Abstract
Effective immunization induces the development of populations of robust effector lymphocytes specific for the immunizing antigens. Amongst them are cytotoxic/CD8+ T lymphocytes, which few will further differentiate into long-lived memory cells persisting in the host and exhibiting improved functional characteristics. The current model is that such memory cells can confer rapid host protection upon cognate antigen-mediated activation and direct killing of infected cells. In this chapter, we discuss work from our group and others that highlight the contribution of inflammatory cytokines to memory CD8+ T cell activation and of cytolysis-independent mechanisms of host protection.
Synonyms: Memory T lymphocytes, Monocytes, Inflammation, Vaccination, Protective immunity
Introduction
Formation of long-lasting antigen-specific memory CD8+ T cells involves complex sets of events that comprise initial activation (priming), proliferation (expansion) and massive apoptosis (contraction), after which the cells that survive give rise to long-lived memory cells (Cui and Kaech, 2010; Harty and Badovinac, 2008; McKinstry et al., 2010). Through this process, memory cells acquire multiple unique functional features which make them able not only to respond to, but also to actively provide different signals ultimately culminating in host protection. While the current view is that cognate antigen is the only essential trigger to reactivate the memory T cells, several evidence in the literature, pointed to an important role of cytokines and chemokines in these processes. Thus, in this chapter, we most specifically present results that our group and others have generated over the past years and that support such evidence.
Non-Cognate Differentiation of Memory CD8+ T Cells Into Robust Effector Cells
Cytokines and Pathways
Initial hints came from early work by the Sprent group showing that CD8+ T cells exhibiting features of memory cells (CD44hi) could proliferate in response to the cytokine IL-15 in vitro and in vivo, and independently of T cell receptor (TCR) triggering (Zhang et al., 1998). A further report from the Forman lab using mice immunized with the intracellular bacterium Listeria monocytogenes (Lm) established that in response to the cytokines IL-12 and IL-18 only, Lm-specific memory but not naive CD8+ T cells were able to differentiate into IFN-γ-secreting effector cells, which could confer some protection to immunized hosts (Berg et al., 2003). Also using Lm as a model, we reported that protection of vaccinated host mice during recall infection was occuring within 6–8 hours post challenge infection (Narni-Mancinelli et al., 2011). Interestingly, control of bacterial growth was correlated with the rapid clustering and activation (IFN-γ+) of memory CD8+ T cells to the red pulp/marginal zone area of the spleen of challenged mice (Bajenoff et al., 2010), within bacteria-containing area where macrophages actively filter the blood (Aoshi et al., 2008). These ‘effector-clusters’ were exclusively formed by memory but not naive T cells, and included rapidly recruited blood-derived innate immune cells such as Ly6C+ ‘inflammatory’ monocytes, the most abundant subset of blood-derived monocytes (Auffray et al., 2009), and neutrophils. Quite unexpectedly, we found that the memory CD8+ T cells underwent comparable activation (as measured by expression of IFN-γ, Granzyme B, CD25, NKG2D etc) whether cognate antigen was present or not (Soudja et al., 2012). Investigating further the underlying mechanisms, we could show that the cytokines IL-18 and IL-15 were major drivers of early memory T cell effector differentiation respectively into IFN-γ-secreting and cytolytic (GrB+) cells. This involved the triggering of the inflammasome (IL-18) and the IRF3/type I IFN (IL-15) pathways. While such rapid memory T cell-differentiation did not require cognate antigen recognition by the memory cells, cellular proliferation and robust secondary expansion did not occur without T cell receptor (TCR) triggering. Similar findings were also reported by other groups (Chu et al., 2013; Kupz et al., 2012).
