinal surgery suggests that it is highly efficacious and is increasingly becoming established as a clinical option to reduce perioperative blood loss from the surgical site.10 11 12 13 14 Similar to cardiac and arthroplasty surgery, considerable postoperative blood loss occurs after complex spinal surgery. The fact that tTXA has been successfully used to attenuate postoperative hemorrhage and blood transfusion requirements in these surgical disciplines provides a rationale for investigating its suggested benefit for postoperative hemostasis in the field of spinal surgery.
Our systematic search revealed a small number of case series on the use of tTXA for spinal surgery. Following, we give a detailed analytical account of the existing published literature (n = 2) as well as relevant ongoing clinical trials (n = 4) on tTXA use in spinal surgery. Subsequently, we discuss strategies for implementation of clinical trials on tTXA in spinal surgery. All analyzed articles and protocols of ongoing clinical trials were written in the English language except where indicated.
Published Literature
A total of 1,631 preliminary articles (RCTs, meta-analyses, and systematic reviews) matched our search criteria (n = 511 in Ovid EMBASE; n = 755 in Ovid MEDLINE; n = 365 in CENTRAL). The title and abstract of these articles were subsequently screened for subject relevance, revealing 76 potentially relevant articles (n = 20 in Ovid EMBASE; n = 29 in Ovid MEDLINE; n = 27 in CENTRAL), which were selected for whole-publication screening. Duplicates and studies using ivTXA (n = 74) were subsequently rejected, resulting in a total of 2 clinical studies on use of tTXA in spinal surgery (Fig. 2), for which data extraction, data analysis, and interpretation (including a judgment on risk of bias) were performed by the reviewers.
Fig. 2.
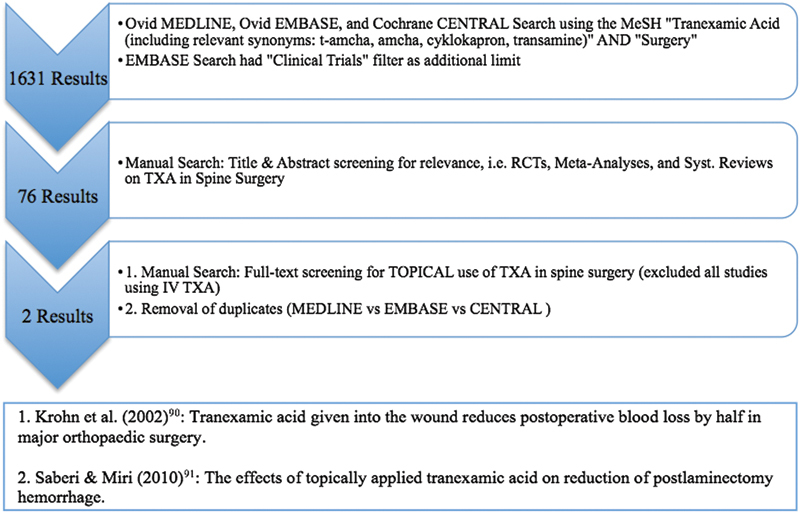
Flow diagram depicting search strategy and study selection process used for systematic review. Abbreviations: RCT, randomized controlled trial; TXA, tranexamic acid.
In a placebo-controlled study, Krohn et al investigated the impact of tTXA on postoperative blood loss as well as the fibrinolytic activity in patients with lower back pain undergoing elective lumbar instrumented spinal fusion surgery (n = 30 patients; 16 received tTXA and 13 received placebo, i.e., saline only).91 tTXA (500 mg in 50 mL saline solution) was applied via irrigation for 2 to 5 minutes before removal of excess fluid and wound closure. The median postoperative blood loss at 18 hours, as measured by drain output, was reduced by half in patients receiving the treatment (252 mL with tTXA versus 525 mL without). Postoperative blood loss thus accounted for 35% of total blood loss, as opposed to 61% in patients receiving placebo, likely demonstrating the locally sustained hemostatic efficacy of tTXA. Following the surgery, tTXA-treated patients also required less autologous blood transfusions from salvaged blood (n = 2 in tTXA group versus n = 9 in placebo group), which was routinely given when blood loss exceeded 150 mL in the first 6 hours postoperatively. The authors also measured markers of fibrinolysis, plasmin/α2-antiplasmin complex and D-dimers, arterially before wound closure and in the drainage tube after wound closure and 1 hour postoperatively. The postoperative increase of both fibrinolytic markers was significantly attenuated in the tTXA-treated patients as compared with controls, suggesting that the observed reduction in blood loss was indeed mediated by the antifibrinolytic activity of tTXA. Although these results indicate the promising hemostatic efficacy of tTXA in major spinal surgery, the sample size was quite small and several methodological uncertainties may exist. Although the patients were assigned randomly to different intervention groups, it is unclear whether the authors used adequate allocation concealment to prevent a potential selection bias. Moreover, the authors did not report any data on complications such as risk for TE and did not measure postoperative TXA plasma levels to assess systemic exposure.
