Abstract
Objective
To determine the relationship between increased triggering receptor expressed on myeloid cells (TREM)-1 and plaque stability in atherosclerotic carotid stenosis.
Methods
The mRNA transcripts and protein for TREM-1, MMP-1, MMP-9, collagen type I (COL1A1) and collagen type III (COL3A1) were analyzed by qPCR and immunofluorescence in both tissues and VSMCs isolated from atherosclerotic carotid plaques of symptomatic and asymptomatic patients with carotid stenosis.
Results
The TREM-1, MMP-1 and MMP-9 mRNA transcripts were significantly increased (TREM-1, p<0.01; MMP-1, p<0.01 and MMP-9, p<0.001) while COL1A1 and COL3A1 mRNA transcripts were decreased (p<0.001) in VSMCs isolated from carotid plaques of symptomatic (S) than asymptomatic (AS) patients. Stimulation of cells with TNF-α further increased the mRNA transcripts of TREM-1, MMPs, COL1A1 and COL3A1. Modulation of TREM-1 by treatment with TREM-1 decoy receptor rTREM-1/Fc, and either TREM-1 antibodies or TREM-1 siRNA attenuated the TNF-α induced expression of MMP-1 and MMP-9 (p<0.01) and COL1A1 and COL3A1 (p<0.01) in S compared to AS VSMCs isolated from carotid plaques. Inhibition of NF-kB (BAY 11-7085), JNK (SP600125) and PI3K (LY294002) signaling pathways decreased the expression of TREM-1 (p<0.01), MMP-1 (p<0.001) and MMP-9 (p<0.01) in TNF-α treated VSMCs isolated from S carotid plaques compared to AS patients.
Conclusion
Increased expression of TREM-1 in S compared to AS patients involving MMP-1 and MMP-9 suggest a potential role of TREM-1 in plaque destabilization. Selective blockade of TREM-1 may contribute to the development of new therapies and promising targets for stabilizing vulnerable atherosclerotic plaques.
Keywords: Atherosclerosis, Extracellular matrix, Inflammation, Matrix metalloproteinases, Transcription factors, Triggering receptor expressed on myeloid cells-1, Tumor necrosis factor-α
1. Introduction
Atherosclerosis is an inflammatory disease and involves multiple processes such as endothelial dysfunction, and matrix alteration. The clinical presentation of atherosclerosis is very broad and inflammation is involved in all stages [1–3]. The stable plaques are rich in VSMC and collagen with few inflammatory cells whereas unstable plaques that are prone to rupture contain few VSMCs, more macrophages and little collagen [4–6]. The stability of atherosclerotic plaques depends on the balance between the matrix formation and degradation of extracellular matrix (ECM) [7, 8]. The activated macrophages and T-lymphocytes present in atherosclerotic plaques secrete matrix metalloproteinases (MMPs) that degrade extracellular matrix proteins, and weaken the fibrous cap, leading to myocardial infarction and stroke. The cells involved in the atherosclerotic plaques secrete inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α)[9], interferon-gamma (IFN-γ) and interleukin-12 (IL-12)[10].
Triggering receptor expressed on myeloid cells (TREM) -1 is an activating receptor that is selectively expressed on neutrophils and monocytes/macrophages and plays an important role in acute and chronic inflammation [11–13] TREM-1 expression was significantly increased in the non-infectious inflammatory diseases such as acute pancreatitis, inflammatory bowel diseases, gout and rheumatoid arthritis and is a diagnostic marker for sepsis with pneumonia [14–18]. Expression of TREM-1 on monocytes release large amounts of pro-inflammatory cytokines including TNF-α and IL-8 and amplify inflammatory responses [19–21]. Blocking of TREM-1 has been shown to attenuate intestinal inflammation, suppressed collagen induced arthritis [19] and reduced TNF-α and IL-1β levels preventing shock and death [18]. TREM-1 expression has been reported in several myeloid and non-myeloid cells and in inflamed human lung, skin and intestinal tissues [22].
At present, it is unclear whether TREM-1 is up-regulated in atherosclerotic plaques, and if it is, whether TREM-1 regulate the stability of collagen in unstable plaques. The main objective of this investigation was to investigate the expression of TREM-1and its association with MMP-1 and MMP-9 to plaque rupture using different approaches in human carotid plaques as well as in isolated VSMCs and examine the effect of inflammatory cytokine TNF-α in symptomatic (S) compared to asymptomatic (AS) patients with carotid stenosis. We have shown that inhibitors of p38, PI3K and JNK MAPK signaling pathways and NF-kB are required for the up-regulation of TREM-1, MMP-1 and MMP-9 expression induced by TNFα, leading to plaque vulnerability in VSMCs isolated from S carotid plaques.
