Abstract
Background
We sought to determine if outcomes with exercise training in heart failure (HF) vary according to ventricular pacing type.
Methods and Results
Heart Failure: A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) randomized 2331 outpatients with HF and LVEF ≤35% to usual care plus exercise training or usual care alone. We examined the relation between outcomes and randomized treatment by ventricular pacing status using Cox proportional hazards modeling. In HF-ACTION 1118 patients (48%) had an implanted cardiac rhythm device; 683 with right ventricular and 435 with biventricular pacemakers. Patients with pacing devices were older, more frequently white, and had lower peak VO2 (p<.001 for all). Peak VO2 improved similarly with training in groups with and without pacing devices. The primary composite endpoint, all-cause death or hospitalization, was reduced only in patients randomized to exercise training without a device (HR 0.79 [95% CI 0.67-.93], p=0.004; RV pacing HR 1.04 [95% CI 0.84–1.28], p=0.74; BiV pacing HR 1.05 [95% CI 0.82–1.34], p=0.72; interaction p=0.058).
Conclusions
Exercise training may improve exercise capacity in patients with implanted cardiac devices. However, the apparent beneficial effects of exercise on hospitalization or death may be attenuated in patients with implanted cardiac devices and requires further study.
Keywords: heart failure, exercise, implanted cardiac pacemaker, mortality
Introduction
There are more than five million persons in the United States with heart failure (HF). Fifty percent or more of HF patients have an implanted cardiac pacemaker or cardioverter defibrillator (ICD). (1) Despite guideline recommendations advocating exercise, there are few longitudinal data on exercise and subsequent outcomes in patients with pacemakers or other implanted cardiac rhythm devices. Furthermore, even less is known about the impact of exercise training in patients with right ventricular pacing versus biventricular pacing. While biventricular pacing has been shown to improve symptoms, survival, and functional status in patients with HF, the efficacy and safety of exercise therapy in patients with existing biventricular pacemakers is not known.
The multicenter Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial randomized patients with symptomatic HF and a left ventricular ejection fraction (LVEF) ≤35% to either usual care plus an exercise program with incremental workout intensity and duration or usual care alone. HF patients randomized to exercise training experienced improved quality of life (HRQoL) and functional status. (2) (3) Exercise training also led to modest reductions in cardiovascular (CV) mortality and HF hospitalization without reaching statistical significance. (3)
The objective of this analysis is to determine if the presence and type of ventricular pacing influences exercise benefit in device patients. We hypothesized that the benefits associated with exercise therapy on functional outcomes and clinical events would be preserved, regardless of pacing status.
Methods
Study Overview
The HF-ACTION trial randomized 2331 outpatients with symptomatic chronic HF (New York Heart Association functional class II – IV) and LVEF 35% or less to exercise training plus usual care or to usual care alone. The rationale, design, and the main results have been published previously. (3, 4) Patients with implantable cardiac devices, including pacemakers, ICDs, and biventricular pacemakers were eligible for enrollment. Patients were excluded if they were unable to exercise or already engaged in a routine exercise program (> 1 session per week) or if they had a major cardiovascular event in the prior six weeks. Patients were excluded if they had fixed-rate pacemakers (not rate-responsive), pacemakers with inability to attain target heart rates, or patients with ICDs and tachyarrhythmia detection zones at or below the target heart rate for exercise training.
The randomized treatment was aerobic exercise training. Treatment started with a goal of three sessions per week of supervised exercise training (walking, treadmill, or cycle ergometer) for a total of 18 sessions with an initial target heart rate of 60% of heart rate reserve (i.e., (peak heart rate – resting heart rate) x 60% + resting heart rate), that was then progressively titrated to 70% of heart rate reserve. Supervised exercise sessions were followed by home-based exercise training five times per week, prescribed at 40 minutes at 60% to 70% of heart rate reserve for the remainder of the trial.
Patients randomized to the usual care arm were not restricted in terms of their activity as per the ACC/AHA Guidelines for the Management of Chronic Heart Failure. (5) Patients were evaluated every three months for the first two years of the trial and then yearly until four years or the end of the trial, whichever came first.
Prior to randomization, subjects underwent a graded exercise test for safety of exercise training and determination of exercise capacity, as measured by peak oxygen uptake (peak VO2). Resting ECG data was collected at the time of the exercise test and included rhythm (sinus, atrial fibrillation or other); ventricular conduction (normal, left bundle branch block, right bundle branch block, intraventricular conduction delay or paced), and QRS interval in milliseconds.
All participating subjects provided informed consent to participate in HF-ACTION as approved by the institutional review board at each participating center.
