Abstract
Study Design Retrospective review.
Objective The purpose of this study was to determine the radiographic impact of a transforaminal lumbar interbody fusion (TLIF) versus a cantilever TLIF technique on segmental lordosis, segmental coronal alignment, and disk height.
Methods A retrospective review was done of all patients undergoing TLIF procedures from 2006 to 2011 by three spine surgeons. Traditional TLIF versus cantilever TLIF results were compared, and radiographic outcomes were assessed.
Results One hundred one patients were included in the study. Patients undergoing the cantilever TLIF procedure had a significantly greater change in segmental lordosis and disk height compared with those who underwent the traditional procedure (p > 0.0001).
Conclusions The cantilever TLIF technique can lead to greater change in segmental lordosis based upon radiographic outcomes.
Keywords: TLIF, lumbar disk disease, lordosis, DDD, spinal fusion
Introduction
Transforaminal lumbar interbody fusion (TLIF) was described by Harms and Jeszenszky in 1998 as an alternative to posterior lumbar interbody fusion (PLIF) and combined anterior/posterior fusion techniques.1 At the time, TLIF offered an approach to the disk space that required less traction on the dura and traversing nerve root than PLIF and avoided the morbidity seen with the anterior approach. Since its introduction, the TLIF procedure has become increasingly popular for treating lumbar pathology.
An early case for interbody fusion was made by Yashiro et al, who showed that PLIF improved the radiographic fusion rate and segmental alignment compared with posterolateral fusion.2 Similarly, the TLIF technique has been utilized with the theory that the interbody device, with additional bone graft, would improve the overall fusion rate compared with posterolateral fusion. The restoration of disk height (DH) and lumbar lordosis are additional goals of the TLIF procedure. The increase in DH can indirectly decompress the neural foramen. Attention to sagittal balance has been stressed in recent literature3; appropriate lordosis has been shown to affect outcomes, including a possible role in adjacent-level degeneration.4 Studies have focused on radiographic outcomes of the TLIF procedure,5 6 showing improvement in DH and segmental lordosis (SL) with TLIF.
The original Harms technique suggested placing the bone graft behind the anterior longitudinal ligament (ALL) and then using two round titanium mesh cages as the interbody device. These cages were to be placed in the middle or posterior third of the body. Additional biomechanical studies have since shown that anterior placement of the interbody device improves load sharing, reduces subsidence, and allows for improved sagittal contour.7 8 This concept has also been suggested in retrospective radiographic reviews.5 6 Variation in the graft position has been coupled with multiple choices for interbody material, shape, and adjunctive grafting material. These TLIF iterations have been discussed throughout the literature,9 10 11 12 but reports consist mostly of patient series without control or comparison groups; the authors are unaware of any direct, head-to-head comparison of TLIF technique with respect to radiographic outcomes and lordosis. The present study was designed to directly compare radiographic outcomes of two different TLIF techniques.
One technique involves placing a kidney-shaped spacer (Fig. 1) as anteriorly as possible (preferably on the apophyseal ring) in the disk space. This technique was introduced by Anand et al and is termed a “cantilever” TLIF, or c-TLIF.12 The second technique involves the use of a straight spacer (Fig. 1) placed obliquely through the disk space. Our goal was to determine if the c-TLIF technique improved radiographic DH and segmental alignment when compared with the straight cage method of TLIF.
Fig. 1.

The kidney-shaped spacer (left) and straight spacer (right).
Materials and Methods
Subjects
After Institutional Review Board approval, a retrospective review was conducted of all TLIF procedures performed at our institution from January 2006 to November 2011. No diagnoses were excluded from the study, as we were concerned primarily with radiographic outcomes. Inclusion criteria consisted of a standing lumbar radiograph prior to surgery, intraoperative radiographs or immediate postoperative imaging, and at least 1-year follow-up with standing lumbar films. One hundred one patients (127 TLIF levels) had adequate follow-up for inclusion in the study. Forty patients (55 spinal levels; 17 males and 23 females) received the kidney-shaped spacer using the c-TLIF technique. The remaining 61 patients (72 spinal levels; 16 males and 45 females) received a straight interbody cage. In the multilevel cases, the same technique was employed at each level. All c-TLIFs were performed by one attending surgeon, and the straight TLIFs were performed by two other attending surgeons at our institution. The operative diagnoses included a variety of processes including degenerative and isthmic spondylolisthesis, degenerative and idiopathic scoliosis, and iatrogenic spinal instability after previous surgeries. Across diagnoses, the indications for TLIF included desire for 360-degree stabilization due to poor bone quality, unstable segment such as mobile degenerative spondylolisthesis, need for additional lordosis, or to preserve foraminal decompression in cases of severe DH loss or asymmetric disk collapse (such as in degenerative scoliosis). The indications for laminectomy in addition to TLIF included central stenosis, often from degenerative spondylolisthesis.