2. Materials and methods
2.1. Study subjects
This research was carried by the approval of the Institutional Review Board of Creighton University and the research protocol was exempted since the carotid endarterectomy specimens were truly anonymized. Patients in both AS and S groups were aged between 50–75 years and included both male and females of any ethnic origin met the guidelines of American Heart Association. The plaques were clinically classified as symptomatic by the surgeon and the symptoms included hemispheric transient ischemic attacks, amaurosis fugax, or stroke [4, 23]. The specimens were collected in the University of Wisconsin solution and transported to the laboratory.
2.2. Isolation of VSMCs
VSMCs were isolated by an established method previously reported in this laboratory [4, 8]. Briefly, the medial layer was digested with trypsin, followed by collagenase type 1A from Clostridium histolyticum, (Sigma C2674, St. Louis, MO, USA) and suspended in smooth muscle cell medium (ScienCell, Carlsbad, CA, USA). The cells from second to fifth passage were used. The VSMCs were confirmed by positive staining for smooth muscle α-actin and caldesmon, as reported previously [24].
2.3. Cell culture and treatment protocol
VSMCs at pre-confluence were incubated in serum-free medium containing TNFα at 10 ng/ml for 24 h.
2.4. Immunofluorescence microscopy
Paraffin sections (5μm) from both S and AS carotid plaques were subjected to immunofluorescence microscopy, as described previously [8] using goat polyclonal antibodies for TREM-1, rabbit antibodies to COL1A1, COL3A1, MMP-1, MMP-9 (Santa Cruz Biotechnology) at 1:50 dilution and mouse monoclonal antibodies to CD68 (eBioscience) at 1:200 dilution. Antibodies to phospho-p38MAPK, phosphor-PI3K, phospho-JNK and NF-kB were obtained from Cell Signaling Technology (Beverly, MA) and used at 1:250 dilutions. The sections were incubated with primary antibodies at room temperature for 2hous, followed by incubation with the matching secondary antibodies conjugated to either Alexa 594 (red) or Alexa 488 (green) (Invitrogen, Grand Island, NY, USA) for 30 min at 1:1000 dilution at room temperature. After washing with PBS, the slides were stained with DAPI, (4, 6-diamidino-2-phenylindole) and the immunofluorescence was observed in an Olympus inverted fluorescent microscope (Olympus BX51). The average fluorescence intensity was quantified in five samples using Image–pro software. Negative controls were stained with isotype IgG
2.5. RNA isolation and real-time qPCR
TRI reagent (Sigma, St Louis, MO, USA) was used to isolate RNA from tissues and cultured VSMCs according to the manufacturer’s instructions and RNA was quantified using Nanodrop (Thermo Scientific, Rockford, IL, USA). The cDNA was prepared from RNA using Improm II reverse transcription kit (Promega, Madison, WI, USA) following the manufacturer’s instructions. QPCR/Real q time PCR was performed using Syber Green Master Mix in a Real-time PCR system model CFX96 (BioRad Laboratories, Hercules, CA, USA). The PCR cycling conditions included 5 min at 95° for initial denaturation, and cycling step (denaturation at 95° C for 30 sec, annealing at different temperatures (55–62° C) for each set of primers and 30 sec and extension at 72° C for 35 cycles) followed by melt curve analysis. Fold expression of mRNA transcripts relative to controls was determined after normalizing to GAPDH. The following primers were used: TREM-1: 5′-AGT TAC AGC CCA AAA CAT GC-3′, sense and 5′-CAG CCC CCA CAA GAG AAT TA-3′; MMP-1: 5′-TGC AAC TCT GAC GTT GAT CCC AGA-3′, sense and 5′-ACT GCACAT GTG TTC TTG AGC TGC-3′; MMP-9: 5′-ATT TCT GCC AGG ACC GCT TCT ACT-3, sense and 5′-CAG TTT GTA TCC GGC AAA CTG GCT-3′, antisense; COL1A1: 5′-CAG TTT GTA TCC GGC AAA CTG GCT-3′, sense and 5′-CAC TTG GGT GTT TGA GCA TGG CCT-3′, antisense; COL3A1: 5′-TATCGA ACA CGC AAG GCT GTG AGA-3′, sense and 5′-GGC CAA CGT CCA CAC CAA ATT CTT-3′, antisense; GAPDH: 5′-GGT GAA GGT CGG AGT CAA CGG ATT TGG TCG-3′, sense and 5′-GGA TCT CGC TCC TGG AAG ATG GTG ATG GG-3′, antisense.
