Abstract
Background
Testing and linkage to care are important determinants of hepatitis C virus (HCV) treatment effectiveness. Public health clinics serve populations at high risk for HCV. We investigated their potential to serve as sites for HCV testing, initiation of and linkage to HCV care.
Methods
Cross-sectional study of patients accessing Sexually Transmitted Infections (STI) care at the Baltimore City Health Department (BCHD) STI clinics, from June 2013 through April 2014. Logistic regression was used to assess factors associated with HCV infection and specialist linkage to care.
Results
Between June 24, 2013 and April 15, 2014, 2681 patients were screened for HCV infection. Overall, 189 (7%) were anti-HCV positive, of whom 185 (98%) received follow-up HCV RNA testing, with 155 (84%) testing RNA positive. Of 155 RNA positive individuals, 138 (89%) returned to the STI clinic for HCV RNA results and initial HCV care including counseling regarding transmission and harm reduction for alcohol, and 132 (85%) were referred to a specialist for HCV care. With provision of patient navigation services, 81(52%) attended an offsite HCV specialist appointment. Alcohol use and lack of insurance coverage were associated with lower rates of specialist linkage (OR 0.4 [95% CI 0.1–0.9] and OR 0.4 [95% CI 0.1–0.9] respectively).
Conclusion
We identified a high prevalence of HCV infection in BCHD STI clinics. With availability of patient navigation services a large proportion of HCV infected patients linked to off-site specialist care.
Keywords: Hazardous alcohol consumption, HCV treatment, Implementation science, rapid testing, sexually transmitted infections clinic
Introduction
Hepatitis C Virus (HCV) infection is a major public health challenge that eclipsed human immunodeficiency virus (HIV) as a cause of mortality in the USA in 2007 (1). There are an estimated 3.2–5 million individuals in the US chronically infected with HCV (2). Recent advances in HCV treatment, including the availability of efficacious all-oral HCV therapies of short duration with minimal side effects, make cure of HCV infection feasible for most people. However, more than half of HCV infected persons in the US are unaware of their infection and thus cannot benefit from these improvements in treatment (3). Additionally, for those who are aware of their HCV positive status, patient, provider, and systems barriers to treatment can impede treatment effectiveness (4–6).
There are major disparities in HCV infection burden in the US. Individuals infected with HCV are more likely to be of non-Hispanic black race/ethnicity (2). HCV positive serostatus has also been associated with high risk sexual behavior, particularly among HIV positive men who have sex with men (MSM). Minority populations are less likely to get tested for HCV (7). Even when diagnosed, minority populations are less likely to be referred for care and when referred are less likely to undergo HCV treatment (8, 9). Since HIV, sexually transmitted infections (STIs), and viral hepatitis share common risk factors, the Centers for Disease Control and Prevention (CDC) has promoted collaboration and service integration as a priority for programs addressing HIV, STIs, and viral hepatitis (10). Additionally, the US Action Plan for the Prevention, Care, and Treatment of Viral Hepatitis calls for improvements in the HCV continuum of care with the goal of curing individuals to reduce the long-term complications of HCV and decrease HCV transmission (11). Previous reports on the progress of patients through the US HCV care continuum suggest that of those who are HCV antibody positive, 22–50% have been HCV tested, 32–38% referred to care, 27% actively engaged in care, 7–15% initiated treatment, and only 5–6% were cured (12–14).
Baltimore has a high burden of HCV infection with previously reported prevalence of 10% among persons attending STI clinics who denied injection drug use, 18% in persons attending emergency departments, and 60–90% among persons who inject drugs (15–17). These groups are all less likely to seek health care in primary care settings, traditionally considered the primary HCV screening sites. However, as many of these individuals regularly seek care in STI clinics (18), STI clinics might serve as effective venues for HCV testing and linkage to care. To investigate the capacity of public health clinics to provide HCV clinical services, we examined the prevalence of HCV, rates of and factors associated with linkage to care at two public health clinics in Baltimore City.
Methods
Study Design
We performed a cross-sectional study involving patients accessing STI care at the Baltimore City Health Department (BCHD) STI clinics, Baltimore, MD from June 2013 through April 2014. These patients were then prospectively followed to assess linkage to HCV care. As part of standard clinical protocol, written informed consent to receive care was obtained from each patient. The Institutional Review Board of the Johns Hopkins University School of Medicine approved the research study.
Setting
There are two BCHD STI clinics serving the largely ethnic minority inner city population of Baltimore. The clinics see an average of 30,000 patient visits a year and operate a walk-in clinic appointment model. Care is provided by nurse practitioners and physician assistants under the supervision of a physician. A wide range of reproductive and sexual health services are provided including comprehensive STI care, HIV testing and counseling, and hepatitis B screening and vaccination. The BCHD STI clinics also have on-site Ryan White-funded HIV treatment clinics which provide continuity for HIV care.