Implanted Device & Pacing Classification
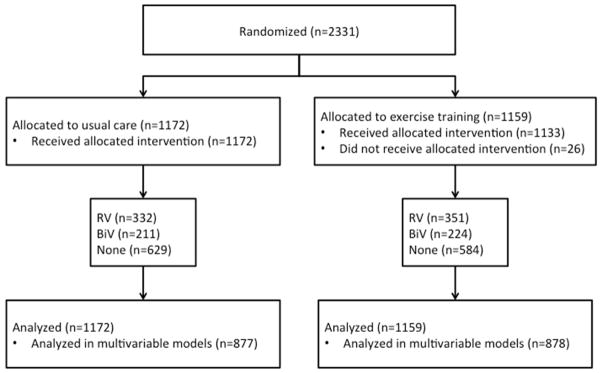
Only patients with an implanted cardiac device were included in the device group (n=1118). For the purpose of this analysis, and based upon the case report form, patients were categorized further according to the type/number of ventricular leads. (3) Patients with single and dual-chamber pacemakers and ICDs were included in the right ventricular lead group (n=683). Patients with biventricular pacemakers (cardiac resynchronization therapy) or biventricular ICDs were included in the biventricular lead group (n=435) (Figure 1). Device interrogation data was not available to identify ventricular pacing burden. All device-related care, including anti-bradycardia programming and anti-tachycardia programming (other than the lower detection limit), were left to the discretion of the patients’ primary ICD physician or electrophysiologist at the enrolling center.
Figure 1. Consort Diagram.
All HF-ACTION subjects with or without implanted cardiac rhythm devices were included in this analysis.
Outcomes
The pre-specified outcomes for this analysis were the same as for the overall HF-ACTION trial. The primary endpoint was the composite of all-cause mortality or all-cause hospitalization. Secondary outcomes included all-cause mortality, the composite of CV death or CV hospitalization, and the composite of CV death or HF hospitalization. (3) In addition to the above pre-specified outcomes, reasons for hospitalization were defined according to device type. Functional outcomes included treadmill exercise duration, peak VO2 and health status (HRQoL) (as measured by the Kansas City Cardiomyopathy Questionnaire [KCCQ] and Beck Depression Inventory II Score (BDI)) at baseline and 3 months.
Statistical Analysis
Baseline characteristics are summarized according to device type using median (25th, 75th percentiles) for continuous variables and percent (number) for categorical variables. Continuous variables were compared across device groups using a Kruskal-Wallis test and categorical variables were compared with a Pearson chi-square test or a Fisher’s exact test.
Functional outcomes are summarized at baseline and 3 months using median (25th, 75th percentiles) for each measure. Due to the high fraction of missing data during follow-up, and the potential for bias as a result, these summaries are descriptive only, and no formal hypothesis testing was undertaken. For these summaries, patients are counted in their baseline device group, and only included in 3-month summaries if they remained in their baseline device group.
Cox proportional hazards modeling was used for each of the pre-specified endpoints (all-cause mortality or hospitalization, all-cause mortality, CV mortality or CV hospitalization, and CV mortality or HF hospitalization). Predictors of each outcome, identified in modeling in the full HF-ACTION cohort, were included in each model for adjustment. These are: Death or all-cause hospitalization: gender, region, LVEF, BUN, presence of severe mitral regurgitation, beta blocker dose, HF symptom stability (KCCQ), and measures from the baseline CPX test (ventricular conduction and Weber class). Death: gender, BMI, LVEF, serum creatinine, CCS angina score, loop diuretic dose, and measures from the baseline CPX test (duration and ventricular conduction). CV death or CV hospitalization: age, gender, race, region, LVEF, BUN, presence of severe mitral regurgitation, on nitrate, KCCQ symptom score, HF symptom stability (KCCQ), and measures from the baseline CPX test (heart rate at peak exercise, ventricular conduction, and Weber class). CV death or HF hospitalization: age, gender, race, region, LVEF, BUN, presence of severe mitral regurgitation, loop diuretic dose, HF symptom stability (KCCQ), and measures from the baseline CPX test (peak VO2, Ve-VCO2 slope, and ventricular conduction). Patients missing any covariates for a particular model were omitted from that model. Each model also included randomized therapy assignment using an intention to treat approach. Device groups were analyzed as time-dependent covariates, to allow patients with device implants during follow-up to enter into the appropriate risk group at the time of implant. A test for interaction was performed in each model to evaluate whether the hazard ratio for randomized therapy was different between device groups. The null hypothesis was that there was no interaction between pacing group and randomized therapy (i.e., usual care plus exercise training versus usual care).
Reasons for hospitalization were summarized by device group (none, RV lead, BiV pacing). For simplicity of presentation and to avoid double-counting of patients, patients were included in their baseline device group. For patients who did not have a device at baseline but who received one during follow-up, any hospitalizations that occurred after the device implant were not counted; similarly, hospitalizations after a device removal were not counted. Patients were counted only once for each hospitalization reason, regardless of the number of hospitalizations for that reason; thus the percentage for a given reason represents the number of patients who were hospitalized at least once for that reason. The categories of reasons for hospitalization were defined in the case report form.