Surgical Technique
All patients were positioned prone on a Jackson frame and had a posterior midline approach to the spine at the appropriate lumbar level. Each patient received a laminectomy when indicated (see above) with the unilateral facetectomy required to perform the TLIF. The nerve roots were identified and protected throughout the TLIF portion of the case. An annulotomy was performed under distraction (if needed), and the TLIF was performed with the aid of fluoroscopy. The disk space was prepared using a combination of curettes and shavers to remove the remaining disk and cartilaginous end plates. Once the disk space was prepared, the interbody spacer was placed according to the attending surgeon's preference. Interbody spacers of both groups were made of either polyetheretherketone (PEEK; with additional autograft bone placed in the center of the graft) or allograft bone. Neither type of graft had any intrinsic lordosis. The most common sizes for the grafts were 9 to 13 mm.
The procedure for the c-TLIF group began by placing autogenous local bone in the disk space behind the ALL. The curved spacer was then placed into the disk space. The spacer was worked around the periphery of the disk space, moving toward its anterior aspect. Final impaction was performed until the spacer abutted the cancellous bone graft and ALL, with its long axis oriented in a medial-lateral direction (Fig. 2A). The TLIF group, likewise, received autogenous graft impacted behind the ALL. The graft used was a straight, rectangular cage with a bullet nose that aids in entering the disk space. The graft was placed in an oblique fashion toward the midline, as anteriorly as possible within the disk space. No cases received two implants at the same level. Both groups received pedicle screw fixation for stabilization of the fusion construct and posterolateral bone grafting (Fig. 2B). Compression along the pedicle screw/rod construct was performed in all cases to improve the sagittal alignment for both groups.
Fig. 2.
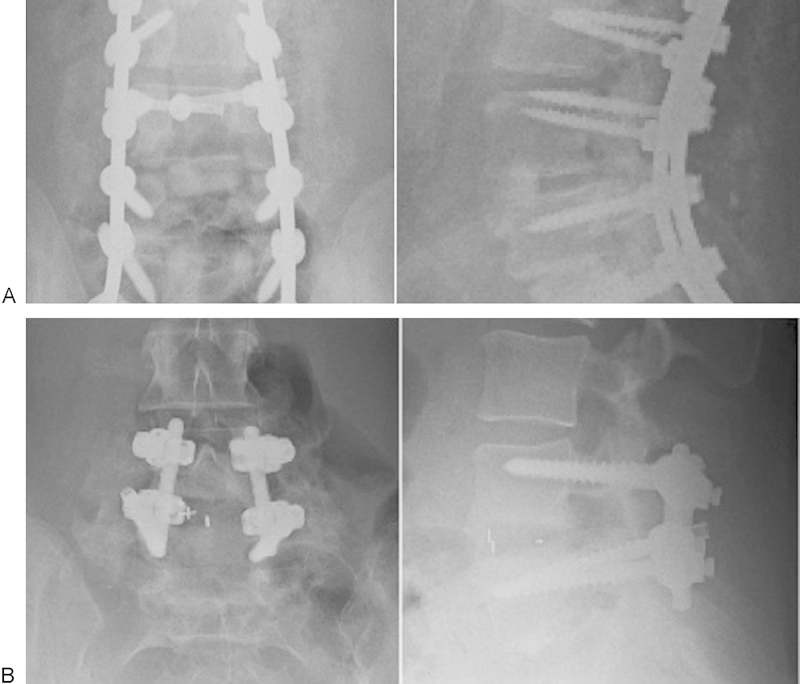
(A) Anteroposterior and lateral radiograph demonstrating anterior placement of the kidney-shaped interbody cage. (B) Anteroposterior and lateral radiograph demonstrating placement of the straight interbody cage.