2.6. Transient transfection
VSMCs were grown to 60–80% confluence without antibiotics. For transient transfection, the cells were transfected with either siRNA targeting TREM-1 or with a scrambled control siRNA using with Lipofectamine 2000 (Life Technologies, Inc., Carlsbad, CA) according to the manufacturer’s protocol. After 24 hours of transfection, cells were stimulated with or without TNFα (10 ng/ml) for 24 hours and mRNA expression of TREM-1, MMP-1, MMP-9, COL1A1 and COL3A1 were quantitated by qRT-PCR.
2.7. TREM-1 blocking
To investigate the role of TREM-1, VSMCs were treated for 24 h with 12 μg/ml of anti-TREM-1 antibodies as well as isotype matched controls in the presence of TNF-α (10 ng/ml). The VSMCs were also treated with recombinant TREM-1/Fc fusion chimera (rTREM-1/Fc) (R & D Systems) at 0.8 μg/ml for 24 h. The mRNA expression of TREM-1, MMP-1 and MMP-9 was analyzed by qRT-PCR.
2.8. Analysis of signaling pathways
Pre-confluent VSMCs were treated for 1 h with inhibitors against p38 (SB 203580), JNK (SP600125), PI3K (Ly294002) and NF-kB (BAY 11-8072) in serum-free medium followed by treatment with TNFα at 10 ng/ml for 24 hours. The mRNA expression of TREM-1, MMP-1 and MMP-9 was analyzed by qRT-PCR.
2.9. Statistical analysis
Differences between treatment groups were analyzed by Student’s t-test. Data are presented as mean ± SD; Statistical differences between the experimental groups were calculated.
3. Results
3.1. Expression of increased TREM-1
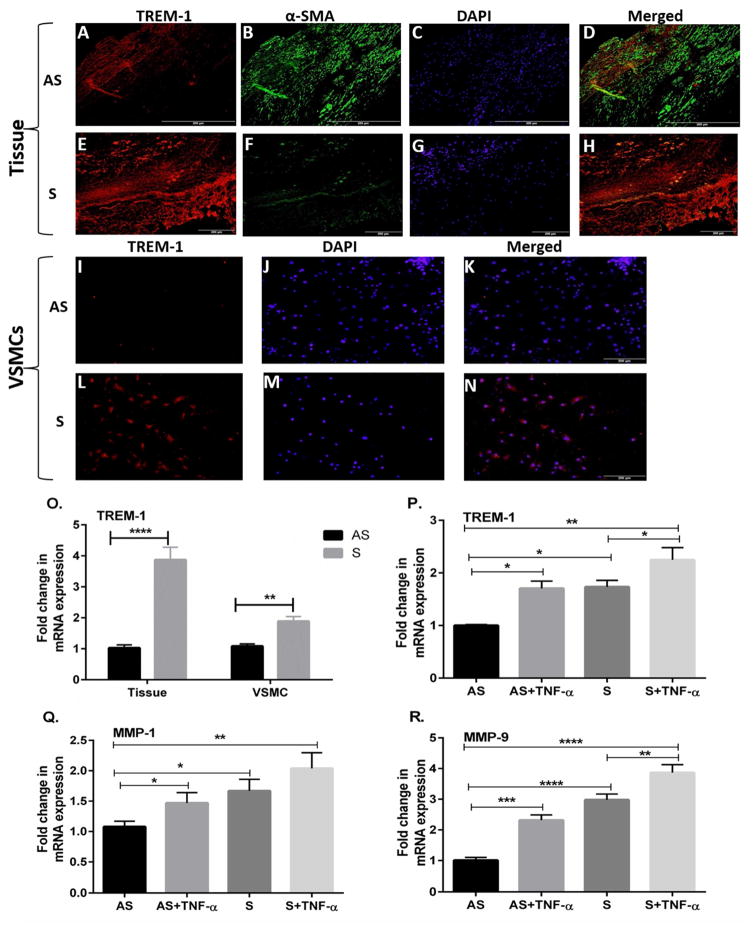
Dual immunofluorescence method with antibodies to TREM-1 and anti-alpha smooth muscle actin (α-SMA), a marker for SMCs was used to demonstrate the expression of TREM-1in carotid plaque with stenosis and VSMCs. The tissue sections and VSMCs were simultaneously incubated with the human α-SMA rabbit polyclonal antibody and a goat polyclonal anti TREM-1antibody. It was observed that theTREM-1 immunofluorescence was greater in S which contained few VSMCs (Fig. 1H) as compared to AS plaques (Fig. 1D). The isolated VSMCs from S carotid plaques also showed greater TREM-1 immunofluorescence (Fig. 1L). Negative controls were incubated with isotype-matched, nonimmune IgG. These results are in agreement with mRNA transcripts in both tissues and VSMCs (Fig. 10).
Fig. 1. TREM-1 is upregulated in carotid tissues and vascular smooth muscle cells (VSMCs).