Primary HCV testing and care
STI clinic attendees between the ages of 18 and 70 years, regardless of prior HCV testing history, were offered a free rapid HCV test at registration. Patients who accepted the rapid HCV test offer were provided pretest counseling and tested using the OraQuick HCV Rapid Antibody test (OraSure Technologies, Bethlehem PA) on blood collected via finger prick or phlebotomy. HCV test results were available in 20 minutes. Given CDC recommendations that persons identified as having HCV infection should receive a brief screening for alcohol use and intervention, a validated 3 question alcohol use disorder identification tool (AUDIT C) was also administered to individuals who received HCV rapid testing (19, 20).
During the clinician encounter, information on alcohol and illicit drug use and sexual practices were routinely collected using a standardized form. All anti-HCV positive individuals received additional HCV posttest counseling from a clinician including the need for confirmatory HCV RNA testing, risk reduction counseling, brief alcohol use counseling with referral for treatment as indicated, at the initial rapid HCV testing visit. HCV RNA testing was performed at a commercial laboratory. Results were received within one week and thus available for patient counseling at the routinely offered walk-in return appointment one or two weeks after the initial encounter.
Individuals were considered to have chronic HCV if they had positive results on both anti-HCV and HCV RNA tests. Primary HCV care at the STI clinic was defined as counseling on HCV transmission and availability of HCV drug treatment, harm reduction for alcohol and assessment for vaccination against hepatitis A and B.
Linkage to Specialty HCV Care
At the time of HCV diagnosis, all chronically infected patients met with a dedicated linkage to care coordinator who provided patient navigation services including insurance evaluation, insurance application assistance if indicated, and assistance with scheduling primary care and HCV specialist appointments, and reminder calls and/or text messages for appointments. Patients were linked to different HCV treating specialists in Baltimore city. Choice of HCV specialist linkage site was dependent primarily on the patient’s insurance and secondarily on patient’s preference. Linkage to care support included phone calls, letters, and field outreach to patients who either did not return to the STI clinic for HCV RNA results or who did not attend at least 1 specialist appointment. If a patient was unresponsive by phone, a letter was sent to the address provided at the baseline visit. If still unresponsive, field outreach by a BCHD outreach worker was used to attempt contact with patients. Other services provided included maintaining contact by phone with patients who did not keep appointments, acting as an intermediary between the patient and primary care provider and specialist clinics and assistance with identification and resolution of any barriers patients experienced in attending appointments. A score to quantify the intensity of outreach effort was created by scaling encounters according to effort required by clinic staff. Phone calls, letters, and home visits regardless of success were weighted as 1, 2, and 3, respectively. Each patient’s outreach effort score was determined as a summation of the weighted encounters. For patients not linking to care after 9 months, phone calls were attempted to assess barriers to linkage to care and provide additional patient navigation services. Barrier to care information was collected using a standardized questionnaire adapted from a previous HCV care barrier study (21). The questionnaire included categorized known barriers to HCV care linkage based on existing literature and an additional open ended response option. Patients could cite more than one barrier. All services at both clinics were mainly provided by one linkage to care coordinator who also served as the primary rapid HCV/HIV tester and one outreach worker.
Data management
Patient data including clinical, social, and demographic information such as past and current injection/non-injection drug and alcohol use and linkage to care efforts were routinely recorded in the clinic electronic medical record system (EMR), INSIGHT. The INSIGHT EMR is designed as a local health department EMR with customizable program specific clinic modules. As such alerts could be placed in the EMR to prompt referral to the HCV coordinator in the event that patients returned to the clinic for any reason. Data were extracted from the electronic medical record system for the purpose of this analysis.
We defined linkage to care by (1) Primary HCV care, repeated attendance at the STI clinic to receive HCV RNA results, HCV counseling, and referral to HCV specialist and (2) Specialty HCV care, attendance at first tertiary HCV specialist appointment. Hazardous drinking is defined as a pattern of alcohol consumption that puts individuals at risk for adverse health events (22). Use of >4 alcoholic drinks in one sitting has been set as a threshold for increased risk of adverse health events by the National Institute of Alcohol Abuse and Alcoholism (23). Engaging in risky sex under the influence of alcohol also puts individuals at risk for the adverse consequence of STI acquisition (24, 25). Based on this, individuals who reported drinking >4 alcoholic drinks at 1 sitting or engaging in risky sex under the influence of alcohol in the preceding 30 days were classified as hazardous drinkers.
Statistical Analyses
Descriptive statistics were used to characterize the study population with respect to demographics and risk behaviors. Proportions were compared using Chi-square tests. Logistic regression was used to assess factors associated with HCV infection and specialist linkage to care. The multivariate analyses included factors previously known to be associated with risk of HCV infection or linkage to HCV care. P values <0.05 were considered to be significant. All analyses were performed using Stata version 13 (Stata Corp, College Station, Texas).
