Abstract
Aedes aegypti (L.) is the main vector of dengue virus and more recently chikungunya virus in Latin America. However, the Asian tiger mosquito Aedes albopictus (Skuse, 1894) is expanding its global range and increasing its role in transmission of these diseases. In this report, we suggest that Ae. albopictus was introduced to the Department of Managua, Nicaragua, in 2010 via two independent routes and demonstrate its dissemination and establishment in urban neighborhoods by 2012. The coexistence of two competent vector species could alter the epidemiology of dengue and chikungunya as well as indicate the need for new strategies aimed at vector control.
Keywords: Aedes albopictus, Nicaragua, introduction, dissemination, dengue
RESUMEN
Aedes aegypti (L.) es el vector principal del virus de dengue y mas recientemente del virus chikungunya an América Latina. Sin embargo, el mosquito tigre asiático Aedes albopictus está ampliando su rango global, y su rol en la transmisión de estas enfermedades está aumentando. En este informe, se describe la introducción de Aedes albopictus en el Departamento de Managua, Nicaragua, en 2010 via dos rutas independientes y se documenta su diseminación y establecimiento en los barrios urbanos en 2012. La co-existencia de dos especies de vectores competentes podría alterar la epidemiología de dengue y chikungunya y sugiere la necesidad de nuevas estrategias de control del vector.
Aedes albopictus (Skuse, 1894) is a mosquito native to Asia, where it is recognized as an important vector of dengue virus (DENV) and other human pathogens (Rai 1991). In Latin America, this species, traditionally considered a secondary vector for dengue (Gratz 2004), is gaining visibility as it disseminates throughout the continent and has been implicated in notorious outbreaks of chikungunya virus (CHIKV; e.g., La Reunion [Renault et al. 2007]) and DENV globally. Aedes aegypti (L.), in contrast, was introduced in the 1600s in the Caribbean and the tropical belt (Brathwaite et al. 2012) and was responsible for outbreaks of yellow fever and dengue in the late 19th to mid-20th centuries. A continent-wide eradication campaign in the 1950s–1960s cleared Ae. aegypti from many Latin American countries (Soper 1963), but by 1980, almost all the countries had been re-infested, including Nicaragua (Brathwaite et al. 2012). Urban yellow fever did not return, but dengue re-emerged and has reached epidemic proportions (Murray et al. 2013).
Although the first record of Ae. aegypti in Nicaragua is not clear, the first documented dengue case was in 1984 (Kouri et al. 1991), and the four serotypes (DENV1–4) are now endemic, causing yearly epidemics (Balmaseda et al. 1999, Guzmán et al. 1996, Harris et al. 2000, Gutierrez et al. 2011, OhAinle et al. 2011). The capital, Managua, is the second largest urban center in Central America, with a population of nearly 2 million. In 2012, the National Dengue Control Program (DCP) of the Ministry of Health (MOH) reported in the Department of Managua one-third of the suspected cases of dengue fever nationwide and almost half of the cases of dengue hemorrhagic fever and dengue shock syndrome.
Ae. albopictus disseminated from Asia to tropical and subtropical regions worldwide (Gratz 2004, Lucientes-Curdi et al. 2014) and was first introduced to the Americas in the 1980s in used tires and bamboo plants shipped from Asia (Hawley et al. 1987, Moore and Mitchell 1997, Womack 1993). Since then, Ae. albopictus has been identified in 20 countries in the Americas, 27 in Europe, and at least 5 in Africa (Reiter 1998, Benedict et al. 2007, Carvalho et al. 2014) and continues to expand its range (Unlu 2013).
The first record of Ae. albopictus in Nicaragua was in 2002, when its larvae were found in five rural communities in the Department of Chinandega, bordering Honduras and El Salvador (E. Lugo, personal communication). In 2003, entomological surveys ostensibly targeting Ae. aegypti were conducted in 1,383 homes in Managua and 842 homes in León, the second largest city, located 50 miles north of Managua and just south of Chinandega; Ae. albopictus was identified in León but was not detected in Managua (Lugo et al. 2005).
