Abstract
Background
Inflammation contributes to the development of depression in a subset of individuals, but risk factors that render certain individuals vulnerable to inflammation-associated depression are undetermined. Drawing from animal studies showing that reduced neuroplasticity mediates effects of inflammation on depression, we hypothesized that individuals genetically predisposed to lower levels of neuroplasticity would be more susceptible to inflammation-associated depression. The current study examined whether the Met allele of the BDNF Val66met polymorphism, which predisposes individuals to reduced levels of brain-derived neurotrophic factor (BDNF), a protein vital for neuroplasticity, moderates the association between inflammation and depressive symptoms.
Methods
Our sample was 112 women with early-stage breast cancer who had recently completed cancer treatment, which can activate inflammation. Participants provided blood for genotyping and assessment of circulating inflammatory markers, and completed a questionnaire assessing depressive symptoms, including somatic, affective, and cognitive dimensions.
Results
There was a significant interaction between C-reactive protein (CRP) and the BDNF Val66met polymorphism in predicting cognitive depressive symptoms (p=.004), such that higher CRP was related to more cognitive depressive symptoms among Met allele carriers, but not among Val/Val homozygotes. Post-hoc longitudinal analyses suggested that, for Met carriers, higher CRP at baseline predicted higher cognitive depressive symptoms across a one-year follow-up period (p<.001).
Conclusion
The BDNF Met allele may be a risk factor for inflammation-associated cognitive depressive symptoms among breast cancer survivors. Women with breast cancer who carry this genotype may benefit from early identification and treatment.
Limitation
BDNF genotype is an indirect measure of BDNF protein levels.
Keywords: inflammation, CRP, BDNF, breast cancer, depression, Beck Depression Inventory-II
Introduction
For most women, receiving a breast cancer diagnosis and undergoing cancer treatment is a stressful experience. In general, this distress tends to decrease over time and is typically resolved shortly after the completion of cancer treatment (Henselmans et al., 2010). However, certain women are more profoundly and persistently impacted, with approximately 25% of breast cancer patients experiencing clinically significant depressive symptoms that persist well beyond the completion of treatment (Donovan et al., 2014). Depression reduces quality of life, and may also lead to shorter survival time in women with breast cancer (Pinquart and Duberstein, 2011).
Substantial evidence implicates inflammation in the development of depression. Elevated inflammatory markers are associated with depression in both clinical and community samples (Howren et al., 2009). Evidence of inflammation as a causal factor for depression comes from studies of patients with Hepatitis C or cancer, who commonly develop major depression after the initiation of IFN-α therapy, which elicits a potent inflammatory response (Capuron and Miller, 2004; Raison et al., 2005). Additionally, administration of inflammatory agents to healthy individuals leads to depressed mood (Reichenberg et al., 2001; Eisenberger et al., 2010). Notably, vaccine studies in healthy individuals have shown that even a mild inflammatory stimulus, one that does not cause a fever or any other symptoms of physical sickness, can lead to depressive symptoms (Wright et al., 2005).
The mechanisms by which peripheral inflammation can access the brain and potentially lead to depressed mood have begun to be elucidated. Pro-inflammatory cytokines can communicate with the brain via various routes, including passage of circulating cytokines through leaky regions in the blood brain barrier, active transport of cytokines via saturable transporters, activation of cytokine-producing cells lining the cerebral vasculature, and binding to cytokine receptors on afferent nerve fibers which then transmit inflammatory signals to the brain (Miller et al., 2013; Dantzer et al., 2008). Recent evidence has also shown that the brain possesses functional lymphatic vessels, which are able to carry immune cells from the cerebrospinal fluid (Louveau et al., 2015). Cytokines and their signaling pathways can then have wide-ranging impacts on neurotransmitter metabolism, neuroendocrine function, and neuroplasticity (Haroon et al., 2012), resulting in a constellation of emotional, cognitive and behavioral changes referred to collectively as “sickness behaviors” (Dantzer et al., 2008). These sickness behaviors include loss of appetite, anhedonia, fatigue, depression, and cognitive impairment (Dantzer et al., 2008). In the case of acute infection, sickness behaviors are thought to be an adaptive response evolved to minimize the spread of infection and promote healing, and are generally dismissed as the temporary and relatively benign byproduct of acute illness (Dantzer and Kelley, 2007). However, in the context of prolonged immune activation, ongoing inflammatory signaling to the brain may lead to more disruptive cognitive and behavioral changes, including the development of clinical or subclinical depression, even in individuals without any prior history of mental disorders (Dantzer et al., 2008).
In the context of cancer, the relationship between inflammation and depressive symptoms may be particularly salient, because factors such as tumor cell burden, tissue destruction, radiation treatments, and chemotherapy can activate inflammatory pathways (Miller et al., 2008). Indeed, studies have demonstrated associations between inflammation and depressive symptoms among cancer patients. For instance, several studies have shown that cancer patients with major depression have higher plasma levels of interleukin-6 (IL-6) compared to cancer patients without depression (Jehn et al., 2006; Musselman et al., 2001; Soygur et al., 2007). A study assessing 61 breast cancer patients prior to the initiation of chemotherapy found an association between C-reactive protein (CRP) and depressive symptoms (Pertl et al., 2013). A longitudinal study found that, among colorectal cancer patients, levels of CRP and tumor necrosis factor-alpha (TNF-α) prior to surgery predicted depressive symptoms post-surgery (Archer et al., 2012).