Innate Immune Cells
An initial study from the Lefrancois group suggested the unexpected importance of CD11chi dendritic cells (DCs) for optimal activation and expansion of memory CD8+ T cells in mice immunized with Lm and two distinct viruses, the vesicular stomatitis and the influenza viruses (Zammit et al., 2005). Our study and that from the Bedoui group investigating the mechanisms of antigen-independent activation of memory CD8+ T cells reported an essential role for different subsets of innate myeloid cells in providing key activating cytokines to the memory cells (Kupz et al., 2012; Soudja et al., 2012). While the latter study showed the implication of the NLRC4 inflammasome inside CD11c+ DCs, leading to IL-18 production, we reported that Ly6C+ monocytes could produce both IL-18 and IL-15. Of note, Ly6C+ monocytes appeared as exclusive providers of IL-15 very early on -by 6–8 hours post infection-, yet other cell types such as macrophages or DCs could compensate as early as 24 hours after the challenge infection. Though substantial differences could account for this discrepancy, including the experimental systems that were used, we favor the idea that both antigen-presenting cell (APC) types are indeed contributing to reactivating the memory cells. As initially proposed by Busch and colleagues (Neuenhahn et al., 2006), and further established by the Murphy lab (Edelson et al., 2011), amongst CD11c+ DCs, the CD8α+ DCs are required to carry live blood bacteria to spleens, thus it is certainly possible that DCs act as initiators while Ly6C+ monocytes, which undergo massive mobilization from the bone-marrow (Serbina and Pamer, 2006), quickly amplify and sustain the cytokinic signals.
In addition to providing activating cytokines and along the lines of the early work from Lefrancois and colleagues (Zammit et al., 2005), we also found that Ly6C+ monocytes contribute to antigen-dependent expansion of the memory CD8+ T cells (Soudja et al., 2012). Selective depletion of Ly6C+ monocytes in immunized mice, substantially decreased memory CD8+ T cell expansion during the challenge infection, as also shown following CD11c+ DC elimination. Both of these observations may indeed be accounted for by indirect ‘inflammatory’ effects, rather than by direct presentation of cognate antigen to the memory cells, as also proposed by Badovinac and Harty (Wirth et al., 2011). The contribution of antigen-presentation by DC and/or Ly6C+ monocytes to memory T cell activation and expansion still remains to be defined.
In summary, all these reports supported the notion that APCs which act as early sentinel cells of the immune system, in particular DCs and Ly6C+ monocytes, play an essential role for optimal cytokine- as well as cognate antigen-mediated activation of memory CD8+ T cells in vivo. This mechanism also contributes to host mechanisms of innate immune protection.
Effective Memory T Cell-Mediated Immunity
Ly6C+ monocytes and CD11c+ DCs are required to promote optimal memory T cell reactivation during a recall infection by acute intracellular pathogens such as Lm or viruses like the lymphochorionmeningitis virus (LCMV Armstrong) or the Vaccinia virus. However, how each of these signals translates into effective protection of the hosts is still incompletely understood. The prominent view proposes that reactivated memory CD8+ T cells rapidly express cytolytic effector functions (such as perforin, Granzyme and Fas) that allow for direct killing of pathogen-infected cells, representing the major mechanism of host protection (Harty et al., 2000). Reactivated memory T cells are also shown to secrete important amounts of proinflammatory cytokines and chemokines such as IFNγ, CCL3, CCL4 and CCL5, which promote immune cell activation and recruitment (Dorner et al., 2002; Sallusto et al., 2000). Ultimately, effective protection of vaccinated hosts likely involves both direct and indirect mechanisms orchestrated by memory T cells, yet we and others have sought to most accurately define the relative contribution of the possible different mechanisms. Of note, which effector mechanisms will be most important will depend on each infection as previously reviewed elsewhere (Harty et al., 2000).
Numerous studies, in particular from the Harty lab, have used both mice lacking essential effector molecules (such as IFN-γ, TNF-α, Perforin, CD95/Fas), as well as adoptive transfer experiments of memory CD8+ T cells purified from mice immunized with Lm as model (Badovinac and Harty, 2000; Harty and Bevan, 1995; White et al., 2000a; White et al., 2000b). Wild-type or knockout immunized mice were challenged and their ability to clear the infection compared. Likewise, memory CD8+ T cells from knockout or WT immunized mice were purified and transferred to naïve recipient mice that were subsequently challenged to assess the contribution of distinct effector mechanisms to host protection. Several major conclusions could be drawn from these studies, specifically that (i) not one but multiple mechanisms accounted for host protection, (ii) all immunized knockout mice listed above turned out to be protected against recall infection and (iii) a mechanism that required TNF-α yet was independent of memory T cell-cytolytic activity and IFN-γ was implicated (White et al., 2000a). Further investigations using potent TNF-α neutralizing reagents in vivo, by several groups including ours, established the importance of TNF-α for the protection of immunized hosts during the recall infection (Narni-Mancinelli et al., 2007; Neighbors et al., 2001). While discrepant with the initial studies using mice lacking TNF-α or its receptor TNFRI (p55) (White et al., 2000a), it did underline that knockout mice can develop compensatory mechanisms that substantially differ from that of WT mice. Alternative explanation, though not exclusive, may be that adoptively transferred memory T cells conferred protection to recipient mice through substantially distinct mechanisms than those of vaccinated mice undergoing the challenge infection.
