Abstract
Recent evidence has shown a complex relationship between the gut microbiota, dietary nutrients, and cardiovascular disease (CVD). Trimethylamine-N-oxide (TMAO) production, initiated by the microbiota, has been associated with CVD events. We sought to test if this association exists in HIV-infected persons. After adjusting for aspirin use and CVD risk factors, HIV-infected men were more likely to have coronary stenosis in the second and third TMAO quartiles compared to the first quartile, but did not differ significantly in the fourth quartile. We found an inverted U-shaped association between TMAO levels and the presence of coronary artery stenosis among HIV-infected men.
Introduction
There is a growing appreciation of the link between dietary nutrients, the intestinal microbiome, and atherosclerosis and cardiovascular disease (CVD) events [1,2]. The gut microbiome is estimated to contain over 100 trillion bacteria that both metabolize and utilize molecules derived from intestinal contents [3,4]. For example, intestinal microbiota metabolize phosphatidylcholine (lecithin), the major dietary source of choline, into trimethylamine, which is then converted by host hepatic enzymes into trimethylamine-N-oxide (TMAO), a metabolite shown to promote atherosclerosis in animal models [1]. In humans, TMAO levels have recently been reported to be predictive of coronary artery disease (CAD) as well as cardiovascular events, independent of traditional cardiovascular risk factors. [1-13].
Among HIV-infected persons, the gut microbiota is altered [14] and there is an increased risk of CVD [15-17]. These findings prompted an investigation into the association of HIV infection and TMAO levels, which found no association [18]. More recently, Haissman et al similarly found to difference in TMAO levels between HIV-infected and uninfected individuals [19]. We therefore designed a case-control study to further explore the association between coronary atherosclerosis and TMAO levels among participants in the Multicenter AIDS Cohort Study (MACS).
Methods
The MACS is a prospective study of HIV-infected and uninfected men who have sex with men. From 2010-2013, participants underwent non-contrast cardiac computed tomography (CT) and coronary CT angiography (CCTA) unless excluded by chronic kidney disease (estimated glomerular filtration rate <60 ml/min/1.73m2), atrial fibrillation, or IV contrast allergy. Participants were between 40-70 years, weighed less than 300 lbs, and without prior heart surgery [20]. We drew our cases (n=51) and controls (n=51) from among the 765 MACS participants who underwent coronary angiography (CCTA). Selection for this case-control control study was limited to participants with a stored fasting serum sample and non-missing data regarding CAD risk factors, including measured blood pressure, fasting serum glucose, fasting lipid panel, and body mass index, self-reported current smoking and use of medications for diabetes, lipid lowering, or hypertension. Covariate data as well as blood samples were collected for all participants at a semiannual MACS visit generally within 6 months prior to the CCTA measurement.
To test our hypothesis that elevated TMAO levels would be independently associated with both CAD and HIV infection, we selected a nested case-control sample population to represent the extremes of atherosclerosis. Cases were defined by the presence of coronary stenosis ≥50% in one or more coronary segments, the measurement of which has been previously described [21,22], whereas controls were defined by the absence of any detectable coronary plaque on CCTA. A total of 105 men met the case definition and 154 met the definition for control. We employed a 1:1 nearest neighbor matching algorithm without replacement to match each case to a control of the same race, HIV serostatus, and age (starting with ±1 year and iteratively expanded in one year increments to a maximum of ±6 years). A program then randomly selected 51 pairs (28 HIV-infected and 23 HIV-uninfected) with a sufficient quantity of stored serum.
TMAO was measured by stable isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) [23]. The lower and upper limits of quantification were 0.05 and >200 μM, respectively, with demonstrated accuracy over this range and with intra-day and inter-day coefficients of variance <10%. TMAO measurements are stable over multiple freeze-thaw cycles of serum [23].