Although there is compelling evidence linking inflammation and depression in the context of cancer, these effects are typically modest and not found in all studies (e.g., Bower et al., 2011). Even in the context of IFN-alpha therapy, major depression only develops in about 30% of initially non-depressed subjects (Lotrich et al., 2013). This suggests that certain individuals may be more vulnerable to depression following an inflammatory stimulus. Emerging evidence suggests that a single nucleotide polymorphism (SNP) in the brain-derived neurotrophic factor (BDNF) gene could be a vulnerability factor for inflammation-induced depression. BDNF is a protein vital for neuroplasticity and neurogenesis, and decreased neurogenesis is one candidate pathway through which inflammation can lead to depressive symptoms (Song and Wang, 2011). A common variant of the BDNF gene, a valine to methionine substitution at codon 66 (Val66Met, rs6265), is associated with reduced activity-dependent secretion of BDNF (Egan et al., 2003), and may increase vulnerability for inflammation-associated depression. Indeed, Lotrich and colleagues examined the relationship between the BDNF Met allele and depressive symptoms in a sample of 209 adults undergoing IFN-α therapy for Hepatitis C (Lotrich et al., 2013). Findings indicated that the Met allele was associated with lower levels of serum BDNF, and that individuals who achieved the lowest BDNF nadirs over the course of IFN-α treatment were the most likely to develop depression (Lotrich et al., 2013). Additionally, Kim et al. (2013; 2015) found that both the BDNF Met allele and higher BDNF gene methylation were associated with suicidal ideation in a sample of 241 breast cancer patients assessed at one year after surgery, though inflammation was not directly measured in these studies.
The present study
Cancer treatments can activate inflammatory processes, and inflammation can drive the development of depression; yet, only a subset of women with breast cancer develop significant and persistent depressive symptoms. The present study examined whether the BDNF Met allele is a vulnerability factor for inflammation-associated depressive symptoms among post-treatment breast cancer survivors. Because proinflammatory cytokines can reduce BDNF (Yirmiya and Goshen, 2011), women who start out with low levels of BDNF (by virtue of carrying a Met allele), may be more vulnerable to further reductions, leading to depressive symptoms. Specifically, we hypothesized that Met carriers would demonstrate a stronger positive relationship between inflammation and depressive symptoms, compared to Val/Val homozygotes.
Additionally, theoretical and empirical work has suggested that depression is not a unitary construct but is rather comprised of different dimensions or clusters of symptoms, each with distinct pathophysiological underpinnings (Capuron and Miller, 2004; Vanheule et al., 2008). These different symptom clusters can manifest independently, operate on distinct time courses, involve disparate neural regions, and be differentially responsive to antidepressant treatment. For example, among patients undergoing IFN-α therapy, a cluster of symptoms including fatigue and abnormal appetite developed within two weeks, whereas another set of symptoms including suicidal thoughts and feelings of guilt did not appear until weeks later, and were more responsive to antidepressant treatment (Capuron et al., 2002). Genetic factors may also influence symptom experience in the context of inflammation; in particular, the BDNF Met allele has been shown to specifically increase reports of suicidal ideation, sadness, and worthlessness among patients undergoing IFN-alpha treatment (Lotrich et al., 2013). Similarly, one study showed that BDNF methylation was specifically associated with suicidal ideation among breast cancer patients, controlling for other depressive symptoms (Kim et al., 2013). Given this evidence, we examined whether the Met allele was a vulnerability factor for specific dimensions of depression.
Methods
Participants
Data were drawn from a larger study on cognitive functioning following cancer therapy, the Mind Body Study (MBS). Women recently diagnosed with breast cancer were primarily identified through the Los Angeles County Surveillance Epidemiology and End Results registry and invited to participate in the study, as previously described (Ganz et al., 2013, 2014, 2016). Eligibility criteria included females aged 21 to 65 years; newly diagnosed with stage 0-IIIA breast cancer; completion of primary breast cancer treatments (surgery, ±radiation, ±chemotherapy) within the past 3 months; had not yet received endocrine therapy (if planned). Exclusion criteria included standard risk factors for preexisting cognitive impairment, including current psychotic-spectrum disorder; prior cancer treatment; autoimmune disease or insulin-dependent diabetes; chronic use of steroid or hormone therapy; and uncontrolled depression (Ganz et al., 2013, 2014, 2016). Exclusions related to age, hormone use, and inflammatory conditions were required due to other MBS study questions regarding the pathophysiology of cognitive dysfunction.
Consenting women were invited to participate in three in-person assessments that were performed at baseline (T1) before the initiation of endocrine therapy if prescribed, 6 months (T2), and 12 months later (T3). All assessments included self-administered questionnaires, neuropsychological testing, and blood draws (Ganz et al., 2013, 2014, 2016). The current study focused primarily on data from the baseline visit, but post hoc analyses also included data from the two follow-up time points. The research was approved by the University of California, Los Angeles Institutional Review Board, and participants provided written informed consent.
Measures
Demographic and clinical information was obtained from self-report and medical records. The Beck Depression Inventory-II (BDI-II) (Beck et al., 1996) assessed the presence and severity of depressive symptoms experienced during the two weeks prior to the study visit, with higher scores indicating more severe symptoms. The BDI-II measures cognitive, affective, and somatic dimensions of depression (Buckley et al., 2001; Vanheule et al., 2008). The cognitive subscale, comprised of nine items from the BDI-II, captures negative thoughts and maladaptive cognitions (e.g., feelings of being a failure, self-dislike, worthlessness, pessimism, guilt). The somatic subscale is comprised of eight items and generally represents the physical symptoms of depression (e.g., loss of energy, changes in sleep, changes in appetite). Finally, the affective subscale, comprised of four items, generally captures anhedonic symptoms (e.g., loss of pleasure, loss of interest). The Pittsburgh Sleep Quality Index (PSQI) (Buysee et al., 1991) was used to assess subjective sleep quality and disturbances over the prior month.