Results
Between June 2013 and April 2014, 6290 patients visited the BCHD STI Clinics and 4399 (70%) of patients were offered a free rapid HCV test regardless of HCV risk factor or prior HCV testing history. HCV testing was not routinely offered to patients enrolled in the BCHD HIV continuity care program or to individuals presenting to the STI clinic for non-clinician encounters. 3466 (79%) accepted and 2681 (77%) had a rapid HCV test performed. Individuals who accepted or declined rapid HCV testing were similar with regards to age; median 28 (IQR 23–39) years and 29 (IQR 23–40) years respectively. Characteristics of both groups was also similar with respect to sex, race distribution, sexual orientation, injection drug use status and number of sexual partners in the preceding 60 days. Compared to individuals who declined the offer of rapid HCV test, acceptance of HCV rapid testing was associated with HIV-uninfected status (OR 3.2 95% CI 1.4–7.2), reporting recent non-injection drug use (OR 2.0 95% CI 1.0–3.7) and hazardous alcohol use (OR 1.5, 95% CI 1.2–1.8). STI clinic attendees who underwent rapid HCV testing had a median age of 30 (IQR 24–42) years. Of these, 2408 (90%) were African American, 165 (6%) Caucasian, 64 (2%) Hispanic and the majority were male (62%).
HCV prevalence
Among 2681 persons tested, 189 (7%) were anti-HCV positive, of whom 185 (98%) received follow-up HCV RNA testing at the same visit. Persons born between 1945 and 1965 were 14.5 times more likely to be HCV infected than those born in other years and 127 (67%) of the 189 anti-HCV positive persons were born in that era. Individuals found to be anti-HCV positive were more likely to be male, non-black, insured and have a history of drug use (injection or non-injection). Men who described themselves as MSM and individuals with hazardous levels of alcohol consumption were significantly less likely to be anti-HCV positive (P<0.05 for all comparisons, Table 1).
Table 1.
Factors associated with HCV antibody positivity.
| Characteristic | No. Screened | HCV Prevalence (%) | OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Age | ||||
| Non-Birth Cohort | 2246 | 2.8 | ||
| Birth Cohorta | 435 | 29.2 | 14.5 (10.5–20.1) | 17.9 (12.0–26.8) |
| Sex | ||||
| Female | 1019 | 5.0 | ||
| Male | 1660 | 8.3 | 1.7 (1.2–2.4) | 2.4 (1.5–3.6) |
| Race/Ethnicity | ||||
| Black | 2408 | 6.6 | ||
| Non-Black | 273 | 11.4 | 1.8 (1.2–2.7) | 1.8 (1.3–2.4) |
| HIV status | ||||
| Negative | 2608 | 6.9 | ||
| Positive | 73 | 11.0 | 1.7 (0.8–3.5) | 1.6 (0.6–4.1) |
| MSM | ||||
| No | 2509 | 7.3 | ||
| Yes | 172 | 2.9 | 0.4 (0.2–0.9) | 0.3 (0.1–0.8) |
| Ever IDU | ||||
| No | 2524 | 2.3 | ||
| Yes | 157 | 84.1 | 228.5 (138.4–377.4) | N/A |
| Current IDU | ||||
| No | 2633 | 5.8 | ||
| Yes | 48 | 77.1 | 54.9 (27.5–109.8) | 76.1 (33.1–174.7) |
| Non-IDU | ||||
| No | 2566 | 5.9 | ||
| Yes | 115 | 33.9 | 8.3 (5.4–12.6) | 3.4 (2.0–6.0) |
| Hazardous alcohol consumptionb | ||||
| No | 1968 | 7.9 | ||
| Yes | 713 | 4.6 | 0.6 (0.4–0.8) | 0.6 (0.4–1.0) |
| Insurance status | ||||
| No | 1419 | 4.5 | ||
| Yes | 1262 | 9.9 | 2.3 (1.7–3.2) | 1.8 (1.2–2.5) |
| >2 sex partners in past 90 days/anonymous sex | ||||
| No | 2112 | 7.2 | ||
| Yes | 569 | 6.5 | 0.9 (0.6–1.3) | |
| Prior STD diagnosis | ||||
| No | 1521 | 6.8 | ||
| Yes | 1160 | 7.4 | 1.1 (0.8–1.5) | |
| STD diagnosis on date of HCV diagnosis | ||||
| No | 2122 | 7.1 | ||
| Yes | 559 | 6.8 | 1.0 (0.7–1.4) |
Birth cohort: Individuals born between 1945 and 1965, as defined by the CDC.
Hazardous alcohol consumption defined as reported use of ≥4 alcoholic drinks in 1 sitting or risky sex under the influence of alcohol in the preceding 30 days
Ever IDU not included in the multivariable analysis
Abbreviations used in the table:
Current IDU: Current (within past 12 months) injection drug use.
Non-IDU: Non injection drug use within the past 12 months.
HIV: Human Immunodeficiency Virus
MSM: Men who have Sex with Men
STD: Sexually Transmitted Disease
HCV: Hepatitis C Virus
Of 185 individuals tested for HCV RNA, 155 (84%) were positive and confirmed to have chronic HCV infection. The median age of HCV RNA positive individuals was 51 (IQR 45–57) years. Although the majority (71%) had public insurance, 18% remained uninsured despite potential eligibility for Medicaid coverage after implementation of the Affordable Care Act in the State of Maryland. Approximately 1 in 5 persons with chronic HCV had hazardous levels of alcohol consumption (Table 2).