SAS version 9.1 (SAS Institute, Inc., Cary, North Carolina) was used for all analyses. The randomized treatment was analyzed according to the intention-to-treat principle. We used a two-tailed significance level of alpha = 0.05 for all statistical testing.
Results
Patient Characteristics
Among 2331 randomized patients, 1118 (48%) had an implanted cardiac rhythm device at study entry. Of all patients with a device at baseline, 683 (61%) had a pacemaker or defibrillator (right ventricular lead) and 435 (39%) had a biventricular pacemaker/defibrillator. The characteristics of the study population according to the type of pacing are shown in Table 1. Patients without implanted cardiac devices were younger and were more frequently female or black compared with the device patients. The patients with right ventricular leads were more likely to have had a prior myocardial infarction; 55% versus 44% in the biventricular pacing patients (p<.001). The median QRS duration was 100 msec in those patients without devices. In paced patients, the median QRS duration was 160 msec in the RV lead group and 144 msec in the BiV group. Abnormal intraventricular conduction was noted in 40% of those without devices, compared with 65% in those with right ventricular leads, and 93% in those with biventricular pacing.
Table 1.
Baseline characteristics of patients according to baseline pacing type
| None | RV | BiV | P-value | ||
|---|---|---|---|---|---|
| Baseline characteristic | (N=1213) | (N=683) | (N=435) | ||
| Age (yrs) | 58 (49, 67) | 61 (52, 70) | 61 (55,70) | <.0001 | |
| Female n (%) | 430 (35) | 137 (20) | 94 (22) | <.0001 | |
| Race | <.0001 | ||||
| Black | 483 (40) | 173 (26) | 93 (22) | ||
| White | 651 (54) | 456 (68) | 319 (74) | ||
| Other | 62 (5) | 41 (6) | 18(4) | ||
| Body Mass Index | 30 (26, 36) | 29 (26, 34) | 30 (26,34) | 0.0058 | |
| Ischemic HF etiology | 512 (42) | 456 (67) | 229 (53) | <.0001 | |
| Prior MI | 409 (34) | 377 (55) | 193 (44) | <.0001 | |
| Severe mitral regurgitation | 129 (12) | 73 (12) | 54 (14) | 0.50 | |
| Functional Classification | <.0001 | ||||
| NYHA Class II | 825 (68) | 423 (62) | 229 (53) | ||
| NYHA Class III/IV | 388 (32) | 260 (38) | 206 (47) | ||
| KCCQ summary score | 70 (51,85) | 68 (51,83) | 65 (49,80) | 0.033 | |
| LVEF | 25 (21,31) | 24 (20,30) | 23 (19,29) | <.0001 | |
| Diabetes | 380 (31) | 224 (33) | 144 (33) | 0.71 | |
| Hypertension | 754 (63) | 389 (57) | 245 (57) | 0.026 | |
| QRS duration | 100 (80,120) | 120 (100,160) | 140 (120,160) | <.0001 | |
| Paced | — | 160 (120, 188) | 144 (120, 160) | 0.0027 | |
| Non-paced | 100 (80, 120) | 110 (90, 122) | 120 (100, 160) | <.0001 | |
| Intraventricular conduction | N=1178 | N=659 | N=426 | ||
| Normal | 717 (61) | 233 (35) | 29 (7) | <.0001 | |
| LBBB | 272 (23) | 78 (12) | 29 (7) | ||
| RBBB | 48 (4) | 29 (4) | 8 (2) | ||
| IVCD | 141 (12) | 127 (19) | 24 (6) | ||
| Paced | NA | 192 (29) | 336 (79) | ||
| Medications | |||||
| Beta blocker | 1153 (95) | 641 (94) | 409 (94) | 0.48 | |
| Beta-blocker dose (carvedilol equivalents, mg) | 38 (13,50) | 25 (13,50) | 25 (13,50) | 0.37 | |
| Digoxin | 476 (39) | 321 (47) | 249 (57) | <.0001 | |
| ACE-I/ARB | 1160 (96) | 634 (93) | 405 (93) | 0.019 | |
| Aldosterone antagonist | 488 (40) | 323 (47) | 240 (55) | <.0001 | |
| Aspirin | 788 (65) | 451 (66) | 254 (58) | 0.021 | |
| Statin | 526 (43) | 371 (54) | 200 (46) | <.0001 | |
| Loop diuretic dose (furosemide equivalents, mg) | 40 (0,80) | 40 (20,80) | 40 (20,80) | <.0001 | |
| Antiarrhythmic Therapy | 61 (5) | 156 (23) | 124 (29) | <.0001 | |
| Amiodarone | 57 (5) | 128 (19) | 106 (24) | <.0001 | |
| Sotalol | 2 (<1) | 20 (3) | 13 (3) | <.0001 | |
| Other | 1 (<1) | 7 (1) | 8 (2) | 0.0001 | |
| 6-min walk distance, meters | 375 (305, 442) | 366 (290, 433) | 363 (290,421) | 0.0025 | |
| Exercise duration on CPX, minutes | 10 (7,12) | 9 (7,12) | 9 (7,11) | 0.0033 | |
| Training heart rate, bpm | 104 (91,114) | 97 (85,108) | 98 (88,109) | <.0001 | |
| Peak VO2, ml/kg/min | 15.0 (11.8, 18.2) | 14.2 (11.1, 17.2) | 13.8 (11.3,16.8) | <.0001 | |
| Creatinine clearance (MDRD) | 71 (56, 86) | 64 (49, 79) | 59 (45,74) | <.0001 | |
| Prior AF/flutter | 166 (14) | 184 (27) | 138 (32) | <.0001 | |
| Prior Sustained VT/VF | 26 (2) | 204 (30) | 114 (26) | <.0001 | |
| Beck Depression Inventory II score | 8 (4,15) | 9 (5,16) | 8 (5,14) | 0.38 | |
Continuous variables show as median (25th, 75th percentiles).