Blood samples for circulating inflammatory markers were collected by venipuncture into EDTA tubes, placed on ice, centrifuged for acquisition of plasma, and stored at -80°C for batch testing. We examined three inflammatory markers that have been investigated in association with cancer-related depression in previous research: (1) IL-6; (2) CRP; and (3) the soluble TNF receptor type II (sTNF-RII) (Archer et al., 2012; Howren et al., 2009; Pertl et al., 2013). IL-6 is a pro-inflammatory cytokine produced by monocytes (among other cell types) that can signal the brain and plays a key role in sickness behavior (Bluthé et al., 2000). CRP is a non-specific acute phase protein produced by cells in the liver in response to stimulation from IL-6, and has been shown to be a sensitive marker of systemic inflammation (Pepys & Hirschfield, 2003). The soluble TNF receptor type II is shed from the cell surface after stimulation by TNF-α and thus can serve as a marker for TNF activity (Diez-Ruiz et al., 1995). Plasma levels of these three inflammatory markers were determined as previously described (Bower et al., 2011; Ganz et al., 2013). Genomic DNA was extracted from peripheral-blood leukocytes and assayed by real-time PCR using a TaqMan SNP genotyping assay (ThermoFisher Scientific) as previously described (Cole et al., 2010).
Statistical analyses
Data analyses were performed using Stata Version 13.1 (StataCorp, College Station, TX, USA). Thirteen participants were missing data for a single item on the BDI-II; we imputed scores for these single items using the mean of the other items from the same subscale.
We used hierarchical multiple linear regression to test interactions between inflammatory markers (at T1) and BDNF genotype in predicting BDI-II total and subscale scores at T1. Blocks of variables were entered in the following sequence: (1) control variables (see below), (2) predictor variables (inflammatory markers and BDNF genotype), (3) the interaction term (e.g., CRP × BDNF genotype). This strategy allowed us to examine the variance specifically attributable to the predictor variables and to the interaction term.
BDNF genotype was treated as a dichotomous variable (0=Val/Val homozygotes; 1 = Met/Met or Val/Met genotypes). CRP was treated as a continuous variable. Control variables included in all analyses were cancer stage, type of cancer treatment received, time since last cancer treatment, age, BMI, and sleep quality (PSQI total score), as these variables can influence inflammation and/or depressive symptoms (Ganz et al., 2013; Irwin et al., 2013; Howren et al., 2009). Cancer stage was treated as a categorical variable with four levels (stages 0, I, II, and III). Type of cancer treatment received was also treated as a categorical variable with four levels (neither radiation nor chemotherapy, radiation only, chemotherapy only, and both chemotherapy and radiation therapy). Time since treatment was calculated as months between the last cancer treatment received (surgery, radiation, and/or chemotherapy) and the baseline visit, and was treated as a continuous variable. Age, BMI, and sleep quality were also treated as continuous variables. Ethnicity and education were not included as covariates given the largely homogeneous demographics of the present sample.
In post hoc analyses, we examined whether the relationship between CRP, BDNF genotype and cognitive depressive symptoms observed in cross-sectional analyses at baseline held across the 1-year assessment period (baseline, 6-month, and 12-month assessments). These analyses were conducted using multilevel growth curve models, specifically random intercept and slope models, fit to the repeated measures data. Time (continuous) was modeled as months since last cancer treatment. BDNF genotype, CRP at T1 and their interaction were modeled as Level 2 (time invariant) predictors affecting the average level of depressive symptoms across the 1-year study period. We included the same covariates as in our regression analyses, with cancer stage, treatment type, and age (at T1) treated as Level 2 (time invariant) covariates, and BMI and sleep quality treated as Level 1 (time-varying) covariates. Because some women received endocrine therapy at T2 and/or T3, we also included endocrine treatment as a time-varying covariate, coded as dichotomous at each assessment (0 = not receiving, 1 = currently receiving). Preliminary analyses revealed that including a random slope did not improve model fit, so our final model included a random intercept and fixed slope. We additionally tested a three-way interaction between CRP, BDNF genotype, and time, to examine whether the interaction between CRP and BDNF differed over time, but this interaction was non-significant (p=.28). Thus, our final model tested whether the interaction between CRP at T1 and BDNF genotype significantly predicted average cognitive depressive symptoms across the three assessments.
Results
Descriptive statistics
Table 1 presents descriptive statistics for sample characteristics and study variables by BDNF genotype at baseline. BDNF genotyping results revealed that 75 participants (67%) were Val/Val genotype; 32 participants (28.6%) were Val/Met genotype, and 5 participants (4.4%) were Met/Met genotype, which did not significantly deviate from Hardy-Weinberg equilibrium (X2 = 0.33, df = 1, p = 0.56). As is typically done in BDNF genotype studies in predominantly Caucasian samples (Herbert et al., 2012), we grouped Val/Met and Met/Met carriers together. Thus, in subsequent analyses, BDNF genotype was a dichotomous variable consisting of two groups: 37 Met allele carriers (33% of the sample) and 75 Val homozygotes. Chi-square, independent group t-tests, and Fisher's exact tests revealed that the two genotype groups did not significantly differ by any demographic or medical characteristics (ps > .05).