Here we present an analysis of the records from the past 10 years of the National Entomological Surveillance system of the DCP in the Department of Managua as well as the results of a detailed entomological survey we conducted in 2012 in 60 randomly selected neighborhood sectors in Managua. The data reveal the introduction of Ae. albopictus into the Department of Managua in 2010, indicate its possible routes of introduction, and document its establishment in the capital. The presence of a second competent vector for the transmission of DENV and potentially the emerging chikungunya virus presents a new challenge for health authorities in Managua and the region.
Materials and Methods
Study Area
Managua, Nicaragua’s capital, is located on the Pacific Coast (12° 9′ N, 86° 16′ W), 55 meters above sea level, and is part of the Department of Managua, which consists of nine municipalities encompassing 150.5 km2 with close to 2 million residents. Managua has a tropical wet and dry climate (Peel et al. 2007) with temperatures averaging 28–32°C (82–90°F) year-round. A marked dry season exists between February and April, while most rainfall occurs between May and December. The dengue season peaks yearly from August to December, when rainfall and humidity levels leading to water accumulation in artificial containers favor mosquito reproduction.
Housing Conditions and Water Storage Practices
Rapid unplanned urbanization after an earthquake in 1972 led to the expansion of Managua. Most houses are single-story buildings constructed of mixed material with concrete foundations and lower walls, wooden upper walls, and metal roofs, and window screens are limited. Most have an interior patio or access to a yard. Although water distribution has increased, most households have intermittent water supply and store water. Large 55-gallon drum barrels or elevated water cisterns are ubiquitous and are key breeding sites for Ae. aegypti in Managua. The city and suburbs are rich in tropical vegetation, including bromeliads and water-containing plants favored by Ae. albopictus (Peña et al. 2003). Plastic waste in the form of bottles, dishes, and bowls are common items in patios and on sidewalks.
Analysis of Entomological Records
The DCP, a division of the Local Integrated Health Care System (SILAIS) of the MOH, conducts annual surveys to assess the impact of government-led vector abatement and fumigation campaigns. Each January, a baseline entomological survey is conducted in all neighborhoods of the SILAIS-Managua health districts, followed by five to six cycles of abatement campaigns year-round and verification surveys. The sampling includes all blocks from each district and a random sample of 10% of the houses in each block. In 2011 and 2012, an average of 34,300 houses were inspected in each cycle.
Samples consisting of immature and mature forms of mosquitoes are routinely sent to the Directorate of Medical Entomology at the National Center for Diagnosis and Reference (CNDR) of the MOH, where specimens are classified using the keys of Darsie and Ward (1981) and recorded in the CNDR and SILAIS databases. We reviewed available records from SILAIS-Managua from 2003–2013 at the CNDR Directorate of Medical Entomology and collected information on the identification of Ae. albopictus, including location, container type, date of collection, and aquatic stage of development (e.g., larval stage I–IV, pupae). The data were analyzed to calculate house, Breteau, and container indices for Ae. aegypti and for Ae. albopictus when possible, and maps of possible routes of Ae. albopictus introduction and dissemination were generated.
Entomological Surveys
In August 2012 during the wet season, in a cluster-randomized controlled trial “Camino Verde: A Green Way to Prevent Dengue,” we conducted entomological surveys in 60 neighborhood sectors randomly selected from a pool of 187, after excluding the top 17 socio-economic status neighborhood sectors of Managua (Andersson 2015). All containers with water (potential breeding sites) such as barrels, plastic bottles, bowls, flower pots and saucers, refuse, etc. located in and around houses were inspected for aquatic mosquito stages in 8,355 households. From each positive container, all larvae and pupae were collected, preserved in 70% ethanol, and transported to the CNDR Directorate of Medical Entomology and classified as above.