Representative immunofluorescence images of triggering receptor expression on myeloid cells (TREM-1) (red) and alpha-smooth muscle actin (α-SMA), (green) expression as visualized by dual immunofluorescence in carotid plaque sections of asymptomatic (AS) (Figs. 1A–D) and symptomatic (S) (Figs. 1E–F) and VSMCs isolated from AS (Figs. 1I–K) and S (Figs. 1L–N). Panels A, E, I and L: TREM-1(red); B, F: α-SMA (green); C, G, J and M: nuclei labeled with DAPI (4, 6-diamidino-2-phenylindole); and Panels D and H are merged images with red and blue. This study demonstrates that TREM-1 expression is increased in both carotid plaques and VSMCs of S compared to AS. Scale bar = 200 μm for all images (N=5). Expression of TREM-1 mRNA is also increased in carotid tissues and VSMC (Fig. 10).
Effect of TNF-α on the expression of TREM-1, MMP-1 and MMP-9 in VSMCs
Cultured VSMCs from AS and S were treated with TNF- α (10 ng/ml) for 24 h and the isolated RNA was subjected to qRT-PCR. Treatment with TNF- α (10 ng/ml) increased the mRNA expression of TREM-1 (Fig. 1P), MMP-1 (Fig. 1Q) and MMP-9 (Fig. 1R) in VSMCs in the AS VSMCs and increased the mRNA expression of TREM-1 (Fig. 1P) and MMP-9 (Fig. 1R) in the S VSMCs. There was no significant effect of TNF- α on MMP-1 mRNA transcripts in the S VSMCs (Fig. 1Q). The results are expressed as fold change in S compared to AS after normalizing to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Data are presented as mean ± SD (N=3). *p<0.05, **p<0.01, ***p<0.001, ****p< 0.0001.
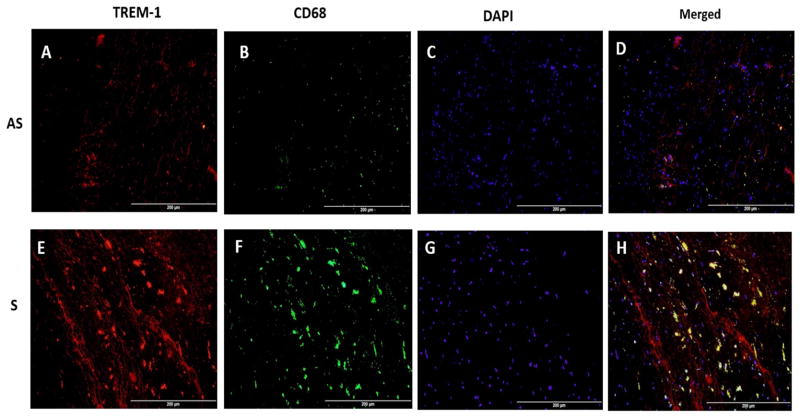
3.2 Expression of CD68, a marker for macrophages and co-localization with TREM-1
The tissue sections were incubated with either mouse monoclonal anti-CD68 antibody or a goat polyclonal anti TREM-1 antibody alone or in combination to examine the co-localization of CD68 and TREM-1 immunopositivity. There was significantly greater immunopositivity to both TREM-1 (Figs. 2A, 2E) and CD68 in the S compared to AS plaques (Figs. 2B, 2F). In the co-localization studies, the immunostaining to CD68 overlapped with that of TREM-1, which is clearly evident in the S plaques (Fig. 2H). However, there were areas, suggestive of smooth muscle cells that were immunopositive to TREM-1 only but not CD68 (Fig. 2D and 2H). Negative controls were incubated with isotype-matched, non-immune IgG. These findings suggest that both smooth muscle cells and macrophages in the plaque express TREM-1.
Fig. 2. Co-localization of TREM-1 and CD68+ Macrophages in carotid plaques.
Representative immunofluorescence images of triggering receptor expression on myeloid cells (TREM-1) (red) and macrophages (CD68) (green) expression as visualized by dual immunofluorescence in carotid plaque sections of asymptomatic (AS) (Figs. 2A–D) and symptomatic (S) (Figs. 2E–F). Panels A, E: TREM-1 (red); B, F: CD68 (green); C, G: nuclei labeled with DAPI (4, 6-diamidino-2-phenylindole); and Panels D and H show merged immunopositivity to both TREM-1 and CD68 in S and AS carotid plaques showing greater co-localization of TREM-1 and macrophages in addition to TREM-1 expression only on smooth muscle cells. Scale bar = 200 μm for all images (N=5).