Table 1. Descriptive statistics for sample characteristics and study variables by genotype at baseline.
| Val/Val (n=75) | Val/Met or Met/Met (n=37) | ||||
|---|---|---|---|---|---|
|
| |||||
| Characteristic | M ± SD or N (%) | Range | M ± SD or N (%) | Range | P |
| Age | 51.4 ± 8.9 | 31 - 66 | 51.2 ± 7.0 | 34 - 64 | .93a |
| BMI | 25.1 ± 4.8 | 19 - 41 | 24.8 ± 4.6 | 18 - 41 | .73a |
| Education | .69b | ||||
| < College degree | 13 (17.3) | 8 (21.6) | |||
| College degree | 26 (34.7) | 10 (27.0) | |||
| > College degree | 36 (48.0) | 19 (51.4) | |||
| Caucasian ethnicity | 63 (84.0) | 31 (83.8) | .48c | ||
| Sleep quality (PSQI) | 7.17 ± 3.5 | 0 - 16 | 7.8 ± 3.6 | 1 - 16 | .39a |
| Stage at diagnosis | .20c | ||||
| 0 | 8 (10.7) | 9 (24.3) | |||
| 1 | 38 (50.1) | 13 (35.1) | |||
| 2 | 25 (33.3) | 12 (32.4) | |||
| 3 | 4 (5.3) | 3 (8.1) | |||
| Surgery type | .85b | ||||
| Lumpectomy | 52 (69.3) | 25 (67.6) | |||
| Mastectomy | 23 (30.7) | 12 (32.4) | |||
| Cancer treatment | .18c | ||||
| Neither radiation nor chemo | 8 (10.7) | 9 (24.3) | |||
| Radiation only | 29 (38.7) | 11 (29.7) | |||
| Chemo only | 10 (13.3) | 2 (5.4) | |||
| Chemo & radiation | 29 (37.3) | 15 (40.5) | |||
| Time since treatment (mos.) | 1.03 ± .94 | .03 - 4 | 1.4 ± 1.2 | .07 – 3.9 | .09a |
| BDI-II total | 8.93 ± 7.4 | 0 - 35 | 9.22 ± 7.5 | 0 - 32 | .85a |
| BDI-II cognitive | 1.99 ± 2.8 | 0 - 17 | 2.05 ± 3.7 | 0 - 19 | .92a |
| BDI-II affective | 1.48 ± 1.7 | 0 - 7 | 1.70 ± 1.8 | 0 - 7 | .53a |
| BDI-II somatic | 5.46 ± 3.9 | 0 -15 | 5.46 ± 3.7 | 0 - 12 | .99a |
| Inflammatory markers | |||||
| IL-6 (pg/mL) | 1.62 ± .95 | .47 – 5.6 | 1.57 ± 1.06 | .44 – 6.16 | .80a |
| CRP (mg/L) | 2.36 ± 3.1 | .1 – 16.8 | 1.78 ± 2.1 | .1 – 7.8 | .30a |
| sTNF-RII (pg/mL) | 2305.9 ± 614.6 | 1305.7 - 3858 | 2298.2 ± 680.8 | 1354.3 – 3546 | .95a |
P-values for independent group t-tests comparing genotype group on continuous variables.
P-values from Chi-squared tests comparing genotype groups on categorical variables.
P-values from Fisher's exact tests comparing genotype groups on categorical variables with cell means <5.
The average score for the total sample on the BDI-II indicated minimal depressive symptoms; however, there was substantial variability and 10.5% of the sample had scores greater than the clinical cutoff (>19) on the BDI-II, indicating that a subset of women were experiencing moderate or severe depressive symptoms at the time of assessment. Levels of CRP, IL-6, and sTNF-RII generally fell in the normal range, but again there was considerable variability in each of these markers. For example, the mean value of CRP (across both genotype groups) was 2.1 mg/L (SD=2.8), but scores ranged widely from 0.1 – 16.8, and 19% of the sample had a CRP level above the clinical cutoff (>3 mg/L), indicating that a sizeable subgroup of women had clinically elevated CRP concentrations in this post-treatment phase. Depressive symptoms and inflammatory markers did not significantly differ between the two genotype groups (ps > .3).
Table 2 presents correlations among study variables at baseline. The BDI-II subscales were significantly correlated with each other (rs = .54 - .66, ps < .001). IL-6 was significantly correlated with CRP (r = .32, p < .001), but neither IL-6 nor CRP were significantly correlated with sTNF-RII (rs = .06 - .12, ns). Age, BMI, time since treatment, cancer stage, treatment type, and PSQI scores were significantly correlated with one or more subscale of the BDI-II and/or inflammatory markers, confirming the need to include these variables as covariates in subsequent analyses.