Radiographic Evaluation
Standing lumbar plain films were obtained for all patients preoperatively and postoperatively. The postoperative radiographs were evaluated at first follow-up (t1), 1-year follow-up (t2), and final follow-up (t3). Each patient also had imaging on the day of surgery (t0), which was done either with intraoperative fluoroscopy or via a portable radiograph in the recovery room. The radiographic parameters of interest were DH, SL, and segmental coronal alignment (SC). DH was measured as the distance (in millimeters) between the supra-adjacent and subjacent end plates at the midpoint of the involved vertebral bodies. The Cobb method was used to measure the segmental coronal and sagittal alignment.
Once the measurements were obtained for all radiographs, the preoperative values were subtracted from their subsequent counterparts. This calculation yielded delta values (ΔDH, ΔSC, ΔSL) for each time point. The mean delta values were calculated for each point and compared between the groups using a two-tailed t test.
Results
One hundred one patients (127 TLIF levels) had adequate follow-up for inclusion in the study. Forty patients (55 spinal levels; 17 males and 23 females) received the kidney-shaped spacer using the c-TLIF technique. The remaining 61 patients (72 spinal levels; 16 males and 45 females) received a straight interbody cage. The distribution of operated levels is found in Table 1. L4–L5 was the most commonly operated level in both groups.
Table 1. Distribution of spinal levels operated and type of cage placed.
| Spinal level | Straight cage | Banana cage |
|---|---|---|
| L1–L2 | 1 | 3 |
| L2–L3 | 5 | 7 |
| L3–L4 | 16 | 11 |
| L4–L5 | 39 | 30 |
| L5–S1 | 11 | 4 |
Both groups had means of 6 weeks for their first follow-up evaluations. The mean final follow-up for patients in the c-TLIF group was 26 months, compared with 22 months for the TLIF group (p = 0.2734).
The mean preoperative SL value was 3.1 degrees in the c-TLIF group and 5.6 degrees in the TLIF group (p = 0.0034). The c-TLIF ΔSL values were 7.8 degrees (t0), 6.5 degrees (t1), 5.5 degrees (t2), and 4.9 degrees (t3). The mean ΔSL values were 4.0 degrees (t0), 1.8 degrees (t1), 0.4 degrees (t2), and 0.1 degrees (t3; Fig. 3). When comparing the two groups, the c-TLIF group showed statistically significantly greater improvement of SL at all points (t1, t2, and t3; p < 0.0001).
Fig. 3.
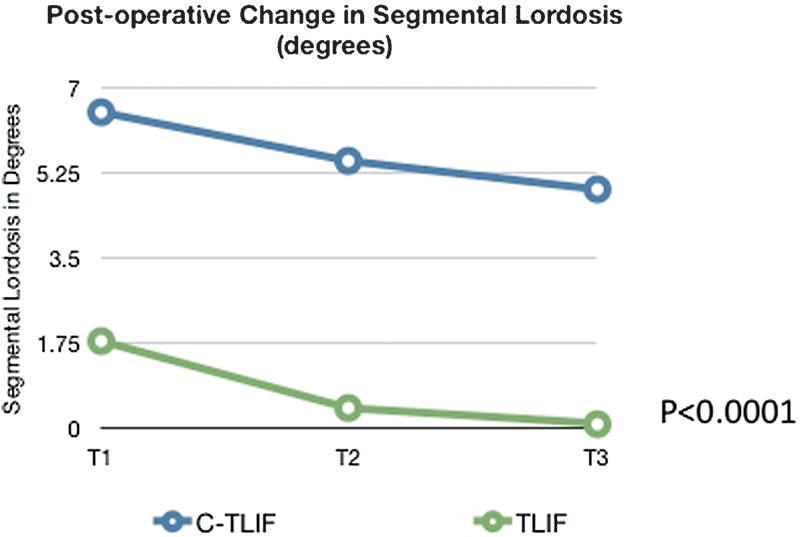
Postoperative change in segmental lordosis (in degrees) between “cantilever” transforaminal lumbar interbody fusion (c-TLIF) and transforaminal lumbar interbody fusion (TLIF) after surgery.