Distributions of absolute TMAO levels and the difference in TMAO levels between a case and its matched control were assessed graphically. The sign test for equality of medians was used to evaluate the difference in TMAO levels between cases and controls. TMAO values in the total sample were divided into four quartiles. We used conditional logistic regression to evaluate the odds of coronary artery stenosis by difference in the quartile of TMAO level within the matched pair. The models were adjusted for aspirin use and the American College of Cardiology/American Heart Association (ACC/AHA) Pooled Cohort Equation, which includes gender, diabetes, age, levels of HDL-cholesterol, total cholesterol, hypertension treatment, systolic blood pressure and race [24]. To explore whether the association between TMAO and coronary artery stenosis differed by HIV serostatus, all analyses were further stratified by the HIV serostatus of the matched pair. As secondary aims, we unmatched the pairs and explored associations between TMAO levels and coronary artery calcium (CAC), ultrasound derived carotid intima-medial thickness (IMT) and the presence of focal carotid plaque [25]. To quantify CAC, the Agatston method was employed using non-contrast CT scans [26]. Finally, we explored the association of TMAO levels with stenosis in 1, 2, or 3 major coronary vessels. Sample selection, matching, and analyses were performed using Stata 13.1 (StataCorp, College Station, TX).
Results
Overall Population
Descriptive characteristics of the cases and controls are shown in Table 1. Cases had a higher ACC/AHA cardiovascular risk score and were significantly more likely than controls to take lipid lowering medication and aspirin. Cases were more likely to have TMAO levels in the third quartile compared to the other quartiles (p=0.02). Among the cases, 75% (n=38), 20% (n=10) and 5% (n=3) had single, double and triple vessel CAD, respectively.
Table 1.
Demographic, Behavioral, and Clinical Characteristics of Cases with Coronary Stenosis >=50% and Matched Controls
| Cases (N=51) | Controls (N=51) | P-Value | Total (n=102) | |
|---|---|---|---|---|
| Age* (years) | 55 (51-58) | 54 (51-59) | 0.81 | 54 (51-58) |
| Race* | 1.00 | |||
| Caucasian (%) | 66.7 | 66.7 | 66.7 | |
| African-American or other (%) | 33.3 | 33.3 | 33.3 | |
| Cohort enrollment period | 0.23 | |||
| pre-2001 (%) | 52.9 | 64.7 | 58.8 | |
| 2001 and later (%) | 47.1 | 35.3 | 41.2 | |
| TMAO quartile | 0.02 | |||
| Q1 (%) | 21.6 | 29.4 | 25.49 | |
| Q2 (%) | 19.6 | 29.4 | 24.51 | |
| Q3 (%) | 39.2 | 11.8 | 25.49 | |
| Q4 (%) | 19.6 | 29.4 | 24.51 | |
| ACC/AHA CVD Risk Score (%) | 8.2 (4.6-14.1) | 5.7 (4.0-10.2) | 0.04 | 6.9 (4.1-12.1) |
| Systolic Blood Pressure (mmHg) | 128 (116-136) | 123 (114-132) | 0.29 | 126 (116-133) |
| On Hypertensive Medications (%) | 35.3 | 27.5 | 0.39 | 31.4 |
| Fasting Glucose (mg/dL) | 101 (92-109) | 98 (90-108) | 0.17 | 99.5 (91-108) |
| On Diabetes Medications (%) | 17.6 | 7.8 | 0.14 | 12.7 |
| Total Cholesterol (mg/dL) | 190 (166-214) | 186 (162-217) | 0.68 | 189.5 (163-216) |
| HDL Cholesterol (mg/dL) | 43.9 (38.9-57.7) | 52.5 (45.5-62.5) | <0.01 | 50.4 (41.5-59.7) |
| On Lipid Lowering Meds (%) | 54.9 | 33.3 | 0.03 | 44.1 |
| Body Mass Index (kg/m2) | 27.0 (23.5-30.8) | 24.8 (22.1-28.5) | 0.08 | 26.0 (22.9-29.3) |
| Current Smoker (%) | 25.5 | 17.6 | 0.34 | 21.6 |
| Cumulative pack yearsa | 10.2 (3.8-41.8) | 8.0 (0.3-21.0) | 0.12 | 9.0 (1.5-27.0) |
| Aspirin use | 45.0 | 18.0 | <0.01 | 31.7 |
| HIV-specific factors | ||||
| HIV-infected* (no. and %) | 28 (54.9) | 28 (54.9) | 56 (54.9) | |
| Viral Load < 50 copies/mL (%) | 71.4 | 89.3 | 0.09 | 80.4 |
| Viral Load log10 copies/mL** | 1.2 (1.0-1.8) | 1.2 (1.0-1.6) | 0.73 | |
| Time on HAART (years) | 9.6 (7.8-12.9) | 9.6 (7.7-12.9) | 0.43 | 9.0 (7.3-12.7) |
| CD4+ T-cell count (cells/mm3) | 667 (417-744) | 562 (434-829) | 0.93 | 614 (424-789) |
| CD4+ T-cell nadir (cells/mm3) | 229 (109-373) | 281 (216-391) | 0.35 | 275 (171-383) |
| History of AIDS (%) | 10.7 | 10.7 | 1.00 | 10.7 |
| Major Coronary Vessel Disease | -- | |||
| Single (%) | 74.5 | -- | -- | |
| Double (%) | 19.6 | -- | -- | |
| Triple (%) | 5.9 | -- | -- |
Matching variable. a) Among current and former smokers.