In a placebo-controlled study, Saberi and Miri assessed the effect of tTXA on postoperative blood loss and on the duration of hospitalization in patients undergoing either unilateral one-level (n = 50) or bilateral two-level (n = 50) decompressive laminectomy.92 In the treatment groups, 250 mg TXA dissolved in 5 mL saline solution was “spray poured” onto the surgical field 5 minutes before wound closure. The blood loss, as assessed by drain output, was significantly reduced at 24 and 48 hours postoperatively in both tTXA-treated study groups, with overall values ranging between 30 and 70% across time points and procedure types for tTXA-related blood loss reduction. In the patient group receiving unilateral one-level laminectomy, the effect was most strongly pronounced during the second postoperative day (24 to 48 hours), as blood loss was reduced by 70% (35 ± 32 mL versus 116.4 ± 43.5 mL) in tTXA-treated patients. In addition, the postoperative duration of hospitalization was reduced (2.16 ± 0.37 versus 2.96 ± 0.89 days) in the tTXA-treated patients, indicating faster patient recovery. The authors did not measure postoperative TXA plasma levels to assess systemic exposure. No data on complications such risk of TE was reported. Several methodological uncertainties may exist. It is unclear whether allocation to intervention groups occurred randomly and was adequately concealed (potential selection bias). Blinding of practitioners during subsequent outcome assessment is not reported (potential detection bias). This study advocates for the use of tTXA in minor spinal procedures such as one- or two-level decompressive laminectomies, where the blood loss is usually not severe. Although it is conceivable to use tTXA in minor spinal surgery, its hemostatic role may be much more pronounced in major multilevel instrumented procedures.
Ongoing Clinical Trails
Out of 285 ongoing clinical trials (n = 125 in ClinicalTrials.gov; n = 147 in WHO ICTRP; n = 13 in ISRCTN registry) matching our search criteria, manual screening by title and protocol revealed a total of 3 ongoing RCTs and 1 prospective cohort study investigating the use of tTXA for spinal surgery (see Table 1 for details).93 94 95 96
Table 1. Ongoing clinical studies for topical TXA in spinal surgery.
| Ongoing clinical trial | Study design | Condition | Inclusion criteria | Key exclusion criteria | Intervention | Outcome measures |
|---|---|---|---|---|---|---|
| Miyanji and Kilb95; University of British Columbia | RCT, double-blind; target sample size: 80 | Major pediatric spine deformity surgery | 8–21 y old; spinal deformity surgery with OP time > 3 h | Dural tear; coagulopathy; history of thromboembolic events; therapeutic anticoagulation requirement; renal impairment | Topical TXA (dose not mentioned in protocol) versus intravenous TXA (15 mg/kg loading dose at time of incision and continuous infusion of 2 mg/kg/h) | 1. Intra-OP blood loss 2. TXA-related complications (reporting at 6-wk visit) |
| Wood94; Massachusetts General Hospital | RCT, double-blind; target sample size: 80 | Posterior approach thoracolumbar spinal surgery | 18–85 y old; elective multilevel spinal surgery with posterior approach to thoracolumbar spine | Dural tear; coagulopathy; history of thromboembolic events; revision procedure where only instrumentation is removed; renal impairment | Topical TXA (single application of 3 g in 100 mL saline) versus placebo (single application of 100 mL saline) | 1. Change in hemoglobin level (first measurement at pre-OP appointment (1 wk before surgery); patient then followed for hospital stay duration (5 d average) 2. Hospital length of stay in days; no. of transfusions during hospitalization |
| Lehman93; Washington University School of Medicine | RCT, double-blind; target sample size: 252 | Complex combat-related spine trauma surgery | 18–75 y old; thoracic/lumbar spinal column trauma requiring surgical fixation (within 21 d postinjury); elective long segment (>5 fusion levels) posterior spinal fusions | Dural tear; coagulopathy; history of thromboembolic events; therapeutic anticoagulation requirement; use of intravenous TXA during prestudy period; trauma penetrating spinal cord/physiological instability; history of seizure; traumatic brain injury; color vision disturbances; renal impairment | Topical TXA (3 g in solution poured in the surgical field and left in contact for 5 min, before removal of excess solution) versus placebo (saline) | 1. Hemoglobin concentration post-OP; blood transfusion requirements 2. Cost analysis using patient cost info during hospital stay; patient QoL (2-y follow-up); surgical site infection (2-wk follow-up); systemic absorption of locally applied drug; complications (up to 4 d post-OP) |
| Xianming and Jun96; The Military General Hospital of Chengdu, China | Prospective cohort study; target sample size: 150 (50 per group) | Posterior thoracolumbar spinal internal fixation operation | 18–70 y old; elective posterior thoracolumbar spinal internal fixation operation | Coagulopathy; history of thromboembolic events; therapeutic anticoagulation requirement; renal impairment | 3 groups: topical TXA (10 mg/kg) versus intravenous TXA (10 mg/kg) versus placebo (saline) | 1. Intra-OP and post-OP (drainage) blood loss; volume of blood transfusion; hemoglobin; fibrinogen; D-dimer; INR; PT; aPTT |
Abbreviations: aPTT, activated partial thromboplastin time; INR, international normalized ratio; OP, operation; PT, prothrombin time; QoL, quality of life; RCT, randomized controlled trial; TXA, tranexamic acid.