Table 2.
Characteristics of individuals diagnosed with chronic HCV.
| Characteristic | Prevalence (n=155) |
|---|---|
| Age: median (IQR) | 51 (45–57 years) |
| Male | 74% |
| Black race | 85% |
| Hazardous alcohol consumptiona | 19% |
| Insurance | |
| Medicaid | 59% |
| Medicare | 12% |
| Private | 11% |
| None | 18% |
| Sex partners in prior 3 months: median (IQR) | 1 (1–2) partners |
| Prior STD clinic visit | 65% |
| Prior STD diagnosis | 57% |
| STD diagnosis at HCV diagnosis visit | 17% |
Hazardous alcohol consumption defined as reported use of ≥4 alcoholic drinks in 1 sitting or risky sex under the influence of alcohol in the preceding 30 days
Abbreviations used in the table:
STD: Sexually Transmitted Disease
HCV: Hepatitis C Virus
Linkage to care
Primary HCV care
Of the 155 individuals identified with chronic HCV, we were able to notify and provide posttest counseling based on HCV antibody results to 154 of them (99%). Most (89%) also returned to the STI clinic for receipt of HCV RNA results and initiation of comprehensive HCV care including further medical education on HCV, alcohol use counseling and referral for alcohol treatment as indicated, and hepatitis B immunization.
Specialty HCV care
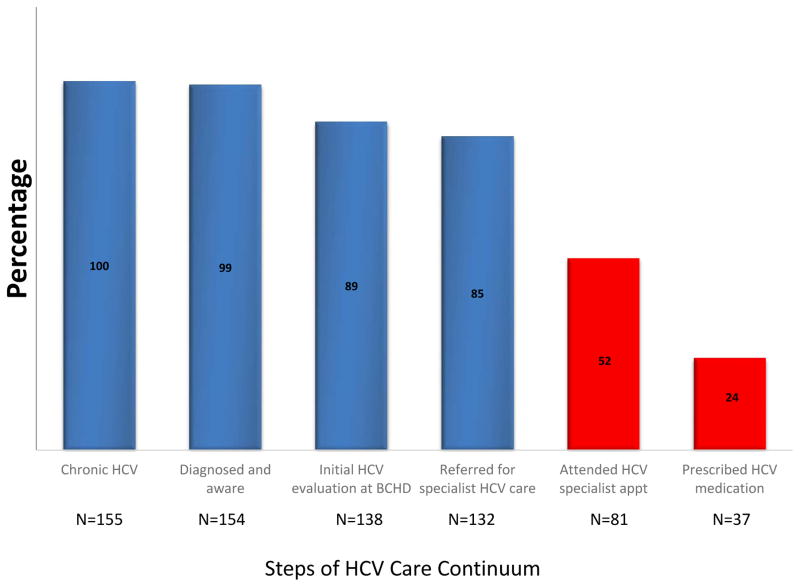
Additionally, 132 (85%) were referred for specialist care at an outside facility. Of the initial 155 chronically HCV infected individuals identified, over half attended their first specialist appointment and 24% have been prescribed HCV antiviral therapy as of July 2015 (Figure 1). The median time from diagnosis to attendance of HCV specialist appointment was 163 (IQR 84–288) days. 35 of 74 individuals who did not link to specialist care could be contacted and were queried about ongoing barriers to care. The most commonly reported barriers to HCV linkage to care cited included not having insurance (12/35), being busy or worried about other things (9/35), not having a primary care provider (6/35), and primary care providers not referring to an HCV specialist (7/35).
Figure 1.
Hepatitis C Virus (HCV) care continuum in the Baltimore City Health Department (BCHD) Sexually Transmitted Infections (STI) clinics.
Percentages in parentheses are percentages of the total population diagnosed with chronic HCV at the BCHD STI clinics between June 2013 and April 2014. Linkage to care follow up through July 2015.
Public health clinic HCV care cascade
In univariate analyses, older individuals born in the CDC-defined “birth cohort” had higher odds of linkage to specialist care (OR 2.4 [95% CI 1.2–4.9]) as did black individuals compared to non-black individuals (OR 2.5 [95% CI 1.0–6.3]). Compared to those with low levels of alcohol consumption, individuals with hazardous levels of alcohol consumption in the preceding 30 days were significantly less likely to attend their specialist appointment (OR 0.3 [95% CI 0.1–0.8]) as were uninsured individuals (OR 0.4[95% CI 0.2–0.9]). Individuals who required significant effort for linkage to care, defined as a linkage intensity score of 9 or greater (indicating they required field outreach) had a lower odds of attendance at a specialist appointment (OR 0.3[95% CI 0.2–0.7]) compared to individuals with a score of 9 or less (Table 3). In multivariable analysis, factors that remained independently associated with lower likelihood of attendance at the first specialist appointment included lack of insurance (OR 0.4 [95% CI 0.2–0.9]) and hazardous levels of alcohol consumption (OR 0.4[95% CI 0.1–0.9]) (Table 3).