Results
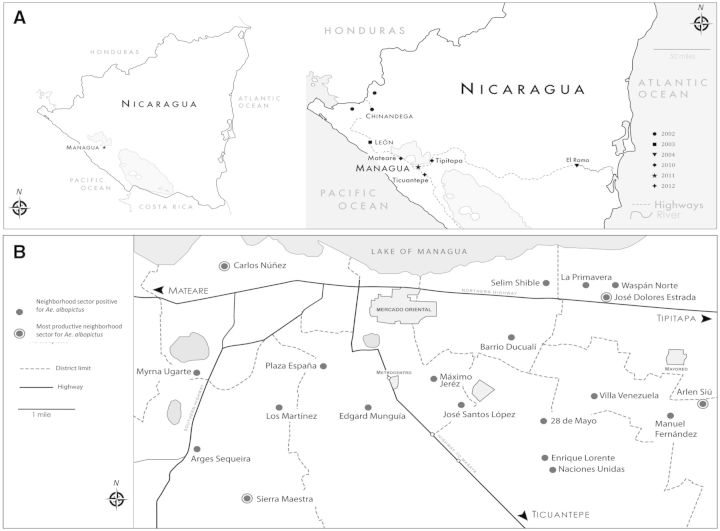
Systematic review of the entomological records from the DCP for the nine municipalities of the Department of Managua from 2003 to 2013 revealed that the first report of Ae. albopictus was on 23 August 2010 in the village of Colonia Agrícola, municipality of Tipitapa, located 22 km northeast of Managua, followed by identification of eight additional Ae. albopictus-positive containers the next month. In September 2010, Ae. albopictus was simultaneously recorded in the municipality of Mateare, situated 25 km northwest of Managua, in the villages of Álvaro Aleman and Sector Occidental (Fig. 1A). In 2011 and 2012, the DCP records document the presence of Ae. albopictus in all nine municipalities of Managua, including the city center, with a total of 309 containers positive for Ae. albopictus larvae or pupae in 2011 and 154 in 2012 (Table 1).
Fig. 1.
(A) Ae. albopictus identifications in Nicaragua from 2003 to 2013 compiled from historical records from the DCP-MOH reveal possible routes of introduction into the Department of Managua. Locations are marked by symbols, with year indicated in key. (B) Neighborhood sectors in Managua where Ae. albopictus was identified in 2012. Four neighborhoods located toward the periphery of urban Managua—Arlen Siu, Sierra Maestra, Carlos Núñez, and José Dolores Estrada—had the most productive containers, with over 73% (275/373) of the registered Ae. albopictus immature forms.
Table 1.
Ae. aegypti and Ae. albopictus sampling in nine municipalities of Managua: data for 2011 and 2012 from the DCP records
| Municipality |
2011 |
2012 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of houses inspected | Number of containers inspected |
Ae. aegypti |
Ae. albopictus |
Number of houses inspected | Number of containers inspected |
Ae. aegypti |
Ae. albopictus |
|||||||||||
| Number of positive houses | Number of positive containers | House indexa | Container indexb | Breteau index | Number of positive containers | Container index | Number of positive houses | Number of positive containers | House index | Container index | Breteau index | Number of positive containers | Container index | |||||
| Ciudad Sandino | 11,765 | 56,296 | 564 | 759 | 4.8 | 1.3 | 6.5 | 40 | 0.07 | 12,851 | 49,655 | 591 | 833 | 4.6 | 1.7 | 6.5 | 18 | 0.04 |
| El Crucero | 1,735 | 12,091 | 59 | 81 | 3.4 | 0.7 | 4.7 | 5 | 0.04 | 1,391 | 10,703 | 72 | 105 | 5.2 | 1.0 | 7.5 | 4 | 0.04 |
| Managua | 111,831 | 573,523 | 3,827 | 5,299 | 3.4 | 0.9 | 4.7 | 125 | 0.02 | 129,217 | 644,914 | 5,879 | 8,406 | 4.5 | 1.3 | 6.5 | 79 | 0.01 |
| Mateare | 5,132 | 27,675 | 283 | 405 | 5.5 | 1.5 | 7.9 | 30 | 0.11 | 5,003 | 27,183 | 329 | 450 | 6.6 | 1.7 | 9.0 | 2 | 0.01 |
| San Francisco Libre | 1,032 | 7,699 | 29 | 39 | 2.8 | 0.5 | 3.8 | 4 | 0.05 | 938 | 6,851 | 36 | 42 | 3.8 | 0.6 | 4.5 | 1 | 0.01 |
| San Rafael del Sur | 7,413 | 52,215 | 501 | 698 | 6.8 | 1.3 | 9.4 | 32 | 0.06 | 7,773 | 49,017 | 543 | 774 | 7.0 | 1.6 | 10.0 | 17 | 0.03 |