The trials intend to use tTXA in elective instrumented spinal surgery for adult and pediatric spinal deformity,93 95 elective instrumented thoracic/lumbar spinal surgery strictly for spinal stenosis,94 or instrumented thoracic/lumbar spinal surgery for combat-related spinal trauma.93 tTXA is compared with saline irrigation placebo or ivTXA or both.93 94 95 96
A tTXA irrigation is typically poured into the surgical field before wound closure, with dosing protocols ranging from 10 mg/kg (i.e., around 1 g) to 3 g of tTXA in saline solution. A generic primary outcome measure of all four studies is the presumed tTXA-related reduction in blood loss, quantified both intra- and postoperatively as well as indirectly by the incidence and/or amount of the blood transfusion requirements. Notably, Miyanji and Kilb only assess intraoperative blood loss and also use an active control group receiving ivTXA in their RCT.95 A definite advantage of this study design is direct intergroup comparison between the topical and intravenous route of TXA administration. The fact that blood loss is quantified only intraoperatively raises an important question about the time point selected for intraoperative tTXA application, left unspecified in the study protocol. Because ivTXA bolus treatment in the active control group occurs before skin incision and is maintained throughout the procedure, we can only speculate that tTXA will be applied in a similar temporal manner (i.e., toward the beginning of the procedure or perhaps as multiple irrigations throughout the procedure).
Beyond the above-mentioned outcome measures, Wood et al will assess the length of hospital stay as an indirect correlate for patient recovery/outcome.94 A limitation of this RCT is the apparent lack of assessment of tTXA-related complications, included in the other studies.
Lehman et al provide the only study protocol reporting a detailed description of the tTXA application procedure and time point,93 using an administration protocol previously used in spinal surgery.91 Moreover, several important secondary outcome measures regarding tTXA safety/systemic exposure and its impact on wound healing, overall treatment cost, and long-term patient quality of life are considered in this RCT.
All study protocols list key exclusion criteria for patients with conditions known or suspected to elevate the risk of developing potential TXA-related side effects, such as a history of TEs and/or coagulopathy, a history of convulsive disorders or dural disruption (due to known epileptogenic/neurotoxic properties of TXA when in direct contact with CNS tissue), and renal impairment.
Discussion
Our review of the literature suggests that use of ivTXA has become an accepted approach in the systemic management of surgical bleeding and trauma-related hemorrhage, reducing both perioperative blood loss and the need for allogenic blood transfusions by one third. For spinal surgery, the available clinical evidence confirms a similar hemostatic benefit and prevalent clinical use of ivTXA in this field.10 11 12 13 14 As TXA has been extensively studied and used clinically as a hemostatic agent since the 1960s, its pharmacology is very well understood, and efficacious intravenous dosing protocols have been established for its clinical use. Serious systemic side effects following intravenous TXA exposure are quite rare but do exist. There remains an unresolved theoretical concern about the potential thrombogenicity of the drug, which has fostered investigations on using the topical route of TXA administration as a potentially safer and more targeted intraoperative hemostatic strategy. We found that tTXA is most widely established in cardiac and arthroplasty surgery, providing at least equal hemostatic benefit particularly in the postoperative period and minimal systemic exposure as compared with ivTXA.
For spinal surgery, our systematic search revealed that very few (albeit promising) published studies exist, reporting at least similar hemostatic efficacy of tTXA in attenuating the postoperative bleeding. As outlined previously, these studies have relatively small sample sizes as well as some methodological limitations; their results have to be interpreted with caution, but they do suggest that tTXA might soon find wider applications in spinal surgery. Beyond these considerations, we cannot completely rule out the possibilities of a publication bias in the medical literature and that other relevant studies may not have been detected by our systematic search strategy, which was limited to selected major international databases and clinical trial registries and was further restricted by the kinds of search terms included. However, we do believe that the four ongoing studies we identified will provide additional insights and may support the sparse but promising available literature on the use of tTXA in spinal surgery. Their combined objectives address several important unanswered questions related to tTXA's hemostatic efficacy (reduction in blood loss and blood transfusion requirements), potential performance difference to ivTXA treatment, safety (in particular risk for TE), impact on patient outcome (hospital stay duration, patient quality of life), and impact on cost. We recommend that all of these should ideally be included in future RCTs investigating this topic. Furthermore, we have identified several research questions and methodological strategies we believe will help with the study and implementation of tTXA for hemostatic use in spinal surgery.
The optimal time point of intraoperative tTXA application in spinal surgery is unknown, and whether tTXA can provide efficacious hemostasis intraoperatively remains to be established. Future RCTs should assess the relative efficacy of different potential tTXA delivery time points, such as after surgical exposure, after osteotomy, after hardware installation, or just before wound closure. Intra- and postoperative blood loss should therefore be included as corresponding outcome measures.
No optimal topical application dosing is established. To our knowledge, only saline-based irrigations of tTXA have been used. The extent of tTXA tissue absorption following a given duration of applied irrigation has not been quantified and should ideally be assessed by collecting corresponding tissue samples after tTXA application for subsequent analysis of residual fibrinolytic activity. Alternative delivery methods, such as using drug-soaked absorbable gelatin compressed sponge, to control bleeding should be explored.