Building on these observations, we further explored the mechanisms of TNF-α-dependent protective immunity. In agreement with prior work (Cook et al., 1999), memory CD8+ T cells could secrete the proinflammatory cytokine CCL3, yet very rapidly following challenge infection, which promoted the differentiation of blood-derived phagocytes -both Ly6C+ monocytes and neutrophils- into TNF-α and reactive oxygen species (ROS) producing cells (Narni-Mancinelli et al., 2007). This oxidative burst enhanced antimicrobial autophagy which was correlated with intracellular pathogen killing (Narni-Mancinelli et al., 2011). Collectively, these results provided solid proof of concept supporting the importance of innate immune effector cells for rapid memory CD8+ T cell-mediated protection in vaccinated hosts. CCL3, however, was released by the memory cells only upon cognate antigen triggering, suggesting that other, possibly earlier, signals were contributing to innate immune cell mobilization.
From prior work by us and others (Berg et al., 2003; Kupz et al., 2012; Soudja et al., 2012), IFN-γ secreted by memory T cells in response to IL-18, IL-12 and IL-15, appeared as a strong candidate. Production of IFN-γ by pathogen-specific memory T cells indeed starts already by 4 hours post recall infection and can occur in a cognate-antigen independent manner. Through series of advanced genetic depletion and bone-marrow chimera experiments, results from our lab provided compelling evidence that IFN-γ from memory T cells, but not other lymphocytes such as NK or NK T cells, was most essential in instructing innate myeloid and lymphoid cell activation and differentiation into robust microbicidal effector cells. IFN-γ signaling to myeloid cells such as Ly6C+ monocytes, neutrophils, macrophages and DCs was required for them to secrete proinflammatory cytokines (TNF-α) and chemokines (CXCL9, CXCL10, CCL2, CXCL1), express the inducible nitric oxid synthase (iNOS), and upregulate costimulatory (CD80, CD86, CD40) and antigen-presenting molecules. While the importance of IFN-γ as a key modulator of immune responses has long been investigated and documented in humans and mice models (Hu and Ivashkiv, 2009), such links to the mechanisms of memory T cell-mediated protection in a relevant in vivo model of vaccination/infection had not been previously established. Analysis of Ly6C+ monocytes genetic expression program in vaccinated mice undergoing challenge infection revealed an overall ‘IFN-γ-skewed’ program, notably with genes encoding for the guanylate binding protein (gbp) GTPases involved in microbial killing (Kim et al., 2011; Yamamoto et al., 2012). Importantly, both Ly6C+ monocytes, CD11c+ DCs and subsets of tissue macrophages contributed to effective protection of vaccinated hosts during the recall infection, in an IFN-γ-dependent manner. Another key aspect to outline that directly results from IFN-γ modulation is the secreted levels of chemokines such as CXCL9, CXCL10 and others, which are essential for immune cell recruitment to infected foci. Elegant studies from the Germain and the von Andrian labs have illustrated the importance of such mechanism orchestrated by memory CD8+ T cells for rapid pathogen containment near the peripheral entry portal of lymph-borne bacterial and viral pathogens (Kastenmuller et al., 2013; Sung et al., 2012).
Interestingly too, we could extend some of our findings to a relevant mucosal model of infection, using mice vaccinated intravaginally by attenuated TK− herpes simplex virus 2 (HSV-2). We found that Ly6C+ monocytes and neutrophils underwent better recruitment and activation during recall infection with WT virulent HSV-2 than that of non-vaccinated counterparts, a finding that is particularly relevant in the context of prior and recent literature on the importance of mucosal resident memory T (TRM) cell-derived IFN-γ for protective immunity against HSV-2 (Ariotti et al., 2014; Gebhardt et al., 2009; Iijima and Iwasaki, 2014; Schenkel et al., 2014; Schenkel et al., 2013). In all of these studies, TRM were reported to carry the very initial immune ‘alarming’ functions, using IFN-γ as the most essential lymphokine.