| Ticuantepe | 4,775 | 33,132 | 160 | 208 | 3.4 | 0.6 | 4.4 | 6 | 0.02 | 5,003 | 30,994 | 250 | 340 | 5.0 | 1.1 | 6.8 | 20 | 0.06 |
| Tipitapa | 13,703 | 79,736 | 699 | 947 | 5.1 | 1.2 | 6.9 | 29 | 0.04 | 14,964 | 79,592 | 860 | 1,217 | 5.7 | 1.5 | 8.1 | 5 | 0.01 |
| Villa Carlos Fonseca | 4,253 | 27,848 | 200 | 269 | 4.7 | 1.0 | 6.3 | 38 | 0.14 | 4,194 | 24,083 | 229 | 304 | 5.5 | 1.3 | 7.2 | 8 | 0.03 |
| Total | 161,639 | 870,215 | 6,322 | 8,705 | 3.9 | 1.0 | 5.4 | 309 | 0.04 | 181,334 | 922,992 | 8,789 | 12,471 | 4.8 | 1.3 | 6.9 | 154 | 0.02 |
Container indices for Ae. albopictus and Ae. aegypti as well as House (HI) and Breteau indices (BI) for Ae. aegypti are reported. Data were not available to calculate HI and BI for Ae. albopictus.
a Number of houses positive for Ae aegypti larvae, pupae, or both, divided by number of houses inspected × 100.
b Number of containers positive for Ae aegypti or Ae alpopictus larvae, pupae, or both (as indicated), divided by number of containers inspected × 100.
In the entomological surveys we conducted in 2012, a total of 33,159 water containers in 8,355 homes were inspected, and 153,326 specimens of mosquito larvae and pupae were collected for analysis. We found 5,054 containers to be positive for Aedes spp., of which 55 contained larvae or pupae of Ae. albopictus, in 20 of the 60 surveyed neighborhoods sectors. In 37 of these containers, Ae. aegypti was also present.
In the municipality of Managua, four neighborhoods located toward the periphery of the urban center were the most productive for Ae. albopictus, with 73% (275/373) of larvae and pupae concentrated in 39 containers: Arlen Siu (50 larvae and 20 pupae in 22 containers), Sierra Maestra (114 larvae and 7 pupae in 2 containers), Carlos Núñez (38 larvae and 16 pupae in 4 containers), and José Dolores Estrada (21 larvae and 9 pupae in 5 containers; Fig. 1B). The 55 containers in which Ae. albopictus was found consisted of 17 refuse items considered “useless” in the home, 7 bowls or cooking pots, 7 barrels for storage of clean water for home use, 7 used tires, 5 buckets, 5 animal drinking containers, 4 flowerpots, 1 car battery case, 1 tree hole, and 1 crack in the concrete floor.
Discussion
Combining analysis of entomological records from the DCP of the nine municipalities in the Department of Managua with a detailed entomological survey of 60 randomly selected neighborhood sectors in the municipality of Managua, we demonstrate that Ae. albopictus arrived to Managua’s periphery in 2010 and by 2012 was found in numerous neighborhoods in the capital city. From 2003, when Ae. albopictus was first reported in León (Lugo et al. 2005), to 2010, no surveys record its presence in the Department of Managua. The first Ae. albopictus documentation in the DCP records for the Department of Managua is in 2010 in the peripheral municipalities of Mateare and Tipitapa, which are adjacent to main highways, suggesting the movement of Ae. albopictus within the Pacific region of the country was effected by land.