The mean preoperative DH was 6.4 mm in the c-TLIF group and 8.4 mm in the TLIF group (p = 0.0003). The c-TLIF mean ΔDH values were 5.6 mm (t1), 4.5 mm (t2), and 3.6 mm (t3). The TLIF group mean ΔDH values were 2.2 mm (t1), 0.8 mm (t2), and 0.2 mm (t3; Fig. 4). When comparing the two groups, the c-TLIF had greater restoration of DH at all points (t1, t2, and t3), which was statistically significant (p < 0.0001).
Fig. 4.
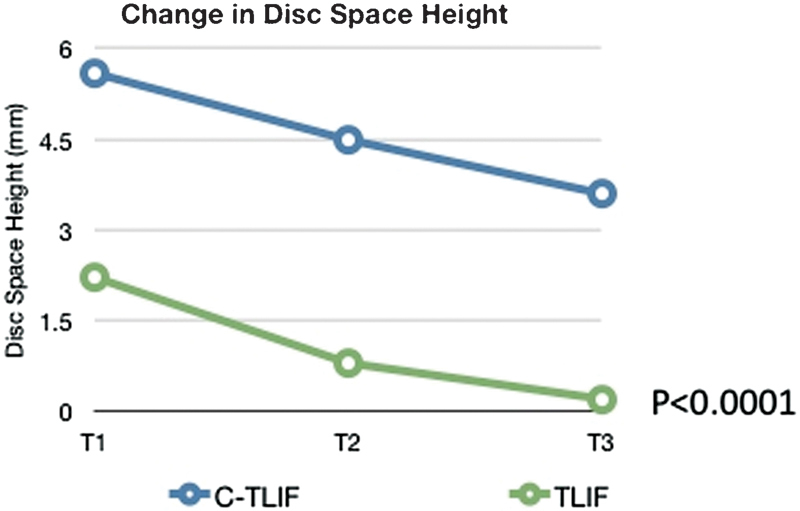
Postoperative change (in millimeters) in disk space height between “cantilever” transforaminal lumbar interbody fusion (c-TLIF) and transforaminal lumbar interbody fusion (TLIF) after surgery.
The mean preoperative SC was 3.1 degrees in the c-TLIF group and 2.1 degrees in the TLIF group (p = 0.0226). The c-TLIF group mean ΔSC values were 2.0 degrees (t0), 1.9 degrees (t1), 1.8 degrees (t2), and 1.6 degrees (t3). The TLIF group mean ΔSC values were 1.2 degrees (t0), 1.1 degrees (t1), 1.0 degrees (t2), and 1.2 degrees (t3). There was no statistical difference between the study groups for SC at any point.
Discussion
Several surgical options are available when deciding to perform a PLIF, TLIF, anterior lumbar interbody fusion, or direct lateral approach for interbody fusion. Material choices include titanium, PEEK, machined allograft bone, and structural autograft, among others. Studies have assessed the radiographic and fusion outcomes related to the implant choice but there is still sufficient evidence to support one type of interbody device over the other options.
The technique of performing the TLIF procedure has been evaluated in the literature as well. Multiple studies have shown that anterior placement of the spacer is more ideal to minimize the chance of graft subsidence.5 6 7 8 Fukuta et al also showed that anterior placement instead of a central location in the vertebral body decreased the chance of nonunion and adjacent-level degeneration.7 Kepler and colleagues noted improved outcomes in back and leg pain with improved lordosis and DH after TLIF.13 The c-TLIF described by Anand and colleagues employs a technique that takes advantage of this concept. A curved graft allows for a more anatomic position on the anterior apophyseal ring and makes passing the graft around the periphery to the anterior disk space easier. Anand et al reviewed 100 consecutive patients and assessed both radiographic and clinical outcomes.12 However, the authors did not compare the c-TLIF to a more conventional method of TLIF.