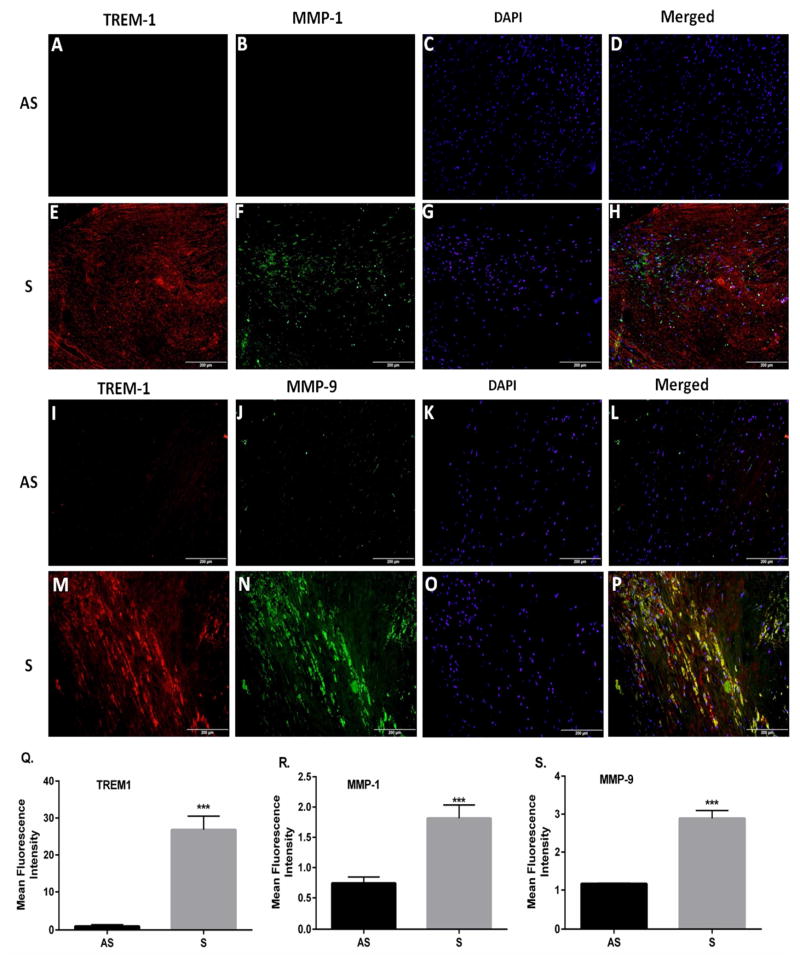
3.3. TREM-1, MMP-1 and MMP-9 protein expression in carotid plaques
To determine if the increased mRNA of TREM-1, MMP-1 and MMP-9 in VSMCs from S is translated into the protein, immunofluorescence was done to detect the TREM-1 protein expression. Immunofluorescence staining was performed to localize TREM-1, MMP-1 and MMP-9 in carotid plaques. Results showed that the TREM-1 intensity was higher in tissues from S (Fig. 3E and 3M) as compared to AS (Fig. 3A and 3I). The immunofluorescence of MMP-1 (Fig. 3F) and MMP-9 (Fig. 3N) was greater in tissue sections from S plaques with carotid stenosis. The dual immunofluorescence analysis also demonstrated significant co-localization of TREM-1 with MMP-1 (Fig. 3H) and MMP-9 (Fig. 3P) in tissue sections from S plaques. However, no appreciable co-localization of MMPs and TREM-1 was observed in the AS plaques (Figs. 3D and 3L). Quantitation of immunofluorescence as measured by image-pro software revealed greater intensity of TREM-1 (Fig. 3Q), MMP-1 (Fig. 3R) and MMP-9 (Fig. 3S) in tissue sections from S patients compared to AS patients.
Fig. 3. Immunofluorescence staining of TREM-1, MMP-1 and MMP-9 in tissue sections of S and AS carotid plaques.
Representative images are shown for TREM-1 (red) and MMP-1 (green) expression as visualized by dual immunofluorescence in sections of asymptomatic (AS; Panels, A–D) and symptomatic plaques (S; Panels, E–F). Co-localization of TREM-1 (red) and MMP-9 (green) are shown for AS (Panels, I–L) and S (Panels, M–P). Panels A, E, I and M: TREM-1(red); B, F, J, and N: MMP-1/MMP-9 (green); C, G, K, and O: nuclei labeled with DAPI; and D, H, L, and P: Co-localization of TREM-1 with MMP-1/MMP-9. It is clear that TREM-1was localized with MMP-1 and MMP-9. The immunofluorescence of TREM-1 (Panel Q), MMP-1 (Panel R) and MMP-9 (Panel S) was quantified using Image-pro software. These are the representative images from 5 individual tissues in each group. Data are presented as mean ± SD; N=5. *p<0.05, ***p<0.001. Scale bar = 200 μm for all images.