HF = heart failure, MI = myocardial infarction, NYHA = New York Heart Association, KCCQ = Kansas City Cardiomyopathy Questionnaire, LVEF = left ventricular ejection fraction, COPD = Chronic obstructive pulmonary disease, CPX = cardiopulmonary exercise test, MDRD = modification of diet in renal disease, VT = ventricular tachycardia, VF = ventricular fibrillation, ACE-I = ace-inhibitor, ARB = angiotensin receptor blockade, LBBB = left bundle branch block, RBBB = right bundle branch block, IVCD = non-specific intraventricular conduction delay, BiV = biventricular.
As reflected in Table 1, patients with devices had greater functional limitation than those without, and those with biventricular pacing had the greatest functional limitation according to New York Heart Association classification (p<.0001), 6-minute walk distance (p=0.0025), and peak VO2 on cardiopulmonary exercise (CPX) testing (p<.0001). In terms of optimal medical therapy, beta-blocker use and dose were similar in all 3 patient groups. There was no difference between groups in the baseline BDI II Score. At baseline, differences in the median KCCQ summary score were statistically significant, but actual differences across groups were small (Table 1). Adherence to the experimental exercise protocol was similar across groups with 67%, 64%, and 62% of the BiV pacing, RV pacing, and no device group patients, respectively, having completed all 36 exercise training sessions. In addition, the total amount of time spent exercising per week (supervised and self-reported) at different time points as well as the median percentage of supervised exercise sessions in which patients achieved target HR was similar across device groups.
All groups, regardless of exercise training or device status, demonstrated improvement in functional capacity over the course of the investigation as measured by CPX testing duration and peak VO2 from baseline to 3 months with the exception of usual care patients with BiV pacemakers who saw no change in peak VO2. Gains in both CPX duration and peak VO2 were greatest in patients randomized to exercise training. Within exercise randomization groups, gains were similar between patients with and without devices. At 3 months with exercise training, peak VO2 improved by an average of 1.1 mL/kg/min (BiV) and 0.8 ml/kg/min (RV) compared to 1.2 mL/kg/min in patients without devices. Similarly, CPX duration increased by 1.5 min (BiV and RV) with exercise training compared to 1.8 min in patients without devices. All groups had some improvement in pro-BNP from baseline to 3 months, with the greatest reductions seen in patients without devices. Exercise training did not lead to greater reduction in pro-BNP than usual care alone (Table 2).
Table 2.