Includes men with undetectable viral loads set at 10 copies/mL. Only 11 men had detectable viral loads (8 cases and 3 controls). For those with detectable viral loads: Cases – 3.4 (2.4-3.6); Controls – 3.5 (2.0-5.0) [p=0.68].
TMAO and HIV
TMAO levels ranged from 0.4 μM to 19.8 μM (median=3.8 μM, IQR=2.4 μM, 6.5 μM), and the distributions, stratified by HIV serostatus and case/control status are shown in Figure 1. The median TMAO levels were slightly greater in HIV-infected (median =4.0 μM, IQR= 2.5 μM, 6.8 μM) compared to HIV-uninfected participants (3.3 μM; IQR; 2.2 μM, 5.8 μM), but this difference was not statistically significant (p=0.31)(Fig1a). The median difference in TMAO levels within matched HIV-infected pairs (TMAOcase-TMAOcontrol) was 1.2 μM (IQR= -2.5, 2.4, p=0.09)(Fig1b). In contrast, there was no meaningful difference in TMAO levels within HIV-uninfected pairs (median difference=0.3 μM, IQR= -1.6 μM, 2.3 μM)(Fig1b). However, the median difference in TMAO levels between HIV-infected and uninfected mean was not statistically significant.
Figure 1.
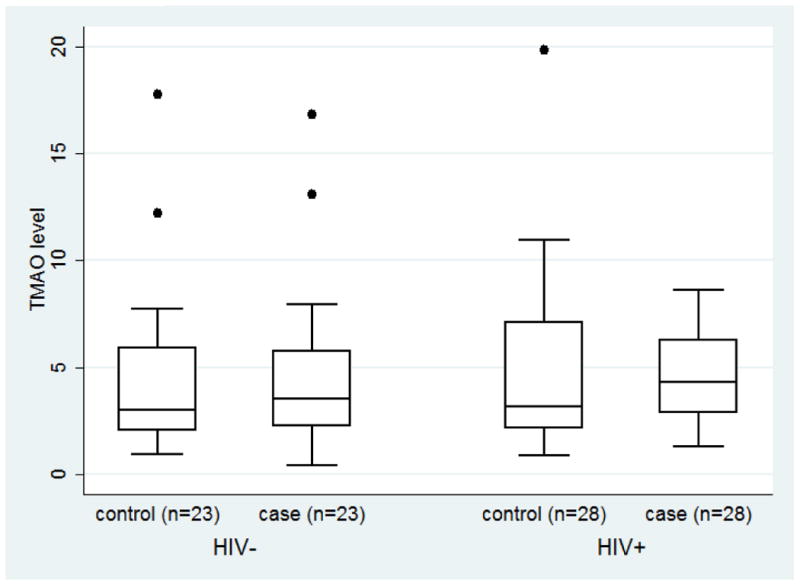
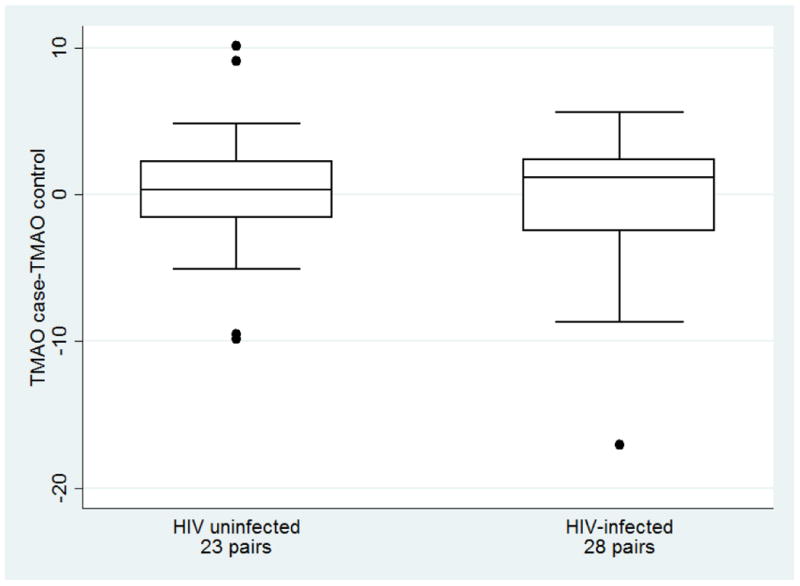
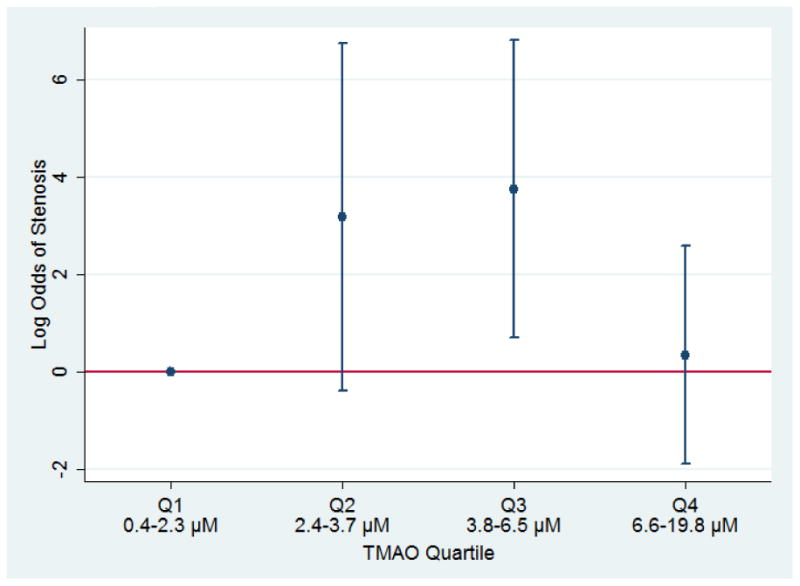
Legend: Panel a: Distribution of TMAO (μM) for men in the MACS with (case) and without (control) coronary stenosis > 50%, by HIV serostatus, 2010-2013. The line within the box delineates the median and the interquartile ranges (25%, 75%) defined the lower and upper bounds of the box. Panel b: Distribution of the difference in the TMAO level between a case and his matched