Experimental studies should assess the tissue absorption and dose–response properties of tTXA. The optimal therapeutic dosing of tTXA should then be identified in RCTs using multiple safe dosing protocols with varying tTXA concentrations. Only Lehman et al have included assessment of systemic exposure following tTXA treatment in their study93; this assessment should be a routine measure to quantify the safety improvement suggested for the topical route of TXA administration. Similarly, follow-up assessment of complication rates should be standardized across different RCTs and include more objective, high-sensitivity measures such as use of Doppler ultrasound for deep vein thrombosis detection and renal function monitoring beyond the perioperative period.
Combinatorial (tTXA + ivTXA) studies may demonstrate additional hemostatic benefit.90 RCTs using this design in spinal surgery are warranted. Moreover, the RCT inclusion criteria should focus on the right type of surgery (i.e., major multilevel spinal surgery with adequate duration [>3 hours] where substantial blood loss is expected to occur).
Apart from assessing perioperative blood loss and blood transfusion requirements, we recommend RCTs should include the rate of postoperative wound complications, such as infection, in their outcome measures. Improved hemostasis under tTXA will likely reduce postoperative hematoma formation, an identified risk factor for bacterial wound infection, and accelerate removal of the blood drainage tube.97 In addition, RCTs should include the duration of hospital stay as a correlate for patient recovery in secondary outcome measures,93 as evidence suggests it may be reduced by tTXA treatment.92 Analogously, potential economic advantages of tTXA (i.e., reduction of patient hospitalization time and blood product use) should be quantified by a cost analysis.93
Conclusion
ivTXA in spine surgery and tTXA in other forms of surgery have become widely accepted hemostatic strategies. tTXA is at the cusp of becoming intensely studied in spinal surgery. We recommend that future trials compare tTXA to ivTXA alone or in combination in major spinal surgery that would generally require blood transfusions. Intraoperative and postoperative blood loss, blood transfusion requirements, length of hospital stay, and adverse effects (specifically TE rate) are important outcome measures. Further research is required to understand the pharmacodynamics of tTXA administration and establish ideal dosing and timing protocols.
Acknowledgments
The authors thank Dr. Mohamad Khazaei for his assistance with translation (Farsi to English) of a research article.
Footnotes
Disclosures Sebastian F. Winter, none Carlo Santaguida, none Jean Wong, none Michael G. Fehlings, none
References
- 1.Tse E Y, Cheung W Y, Ng K F, Luk K D. Reducing perioperative blood loss and allogeneic blood transfusion in patients undergoing major spine surgery. J Bone Joint Surg Am. 2011;93(13):1268–1277. doi: 10.2106/JBJS.J.01293. [DOI] [PubMed] [Google Scholar]
- 2.Elgafy H Bransford R J McGuire R A Dettori J R Fischer D Blood loss in major spine surgery: are there effective measures to decrease massive hemorrhage in major spine fusion surgery? Spine (Phila Pa 1976) 201035(9, Suppl):S47–S56. [DOI] [PubMed] [Google Scholar]
- 3.Neilipovitz D T. Tranexamic acid for major spinal surgery. Eur Spine J. 2004;13 01:S62–S65. doi: 10.1007/s00586-004-0716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tate D E Jr, Friedman R J. Blood conservation in spinal surgery. Review of current techniques. Spine (Phila Pa 1976) 1992;17(12):1450–1456. doi: 10.1097/00007632-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Zollo R A, Eaton M P, Karcz M, Pasternak R, Glance L G. Blood transfusion in the perioperative period. Best Pract Res Clin Anaesthesiol. 2012;26(4):475–484. doi: 10.1016/j.bpa.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Marik P E, Corwin H L. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36(9):2667–2674. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann A, Ozawa S, Farrugia A, Farmer S L, Shander A. Economic considerations on transfusion medicine and patient blood management. Best Pract Res Clin Anaesthesiol. 2013;27(1):59–68. doi: 10.1016/j.bpa.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Ker K, Prieto-Merino D, Roberts I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013;100(10):1271–1279. doi: 10.1002/bjs.9193. [DOI] [PubMed] [Google Scholar]