Table 2. Correlations among study variables at baseline.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. BDI-II total | — | ||||||||||||
| 2. BDI-II cognitive | .83c | — | |||||||||||
| 3. BDI-II affective | .81c | .57c | — | ||||||||||
| 4. BDI-II somatic | .90c | .54c | .66c | — | |||||||||
| 5. IL-6 (pg/mL) | -.005 | .06 | -.02 | -.05 | — | ||||||||
| 6. CRP (mg/L) | .02 | .05 | -.03 | .02 | .32c | — | |||||||
| 7. sTNF-RII (pg/mL) | .06 | .08 | -.01 | .05 | .12 | .06 | — | ||||||
| 8. BDNF genotype | .02 | .01 | .06 | -.003 | -.02 | -.10 | -.01 | — | |||||
| 9. Age | -.18 | -.18 | -.13 | -.15 | .12 | -.06 | .25b | -.01 | — | ||||
| 10. BMI | .16 | .22a | .13 | .07 | .29b | .36c | .21a | -.03 | .22a | — | |||
| 11. Time since treatment | -.15 | -.09 | -.06 | -.19 | -.16 | -.11 | -.22a | .16 | -.04 | -.08 | — | ||
| 12. Cancer stage | .22a | .12 | .10 | .25b | -.01 | .07 | .28b | -.05 | -.03 | .08 | -.17 | — | |
| 13. Treatment type | .32c | .16 | .20a | .34c | .07 | .10 | .29b | -.06 | -.10 | .008 | -.3c | .62c | — |
| 13. PSQI | .50 c | .34 c | .36 c | .54c | .03 | .04 | .04 | .08 | .08 | -.01 | -.15 | .12 | .21a |
p<.05.
p<.01.
p<.001.
Note. BDNF genotype was dummy coded 0 = Val/Val, 1 = Val/Met or Met/Met. Cancer stage was dummy coded 0 = stage 0, 1 = stage I, 2 = stage II, 3 = stage III. Treatment type was dummy coded 0 = neither radiation nor chemotherapy, 1 = radiation only, 2 = chemotherapy only, 3 = chemotherapy and radiation. Coefficients measuring associations between two continuous variables are Pearson product-moment correlations. Coefficients involving BDNF genotype are point biserial correlations. Coefficients measuring associations between cancer stage and treatment type, or between cancer stage/treatment type and continuous variables, are Spearman's rank-order correlations.
Hierarchical multiple regression analyses
A three-step approach was used to evaluate the unique contribution of each set of predictors: covariates were entered in step 1, main effects of inflammation and BDNF genotype in step 2, and finally the interaction term (inflammatory marker × BDNF genotype) in step 3. Separate models were used for each of the three inflammatory markers. Additionally, separate models were used to predict the BDI-II total score and each of the three BDI-II subscales (cognitive, affective, and somatic symptoms). Models including IL-6 as the inflammatory marker revealed no significant main effects of IL-6 or BDNF genotype on depressive symptoms (BDI-II total or subscale scores) (ps > .3), and no significant interaction between IL-6 and BDNF genotype (ps >. 3). Similarly, models examining sTNF-RII as the inflammatory marker revealed no significant main effects sTNF-RII or BDNF genotype on depressive symptoms (ps >.3), and no significant interaction between sTNF-RII and BDNF genotype (ps > .2). A model examining CRP revealed no significant main effects of CRP or BDNF genotype on depressive symptoms (ps >.19), and no significant interaction between BDNF genotype and CRP in predicting BDI-II total scores, or the affective or somatic subscales (ps > .17). However, there was a significant interaction between BDNF genotype and CRP in predicting scores on the BDI-II cognitive subscale (Table 3). At step 1, cancer stage and treatment type were unrelated to cognitive depressive symptoms (ps > .11). Older age was significantly associated with fewer cognitive depressive symptoms (p = .001). Higher BMI was associated with more cognitive depressive symptoms (p = .003), as was greater sleep disturbance (p < .001). Together these variables accounted for 28% of the variance in cognitive depression scores (F (10, 101)= 3.9, p = .0002). At step 2, neither BDNF genotype nor CRP were significantly related to depressive symptoms (ps > .3), and the inclusion of these variables did not significantly improve the predictive ability of the model (ΔR2 = .006, F (2, 99)= 0.39, p = .68). At step 3, the interaction between BDNF genotype and CRP was significantly related to cognitive depressive symptoms (b = .72, t(98) = 2.94, p = .004), and the interaction accounted for an additional 6% of the variance (F (1, 98) = 8.65, p = .004). The interaction term remained statistically significant after Bonferroni correction for 12 comparisons (p = .048).
Table 3. Hierarchical multiple regression analysis predicting cognitive depressive symptoms at baseline.
| Variable | β | T | R2 | df | F | ΔR2 |
|---|---|---|---|---|---|---|
| Step 1 | .28 | 10, 101 | 3.9*** | -- | ||
| Cancer stagea | ||||||
| Stage 1 | .45 | .52 | ||||
| Stage II | 1.62 | 1.53 | ||||
| Stage III | -.36 | -.25 | ||||
| Cancer treatmentb | ||||||
| Radiation only | -1.29 | -1.26 | ||||
| Chemotherapy only | -1.86 | -1.37 | ||||
| Chemo & radiation | -1.83 | -1.60 | ||||
| Time since treatment | -.4 | -1.18 | ||||
| Age | -.12 | -3.44** | ||||
| BMI | .18 | 3.10** | ||||
| PSQI | .33 | 4.03*** | ||||
| Step 2 | .28 | 2, 99 | 0.39 | .006 | ||
| BDNF genotype | -.11 | -.19 | ||||
| CRP | -.09 | -.88 | ||||
| Step 3 | .34 | 1, 98 | 8.65** | .06 | ||
| CRP*BDNF genotype | .72 | 2.94** |
Note. BMI = Body mass index; PSQI = Pittsburgh Sleep Quality Index; BDNF = Brain derived neurotrophic factor; CRP = C-reactive protein.
p <.05.
p <.01.
p <.001.
The reference group is Stage 0.
The reference group is the group that received neither radiation nor chemotherapy.