In summary, results from several studies largely conducted in our lab support the notion that indirect mechanisms of protection, distinct from the ‘usual’ cytolytic effector mechanisms expressed by CD8+ T cells, are significant contributors to the protection of vaccinated hosts during virulent pathogen infection. This mechanism implicates the coordinated orchestration of innate immune cell activation and differentiation by the memory T cells. Altogether, this body of work reveals the importance for such indirect mechanisms of protection, and further emphasizes their need to be accounted for when evaluating vaccine efficacy.
Conclusion
This book chapter summarizes recent developments from our groups and others investigating mechanisms of in vivo memory T cell-activation and effective host protection that are taking place in vaccinated hosts during an acute challenge infection (See also summary Figure). Current evidence reveal the complexity of these processes and suggest the implication of multiple steps of innate and adaptive cell cross-talks that ultimately lead to microbial pathogen containment and killing, and host protection. Cognate memory T cell antigens, cytokines and chemokines, and the differentiation of robust microbicidal innate and adaptive effector cells are all contributing to an optimal and effective immune response. Further studies, in particular using relevant models of mucosal immunizations, will be necessary to fully dissect and understand these mechanisms, and harness them for potential therapies.
Figure.
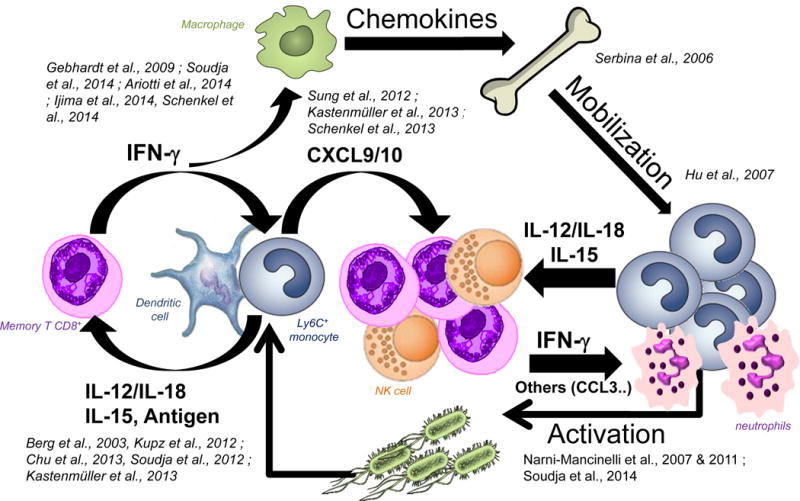
Crosstalk Between Memory T cells and Innate Immune Cells Occurring During Recall Infection of Vaccinated Hosts
Contributor Information
Grégoire Lauvau, Email: gregoire.lauvau@einstein.yu.edu, Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, NY, USA.
Saïdi M’Homa Soudja, Email: saidi.soudja@inserm.fr, Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, NY, USA.