- 9.Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. doi: 10.1136/bmj.e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badeaux J, Hawley D. A systematic review of the effectiveness of intravenous tranexamic acid administration in managing perioperative blood loss in patients undergoing spine surgery. J Perianesth Nurs. 2014;29(6):459–465. doi: 10.1016/j.jopan.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Cheriyan T, Maier S P II, Bianco K. et al. Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J. 2015;15(4):752–761. doi: 10.1016/j.spinee.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Yang B, Li H, Wang D, He X, Zhang C, Yang P. Systematic review and meta-analysis of perioperative intravenous tranexamic acid use in spinal surgery. PLoS ONE. 2013;8(2):e55436. doi: 10.1371/journal.pone.0055436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z-J, Fu X, Xing D, Zhang H-F, Zang J-C, Ma X-L. Is tranexamic acid effective and safe in spinal surgery? A meta-analysis of randomized controlled trials. Eur Spine J. 2013;22(9):1950–1957. doi: 10.1007/s00586-013-2774-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F, Wang K, Li F-N. et al. Effectiveness of tranexamic acid in reducing blood loss in spinal surgery: a meta-analysis. BMC Musculoskelet Disord. 2014;15:448. doi: 10.1186/1471-2474-15-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ipema H J, Tanzi M G. Use of topical tranexamic acid or aminocaproic acid to prevent bleeding after major surgical procedures. Ann Pharmacother. 2012;46(1):97–107. doi: 10.1345/aph.1Q383. [DOI] [PubMed] [Google Scholar]
- 16.Moher D Liberati A Tetzlaff J Altman D G; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement Ann Intern Med 20091514264–269., W64 [DOI] [PubMed] [Google Scholar]
- 17.Okamoto S, Okamoto U. Amino-methyl-cyclohexane-carboxylic acid: AMCHA. A new potent inhibitor of the fibrinolysis. Keio J Med. 1962;11(3):105–115. [Google Scholar]
- 18.Melander B, Gliniecki G, Granstrand B, Hanshoff G. Biochemistry and toxicology of amikapron; the antifibrinolytically active isomer of AMCHA. (A comparative study with epsilon-aminocaproic acid) Acta Pharmacol Toxicol (Copenh) 1965;22(4):340–352. doi: 10.1111/j.1600-0773.1965.tb01829.x. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto S, Sato S, Takada Y, Okamoto U. An active stereo-isomer (trans-form) of AMCHA and its antifibrinolytic (antiplasmic) action in vitro and in vivo. Keio J Med. 1964;13:177–185. doi: 10.2302/kjm.13.177. [DOI] [PubMed] [Google Scholar]
- 20.Royston D The current place of aprotinin in the management of bleeding Anaesthesia 20157010146–49., e17 [DOI] [PubMed] [Google Scholar]
- 21.WHO Expert Committee on the Selection and Use of Essential Medicines Summary of the report of the 18th meeting of the WHO Expert Committee on the Selection and Use of Essential Medicines March 2011. Available at: http://www.who.int/selection_medicines/committees/TRS_web_summary.pdf. Accessed March 20, 2015
- 22.McCormack P L. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72(5):585–617. doi: 10.2165/11209070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22–25. [PubMed] [Google Scholar]
- 24.Dunn C J, Goa K L. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57(6):1005–1032. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 25.Andersson L, Nilsson I M, Niléhn J E, Hedner U, Granstrand B, Melander B. Experimental and clinical studies on AMCA, the antifibrinolytically active isomer of p-aminomethyl cyclohexane carboxylic acid. Scand J Haematol. 1965;2(3):230–247. doi: 10.1111/j.1600-0609.1965.tb01300.x. [DOI] [PubMed] [Google Scholar]
- 26.Cyklokapron (tranexamic acid) [package insert] Auckland, New Zealand: Pfizer New Zealand Ltd; 2013
- 27.Horrow J C, Van Riper D F, Strong M D, Grunewald K E, Parmet J L. The dose-response relationship of tranexamic acid. Anesthesiology. 1995;82(2):383–392. doi: 10.1097/00000542-199502000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Andersson L, Nilsoon I M, Colleen S, Granstrand B, Melander B. Role of urokinase and tissue activator in sustaining bleeding and the management thereof with EACA and AMCA. Ann N Y Acad Sci. 1968;146(2):642–658. doi: 10.1111/j.1749-6632.1968.tb20322.x. [DOI] [PubMed] [Google Scholar]
- 29.Murkin J M, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350–353. doi: 10.1213/ANE.0b013e3181c92b23. [DOI] [PubMed] [Google Scholar]
- 30.Schlag M G, Hopf R, Redl H. Convulsive seizures following subdural application of fibrin sealant containing tranexamic acid in a rat model. Neurosurgery. 2000;47(6):1463–1467. [PubMed] [Google Scholar]
- 31.Schlag M G, Hopf R, Zifko U, Redl H. Epileptic seizures following cortical application of fibrin sealants containing tranexamic acid in rats. Acta Neurochir (Wien) 2002;144(1):63–69. doi: 10.1007/s701-002-8275-z. [DOI] [PubMed] [Google Scholar]
- 32.Lecker I, Wang D-S, Romaschin A D, Peterson M, Mazer C D, Orser B A. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest. 2012;122(12):4654–4666. doi: 10.1172/JCI63375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cravens G T, Brown M J, Brown D R, Wass C T. Antifibrinolytic therapy use to mitigate blood loss during staged complex major spine surgery: postoperative visual color changes after tranexamic acid administration. Anesthesiology. 2006;105(6):1274–1276. doi: 10.1097/00000542-200612000-00029. [DOI] [PubMed] [Google Scholar]