Changes in Cardiopulmonary Exercise Testing Parameters and Quality of Life by Pacing and Exercise Group
| Parameter | No device | RV pacing | BiV pacing | |||
|---|---|---|---|---|---|---|
| Usual care n=629 |
Exercise n=584 |
Usual Care n=332 |
Exercise n= 351 |
Usual Care n= 211 |
Exercise n=224 |
|
| CPX duration (min) at baseline | 10.0 n=620 (7.0, 12.8) |
9.8 n=580 (7.0, 12.0) |
9.5 n=330 (7.0, 12.0) |
9.3 n=346 (6.5, 12.0) |
9.0 n=210 (6.7, 11.1) |
9.1 n=223 (6.6, 11.6) |
| At 3 months | 10.5 n=466 (7.5, 13.3) |
11.6 n=495 (9.0, 14.5) |
9.8 n=274 (7.4, 12.3) |
10.8 n=300 (8.3, 13.7) |
9.3 n=167 (7.5, 11.7) |
10.6 n=192 (8.1, 14.0) |
| Peak VO2 at baseline (ml/kg/min) | 15.1 n=613 (12.1, 18.7) |
14.7 n=580 (11.5, 18.0) |
14.1 n=325 (12.2, 17.1) |
14.3 n=335 (11.0, 17.4) |
13.9 n=205 (11.3, 16.6) |
13.8 n=217 (11.3, 16.9) |
| At 3 months | 15.6 n=456 (12.4, 19.4) |
15.9 n=489 (12.6, 19.1) |
14.5 n=269 (11.5, 17.5) |
15.1 n=288 (11.7, 18.4) |
13.9 n=162 (11.7, 16.8) |
14.9 n=187 (12.1, 18.3) |
| KCCQ overall at baseline | 70 n=628 (51, 85) |
69 n=584 (51, 85) |
68 n=332 (51, 84) |
67 n=351 (52, 82) |
67 n=211 (53, 82) |
63 n=224 (47, 79) |
| At 3 months | 75 n=498 (56, 89) |
76 n=511 (59, 90) |
72 n=282 (55, 86) |
73 n=317 (58, 88) |
71 n=185 (55, 82) |
72 n=209 (52, 85) |
| Pro-BNP at baseline (pg/ml) | 621.4 n=373 (278.4, 1484) |
582.4 n=357 (221.7, 1313) |
980.7 n=194 (459.7, 2069) |
924.1 n=207 (406.7, 2171) |
1315 n=120 (509.6, 2694) |
1236 n=132 (528.7, 3023) |
| At 3 months | 558.0 n=272 (219.9, 1581) |
480.8 n=260 (183.7, 1264) |
959.8 n=144 (418.9, 1949) |
998.2 n=159 (410.2, 2399) |
881.5 n=93 (367.3, 1975) |
1197 n=116 (522.5, 2801) |
Continuous variables show as median (25th, 75th percentiles). These data represent the overall results and are not adjusted for missing data.
Overall HRQoL improved in all groups at 3 months, with greater increases in those undergoing exercise training as compared to usual care subjects in the same device group. Importantly, as noted in Table 2, there was considerable drop off in the number of patients evaluated over time.
Site of Ventricular Pacing and Hospitalizations
Reasons for hospitalization during the course of the trial according to the device group are shown in Table 3. One-third of those with BiV pacing had an admission for HF during the trial compared with 26% with right ventricular pacing. Hospitalization for stroke was more common in those patients without a device (2.5%) compared with the right ventricular lead patients (1.6%) and the biventricular pacing patients (1.1%), although follow-up duration was slightly longer in patients without a device, allowing more time for events to occur. Hospitalization for arrhythmia was more frequent in patients with devices (RV lead 16.4%, biventricular pacing 16.6%) compared to those without devices (5.9%). Patients with devices had more non-cardiovascular hospitalizations compared to patients without devices (26% vs. 15%) (Table 3).
Table 3.
Reasons for hospitalization according to type of ventricular pacing
| Reason for Hospitalization | No device | RV pacing | BiV pacing |
|---|---|---|---|
| N=1213 | N=683 | N=435 | |
| Heart failure | 212 (17.5) | 180 (26.4) | 146 (33.6) |
| Ischemic heart disease | 107 (8.8) | 73 (10.7) | 33 (7.6) |
| Myocardial infarction | 28 (2.3) | 16 (2.3) | 6 (1.4) |
| Unstable Angina | 57 (4.7) | 41 (6.0) | 22 (5.1) |
| Other CAD | 22 (1.8) | 16 (2.3) | 5 (1.1) |
| Arrhythmia | 72 (5.9) | 112 (16.4) | 72 (16.6) |
| Atrial fibrillation | 19 (1.6) | 22 (3.2) | 18 (4.1) |
| Other supraventricular arrhythmia | 14 (1.2) | 3 (0.4) | 3 (0.7) |
| Bradycardia | 10 (0.8) | 3 (0.4) | 2 (0.5) |
| Ventricular arrhythmia | 17 (1.4) | 24 (3.5) | 11 (2.5) |
| ICD firing | 0 (0.0) | 56 (8.2) | 36 (8.3) |
| Cardiac arrest | 11 (0.9) | 4 (0.6) | 2 (0.5) |
| Cardiovascular procedure | 213 (17.6) | 101 (14.8) | 68 (15.6) |
| Hypertension requiring inpatient treatment | 5 (0.4) | 3 (0.4) | 0 (0) |
| Peripheral vascular disease | 5 (0.4) | 4 (0.6) | 3 (0.7) |
| Presyncope/hypotension | 17 (1.4) | 17 (2.5) | 23 (5.3) |
| Syncope | 24 (2.0) | 33 (4.8) | 17 (3.9) |
| Stroke | 30 (2.5) | 11 (1.6) | 5 (1.1) |
| Transient ischemic attack | 11 (0.9) | 11 (1.6) | 4 (0.9) |
| Noncardiovascular | 186 (15.3) | 176 (25.8) | 116 (26.7) |
Exercise Training and Adjusted Outcomes according to Ventricular Pacing Status
The outcomes associated with exercise training according to device type and the corresponding interaction tests are shown in Table 4. The primary endpoint, all-cause death or hospitalization, was reduced only in patients randomized to exercise training without a device (HR 0.79 [95% CI 0.67–93], p=0.004), although the confidence intervals overlapped in all 3 groups (RV pacing HR 1.04 [95% CI 0.84–1.28], p=0.74; BiV pacing HR 1.05 [95% CI 0.82–1.34], p=0.72). There was a borderline significant interaction regarding the effect of training between device versus non-device groups for the composite primary outcome of all-cause death or hospitalization (p=0.058).