Table 3.
Unadjusted and adjusted odds ratios for attendance at 1st HCV treating specialist appointment (n=155).
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Age | ||
| Birth Cohorta member vs. Non-Birth Cohort | 2.4(1.2–4.9) | 1.7 (0.7–3.8) |
| Sex | ||
| Male vs Female | 1.8 (0.9–3.7) | 1.7 (0.7–3.8) |
| Race/Ethnicity | ||
| Non-Black vs. Black | 2.5 (1.0–6.3) | 2.4 (0.9–6.5) |
| HIV status | ||
| Positive vs. Negative | 1.9 (0.3–10.5) | 2.4 (0.4–15.7) |
| Drug use | ||
| Ever IDU | 0.8 (0.4–1.7) | N/A |
| Current IDU (past 12 months) | 0.8 (0.3–1.9) | 1.3 (0.5–3.5) |
| Non-IDU (past 12 months) | 0.6 (0.3–1.3) | N/A |
| Hazardous alcohol consumption*b | ||
| Yes vs. No | 0.3 (0.1–0.8) | 0.4 (0.1–0.9) |
| Health insurance* | ||
| No vs Yes | 0.4 (0.1-0.2-0.9) | 0.4 (0.1–0.9) |
| Linkage to care effort scorec | ||
| >9 vs. 0–9 | 0.3 (0.2–0.7) | NA |
Birth Cohort: Individuals born between 1945 and 1965, as defined by the CDC.
Hazardous alcohol consumption defined as reported use of ≥4 alcoholic drinks in 1 sitting or risky sex under the influence of alcohol in the preceding 30 days.
Linkage to care effort score: The linkage to care effort score is a summation of the weighted outreach efforts for each patient. A score for outreach effort was created by scaling encounters according to effort required. Phone calls, letters, and home visits were weighted as 1, 2, and 3, respectively.
Statistically significant at P<0.05 in the multivariable analysis
Ever-IDU, non-IDU and Linkage to care effort score were not included in the multivariable analysis
Abbreviations used in the table:
Ever IDU: History of injection drug use more than 12 months ago.
Current IDU: Current (within the past 12 months) injection drug use
Non-IDU: Non injection drug use within the past 12 months
HIV: Human Immunodeficiency Virus
MSM: Men who have Sex with Men
STD: Sexually Transmitted Disease
HCV: Hepatitis C Virus
Discussion
In this urban public health clinic, we found an HCV prevalence of 7% in a general STI clinic population in Baltimore. Moreover, we found that nearly 1 out of 3 persons born between 1945 and 1965, the so-called “birth cohort,” was HCV antibody positive, a prevalence that is almost 9 times the estimated U.S. national prevalence of 3.3% for this cohort (26). The 29% prevalence of HCV in the CDC-defined “birth cohort” reinforces the importance of the birth cohort-based recommendations for HCV screening. Through on-site testing in these STI clinics, we were able to test a large number of urban minority medically underserved patients who do not routinely access the health care system through more traditional means such as primary care clinics. We also found evidence that these HCV infected persons could receive initial HCV care in the public health clinics, and that with referral assistance, over half of patients attended a specialist clinic appointment.
The HCV prevalence found in our study was much higher than national estimates but consistent with HCV prevalence of 18%–42% reported from other studies of STI clinic based HCV testing among high risk individuals (27, 28). The exceedingly high rates of hepatitis C in those born between 1945 and 1965 may be due to the introduction of heroin into Baltimore in the 1950s, and ongoing high rates of IDU in Baltimore City (prevalence of injection drug use of 336/10,000 population aged 15–64)(29, 30). Lower prevalence of HCV among MSM and individuals with hazardous alcohol use, reflect the predominantly younger STD clinic population tested, the majority of whom lacked other HCV infection risk factors.
Although HCV testing in STI clinics has been demonstrated to be high-yield and cost-effective in North America (27, 31, 32), the success of these programs has been limited by low rates of linkage to care (24). To the best of our knowledge this is the first study to report the impact of on-site patient navigation services on increasing rates of HCV linkage to care in public health clinic settings.
US national data suggest low rates of HCV status awareness (3). By instituting a rapid HCV test protocol, we could provide HCV antibody results, follow-up HCV RNA testing, HCV transmission education, and alcohol use counseling to 99% of individuals found to be anti-HCV positive on the same day of testing. However, there is no point-of-care test for HCV RNA. Thus, it is very significant that we were able to bring 89% of persons to complete the HCV testing process and receive their HCV RNA results and initiation of primary HCV care. The individuals diagnosed with chronic HCV were able to benefit from low-cost interventions that may reduce the progression of liver disease, such as alcohol screening (19% reported hazardous alcohol use), a brief intervention to reduce alcohol use and referral to treatment if indicated [Screening, Brief Intervention, and Referral to Treatment (SBIRT)], and immunization against hepatitis B (33, 34). Our program likely benefited from the experience that public health clinics and local health departments such as BCHD have in conducting contact investigations, linking patients to care, and working with populations disproportionately affected by STIs, HIV and hepatitis. Other public health clinics with similar expertise have the potential to replicate these high rates of return for HCV care.