- 34.Odabaş A R, Cetinkaya R, Selçuk Y, Kaya H, Coşkun U. Tranexamic-acid-induced acute renal cortical necrosis in a patient with haemophilia A. Nephrol Dial Transplant. 2001;16(1):189–190. doi: 10.1093/ndt/16.1.189. [DOI] [PubMed] [Google Scholar]
- 35.Koo J R, Lee Y K, Kim Y S, Cho W Y, Kim H K, Won N H. Acute renal cortical necrosis caused by an antifibrinolytic drug (tranexamic acid) Nephrol Dial Transplant. 1999;14(3):750–752. doi: 10.1093/ndt/14.3.750. [DOI] [PubMed] [Google Scholar]
- 36.Ker K, Roberts I. Tranexamic acid for surgical bleeding. BMJ. 2014;349:g4934. doi: 10.1136/bmj.g4934. [DOI] [PubMed] [Google Scholar]
- 37.Shakur H, Roberts I, Bautista R. et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 38.Poeran J, Rasul R, Suzuki S. et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829. doi: 10.1136/bmj.g4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunt B J The current place of tranexamic acid in the management of bleeding Anaesthesia 20157010150–53., e18 [DOI] [PubMed] [Google Scholar]
- 40.Tengborn L, Blombäck M, Berntorp E. Tranexamic acid—an old drug still going strong and making a revival. Thromb Res. 2015;135(2):231–242. doi: 10.1016/j.thromres.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Ackery A, Rizoli S. Tranexamic acid for trauma-related hemorrhage. CMAJ. 2014;186(15):E587. doi: 10.1503/cmaj.131741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sentilhes L, Lasocki S, Ducloy-Bouthors A S. et al. Tranexamic acid for the prevention and treatment of postpartum haemorrhage. Br J Anaesth. 2015;114(4):576–587. doi: 10.1093/bja/aeu448. [DOI] [PubMed] [Google Scholar]
- 43.Lumsden M A, Wedisinghe L. Tranexamic acid therapy for heavy menstrual bleeding. Expert Opin Pharmacother. 2011;12(13):2089–2095. doi: 10.1517/14656566.2011.598857. [DOI] [PubMed] [Google Scholar]
- 44.Huang F, Wu D, Ma G, Yin Z, Wang Q. The use of tranexamic acid to reduce blood loss and transfusion in major orthopedic surgery: a meta-analysis. J Surg Res. 2014;186(1):318–327. doi: 10.1016/j.jss.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 45.Stoicea N, Bergese S D, Ackermann W. et al. Current status of blood transfusion and antifibrinolytic therapy in orthopedic surgeries. Front Surg. 2015;2:3. doi: 10.3389/fsurg.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eubanks J D. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132–138. [PubMed] [Google Scholar]
- 47.Dhir A. Antifibrinolytics in cardiac surgery. Ann Card Anaesth. 2013;16(2):117–125. doi: 10.4103/0971-9784.109749. [DOI] [PubMed] [Google Scholar]
- 48.Liu J-M, Peng H-M, Shen J-X, Qiu G-X. [A meta-analysis of the effectiveness and safety of using tranexamic acid in spine surgery] Zhonghua Wai Ke Za Zhi. 2010;48(12):937–942. [PubMed] [Google Scholar]
- 49.Wong J, El Beheiry H, Rampersaud Y R. et al. Tranexamic acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg. 2008;107(5):1479–1486. doi: 10.1213/ane.0b013e3181831e44. [DOI] [PubMed] [Google Scholar]
- 50.Baldus C R, Bridwell K H, Lenke L G, Okubadejo G O. Can we safely reduce blood loss during lumbar pedicle subtraction osteotomy procedures using tranexamic acid or aprotinin? A comparative study with controls. Spine (Phila Pa 1976) 2010;35(2):235–239. doi: 10.1097/BRS.0b013e3181c86cb9. [DOI] [PubMed] [Google Scholar]
- 51.Farrokhi M R, Kazemi A P, Eftekharian H R, Akbari K. Efficacy of prophylactic low dose of tranexamic acid in spinal fixation surgery: a randomized clinical trial. J Neurosurg Anesthesiol. 2011;23(4):290–296. doi: 10.1097/ANA.0b013e31822914a1. [DOI] [PubMed] [Google Scholar]
- 52.Endres S, Heinz M, Wilke A. Efficacy of tranexamic acid in reducing blood loss in posterior lumbar spine surgery for degenerative spinal stenosis with instability: a retrospective case control study. BMC Surg. 2011;11:29. doi: 10.1186/1471-2482-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Q, Liu J, Fan R. et al. Tranexamic acid reduces postoperative blood loss of degenerative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trial. Eur Spine J. 2013;22(9):2035–2038. doi: 10.1007/s00586-013-2836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neilipovitz D T, Murto K, Hall L, Barrowman N J, Splinter W M. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg. 2001;93(1):82–87. doi: 10.1097/00000539-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 55.Shapiro F, Zurakowski D, Sethna N F. Tranexamic acid diminishes intraoperative blood loss and transfusion in spinal fusions for duchenne muscular dystrophy scoliosis. Spine (Phila Pa 1976) 2007;32(20):2278–2283. doi: 10.1097/BRS.0b013e31814cf139. [DOI] [PubMed] [Google Scholar]
- 56.Xu C, Wu A, Yue Y. Which is more effective in adolescent idiopathic scoliosis surgery: batroxobin, tranexamic acid or a combination? Arch Orthop Trauma Surg. 2012;132(1):25–31. doi: 10.1007/s00402-011-1390-6. [DOI] [PubMed] [Google Scholar]