control, by HIV serostatus, 2010-2013. Panel c: Log odds (with 95% confidence intervals) of coronary artery stenosis ≥ 50%, by TMAO quartiles among 28 HIV-infected matched pairs from the MACS, 2010-2013. Quartile 1 (Q1) is the reference category; TMAO quartile ranges were defined by the TMAO values for the overall sample.
TMAO and Coronary Stenosis
We then determined that the association between TMAO and coronary stenosis was nonlinear in the overall cohort and, in stratified analysis, limited to the HIV-infected pairs. After adjusting for aspirin use and ACC/AHA risk score, the log odds of stenosis were 3.2 fold greater for HIV-infected men in the second TMAO quartile (95% CI: -0.39, 6.74, p=0.08) and 3.7 fold greater for HIV-infected men in the third compared to the first TMAO quartile (95% CI: 0.69, 6.81; p=0.02); however, the log odds of stenosis did not differ between the men in the fourth compared to the first TMAO quartile (Fig1c). The association between the third quartile of TMAO and stenosis in HIV-infected men was robust to sequential adjustment by potential confounding factors, including cohort enrollment calendar period, smoking status, BMI, fasting serum glucose adjusted for the use of medication to treat hyperglycemia, systolic blood pressure adjusted for use of anti-hypertension medications, and serum lipids (total and HDL cholesterol levels) adjusted for the use of lipid lowering medications. Further, TMAO levels were not higher in men with double or triple coronary vessel disease compared to those with single vessel disease.