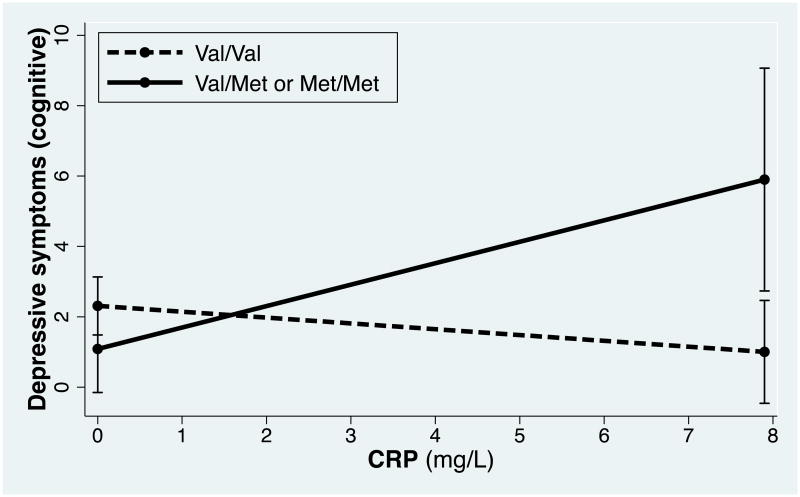
Tests of simple slopes showed that, among Met allele carriers, CRP was significantly positively associated with the BDI-II cognitive subscale (b = .52, t(98) = 2.23, p = .028), such that higher CRP was related to more cognitive depressive symptoms. Among Val homozygotes, the relationship between CRP and the BDI-II cognitive subscale was not significant, and trended in the opposite direction (b = -.21, t(98) = -1.87, p = .07). These results are presented graphically in Figure 1. Because very high levels of CRP could reflect an acute infection or illness rather than chronically elevated levels, we re-ran analyses excluding participants with CRP levels equal to or greater than 10 mg/L (n = 3). Results were not significantly changed after the exclusion of these participants.
Fig. 1.
The cross-sectional relationship between CRP and cognitive depressive symptoms at baseline as a function of BDNF genotype. At baseline, CRP was significantly, positively related to cognitive depressive symptoms for Met allele carriers (b = .52, t(98) = 2.23, p = .028), but not for Val/Val homozygotes (b = -.21, t(98) = -1.87, p = .07).
In post hoc analyses, we examined whether the cross-sectional relationship observed at baseline held across subsequent assessments (the 6- and 12-month follow-ups) (see Supplementary Table 1 for descriptive statistics of the BDI-II scales at the follow-up assessments). Specifically, we aimed to test whether, among BDNF Met allele carriers, higher CRP levels following the completion of initial cancer treatment (at baseline) would predict a higher average level of cognitive depressive symptoms across the three assessment points. Results revealed that this interaction was significant (Supplementary Table 2; b = .71, z = 3.57, p < .001). Tests of the simple slopes revealed that, among Met allele carriers, CRP at T1 was significantly associated with cognitive depressive symptoms across the 1-year assessment period (b = .53, z = 2.87, p = .004). The same pattern of results was observed in sensitivity analyses in which CRP was log-transformed or entered as a time-varying predictor.
Discussion
In a sample of 112 breast cancer survivors, CRP was positively associated with cognitive depressive symptoms among women carrying a BDNF Met allele, but not among Val/Val homozygotes. The positive association between CRP levels and cognitive depressive symptoms among Met carriers held at treatment completion and across the 1-year follow-up. These findings suggest that women with both the Met allele and elevated CRP at baseline had, on average, higher cognitive depressive symptoms over time relative to those with the Val/Val genotype and comparable baseline levels of CRP. These findings provide preliminary support for the BDNF Met allele as a risk factor for inflammation-associated cognitive depression among breast cancer survivors.
Prior work identified the BDNF Met allele as a vulnerability factor for inflammation-associated depressive symptoms among Hepatitis C patients undergoing IFN-α treatment (Lotrich et al., 2013). Our study extends these findings to a sample of early-stage, post-treatment breast cancer survivors. Additionally, one prior study had shown an association between the BDNF Met allele and suicidal ideation among breast cancer survivors (Kim et al., 2013); our study builds on this work by examining the interaction between the BDNF Met allele and inflammation in predicting depressive symptoms. Inflammatory cytokines reduce the production of BDNF (Yirmiya and Goshen, 2011). We speculate that, for Met allele carriers, who start out with lower levels of activity-dependent BDNF, the result of inflammatory activity may be critically low BDNF levels, leading to depressive symptoms.
On average, levels of depressive symptoms were modest in the current sample, although a subset of women demonstrated clinically significant depressive symptoms. These findings were consistent with our expectations and with prior work showing that while the majority of women have typically recovered to baseline psychological functioning shortly after the completion of cancer treatment, a subgroup of women experience persistent psychological distress well into the survivorship period (Donovan et al., 2014; Henselmans et al., 2010). The current study attempted to better characterize this vulnerable subgroup, and findings suggest that the combination of BDNF genotype and systemic inflammation at treatment completion may have a contributing role.
In the present study, among Met allele carriers, inflammation was specifically associated with the cognitive symptoms of depression. Consistent with this, Gimeno et al. (2009), examining data from a prospective study of British civil servants, found an association between CRP at baseline and cognitive depressive symptoms 12 years later. Additionally, the Met allele has been associated with higher cognitive depressive symptoms on the BDI-II among individuals receiving IFN-α treatment (Lotrich et al., 2013). Why would CRP and BDNF genotype interact to uniquely predict cognitive depressive symptoms, as opposed to other dimensions of depression? Cytokines access the brain and can influence virtually every system (and, therefore, symptom) relevant to depression (Haroon et al., 2012). However, the effect of inflammation on the BDNF pathway may be particularly relevant to the cognitive dimension of depression. Indeed, studies have shown that the BDNF polymorphism is associated with greater neural activation to negative stimuli (Molendijk et al., 2012) and rumination (Beevers et al., 2010). Such processes (negative bias, rumination) are thought to give rise to the distorted thoughts and cognitions that characterize the cognitive dimension of depression (Disner et al., 2011), and may not be as relevant for the other symptom clusters.