- 57.Sethna N F, Zurakowski D, Brustowicz R M, Bacsik J, Sullivan L J, Shapiro F. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. 2005;102(4):727–732. doi: 10.1097/00000542-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Grant J A, Howard J, Luntley J, Harder J, Aleissa S, Parsons D. Perioperative blood transfusion requirements in pediatric scoliosis surgery: the efficacy of tranexamic acid. J Pediatr Orthop. 2009;29(3):300–304. doi: 10.1097/BPO.0b013e31819a85de. [DOI] [PubMed] [Google Scholar]
- 59.Verma K, Errico T J, Vaz K M, Lonner B S. A prospective, randomized, double-blinded single-site control study comparing blood loss prevention of tranexamic acid (TXA) to epsilon aminocaproic acid (EACA) for corrective spinal surgery. BMC Surg. 2010;10:13. doi: 10.1186/1471-2482-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhawale A A, Shah S A, Sponseller P D. et al. Are antifibrinolytics helpful in decreasing blood loss and transfusions during spinal fusion surgery in children with cerebral palsy scoliosis? Spine (Phila Pa 1976) 2012;37(9):E549–E555. doi: 10.1097/BRS.0b013e31823d009b. [DOI] [PubMed] [Google Scholar]
- 61.Yagi M, Hasegawa J, Nagoshi N. et al. Does the intraoperative tranexamic acid decrease operative blood loss during posterior spinal fusion for treatment of adolescent idiopathic scoliosis? Spine (Phila Pa 1976) 2012;37(21):E1336–E1342. doi: 10.1097/BRS.0b013e318266b6e5. [DOI] [PubMed] [Google Scholar]
- 62.Khurana A, Guha A, Saxena N, Pugh S, Ahuja S. Comparison of aprotinin and tranexamic acid in adult scoliosis correction surgery. Eur Spine J. 2012;21(6):1121–1126. doi: 10.1007/s00586-012-2205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsutsumimoto T, Shimogata M, Ohta H, Yui M, Yoda I, Misawa H. Tranexamic acid reduces perioperative blood loss in cervical laminoplasty: a prospective randomized study. Spine (Phila Pa 1976) 2011;36(23):1913–1918. doi: 10.1097/BRS.0b013e3181fb3a42. [DOI] [PubMed] [Google Scholar]
- 64.Bednar D A, Bednar V A, Chaudhary A, Farrokhyar F. Tranexamic acid for hemostasis in the surgical treatment of metastatic tumors of the spine. Spine (Phila Pa 1976) 2006;31(8):954–957. doi: 10.1097/01.brs.0000209304.76581.c5. [DOI] [PubMed] [Google Scholar]
- 65.Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, El-Dawlatly A. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine (Phila Pa 1976) 2008;33(24):2577–2580. doi: 10.1097/BRS.0b013e318188b9c5. [DOI] [PubMed] [Google Scholar]
- 66.Colomina M J, Bagó J, Vidal X, Mora L, Pellisé F. Antifibrinolytic therapy in complex spine surgery: a case-control study comparing aprotinin and tranexamic acid. Orthopedics. 2009;32(2):91. [PubMed] [Google Scholar]
- 67.Patatanian E, Fugate S E. Hemostatic mouthwashes in anticoagulated patients undergoing dental extraction. Ann Pharmacother. 2006;40(12):2205–2210. doi: 10.1345/aph.1H295. [DOI] [PubMed] [Google Scholar]
- 68.Tibbelin A, Aust R, Bende M. et al. Effect of local tranexamic acid gel in the treatment of epistaxis. ORL J Otorhinolaryngol Relat Spec. 1995;57(4):207–209. doi: 10.1159/000276741. [DOI] [PubMed] [Google Scholar]
- 69.Zahed R, Moharamzadeh P, Alizadeharasi S, Ghasemi A, Saeedi M. A new and rapid method for epistaxis treatment using injectable form of tranexamic acid topically: a randomized controlled trial. Am J Emerg Med. 2013;31(9):1389–1392. doi: 10.1016/j.ajem.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 70.Wong J, Abrishami A, El Beheiry H. et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503–2513. doi: 10.2106/JBJS.I.01518. [DOI] [PubMed] [Google Scholar]
- 71.Patel J N, Spanyer J M, Smith L S, Huang J, Yakkanti M R, Malkani A L. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528–1531. doi: 10.1016/j.arth.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 72.Georgiadis A G Muh S J Silverton C D Weir R M Laker M W A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty J Arthroplasty 201328(8, Suppl):78–82. [DOI] [PubMed] [Google Scholar]
- 73.Konig G, Hamlin B R, Waters J H. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473–1476. doi: 10.1016/j.arth.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang C-H, Chang Y, Chen D W, Ueng S WN, Lee M S. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1552–1557. doi: 10.1007/s11999-013-3446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilbody J, Dhotar H S, Perruccio A V, Davey J R. Topical tranexamic acid reduces transfusion rates in total hip and knee arthroplasty. J Arthroplasty. 2014;29(4):681–684. doi: 10.1016/j.arth.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad F S, Mason J M. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005–1015. doi: 10.1302/0301-620X.96B8.33745. [DOI] [PubMed] [Google Scholar]