Secondary Outcomes
Among the 46 HIV-infected and -uninfected men with CAC (5 cases with stenosis had CAC scores of 0), TMAO was not significantly associated with Agatston score (p=0.58). Median TMAO levels were not statistically significantly higher among those with carotid plaque present (4.4, IQR 2.7, 7.6 vs 3.7 IQR 2.4, 6.2, p=0.36), among the 93 men with available ultrasound data. Furthermore, there were no significant associations between TMAO levels (on the natural log linear scale or in quartiles) and carotid IMT in the total sample (p=0.46). Neither CAC, carotid plaque presence, nor carotid IMT showed a significant association with either strata of HIV serostatus (data not shown).
Discussion
In our sample, TMAO levels demonstrated an unexpected inverted U shaped association with coronary artery stenosis among HIV-infected participant pairs, but not associated with stenosis among the HIV-uninfected pairs. TMAO levels were not associated with CAC, carotid plaque or IMT overall or within either HIV serostatus groups in the unmatched sample. These findings contrast with those from the large (> 4000 subject) prospective study by Tang et al among subjects undergoing elective cardiac evaluations in which there were incremental risks of cardiovascular events by increasing TMAO quartile [2]. Indeed, numerous additional studies have now showed an association between TMAO with either atherosclerotic burden, prevalent CVD, or incident adverse cardiac events [3-13].
In addition to including men with HIV, our study sample was younger with lower rates of hypertension, diabetes, and multiple vessel disease than these cohorts. The mechanism accounting for the inverted U shaped association between TMAO levels and coronary stenosis among HIV-infected participant pairs requires investigation. However, given the reliance of TMAO on gut micriobiota and the gut's known alteration in HIV-infected persons [14], altered absorption and metabolism of TMAO precursors could be playing a role. Although, measurement bias, chance results, or unrecognized confounding cannot be ruled out, nonlinear and inverted U-shaped relationships have been documented with certain biologic systems, including bisphenol A and atherosclerotic plaque, organic pollutants and diabetes, lipoproteins and aging, and physiologically with epinephrine and exposure to chromogranin A [27-30]. Our study is not the first analysis reporting equivocal relationships between TMAO and atherosclerosis. Srinivasa and colleagues found no association between TMAO levels and coronary plaque burden in a group of HIV-infected and uninfected participants, instead finding that levels of its biologic precursor, trimethylamine (TMA), were significantly associated with calcified plaque burden independent of cardiovascular risk factors [18]. Haissman et al similarly did not find differences in TMAO levels between those with and without HIV [19]. However, they did find elevated levels of TMAO in HIV-infected individuals with myocardial perfusion defects on stress testing, which may be consistent with our results as perfusion defects typically occur with coronary stenosis.
The present results should be interpreted with caution due to the small sample size and the cross-sectional measurement of coronary artery stenosis and TMAO levels. While the cases had worse cardiovascular profiles than the controls, the levels of many established risk factors for stenosis were not significantly different between the cases and controls in our sample, potentially because the sample size is small, and the population examined relatively young with cases having less advanced CAD compared to other study populations. Further, antibiotic use in the month prior and dietary intake during the several days prior to sample collection has been shown to affect TMAO levels [2], and these factors were not assessed in our study. A standardized protocol for diet and other relevant exposures prior to collection might help to minimize variability in the measurement of TMAO, and improve comparability between studies. Future studies should investigate the relationship of TMAO with coronary plaque morphology. Since numerous studies now have shown an association between TMAO with CVD and incident adverse events [1-13], additional larger studies are needed to determine the relationship between TMAO and HIV, especially exploring whether there is an association between TMAO and plaque-destabilization or the initiation and progression of atherosclerosis in HIV-infected subjects.