Although specialist linkage to care rates observed were still suboptimal, they were almost twice those previously reported and likely reflect the additional services and support provided to help individuals link to care. This is supported by findings from Coyle et al in their study of HCV testing and care at four federally qualified health centers in Philadelphia, Pennsylvania, in which they noted an increase in HCV linkage to care rates by 29% with addition of an HCV linkage to care coordinator (35).
The reasons for failure to link to specialist HCV care in a subset of our population includes hazardous alcohol consumption. This finding is concerning not only because of the negative impact on linkage to care but also because alcohol use is associated with accelerated progression to liver cirrhosis and end stage liver disease (36). This finding is also consistent with previous studies suggesting that competing health priorities including substance abuse and environmental factors such as lack of drug treatment and social support may limit uptake of HCV treatment (4, 37). Additional interventions may be needed to improve HCV care in substance using populations who are also at highest risk for poor HCV-related health outcomes. This finding also provides further rationale for screening for alcohol use as recommended in CDC guidelines for HCV testing and developing additional interventions for alcohol abuse treatment for HCV infected individuals (20). In light of the finding that HCV-related mortality is significantly higher in HCV-infected persons with alcohol disorders, persons with concurrent alcohol and HCV disease should be prioritized for HCV intervention. Of note, although current HCV treatment guidelines do not recommend withholding HCV treatment from persons drinking alcohol, several state Medicaid programs put restrictions on access to HCV drug therapy in persons who use alcohol (38)
Beyond substance use, lack of insurance was a barrier to HCV linkage. National data suggest that HCV infected individuals are less likely to be insured. HCV infected uninsured individuals are also more likely to report alcohol abuse and less education (39). Therefore, there is a clear need for interventions to reach these individuals and provide comprehensive services that link them to health insurance and other social services, such as substance use treatment. Given the complexity of coordinating care in a fragmented medical and social service system, these interventions will likely require patient navigation as a component.
In our study, we were able to link most of our patients to HCV care with relatively minimal interventions such as reminder calls and text messages. These simple and relatively low cost interventions have the potential to significantly increase rates of HCV care linkage and can be feasibly implemented in similar urban settings caring for underserved populations. Most of the patients who did not link to specialist care were lost to follow-up. Those patients that required the most intense linkage to care support were 60% less likely to attend the first HCV specialist appointment compared to patients who required relatively little effort for linkage. To improve HCV treatment outcomes for the majority of HCV infected individuals, it will be critical to identify optimal approaches to access hard-to-reach individuals and motivate them to engage in care. Interestingly, we found that one quarter of HCV infected patients who did not attend their first scheduled off-site HCV specialist appointment were seen at the STI clinics three or more times in the nine months after their HCV diagnosis. This suggests that even among hard to reach individuals there are multiple opportunities for continued contact and reinforcement of messaging regarding the importance of seeking treatment for HCV. An optimal strategy to improve treatment rates in this population may be to provide integrated on-site HCV treatment at the STI clinic. Other studies support the co-localization of HCV testing and treatment services at sites serving individuals with a high prevalence of HCV infection (35, 40). The availability of simple oral regimens for HCV has made it feasible for trained mid-level providers at public health clinics to offer treatment on site. Given their extensive experience working with underserved populations and the public health infrastructure for linkage to care, this could have a major impact on HCV treatment uptake and cure.
Our study is limited by our inability to test all individuals presenting for care at the STI clinics for HCV. Additionally, the small sample size of HIV/HCV co-infected individuals or individuals reporting drug use make it difficult to make assertions about the impact of these co-morbidities on HCV linkage to care. The patients in this study were also identified at two STI clinics in Baltimore City, thus limiting the generalizability of our findings. However, we believe that our STI patient population may be similar to other STI clinic populations in large urban areas in the US.
In summary, we found a high HCV prevalence in urban public health clinics in Baltimore. These rates were especially high among persons born between 1945 and 1965, the so-called “birth cohort.” We also found evidence that these HCV infected persons could receive care in this setting and that with referral assistance, the proportion who attended a remote specialist clinic appointment was almost twice the national average. Efforts are needed to identify alternate HCV testing sites and linkage to care interventions for populations who have a high HCV burden and do not access the health care system through traditional means such as primary care clinics. Additional studies are needed to Additional studies are needed to determine the feasibility and efficacy of providing HCV treatment at public health settings, such as STI clinics.
Footnotes
Statement of Interests
This study was funded the CDC Foundation/Viral Hepatitis Action Coalition, and in part by NIH grant numbers [R3701380] and [K24 DA034621/DA/NIDA].