- 77.Sa-Ngasoongsong P, Channoom T, Kawinwonggowit V. et al. Postoperative blood loss reduction in computer-assisted surgery total knee replacement by low dose intra-articular tranexamic acid injection together with 2-hour clamp drain: a prospective triple-blinded randomized controlled trial. Orthop Rev (Pavia) 2011;3(2):e12. doi: 10.4081/or.2011.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ker K, Beecher D, Roberts I. Topical application of tranexamic acid for the reduction of bleeding. Cochrane Database Syst Rev. 2013;7:CD010562. doi: 10.1002/14651858.CD010562.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz N G, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937–1944. doi: 10.2106/JBJS.N.00060. [DOI] [PubMed] [Google Scholar]
- 80.Wang H, Shen B, Zeng Y. Comparison of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled and prospective cohort trials. Knee. 2014;21(6):987–993. doi: 10.1016/j.knee.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 81.De Bonis M, Cavaliere F, Alessandrini F. et al. Topical use of tranexamic acid in coronary artery bypass operations: a double-blind, prospective, randomized, placebo-controlled study. J Thorac Cardiovasc Surg. 2000;119(3):575–580. doi: 10.1016/s0022-5223(00)70139-5. [DOI] [PubMed] [Google Scholar]
- 82.Almer S, Andersson T, Ström M. Pharmacokinetics of tranexamic acid in patients with ulcerative colitis and in healthy volunteers after the single instillation of 2 g rectally. J Clin Pharmacol. 1992;32(1):49–54. doi: 10.1002/j.1552-4604.1992.tb03787.x. [DOI] [PubMed] [Google Scholar]
- 83.Sindet-Pedersen S, Ramström G, Bernvil S, Blombäck M. Hemostatic effect of tranexamic acid mouthwash in anticoagulant-treated patients undergoing oral surgery. N Engl J Med. 1989;320(13):840–843. doi: 10.1056/NEJM198903303201305. [DOI] [PubMed] [Google Scholar]
- 84.Roberts I, Shakur H, Coats T. et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17(10):1–79. doi: 10.3310/hta17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moskal J T, Harris R N, Capps S G. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365–368. doi: 10.1016/j.arth.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 86.Tuttle J R, Ritterman S A, Cassidy D B, Anazonwu W A, Froehlich J A, Rubin L E. Cost benefit analysis of topical tranexamic acid in primary total hip and knee arthroplasty. J Arthroplasty. 2014;29(8):1512–1515. doi: 10.1016/j.arth.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 87.Slover J, Bosco J. Cost analysis of use of tranexamic acid to prevent major bleeding complications in hip and knee arthroplasty surgery. Am J Orthop. 2014;43(10):E217–E220. [PubMed] [Google Scholar]
- 88.Alshryda S, Mason J, Sarda P. et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H) J Bone Joint Surg Am. 2013;95(21):1969–1974. doi: 10.2106/JBJS.L.00908. [DOI] [PubMed] [Google Scholar]
- 89.Alshryda S, Mason J, Vaghela M. et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K) J Bone Joint Surg Am. 2013;95(21):1961–1968. doi: 10.2106/JBJS.L.00907. [DOI] [PubMed] [Google Scholar]
- 90.Spegar J, Vanek T, Snircova J. et al. Local and systemic application of tranexamic acid in heart valve surgery: a prospective, randomized, double blind LOST study. J Thromb Thrombolysis. 2011;32(3):303–310. doi: 10.1007/s11239-011-0608-3. [DOI] [PubMed] [Google Scholar]
- 91.Krohn C D, Sørensen R, Lange J E, Riise R, Bjørnsen S, Brosstad F. Tranexamic acid given into the wound reduces postoperative blood loss by half in major orthopaedic surgery. Eur J Surg Suppl. 2003;588(588):57–61. [PubMed] [Google Scholar]
- 92.Saberi H, Miri S M. The effects of topically applied tranexamic acid on reduction of post-laminectomy hemorrhage. Tehran University Medical Journal. 2010;68(9):527–533. [Google Scholar]
- 93.Lehman R A; Washington University School of Medicine. Topical application of tranexamic acid to reduce blood loss during complex combat-related spine trauma surgery Bethesda, MD: National Library of Medicine; 2000. Available at: https://clinicaltrials.gov/ct2/show/NCT02314988. Accessed March 20, 2015
- 94.Wood K B; Massachusetts General Hospital. Topical application of tranexamic acid to reduce postoperative blood loss in posterior approach spinal surgery Bethesda, MD: National Library of Medicine; 2000. Available at: https://clinicaltrials.gov/ct2/show/NCT02063035. Accessed March 20, 2015
- 95.Miyanji F Kilb B; University of British Columbia. Topical tranexamic acid in major paediatric spine deformity surgery: a randomized controlled trial Bethesda, MD: National Library of Medicine; 2000. Available at: https://clinicaltrials.gov/ct2/show/NCT02093988. Accessed March 20, 2015]
- 96.Xianming P Jun S; The Military General Hospital of Chengdu, PLA. Topical vs intravenous administration of tranexamic acid in posterior thoracolumbar spinal internal fixation operation: a double-blind randomized controlled study. Chinese Clinical Trial Register Chengdu, Sichuan: Ministry of Health; 2015. Available at: http://www.chictr.org.cn/showproj.aspx?proj=10334. Accessed March 20, 2015]
- 97.Cheung E V, Sperling J W, Cofield R H. Infection associated with hematoma formation after shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(6):1363–1367. doi: 10.1007/s11999-008-0226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