This study was designed to compare the c-TLIF to the straight cage method because it was felt anecdotally that there was more lordosis and DH achieved using c-TLIF. All diagnoses, including degenerative scoliosis, were included in the study and we therefore evaluated the coronal alignment of the TLIFs performed as well. Our study showed that the c-TLIF method was significantly better at improving sagittal plane deformity and indirect decompression of the foramen due to increasing DH. The coronal alignment was not affected by technique choice. The c-TLIF method showed an improvement of lordosis from 3 to 8 degrees at final follow-up. This change was consistent with findings in the study by Anand et al (2 to 9 degrees).12 In comparison, the SL was essentially unchanged from preoperative value (0.1 degree) at final follow-up of the TLIF group. Both groups demonstrated an increase in disk space height, but again, the c-TLIF group was superior, as the TLIF group was nearly at baseline (improved 0.2 mm) at final follow-up.
Of note, improvements in sagittal alignment and disk space height demonstrated some regression over time. The greatest drop-off in both groups occurred between t0 and t1, which may be due to how imaging was obtained for patients at these times. Images for t0 included either intraoperative lateral fluoroscopic images or portable lateral radiographs obtained in the recovery room. In the former, the patient was ideally positioned prone on the operating table (which is designed to maximize lordosis), and in the latter, the patient was lying supine in a bed. In either case, the t0 images were obtained without any weight bearing through the spine. In contrast, images at t1 and beyond were upright lateral radiographs, which may explain the substantial decrease in ΔSL and ΔDH noted between the initial points. Settling of the interbody grafts over time may explain the remainder of the lost improvement. One possible explanation for the greater regression toward baseline seen in the TLIF group is that the placement of a c-TLIF graft anteriorly on the ring apophysis provides stronger support and resistance to subsidence than a graft placed obliquely across the center of the disk space. Both the groups showed similar correction of coronal alignment, with those improvements maintained over time. Of note, the TLIF group demonstrated a change in SC that trended down, but had an increase from 1.0 degrees to 1.2 degrees at the t2 and t3 points, respectively. This result likely relates to measurement error.
Our study is not without limitations. The Cobb method was performed using digital radiology to provide the measurements for SL and SC. There is some variability in the Cobb method, which could have introduced measurement errors. In addition, investigators making the measurements were not blinded to the type of interbody device, which could potentially introduce bias. We strove to minimize these effects by utilizing a standardized technique of measurement, repeating the measurements, and having all images measured by the same individuals, who were not the operating surgeons. The straight cage group had a larger preoperative SL and DH, which could potentially give the c-TLIF group an advantage in having more opportunity for improvement. However, it can also be more difficult to prepare a collapsed disk space while performing interbody fusions, which could also lead to less correction. Because the two techniques under comparison were done by different surgeons, one concern could be raised that the surgeon for the c-TLIF group was simply more committed to placing a larger graft in the interspace. However, the subsidence from points t1 to t3 are similar in both groups; furthermore, it is postulated that lordosis achieved, rather than simply disk space height, is the main value of the technique. Finally, this study was purely radiographic; clinical outcomes were not considered, nor did we assess fusion or complication rates of either procedure. Therefore, we cannot comment on the potential clinical benefit of either technique.
Conclusions
In conclusion, we found that the technique for TLIF does have an effect on postoperative sagittal balance and DH. Placement of a kidney-shaped spacer anteriorly appears to improve SL and increase DH when compared with a straight cage placed obliquely. Either technique can provide modest improvement in coronal alignment. Further study is needed to determine if the radiographic outcomes correspond to improved clinical outcomes.
Acknowledgments
The authors wish to acknowledge Nina Clovis, Suzanne Danley, Sheila Rye, and Gerald Hobbs, PhD for their assistance with the preparation of this paper. No funding was received for this study.