Table 4.
Effects of exercise vs control group assignment on outcomes by pacing status
| All-cause death or hospitalization | All-cause death | CV death or CV hospitalization | CV death or HF hospitalization | |
|---|---|---|---|---|
| Comparisons of treatment effect among device groups (interaction tests) | ||||
| Any difference among device groups (overall test) | P=0.058 | P=0.33 | P=0.19 | P=0.97 |
| Treatment effect by device group* | ||||
| BiV pacing | ||||
| Exercise training vs. usual care — HR(CI) | 1.05 (0.82, 1.34) P=0.72 |
1.18 (0.79, 1.77) P=0.41 |
1.11 (0.85, 1.46) P=0.44 |
0.89 (0.64, 1.25) P=0.51 |
| RV lead | ||||
| Exercise training vs. usual care — HR(CI) | 1.04 (0.84, 1.28) P=0.74 |
0.94 (0.65, 1.35) P=0.74 |
0.92 (0.73, 1.15) P=0.46 |
0.85 (0.65, 1.12) P=0.24 |
| No device | ||||
| Exercise training vs. usual care — HR(CI) | 0.79 (0.67, 0.93) P=0.0045 |
0.79 (0.55, 1.13) P=0.20 |
0.82 (0.69, 0.99) P=0.034 |
0.88 (0.67, 1.16) P=0.37 |
Hazard ratios and p-values are from adjusted models with device groups included as time-dependent covariates.
Similar trends were observed for the secondary endpoints of all-cause death and the composite endpoint of CV death or CV hospitalization. For both of these endpoints, the greatest risk reduction with exercise therapy was observed in patients without devices (Table 4). However, as with the primary endpoint, the confidence intervals overlapped across all three groups. Interaction tests for reduction in all-cause death and device type (p=0.33) and reduction in CV death or CV hospitalization (p=0.19) did not meet statistical significance. For the other secondary endpoint, CV death or HF hospitalization, there were also overlapping confidence intervals across all three groups (p for interaction 0.97).
As Figure 1 illustrates, 25% of patients were omitted due to missing covariate values (98 BiV pacing, 164 RV pacing, and 314 no device). These omitted patients had an overall event rate that was similar to that of patients included in the model (63% versus 68%, respectively), and their risk of an event was not significantly different (p=0.45 by unadjusted Cox model).
Discussion
There are three major findings from this exploratory analysis of the HF-ACTION trial that address exercise training in patients with symptomatic HF and implanted cardiac devices. First, exercise training in patients with HF and implanted cardiac devices appears to be safe and does not lead to increased mortality or hospitalization. Second, improvement in aerobic exercise capacity and HRQoL are generally similar in patient groups with and without an implanted device. Finally, the aggregate data from HF-ACTION suggest that the beneficial effect of exercise training on clinical events observed in those without devices may be attenuated in patients with devices. In particular, this last finding lends itself to future investigation into potential interactions of implanted devices, ventricular pacing, and exercise tolerance in HF patients.
Current clinical guidelines recommend implanted devices for symptomatic HF patients with reduced LV function, with the specific device type depending on several important clinical factors, including functional status, intraventricular conduction pattern, and degree of QRS prolongation. (6) Registry data suggest that more than half of all HF patients have an implanted cardiac device, which is consistent with the HF-ACTION cohort. (1) Prior studies examining the safety and efficacy of exercise training in patients with implanted cardiac devices have been limited by small sample sizes and the use of surrogate physiologic endpoints, and have been relatively focused on the occurrence of arrhythmia. (7–11) Thus, despite the ubiquity of device therapy in patients with chronic HF, little is known about the impact of device therapy on the benefits of exercise training.