Contributor Information
Oluwaseun Falade-Nwulia, Email: ofalade1@jhmi.edu.
Shruti H. Mehta, Email: smehta@jhu.edu.
Jackline Lasola, Email: jackline.lasola@gmail.com.
Carl Latkin, Email: carl.latkin@jhu.edu.
Alex Niculescu, Email: awill185@jhmi.edu.
Cristi O’Connor, Email: coconn10@jhu.edu.
Patrick Chaulk, Email: Patrick.chaulk@baltimorecity.gov.
Khalil Ghanem, Email: kghanem@jhmi.edu.
Kathleen R Page, Email: kpage2@jhmi.edu.
Mark S. Sulkowski, Email: msulkowski@jhmi.edu.
David L. Thomas, Email: dthomas@jhmi.edu.
References
- 1.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Annals of internal medicine. 2012 Feb 21;156(4):271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Annals of internal medicine. 2006 May 16;144(10):705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012 Jun;55(6):1652–61. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. Journal of general internal medicine. 2005 Aug;20(8):754–8. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afdhal NH, Zeuzem S, Schooley RT, et al. The new paradigm of hepatitis C therapy: integration of oral therapies into best practices. Journal of viral hepatitis. 2013 Nov;20(11):745–60. doi: 10.1111/jvh.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeremski M, Zibbell JE, Martinez AD, Kritz S, Smith BD, Talal AH. Hepatitis C virus control among persons who inject drugs requires overcoming barriers to care. World journal of gastroenterology : WJG. 2013 Nov 28;19(44):7846–51. doi: 10.3748/wjg.v19.i44.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tohme RA, Xing J, Liao Y, Holmberg SD. Hepatitis C testing, infection, and linkage to care among racial and ethnic minorities in the United States, 2009–2010. American journal of public health. 2013 Jan;103(1):112–9. doi: 10.2105/AJPH.2012.300858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen GC, Thuluvath PJ. Racial disparity in liver disease: Biological, cultural, or socioeconomic factors. Hepatology. 2008 Mar;47(3):1058–66. doi: 10.1002/hep.22223. [DOI] [PubMed] [Google Scholar]
- 9.Melia MT, Muir AJ, McCone J, et al. Racial differences in hepatitis C treatment eligibility. Hepatology. 2011 Jul;54(1):70–8. doi: 10.1002/hep.24358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Services UDoHaH. Program Collaboration and Service Integration: Enhancing the Prevention and Control of HIV/AIDS, Viral Hepatitis, Sexually Transmitted Diseases and Tuberculosis in the United States. http://wwwcdcgov/nchhstp/programintegration/docs/207181-C_NCHHSTP_PCSI%20WhitePaper-508cpdf.
- 11.Services. UDoHaH. Action plan for the prevention, care, and treatment of viral hepatitis. Department ofHealth and Human Services; 2014–2016. pp. 1–100. [Google Scholar]
- 12.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. The New England journal of medicine. 2013 May 16;368(20):1859–61. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yehia BR, Schranz AJ, Umscheid CA, Lo Re V., 3rd The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PloS one. 2014;9(7):e101554. doi: 10.1371/journal.pone.0101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viner K, Kuncio D, Newbern EC, Johnson CC. The continuum of hepatitis C testing and care. Hepatology. 2014 Oct 28; doi: 10.1002/hep.27584. [DOI] [PubMed] [Google Scholar]
- 15.Thomas DL, Cannon RO, Shapiro CN, Hook EW, 3rd, Alter MJ, Quinn TC. Hepatitis C, hepatitis B, and human immunodeficiency virus infections among non-intravenous drug-using patients attending clinics for sexually transmitted diseases. The Journal of infectious diseases. 1994 May;169(5):990–5. doi: 10.1093/infdis/169.5.990. [DOI] [PubMed] [Google Scholar]
- 16.Kelen GD, Green GB, Purcell RH, et al. Hepatitis B and hepatitis C in emergency department patients. The New England journal of medicine. 1992 May 21;326(21):1399–404. doi: 10.1056/NEJM199205213262105. [DOI] [PubMed] [Google Scholar]
- 17.Mehta SH, Astemborski J, Kirk GD, et al. Changes in blood-borne infection risk among injection drug users. The Journal of infectious diseases. 2011 Mar 1;203(5):587–94. doi: 10.1093/infdis/jiq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celum CL, Bolan G, Krone M, et al. Patients attending STD clinics in an evolving health care environment. Demographics, insurance coverage, preferences for STD services, and STD morbidity. Sexually transmitted diseases. 1997 Nov;24(10):599–605. doi: 10.1097/00007435-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcoholism, clinical and experimental research. 2007 Jul;31(7):1208–17. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2012 Aug 17;61(RR-4):1–32. [PubMed] [Google Scholar]
- 21.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. Journal of community health. 2008 Jun;33(3):126–33. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993 Jun;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 23.Alcoholism NIoAAa. The Physicians’ Guide to Helping Patients With Alcohol Problems. Washington, DC: Government Printing Office; 1995. Publication NIH 95-3769. [Google Scholar]