References
- Aoshi T, Zinselmeyer BH, Konjufca V, Lynch JN, Zhang X, Koide Y, Miller MJ. Bacterial entry to the splenic white pulp initiates antigen presentation to CD8+ T cells. Immunity. 2008;29:476–486. doi: 10.1016/j.immuni.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, Jacobs H, Haanen JB, Schumacher TN. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Harty JT. Adaptive immunity and enhanced CD8+ T cell response to Listeria monocytogenes in the absence of perforin and IFN-gamma. J Immunol. 2000;164:6444–6452. doi: 10.4049/jimmunol.164.12.6444. [DOI] [PubMed] [Google Scholar]
- Bajenoff M, Narni-Mancinelli E, Brau F, Lauvau G. Visualizing early splenic memory CD8+ T cells reactivation against intracellular bacteria in the mouse. PLoS One. 2010;5:e11524. doi: 10.1371/journal.pone.0011524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T, Tyznik AJ, Roepke S, Berkley AM, Woodward-Davis A, Pattacini L, Bevan MJ, Zehn D, Prlic M. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell reports. 2013;3:701–708. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DN, Smithies O, Strieter RM, Frelinger JA, Serody JS. CD8+ T cells are a biologically relevant source of macrophage inflammatory protein-1 alpha in vivo. J Immunol. 1999;162:5423–5428. [PubMed] [Google Scholar]
- Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev. 2010;236:151–166. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner BG, Scheffold A, Rolph MS, Huser MB, Kaufmann SH, Radbruch A, Flesch IE, Kroczek RA. MIP-1alpha, MIP-1beta, RANTES, and ATAC/lymphotactin function together with IFN-gamma as type 1 cytokines. Proc Natl Acad Sci U S A. 2002;99:6181–6186. doi: 10.1073/pnas.092141999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson BT, Bradstreet TR, Hildner K, Carrero JA, Frederick KE, Kc W, Belizaire R, Aoshi T, Schreiber RD, Miller MJ, et al. CD8alpha(+) Dendritic Cells Are an Obligate Cellular Entry Point for Productive Infection by Listeria monocytogenes. Immunity. 2011;35:236–248. doi: 10.1016/j.immuni.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmuller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. Peripheral Prepositioning and Local CXCL9 Chemokine-Mediated Guidance Orchestrate Rapid Memory CD8(+) T Cell Responses in the Lymph Node. Immunity. 2013 doi: 10.1016/j.immuni.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332:717–721. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- Kupz A, Guarda G, Gebhardt T, Sander LE, Short KR, Diavatopoulos DA, Wijburg OL, Cao H, Waithman JC, Chen W, et al. NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8(+) T cells. Nat Immunol. 2012 doi: 10.1038/ni.2195. [DOI] [PubMed] [Google Scholar]
- McKinstry KK, Strutt TM, Swain SL. The potential of CD4 T-cell memory. Immunology. 2010;130:1–9. doi: 10.1111/j.1365-2567.2010.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narni-Mancinelli E, Campisi L, Bassand D, Cazareth J, Gounon P, Glaichenhaus N, Lauvau G. Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. J Exp Med. 2007;204:2075–2087. doi: 10.1084/jem.20070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narni-Mancinelli E, Soudja SM, Crozat K, Dalod M, Gounon P, Geissmann F, Lauvau G. Inflammatory Monocytes and Neutrophils Are Licensed to Kill During Memory Responses In Vivo. PLoS Pathog. 2011;29 doi: 10.1371/journal.ppat.1002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors M, Xu X, Barrat FJ, Ruuls SR, Churakova T, Debets R, Bazan JF, Kastelein RA, Abrams JS, O’Garra A. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on Interferon gamma production. J Exp Med. 2001;194:343–354. doi: 10.1084/jem.194.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenhahn M, Kerksiek KM, Nauerth M, Suhre MH, Schiemann M, Gebhardt FE, Stemberger C, Panthel K, Schroder S, Chakraborty T, et al. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–630. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory Monocytes Activate Memory CD8(+) T and Innate NK Lymphocytes Independent of Cognate Antigen during Microbial Pathogen Invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JH, Zhang H, Moseman EA, Alvarez D, Iannacone M, Henrickson SE, de la Torre JC, Groom JR, Luster AD, von Andrian UH. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. 2012;150:1249–1263. doi: 10.1016/j.cell.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DW, Badovinac VP, Fan X, Harty JT. Adaptive immunity against Listeria monocytogenes in the absence of type I tumor necrosis factor receptor p55. Infect Immun. 2000a;68:4470–4476. doi: 10.1128/iai.68.8.4470-4476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DW, Badovinac VP, Kollias G, Harty JT. Cutting edge: antilisterial activity of CD8+ T cells derived from TNF-deficient and TNF/perforin double-deficient mice. J Immunol. 2000b;165:5–9. doi: 10.4049/jimmunol.165.1.5. [DOI] [PubMed] [Google Scholar]
- Wirth TC, Martin MD, Starbeck-Miller G, Harty JT, Badovinac VP. Secondary CD8+ T-cell responses are controlled by systemic inflammation. Eur J Immunol. 2011;41:1321–1333. doi: 10.1002/eji.201040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Okuyama M, Ma JS, Kimura T, Kamiyama N, Saiga H, Ohshima J, Sasai M, Kayama H, Okamoto T, et al. A cluster of interferon-gamma-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity. 2012;37:302–313. doi: 10.1016/j.immuni.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]