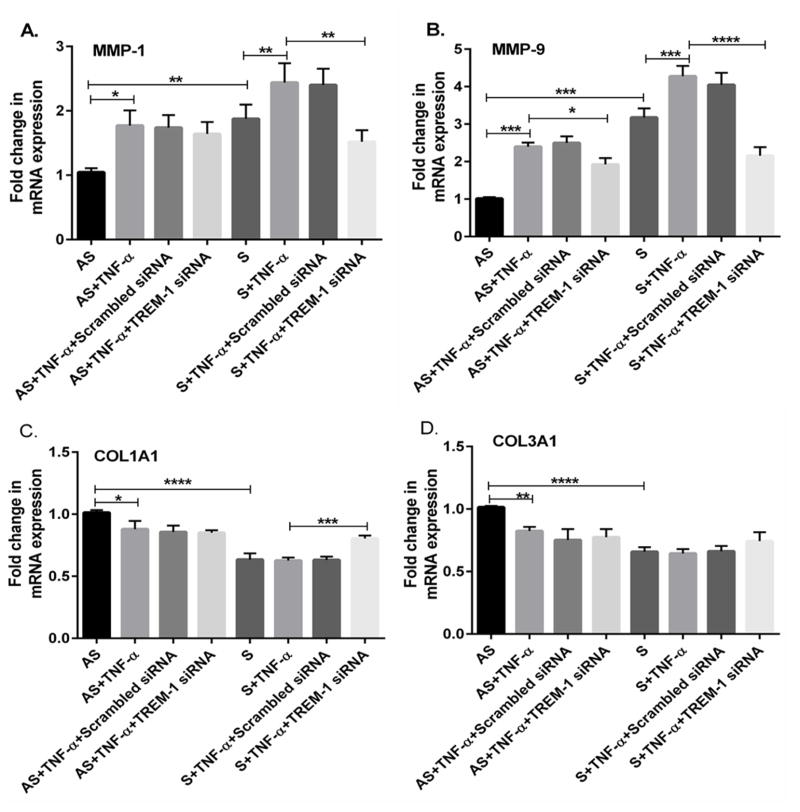
3.4. Effect of TREM-1 siRNA transfection
To confirm the role of TREM-1 on the expression of MMPs and collagens, we examined whether attenuation of TREM-1 in TNF-α treated cells decreases the expression of MMP-1 (p<0.01) and MMP-9 (p<0.001) and upregulate collagen genes (p<0.001). Results demonstrated that both MMP-1 and MMP-9 mRNA transcripts were significantly increased in S compared to AS (Figs. 4A, 4B). Treatment with non-specific control siRNA did not show any significant changes in either MMP-1 or MMP-9 mRNA transcripts in both AS and S VSMCs cultures. However, TREM-1 siRNA transfection significantly decreased the expression of MMP-1 (Fig. 4A, p<0.01) and MMP-9 (Fig. 4, p<0.001) and increased COL1A1 (Fig. 4C) mRNA transcripts in VSMCs from S. Treatment with TNF-α further increased the MMP-1 and MMP-9 mRNA transcripts (Figs. 4A and 4B) and decreased the expression of COL1A1 and COL3A1 (Figs. 4C and 4D) mRNA transcripts in VSMCs from S.
Fig 4. TREM-1 siRNA transfection attenuates the expression of MMP-1, MMP-9, COL1A1 and COL3A1 in TNF-α-treated plaque VSMCs.
VSMCs isolated from AS and S carotid plaques were transfected with either TREM-1 siRNA (40 nM) or scrambled control siRNA (40 nM) using lipofectamine 2000 (Life Technologies, Inc., Carlsbad, CA). After transfection, cells were stimulated with or without TNF-α followed by RNA isolation, and subjected to RT-PCR. Figure 4 shows the mRNA expression of MMP-1 (Fig. 4A), MMP-9 (Fig. 4B), COL1A1 (Fig. 4C) and COL3A1 (Fig. 4D). The relative expressions of the genes were compared withglyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the results were expressed as fold change in S compared to AS group (assigned as one fold). Data are presented as mean ± SD (N=3); *p<0.05, **p<0.01, ***p<0.001, ****p< 0.0001.