Mateare is 25 km northwest of the capital on the road from León, while Tipitapa is on the main highway that links Managua with El Rama, a river port city that communicates by river travel with the Caribbean coast. According to reports from the CNDR Directorate of Medical Entomology, Ae. albopictus was first observed in El Rama in 2004. These early reports suggest that Ae. albopictus traveled southeast from León and west from El Rama into Managua through highways in parallel events. Since 2011, Ae. albopictus has been found in all of Managua’s municipalities (Table 1); however, our detailed entomological surveys in 2012 demonstrated greatest abundance in peripheral neighborhoods of the city of Managua with semiurban conditions, consistent with previous reports (Rey and O'Connel 2014). This evidence for the movement and invasion of Ae. albopictus to Managua from ports via roads reinforces the relevance of human-mediated transport through water and land routes in the geographic dispersion of vectors across relatively short periods of time (Lounibos 2002). The importance of human interventions or anthropogenic forces in Aedes evolution and passive transport has been recently documented with genetic studies (Brown et al. 2014). In the future, our work could expand to include a phylogeography component to better understand Aedes introductions and gene flow in Nicaragua.
The DCP sampling strategy is consistent year-to-year in terms of coverage and number of houses inspected. However, the surveys are directed toward control efforts against Ae. aegypti and might be heterogeneous in relation to potential Ae. albopictus breeding sites. Thus, the decrease in Ae. albopictus indices observed in DCP records between 2011 and 2012 could potentially be explained by the type of containers inspected (e.g., more water storage barrels than “useless” containers or outdoor breeding sites surveyed in 2012 compared with 2011), as 2012 had a larger infestation of Ae. aegypti in Managua. Although insufficient systematically collected data exist to make comparisons of relative indices from year to year, the data clearly demonstrate the presence and geographical distribution of Ae. albopictus in urban Managua and its coexistence with Ae. aegypti in several container types. While Ae. albopictus is more ubiquitous in natural breeding sites, its immature forms can cohabit and compete with other container-breeding mosquitoes (Rey and O'Connel 2014).
A major impact of the establishment of Ae. albopictus in Managua relates to its competence for transmitting the four DENV serotypes, particularly as a vehicle for maintaining their endemicity during interepidemic periods (Gratz 2004), as it has been suggested that Ae. albopictus females transmit DENV to their progeny more efficiently than Ae. aegypti females (Gokhal et al 2001; Martins et al. 2012). Additionally, evidence indicates that Ae. albopictus males are capable of transmitting DENV to female mosquitoes during mating. Ae. albopictus females can produce eggs without a bloodmeal, leading to increased distribution and population density (Lambrechts et al. 2010).
Although Ae. aegypti continues to be more prevalent and is the main vector for DENV in urban areas, changing ecosystems (e.g., climate change, construction of a new interoceanic canal in Nicaragua) are enabling dengue outbreaks outside the boundaries of urban centers, in the periphery, and in rural communities where Ae. albopictus is better adapted. Its adaptability makes it particularly important in Managua, a densely populated city with large bodies of water and tropical vegetation, an ecosystem favorable to Ae. albopictus. Recently, the emergence of chikungunya virus in the Americas, specifically in the Caribbean and Central America, including Nicaragua, has put government and health authorities in the region on high alert. Although the current outbreak strain is transmitted by Ae. aegypti, other chikungunya virus strains are adapted to Ae. albopictus (Tsetsarkin et al. 2007, Vazeille et al. 2007), which is becoming established rapidly in the region. Furthermore, arboviruses can adapt their vector competence, and displacement of Ae. aegypti by Ae. albopictus could in the future be accompanied in the Americas by greater DENV adaptation to this invasive and increasingly abundant mosquito vector species (Lambrechts et al. 2010). Thus, the human-mediated transport and the dissemination and adaptability of Ae. albopictus linked to environmental changes in urban infrastructure warrant the close monitoring of this species in previously uncolonized regions as another player to consider in the spread of arboviruses in the Americas.