There are limited data regarding the impact of exercise training in HF patients with right ventricular pacing. Prior work indicates that right ventricular pacing can have deleterious effects, including an increased risk of HF, worsening left ventricular dysfunction, and mortality. Single-chamber ventricular pacing has been shown to lead to dyssynchronous activation of the ventricles (12) which may partially explain baseline differences in peak VO2 by conduction pattern in the HF-ACTION population. (13) Despite these potential drawbacks, patients with single chamber ICDs did experience improvement in peak VO2, and recent analyses from the HF-ACTION cohort suggest that exercise training does not increase the risk of defibrillator shock. (14)
The finding of improved exercise capacity in HF–ACTION participants with BiV devices was expected. In a study of 50 patients with HF and cardiac resynchronization devices (CRT/CRT-D), patients randomized to exercise training several months after CRT implantation experienced a further 7% increase in peak VO2, a 30% increase in exercise duration, improved functional capacity (NYHA Class I versus II), and significant improvement in quality of life. (11) Evidence suggests that this improvement in exercise tolerance may, in part, be due to a decreased resting heart rate and better heart rate adaptation. (15) Although prior studies have not directly compared the functional benefits of exercise training in right ventricular pacing patients versus biventricular paced patients, the findings from the current analysis suggests that the response to exercise training in terms of both changes in aerobic capacity and clinical outcomes is similar. It is possible, however, that the improvements in exercise tolerance may vary amongst patients with BiV devices based on whether they achieve significant improvements in LV remodeling, change in LVEF, or other measures of response to BiV pacing; our analysis provides no such discrimination within the BiV group.
The hypothesis for the current analysis was based on the concept that the effect of exercise training on risk of a cardiovascular event would be independent of a participant’s device status at the time of enrollment. Although the current analysis did not reject the hypothesis, a significant risk reduction in mortality and hospitalization with exercise therapy was observed only in the patients without implanted devices (p for interaction 0.058) despite similar changes in CPX duration and peak VO2 between groups with and without implanted devices. There are several possible explanations for these results. First, the benefits of exercise training in HF patients on all-cause mortality and hospitalization (the primary outcome in the HF-ACTION study) may be greatest in those patients without ventricular pacing. The significant favorable effect of exercise training on events in those without a device was seemingly abrogated by the presence of right ventricular or biventricular pacing, suggesting that pacing-induced ventricular activation may counteract any cardioprotective effects from gains in myocardial remodeling and cardiorespiratory fitness as demonstrated by improvements in peak VO2 and CPX duration Alternatively, the risk reduction observed in those patients without devices may be due to factors associated with receipt of a device (e.g., more severe disease) rather than the ill effects of pacing. This is particularly true in patients with ICD-only devices (i.e., no CRT), where one would expect pacing burden to be minimal given the known risks of RV pacing. (16) Moreover, the patients with ventricular pacing were older and sicker, with more symptomatic HF, more electrical dyssynchrony, and worse renal function. However, in previous HF-ACTION analyses; there was no consistent interaction between HF severity and effect of exercise training on outcomes. (3) The three criteria for HF severity were NYHA class, CPX duration, and a multivariate-derived risk score using significant predictors of the primary outcome. (17) These findings suggest that something intrinsic to ventricular pacing may have attenuated the trend to improved outcomes seen in those without devices. Future studies are needed to address this hypothesis.
Limitations
There are several pertinent limitations to consider when evaluating the results of this study; in general, these analyses should be interpreted with caution and are meant to be hypothesis generating. First, HF-ACTION was a randomized controlled trial of exercise training, and therefore represents a select population of HF patients. Second, HF-ACTION only collected data regarding device-type and one snapshot of paced versus non-paced status; ventricular pacing burden data were not available. Third, we considered device-type as a time-dependent covariate; therefore, device status was a post-randomization variable in many patients. Fourth, as with any retrospective study, we cannot exclude the possibility that differences in the clinical outcomes among the three groups were due to residual confounding despite multivariable adjustment. Fifth, in regard to the secondary quality of life and exercise testing parameters, there was considerable missing data especially at later time points, and no analysis was done to evaluate the potential impact of those missing data on outcomes. For example, since the BiV group had worse heart failure at baseline as measured by NYHA class and baseline peak VO2, greater mortality may have resulted in selection of surviving patients with the most cardiac reserve and greatest potential for improvement in peak VO2. Also, differences in mortality by device status could affect the results of the analysis of cause-specific hospitalizations by device group. Moreover, the well-established benefits of ICDs in heart failure patients may have substantially reduced the death and hospitalization event rates regardless of exercise training, so a larger cohort may have increased our power to detect a small yet statistically significant difference in the groups we studied. As such, our analyses may have erroneously accepted the null hypothesis that the effect of exercise training on risk of a cardiovascular event was independent of a participant’s device status at the time of enrollment. Finally, HF-ACTION enrolled a heterogeneous population of device patients, who likely had wide differences in anti-bradycardia programming, factors known to influence survival. (16) However, the diversity of the enrolled population is also a strength of our analysis, as it enhances the generalizability of these results. Additional study strengths include the large patient population, rigorous exercise testing protocol, relatively long-term follow-up, and the use of clinically meaningful endpoints including all-cause death or hospitalization.