Footnotes
Disclosures James W. Rice, none Cara L. Sedney, none Scott D. Daffner, none Justin W. Arner, none Sanford E. Emery, none John C. France, none
References
- 1.Harms J G, Jeszenszky D. [Die posterior, lumbale, interkorporelle Fusion in unilateraler transforaminaler Technik.] Oper Orthop Traumatol. 1998;10(2):90–102. doi: 10.1007/s00064-006-0112-7. [DOI] [PubMed] [Google Scholar]
- 2.Yashiro K, Homma T, Hokari Y, Katsumi Y, Okumura H, Hirano A. The Steffee variable screw placement system using different methods of bone grafting. Spine (Phila Pa 1976) 1991;16(11):1329–1334. doi: 10.1097/00007632-199111000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Booth K C, Bridwell K H, Lenke L G, Baldus C R, Blanke K M. Complications and predictive factors for the successful treatment of flatback deformity (fixed sagittal imbalance) Spine (Phila Pa 1976) 1999;24(16):1712–1720. doi: 10.1097/00007632-199908150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Akamaru T, Kawahara N, Tim Yoon S. et al. Adjacent segment motion after a simulated lumbar fusion in different sagittal alignments: a biomechanical analysis. Spine (Phila Pa 1976) 2003;28(14):1560–1566. [PubMed] [Google Scholar]
- 5.Kwon B K, Berta S, Daffner S D. et al. Radiographic analysis of transforaminal lumbar interbody fusion for the treatment of adult isthmic spondylolisthesis. J Spinal Disord Tech. 2003;16(5):469–476. doi: 10.1097/00024720-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Recnik G, Košak R, Vengust R. Influencing segmental balance in isthmic spondylolisthesis using transforaminal lumbar interbody fusion. J Spinal Disord Tech. 2013;26(5):246–251. doi: 10.1097/BSD.0b013e3182416f5c. [DOI] [PubMed] [Google Scholar]
- 7.Fukuta S, Miyamoto K, Hosoe H, Shimizu K. Kidney-type intervertebral spacers should be located anteriorly in cantilever transforaminal lumbar interbody fusion: analyses of risk factors for spacer subsidence for a minimum of 2 years. J Spinal Disord Tech. 2011;24(3):189–195. doi: 10.1097/BSD.0b013e3181e9f249. [DOI] [PubMed] [Google Scholar]
- 8.Faundez A A, Mehbod A A, Wu C, Wu W, Ploumis A, Transfeldt E E. Position of interbody spacer in transforaminal lumbar interbody fusion: effect on 3-dimensional stability and sagittal lumbar contour. J Spinal Disord Tech. 2008;21(3):175–180. doi: 10.1097/BSD.0b013e318074bb7d. [DOI] [PubMed] [Google Scholar]
- 9.Cutler A R, Siddiqui S, Mohan A L, Hillard V H, Cerabona F, Das K. Comparison of polyetheretherketone cages with femoral cortical bone allograft as a single-piece interbody spacer in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2006;5(6):534–539. doi: 10.3171/spi.2006.5.6.534. [DOI] [PubMed] [Google Scholar]
- 10.Gödde S, Fritsch E, Dienst M, Kohn D. Influence of cage geometry on sagittal alignment in instrumented posterior lumbar interbody fusion. Spine (Phila Pa 1976) 2003;28(15):1693–1699. doi: 10.1097/01.BRS.0000083167.78853.D5. [DOI] [PubMed] [Google Scholar]
- 11.Groth A T, Kuklo T R, Klemme W R, Polly D W, Schroeder T M. Comparison of sagittal contour and posterior disc height following interbody fusion: threaded cylindrical cages versus structural allograft versus vertical cages. J Spinal Disord Tech. 2005;18(4):332–336. doi: 10.1097/01.bsd.0000163037.17634.89. [DOI] [PubMed] [Google Scholar]
- 12.Anand N, Hamilton J F, Perri B, Miraliakbar H, Goldstein T. Cantilever TLIF with structural allograft and RhBMP2 for correction and maintenance of segmental sagittal lordosis: long-term clinical, radiographic, and functional outcome. Spine (Phila Pa 1976) 2006;31(20):E748–E753. doi: 10.1097/01.brs.0000240211.23617.ae. [DOI] [PubMed] [Google Scholar]
- 13.Kepler C K, Rihn J A, Radcliff K E. et al. Restoration of lordosis and disk height after single-level transforaminal lumbar interbody fusion. Orthop Surg. 2012;4(1):15–20. doi: 10.1111/j.1757-7861.2011.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