In conclusion, we found an inverted U-shaped association between TMAO levels and the presence of coronary artery stenosis among HIV-infected, but not among HIV–uninfected men in this small study. Our results are limited by sample size and cross-sectional design, but raise questions regarding the metabolism and contribution to disease pathogenesis of TMAO among different hosts. Additional prospective studies in HIV-infected and -uninfected populations, with TMAO measured longitudinally and prior to disease occurrence, are needed to further understand the relationship between TMAO and CVD across different populations, and to understand underlying causal pathways. Given the growing interest in the role of TMAO in cardiovascular events, the contribution of TMAO to increased cardiovascular risk among HIV-infected persons deserves further study.
Acknowledgments
Funding sources: The MACS CVD study is funded by NHLBI: RO1 HL095129 (Post). The TMAO study is funded by a subaward to CLS through NIAID P30AI094189 (Chaisson). Data in this manuscript were collected by the MACS with centers (Principal Investigators) at: Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI). MACS data collection is also supported by UL1 TR 001079 (JHU CTSA). Website located at http://www.statepi.jhsph.edu/macs/macs.html. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health
Footnotes
Conflicts of interest: Dr. Hazen is named as co-inventor on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics or therapeutics. Dr. Hazen reports having been paid as a consultant for the following companies: Esperion, and Procter & Gamble. Dr. Hazen reports receiving research funds or support from Abbott, Astra Zeneca, Cleveland Heart Lab, Procter & Gamble, Pfizer Inc., Roche and Takeda. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Cleveland Heart Lab., Siemens, Esperion, Frantz Biomarkers, LLC. All other authors have no conflicting interests to declare.
References
- 1.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidycholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 5.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Tang WH, Buffa JA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35:904–910. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang WH, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lever M, George PM, Slow S, et al. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observation study. PLoS One. 2014;9:e1114969. doi: 10.1371/journal.pone.0114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang WH, Wang Z, Shrestha K, et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21:91–96. doi: 10.1016/j.cardfail.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine-N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troseid M, Ueland T, Hov JF, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717–726. doi: 10.1111/joim.12328. [DOI] [PubMed] [Google Scholar]
- 12.Mente A, Chalcraft K, Ak H, et al. The relationship between trimethylamine-N-oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can J Cardiol. 2015 doi: 10.1016/j.cjca.2015.06.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Stubbs JR, House JA, Ocque AJ, et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014111063. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinh DM, Volpe GE, Duffalo C, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2014;211:19–27. doi: 10.1093/infdis/jiu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palella FJ, Jr, Baker RK, Moorman AC, et al. Morality in the highly active antiretroviral therapy era: changing cause of death and disease the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 17.Friedberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivasa S, Fitch KV, Lo J, et al. Plaque burden in HIV-infected patients is associated with serum intestinal microbiota-generated trimethylamine. AIDS. 2015;29:443–452. doi: 10.1097/QAD.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haissman JM, Knudsen A, Hoel H, et al. Microbiota-dependent marker TMAO is elevated in silent ischemia but not associated with first-time myocardial infarction in HIV infection. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000843. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The multicenter AIDS cohort study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 21.Hacioglu Y, Gupta M, Choi TY, et al. Use of cardiac CT angiography imaging in an epidemiology study – the methodology of the multicenter aids cohort study cardiovascular disease substudy. Anadolu Kardiyol Derg. 2013;13:207–214. doi: 10.5152/akd.2013.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–467. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen Sl. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. doi: 10.1016/j.ab.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantiification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990:15, 827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 27.Lind PM, Lind L. Circulationg levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis. 2011;218:207–213. doi: 10.1016/j.atherosclerosis.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study. Environ Health Perspect. 2010;118:1235–1242. doi: 10.1289/ehp.0901480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park YM, Sui X, Liu J, et al. The effect of cardiorespiratory fitness on age-related lipids and lipoproteins. J Am Coll Cardiol. 2105;65:2091–2100. doi: 10.1016/j.jacc.2015.03.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaingankar SM, Li Y, Biswas N, et al. Effects of chromogranin A deficiency and excess in vivo: biphasic blood pressure and catecholamine responses. J Hypertens. 2010;28:817–825. doi: 10.1097/HJH.0b013e328336ed3e. [DOI] [PMC free article] [PubMed] [Google Scholar]