3.5. TREM-1 blocking studies
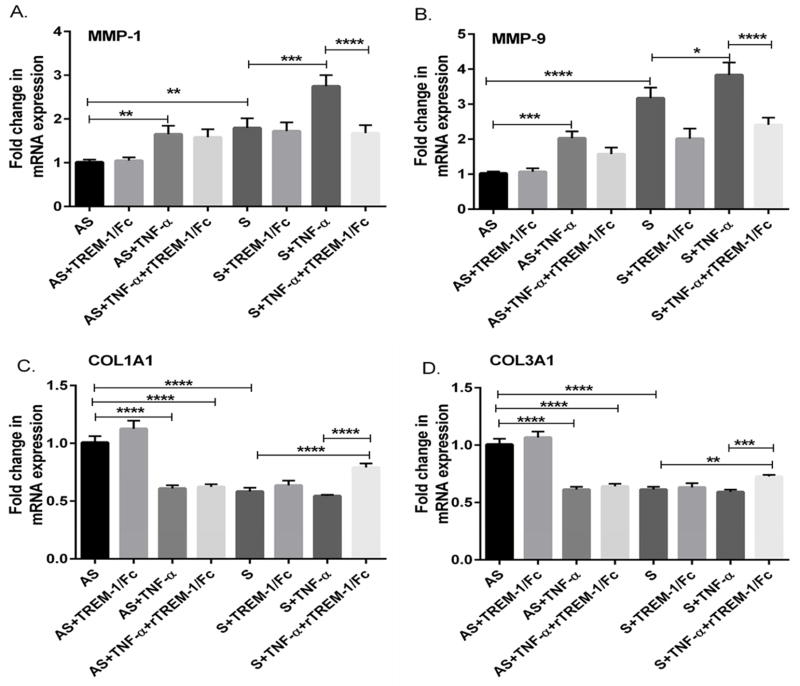
To investigate the role of TREM-1 in the TNF-α induced change, VSMCs were treated with TREM-1 antibody and the changes in MMP-1, MMP-9, COL1A1 and COL3A1 were studied. Treatment with TREM-1 antibody significantly reduced the expression of MMP-1 and -9 (Supplemental Fig 1A, p<0.01; and Fig 1B, p<0.001) and increased the mRNA expression of COL1A1 and COL3A1 (Supplemental Figs. 1C and 1D; p<0.01). In addition, we also investigated the effect of blocking TREM-1 with TREM-1 fusion protein (rTREM-1/Fc) on changes induced by TNF-α treatment. Treatment with TREM-1 fusion protein (rTREM-1/Fc) decreased the expression of MMP-1 and MMP-9 (Figs. 5A and 5B) and increased the mRNA expression of COL1A1 and COL3A1 (Figs. 5C and 5D). These results suggest that decreasing the TREM-1 with TREM-1 antibody, siRNA or TREM-1 fusion protein (rTREM-1/Fc) significantly attenuated the changes induced by TNF-α on MM-1 and MMP-9 and COL1A1 and COL3A1 genes.
Fig 5. Blocking TREM-1 attenuates the TNF-α-mediated increase in the expression of MMP-1, MMP-9, COL1A1 and COL3A1.
VSMCs isolated from AS and S carotid plaques were treated with recombinant rTREM-1/Fc (TREM-1/FC) at 0.8 μg/ml for 24 h in the presence or absence of TNF-α. The RNA isolated from the VSMCs cells was subjected qPCR and the results on mRNA expression are shown in Figure 5 (Panel A, MMP-1; Panel B, MMP-9; Panel C, COL1A1; and Panel D, COL3A1). Data were normalized to GAPDH and are expressed as fold change in S compared to AS group (assigned as one fold). Data are presented as mean ± SD (N=3); *p<0.05, **p<0.01, ***p<0.001, ****p< 0.0001.
3.6. MAPK and NF-kB signaling pathways
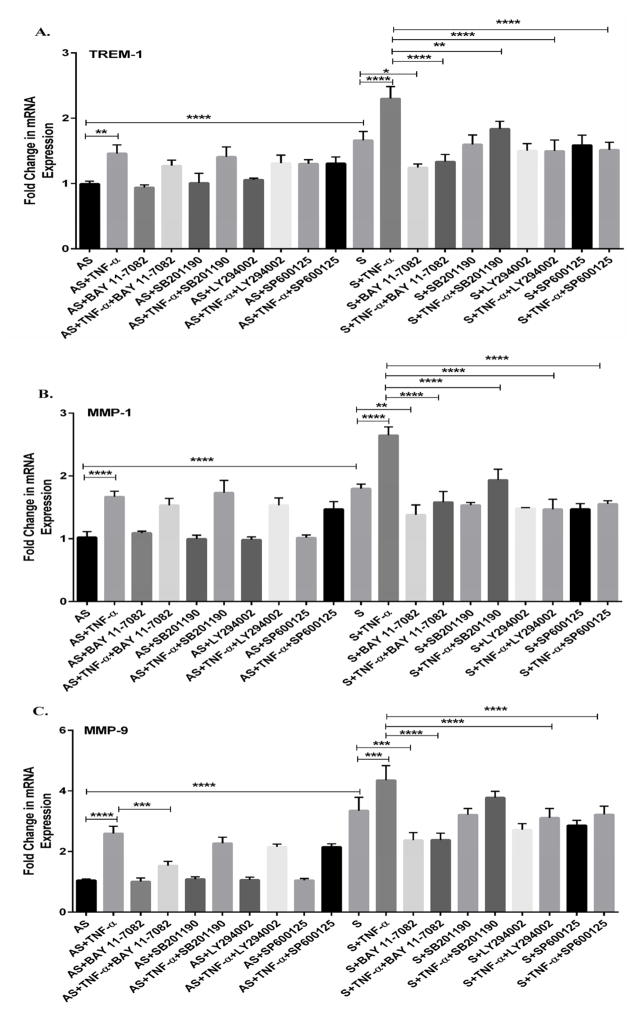
We have demonstrated increased expression of TREM-1, MMP-1 and MMP-9 in TNF-α treated VSMCs isolated from S plaques compared to AS, however the signaling pathways involved are not known. Therefore, we studied the role of MAPK and NF-kB signaling pathways using specific inhibitors (SB 202190 to inhibit p38-MAPK, LY294002 to inhibit PI3K-MAPK, and SP600125 to inhibit JNK-MAPK and BAY11-8075 to inhibit NF-kB) in VSMCs from S and AS without or with TNF-α (10 ng/ml) for 24 h. There was no significant effect of SB202190, LY294002 or SP600125 alone on the expression of TREM-1 (Fig. 6A), MMP-1 (Fig. 6B) and MMP-9 (Fig. 6C) in VSMCs isolated from either AS or S. Also, NF-kB inhibitor (BAY11-8075) by itself did not elicit any significant effect on TREM-1, MMP-1 and MMP-9 in VSMCs isolated from AS patients. In