- 24.Cook RL, Clark DB. Is there an association between alcohol consumption and sexually transmitted diseases? A systematic review. Sexually transmitted diseases. 2005 Mar;32(3):156–64. doi: 10.1097/01.olq.0000151418.03899.97. [DOI] [PubMed] [Google Scholar]
- 25.Hutton HE, McCaul ME, Santora PB, Erbelding EJ. The relationship between recent alcohol use and sexual behaviors: gender differences among sexually transmitted disease clinic patients. Alcoholism, clinical and experimental research. 2008 Nov;32(11):2008–15. doi: 10.1111/j.1530-0277.2008.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Ward JW. Hepatitis C virus testing of persons born during 1945–1965: recommendations from the Centers for Disease Control and Prevention. Annals of internal medicine. 2012 Dec 4;157(11):817–22. doi: 10.7326/0003-4819-157-9-201211060-00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subiadur J, Harris JL, Rietmeijer CA. Integrating viral hepatitis prevention services into an urban STD clinic: Denver, Colorado. Public health reports. 2007;122( Suppl 2):12–7. doi: 10.1177/00333549071220S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmerman R, Finley C, Rabins C, McMahon K. Integrating viral hepatitis prevention into STD clinics in Illinois (excluding Chicago), 1999–2005. Public health reports. 2007;122( Suppl 2):18–23. doi: 10.1177/00333549071220S204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.JJ Everybody Must Get Stoned: The Origins of Modern Drug Culture in Baltimore. Maryland Historical Magazine. 1996;91(2):132–55. [Google Scholar]
- 30.Brady JE, Friedman SR, Cooper HL, Flom PL, Tempalski B, Gostnell K. Estimating the prevalence of injection drug users in the U.S. and in large U.S. metropolitan areas from 1992 to 2002. Journal of urban health : bulletin of the New York Academy of Medicine. 2008 May;85(3):323–51. doi: 10.1007/s11524-007-9248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romanowski B, Preiksaitis J, Campbell P, Fenton J. Hepatitis C seroprevalence and risk behaviors in patients attending sexually transmitted disease clinics. Sexually transmitted diseases. 2003 Jan;30(1):33–8. doi: 10.1097/00007435-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Honeycutt AA, Harris JL, Khavjou O, Buffington J, Jones TS, Rein DB. The costs and impacts of testing for hepatitis C virus antibody in public STD clinics. Public health reports. 2007;122( Suppl 2):55–62. doi: 10.1177/00333549071220S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dieperink E, Ho SB, Heit S, Durfee JM, Thuras P, Willenbring ML. Significant reductions in drinking following brief alcohol treatment provided in a hepatitis C clinic. Psychosomatics. 2010 Mar-Apr;51(2):149–56. doi: 10.1176/appi.psy.51.2.149. [DOI] [PubMed] [Google Scholar]
- 34.Gunn RA, Murray PJ, Ackers ML, Hardison WG, Margolis HS. Screening for chronic hepatitis B and C virus infections in an urban sexually transmitted disease clinic: rationale for integrating services. Sexually transmitted diseases. 2001 Mar;28(3):166–70. doi: 10.1097/00007435-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Coyle C, Viner K, Hughes E, et al. Identification and Linkage to Care of HCV-Infected Persons in Five Health Centers - Philadelphia, Pennsylvania, 2012–2014. MMWR Morbidity and mortality weekly report. 2015 May 8;64(17):459–63. [PMC free article] [PubMed] [Google Scholar]
- 36.Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998 Jun;27(6):1717–22. doi: 10.1002/hep.510270635. [DOI] [PubMed] [Google Scholar]
- 37.Blasiole JA, Shinkunas L, Labrecque DR, Arnold RM, Zickmund SL. Mental and physical symptoms associated with lower social support for patients with hepatitis C. World journal of gastroenterology : WJG. 2006 Aug 7;12(29):4665–72. doi: 10.3748/wjg.v12.i27.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid Reimbursement of Sofosbuvir for the Treatment of Hepatitis C Virus Infection in the United States. Annals of internal medicine. 2015 Aug 4;163(3):215–23. doi: 10.7326/M15-0406. [DOI] [PubMed] [Google Scholar]
- 39.Stepanova M, Kanwal F, El-Serag HB, Younossi ZM. Insurance status and treatment candidacy of hepatitis C patients: analysis of population-based data from the United States. Hepatology. 2011 Mar;53(3):737–45. doi: 10.1002/hep.24131. [DOI] [PubMed] [Google Scholar]
- 40.Stein MR, Soloway IJ, Jefferson KS, Roose RJ, Arnsten JH, Litwin AH. Concurrent group treatment for hepatitis C: implementation and outcomes in a methadone maintenance treatment program. Journal of substance abuse treatment. 2012 Dec;43(4):424–32. doi: 10.1016/j.jsat.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]