CRP was the only inflammatory marker of the three examined that was significantly associated with depressive symptoms among Met allele carriers. In contrast to an instantaneous marker of inflammatory activity such as IL-6, CRP is a downstream inflammatory marker that may provide a more stable and time-integrated signal of chronic inflammation (Bower and Lamkin, 2013; Pepys and Hirschfield, 2003), and thus may be more relevant in the maintenance of depressive symptoms in the post-treatment phase. Although sTNF-RII is also a downstream marker, in our studies of breast cancer patients, sTNF-RII has been more closely linked to fatigue (Bower et al., 2011).
Limitations
One limitation of the present study is that we did not collect data prior to cancer diagnosis and treatment; thus, we can only conjecture that elevations in CRP and/or depressive symptoms were triggered by cancer diagnosis and treatment. It is possible that the observed elevations may have preceded cancer diagnosis and/or treatment. While prospective studies assessing women prior to cancer diagnosis are challenging to carry out, our lab is currently conducting work assessing women prior to the start of cancer treatment (immediately following breast cancer diagnosis and surgery), which will allow us to more carefully examine mood and inflammatory responses to cancer treatment.
The present study focused on BDNF genotype, but did not assess BDNF gene expression and/or circulating BDNF protein levels. There are several intervening biological steps between an individual's genotype and its functional outcome, so an individual's BDNF genotype does not necessarily determine BDNF protein levels. Although prior work has shown an association between the Val66Met polymorphism and circulating levels of BDNF (e.g., Lotrich et al., 2013; Minelli et al., 2011), suggesting that BDNF genotype may serve as a reasonable proxy for protein levels at least in some samples, other studies have not found this association (e.g., Terracciano et al., 2010). Thus, future work would benefit from more direct measurements of BDNF.
Although we considered a number of potential confounders, we did not have information on physical activity, which has been shown to influence both inflammation and depressive symptoms (Schuch et al., 2016) and thus would have been a reasonable control variable to include in analyses. Of note, our results did hold controlling for body mass index. Finally, replication of this finding is needed in order to identify whether our findings would generalize to more diverse samples of cancer survivors (our sample was relatively homogeneous with regard to race and education), or to non-cancer populations.
Conclusions
The present study identifies a potential vulnerability factor for inflammation-associated cognitive depressive symptoms among breast cancer survivors. Inflammation persisting into the post-treatment survivorship period, in combination with a BDNF Met allele, may amount to a “double hit” that leaves some women particularly vulnerable to depression. The present study may thus help to identify a subset of breast cancer survivors who may benefit from early identification and treatment.
Supplementary Material
Highlights.
Does BDNF SNP moderate inflammation-depression link in breast cancer survivors?
CRP was associated with cognitive depressive symptoms, among Met allele carriers only
BDNF SNP a risk factor for inflammation-based depression in breast cancer survivors
Acknowledgments
This research was supported by funding from the National Cancer Institute (R01 CA 109650) (PAG), the Breast Cancer Research Foundation (PAG), the OAIC Inflammatory Biology Core (NIH/NIA P30- AG028748), the USC-UCLA Biodemography Center's Social Genomics Core (NIH/NIA P30-AG017265) and the California Center for Population Research at UCLA (CCPR) with training support from the National Institute on Aging (T32-AG033533) and from the National Institute of General Medical Sciences (T32 GM 08903) (LND). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Ganz would like to disclose that she is a member of the Scientific Advisory Board of the Breast Cancer Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archer JA, Hutchison IL, Dorudi S, Stansfeld SA, Korszun A. Interrelationship of depression, stress and inflammation in cancer patients: A preliminary study. J Affect Disord. 2012;143:39–46. doi: 10.1016/j.jad.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory-II. San Antonio, TX: Psychol. Corp; 1996. pp. 1–82. [Google Scholar]
- Beevers CG, Wells TT, Mcgeary JE. The BDNF Val66Met polymorphism is associated with rumination in healthy adults. Emotion. 2010;9:579–584. doi: 10.1037/a0016189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthé RM, Michaud B, Poli V, Dantzer R. Role of IL-6 in cytokine-induced sickness behavior: A study with IL-6 deficient mice. Physiol Behav. 2000;70:367–73. doi: 10.1016/S0031-9384(00)00269-9. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–22. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30:S48–S57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TC, Parker JD, Heggie J. A psychometric evaluation of the BDI-II in treatment-seeking substance abusers. J Subst Abuse Treat. 2001;20:197–204. doi: 10.1016/S0740-5472(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yaeger AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–52. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: Lessons from interferon-α. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Cole SW, Arevalo JMG, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci U S A. 2010;107:5681–6. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Connor JCO, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Ruiz A, Tilz GP, Zangerle R, Baier-Bitterlich G, Wachter H, Fuchs D. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur J Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EaP, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Donovan KA, Gonzalez BD, Small BJ, Andrykowski MA, Jacobsen PB. Depressive symptom trajectories during and after adjuvant treatment for breast cancer. 2014;47:292–302. doi: 10.1007/s12160-013-9550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24:558–63. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DHS, Geist C, Breen EC, Irwin MR, Cole SW. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30(Suppl 1):S99–108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Petersen L, Castellon Sa, Bower JE, Silverman DHS, Cole SW, Irwin MR, Belin TR. Cognitive Function After the Initiation of Adjuvant Endocrine Therapy in Early-Stage Breast Cancer: An Observational Cohort Study. J Clin Oncol. 2014;32:3559–3567. doi: 10.1200/JCO.2014.56.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Petersen L, Bower JE, Crespi CM. Impact of adjuvant endocrine therapy on quality of life and symptoms: Observational data over 12 months from the Mind-Body Study. Advance online publication. 2016 doi: 10.1200/JCO.2015.64.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Kivimäki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe a, Kumari M, Lowe GDO, Rumley a, Marmot MG, Ferrie JE. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39:413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henselmans I, Helgeson VS, Seltman H, de Vries J, Sanderman R, Ranchor AV. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychol. 2010;29:160–8. doi: 10.1037/a0017806. [DOI] [PubMed] [Google Scholar]
- Herbert J, Ban M, Brown GW, Harris TO, Ogilvie A, Uher R, Craig TKJ. Interaction between the BDNF gene Val/66/Met polymorphism and morning cortisol levels as a predictor of depression in adult women. Br J Psychiatry. 2012;201:313–9. doi: 10.1192/bjp.bp.111.107037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Omstead RE, Ganz PA, Haque R. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behav Immun. 2013;15:S58–S67. doi: 10.1016/j.bbi.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehn CF, Kuehnhardt D, Bartholomae A, Pfeiffer S, Krebs M, Regierer AC, Schmid P, Possinger K, Flath BC. Biomarkers of depression in cancer patients. Cancer. 2006;107:2723–2729. doi: 10.1002/cncr.22294. [DOI] [PubMed] [Google Scholar]
- Kim J, Jang J, Stewart R, Kim S, Kim S, Kang H, Shin I, Park M, Yoon J, Yoon J. Determinants of suicidal ideation in patients with breast cancer. Psychooncology. 2013;22:2848–2856. doi: 10.1002/pon.3367. [DOI] [PubMed] [Google Scholar]
- Kim J, Kang H, Kim S, Kim S, Shin I, Kim H. BDNF promotor methylation associated with suicidal ideation in patients with breast cancer. Int J Psychiatry Med. 2015;49:75–94. doi: 10.1177/0091217415574439. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Albusaysi S, Ferrell RE. Brain-Derived Neurotrophic Factor Serum Levels and Genotype: Association with Depression during Interferon-alpha Treatment. Neuropsychopharmacology. 2013;38:985–995. doi: 10.1038/npp.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–354. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud RAK, El-Gendi HI, Ahmed HH. Serum neopterin, tumor necrosis factor-a and soluble tumor necrosis factor receptor II (p75) levels and disease activity in Egyptian female patients with systemic lupus erythematosus. Clin Biochem. 2005;38:134–141. doi: 10.1016/j.clinbiochem.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Zanardini R, Bonvicini C, Sartori R, Pedrini L, Gennarelli M, Bocchio-Chiavetto L. BDNF serum levels, but not BDNF Val66Met genotype, are correlated with personality traits in healthy subjects. Eur Arch Psychiatry Clin Neurosci. 2011;261:323–329. doi: 10.1007/s00406-011-0189-3. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, van Tol MJ, Penninx BWJH, van der Wee NJA, Aleman A, Veltman DJ, Spinhoven P, Elzinga BM. BDNF val66met affects hippocampal volume and emotion-related hippocampal memory activity. Transl Psychiatry. 2012;2:e74. doi: 10.1038/tp.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI200318921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertl MM, Hevey D, Boyle NT, Hughes MM, Collier S, O'Dwyer AM, Harkin A, Kennedy MJ, Connor TJ. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav Immun. 2013;34:108–119. doi: 10.1016/j.bbi.2013.07.177. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Duberstein PR. Depression and cancer mortality: A meta-analysis. Psychol Med. 2011;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-α. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg a, Yirmiya R, Schuld a, Kraus T, Haack M, Morag a, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–52. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Schuch FB, Deslandes CA, Stubbs B, Gosmann PN, da Silva CTB, Fleck MPdA. Neurobiological effects of exercise on major depressive disorder: A systematic review. Neurosci Biobehav Rev. 2016;61:1–11. doi: 10.1016/j.neubiorev.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2011;35:760–768. doi: 10.1016/j.pnpbp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Soygur H, Palaoglu O, Akarsu ES, Cankurtaran ES, Ozalp E, Turhan L, Ayhan IH. Interleukin-6 levels and HPA axis activation in breast cancer patients with major depressive disorder. Prog Neuro-Psychopharmacology Biol Psychiatry. 2007;31:1242–1247. doi: 10.1016/j.pnpbp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Terracciano A, Bronwen M, Ansari D, Tanaka T, Ferrucci L, Maudsley S, Mattson MP, Costa PT., Jr Plasma BDNF concentration, Val66Met genetic variant, and depression-related personality traits. Genes Brain Behav. 2010;9:512–518. doi: 10.1111/j.1601-183X.2010.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheule S, Desmet M, Groenvynck H, Rosseel Y, Fontaine J. The factor structure of the Beck Depression Inventory-II: an evaluation. Assessment. 2008;15:177–187. doi: 10.1177/1073191107311261. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–50. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.