Conclusions
In a large, contemporary HF population receiving guideline-based therapy, including implanted cardiac devices, exercise training was safe, improved exercise capacity and HRQoL, and did not lead to increased mortality or hospitalization. However, the aggregate data from HF-ACTION suggest that the risk reduction observed with exercise training may be abrogated by ventricular pacing. These hypothesis- generating data highlight the need for further investigation into the interaction between cardiac pacing and exercise training on clinical events in HF populations.
Acknowledgments
Funding Sources: HF-ACTION trial was funded by the National Institutes of Health [5U01HL063747 to CMO, RU01HL066482 to ILP, and 5UO1HL06694 to SJK]. This work was supported by a grant from Boston Scientific to JPP, DJW, and CMO.
Footnotes
Conflict of Interest: Dr. Piccini reports receiving grants for clinical research from GE Healthcare, Johnson & Johnson, and serves as a consultant to Johnson & Johnson, Forest Laboratories, Medtronic, and Spectranetics. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI, NIH, or the Department of Health and Human Services.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heywood JT, Fonarow GC, Yancy CW, Albert NM, Curtis AB, Gheorghiade M, et al. Comparison of medical therapy dosing in outpatients cared for in cardiology practices with heart failure and reduced ejection fraction with and without device therapy: report from IMPROVE HF. Circ Heart Fail. 2010;3(5):596–605. doi: 10.1161/CIRCHEARTFAILURE.109.912683. [DOI] [PubMed] [Google Scholar]
- 2.Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1451–9. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bensimhon DR, Adams GL, Whellan DJ, Pagnanelli RA, Trimble M, Lee BA, et al. Effect of exercise training on ventricular function, dyssynchrony, resting myocardial perfusion, and clinical outcomes in patients with heart failure: a nuclear ancillary study of Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION); design and rationale. Am Heart J. 2007;154(1):46–53. doi: 10.1016/j.ahj.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 5.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 6.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61(3):e6–75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58(10):1001–6. doi: 10.1016/j.jacc.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Vanhees L, Kornaat M, Defoor J, Aufdemkampe G, Schepers D, Stevens A, et al. Effect of exercise training in patients with an implantable cardioverter defibrillator. Eur Heart J. 2004;25(13):1120–6. doi: 10.1016/j.ehj.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 9.Vanhees L, Schepers D, Heidbuchel H, Defoor J, Fagard R. Exercise performance and training in patients with implantable cardioverter-defibrillators and coronary heart disease. Am J Cardiol. 2001;87(6):712–5. doi: 10.1016/s0002-9149(00)01488-0. [DOI] [PubMed] [Google Scholar]
- 10.Fitchet A, Doherty PJ, Bundy C, Bell W, Fitzpatrick AP, Garratt CJ. Comprehensive cardiac rehabilitation programme for implantable cardioverter-defibrillator patients: a randomised controlled trial. Heart. 2003;89(2):155–60. doi: 10.1136/heart.89.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patwala AY, Woods PR, Sharp L, Goldspink DF, Tan LB, Wright DJ. Maximizing patient benefit from cardiac resynchronization therapy with the addition of structured exercise training: a randomized controlled study. J Am Coll Cardiol. 2009;53(25):2332–9. doi: 10.1016/j.jacc.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 12.Bashore TM, Stine RA, Shaffer PB, Bush CA, Leier CV, Schaal SF. The noninvasive localization of ventricular pacing sites by radionuclide phase imaging. Circulation. 1984;70(4):681–94. doi: 10.1161/01.cir.70.4.681. [DOI] [PubMed] [Google Scholar]
- 13.Pina IL, Kokkinos P, Kao A, Bittner V, Saval M, Clare B, et al. Baseline differences in the HF-ACTION trial by sex. Am Heart J. 2009;158(4 Suppl):S16–23. doi: 10.1016/j.ahj.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piccini JP, Hellkamp AS, Whellan DJ, Ellis SJ, Keteyian SJ, Kraus WE, et al. Exercise training and implantable cardioverter defibrillator shocks in patients with heart failure: Results from HF-ACTION. JACC Heart failure. 2013;1(2):142–8. doi: 10.1016/j.jchf.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auricchio A, Kloss M, Trautmann SI, Rodner S, Klein H. Exercise performance following cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. Am J Cardiol. 2002;89(2):198–203. doi: 10.1016/s0002-9149(01)02200-7. [DOI] [PubMed] [Google Scholar]
- 16.Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288(24):3115–23. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- 17.Whellan DJ, Nigam A, Arnold M, Starr AZ, Hill J, Fletcher G, et al. Benefit of exercise therapy for systolic heart failure in relation to disease severity and etiology-findings from the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training study. Am Heart J. 2011;162(6):1003–10. doi: 10.1016/j.ahj.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]