Acknowledgments
We thank Amy Morrison for helpful advice and thoughtful review of the manuscript. This work was supported in part by the UBS Optimus Foundation, grant “Camino Verde: Green road to dengue control in Nicaragua” (E.H.).
References Cited
- Andersson N., Nava-Aguilera E., Arostegui J., Morales-Perez A., Suazo-Laguna H., Legorreta-Soberanis J., Hernandez-Alvarez C., Fernandez-Salas I., Balmaseda A., Cortés-Guzmán A. J., et al. 2015. Camino Verde (Green Way) to Dengue Prevention: a pragmatic cluster-randomised controlled trial of evidence-based community mobilisation in Nicaragua and Mexico. BMJ. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmaseda A., Sandoval E., Pérez L., Gutiérrez C. M., Harris E. 1999. Application of molecular typing techniques in the 1998 dengue epidemic in Nicaragua. Am. J. Trop. Med. Hyg. 61: 893–897. [DOI] [PubMed] [Google Scholar]
- Benedict M. Q., Levine R. S., Hawley W. A., Lounibos P. 2007. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoo. Dis. 7: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brathwaite D. O., San Martín J. L., Montoya R. H., del Diego J., Zambrano B., Dayan G. 2012. The history of dengue outbreaks in the Americas. Am. J. Trop. Med. Hyg. 87: 584–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Evans B. R., Zheng W., Obas V., Barrera-Martinez L., Egizi A., Zhao H., Caccone A., Powell J. R. 2014. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution 68: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho R. G., Lourenço-de-Oliveira R., Braga I. A. 2014. Updating the geographical distribution and frequency of Aedes albopictus in Brazil with remarks regarding its range in the Americas. Mem. Inst. Oswaldo Cruz. 109: 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsie R. F., Ward R. A. 1981. Identification and geographical distribution of the mosquitoes of North America, North of Mexico. Mosq. Syst. Suppl. 1: 1–313 [Google Scholar]
- Gokhal M. D., Barde P. V., Sapkal G. N., Gore M. M., Mourya D. T. 2001. Vertical transmission of Dengue-2 virus through Aedes albopictus mosquitoes. J. Commun. Dis. 33: 212–215 [PubMed] [Google Scholar]
- Gratz N. G. 2004. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 18: 215–227. [DOI] [PubMed] [Google Scholar]
- Gutierrez G., Standish K., Narvaez F., Perez M. A., Elizondo D., Ortega O., Nuñez A., Kuan G., Balmaseda A., Harris E. 2011. Unusual dengue virus 3 epidemic in Nicaragua, 2009. PLoS Negl. Trop. Dis. 5: e1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán M. G., Vásquez S., Martínez E., Alvarez M., Rodríguez R., Kourí G., de los Reyes J., Acevedo F. 1996. Dengue en Nicaragua, 1994: Reintroducción del serotipo 3 en las Américas. Bol. Of. Sanit. Panam. 121: 102–110. [PubMed] [Google Scholar]
- Harris E., Videa E., Perez L., Sandoval E., Tellez Y., Perez M. L., Cuadra R., Rocha J., Idiaquez W., Alonso R. E., et al. 2000. Clinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in Nicaragua. Am. J. Trop. Med. Hyg. 63: 5–11. [DOI] [PubMed] [Google Scholar]
- Hawley W. A., Reiter P., Copeland R. S., Pumpuni C. B., Craig G. B., Jr 1987. Aedes albopictus in North America: Probable introduction in used tires from northern Asia. Science 236: 1114–1116. [DOI] [PubMed] [Google Scholar]
- Kouri G., Valdez M., Arguello L., Guzmán M. G., Valdes L., Soler M., Bravo J. 1991. Dengue epidemic in Nicaragua, 1985. Rev. Inst. Med. Trop. Sao Paulo 33: 365–371. [PubMed] [Google Scholar]
- Lambrechts L., Scott T., Gubler D. 2010. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 4: e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos L. P. 2002. Invasions by insect vectors of human disease. Ann Rev Entomol. 47: 233–266. [DOI] [PubMed] [Google Scholar]
- Lucientes-Curdi J., Molina-Moreno R., Amela-Heras C., Simon-Soria F., Santos-Sanz S., Sánchez-Gómez A., Suárez-Rodriguez B., Sierra-Moros M. J. 2014. Dispersion of Aedes albopictus in the Spanish Mediterranean Area. Eur. J. Public Health 24: 637–640. [DOI] [PubMed] [Google Scholar]
- Lugo E., Valle S., Delgado M., Espinoza P., Rodriguez D., Lopez M. M., Guadamuz J. R., Danilo-Mendoza C., Juarez S., Salgado M., et al. 2005. Identification of Aedes albopictus in urban Nicaragua. J. Am. Mosq. Control Assoc. 21: 325–327. [DOI] [PubMed] [Google Scholar]
- Martins V. E., Alencar C. H., Kamimura M. T., Kamimura M. T., de Carvalho Araujo F. M., De Simone S. G., Dutra R. F., Guedes M. I. 2012. Occurrence of natural vertical transmission of dengue-2 and dengue-3 viruses in Aedes aegypti and Aedes albopictus in Fortaleza, Ceara, Brazil. PLoS One 7: e41386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. G., Mitchell C. J. 1997. Aedes albopictus in the United States: Ten-year presence and public health implications. Emerg. Infect. Dis. 3: 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E. A., Quam M., Wilder-Smith A. 2013. Epidemiology of dengue: Past, present and future prospects. Clin. Epidemiol. 5: 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OhAinle M., Balmaseda A., Macalalad A. R., Tellez Y., Zody M. C., Saborio S., Nunez A., Lennon N. J., Birren B. W., Gordon A., et al. 2011. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci. Transl. Med. 3: 114ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel M. C., Finlayson B. L., McMahon T. A. 2007. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 11: 1633–1644. [Google Scholar]
- Peña C., Gonzalvez G., Chadee D. D. 2003. Seasonal prevalence and container preferences of Aedes albopictus in Santo Domingo, Dominican Republic. J. Vector Ecol. 28: 208–212 [PubMed] [Google Scholar]
- Rai K. 1991. Aedes albopictus in the Americas. Ann. Rev. Entomol. 36: 459–484 [DOI] [PubMed] [Google Scholar]
- Reiter P. 1998. Aedes albopictus and the world trade in used tires, 1988–1995: The shape of things to come? J. Am. Mosq. Control Assoc. 14: 83–94. [PubMed] [Google Scholar]
- Renault P., Solet J. -L., Sissoko D., Balleydier E., Larrieu S., Filleul L., Lassalle C., Thiria J., Rachou E., De Valk H., et al. 2007. A major epidemic of chikungunya virus infection in Reunion Island, France, 2005–2006. Am. J. Trop. Med. Hyg. 77: 727–731. [PubMed] [Google Scholar]
- Rey J., O'Connel S. M. 2014. Oviposition by Aedes aegypti and Aedes albopictus: Influence of congeners and of oviposition site characteristics. J. Vector Ecol. 39: 190–196. [DOI] [PubMed] [Google Scholar]
- Soper F. L. 1963. The elimination of urban yellow fever in the Americas through the eradicaiton of Aedes aegypti. Am. J. Public Health 53: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin K. A., Vanlandingham D. L., McGee C. E., Higgs S. 2007. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 3: e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unlu I., Farajollahi A., Strickman D., Fonseca D. M. 2013. Crouching tiger, hidden trouble: Urban sources of Aedes albopictus (Diptera: Culicidae) refractory to source-reduction. PLoS ONE 8: e77999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeille M., Moutailler S., Coudrier D., Rousseaux C., Khun H., Huerre M., Thiria J., Dehecq J. S., Fontenille D., Schuffenecker I., et al. 2007. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus . PLoS One 2: e1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack M. L. 1993. Distribution, abundance and bionomics of Aedes albopictus in southern Texas. J. Am. Mosq. Control Assoc. 9: 367–369. [PubMed] [Google Scholar]