contrast, NF-kB inhibitor (BAY11-8075) alone significantly attenuated the expression of TREM-1, MMP-1 and MMP-9 in VSMCs isolated from S patients (Fig. 6A–C). Also, the inhibitors of p38, PI3K, JNK and NF-kB significantly decreased TNF-α-induced mRNA expression of TREM-1 in VSMCs from S compared to AS (Fig. 6A). Also, treatment with p38, PI3K, JNK and NF-kB inhibitors decreased the mRNA expression of MMP-1 (Fig. 6B) and MMP-9 (Fig. 6C) in TNF-α treated VSMCs from S compared to AS.
Fig 6. Induction of TREM-1 by TNF-α involves the activation of MAPK and NF-kB signaling pathways.
The VSMCs isolated from AS and S carotid plaques with carotid stenosis were treated without or with TNFα alone or in combination with selective inhibitors of p38 MAPK (SB 202190), PI3K (LY294002), JNK (SP600125) and NF-kB (BAY 11-8072) for 24 hours. The RNA isolated from the VSMCs cells was subjected qPCR and the results on mRNA expression are shown in Figure 6 (Panel A, TREM-1; Panel B, MMP-1; and Panel C, MMP-9). Data were normalized to GAPDH and are expressed as fold change compared to AS group (assigned as one-fold). Data are presented as mean ± SD (N=3); *p<0.05, **p<0.01, ***p<0.001, ****p< 0.0001.
3.7 Increased p-p38 MAP, p-JNK MAPK and NF-kB p65 immunofluorescence staining in tissue sections from symptomatic plaques
The decreased mRNA expression of TREM-1, MMPs and collagen genes in TNF-α-treated VSMCs in the presence of specific inhibitors of MAPK and NF-kB suggested that p38, PI3K, JNK and NF-kB signaling pathways attenuated the changes induced by TNF-α in VSMCs isolated from S and AS plaques with carotid stenosis (Figs. 6A–C). These results were also confirmed by dual immunofluorescence staining (Supplemental Fig. 2). Dual Immunofluorescence analysis revealed TREM-1 was co-localized with p38 (Supplemental Fig. 2H), TREM-1 with JNK (Supplemental 2P) and TREM-1 with NF-kB p65 (Supplemental Fig. 2X) in S as compared to AS carotid plaques. Quantification of the fluorescence intensity revealed a significant increase in p38 (Fig. Supplemental Fig. 2a, p<0.01), JNK (Supplemental Fig. 2b, p<0.01) and NF-kB (Supplemental Fig. 2c, p<0.001) in VSMCs from S plaques. The immunofluorescence studies support the results on the mRNA expression of TREM-1, MMP-1 and MMP-9 induced by TNF-α in VSMC.
4. Discussion
Production of inflammatory cytokines by macrophages contribute to the pathogenesis of atherosclerosis [25]. TREM-1 is expressed on monocytes/macrophages, neutrophils is increased by various stimuli such as TLR ligands [19, 26, 27] and secrete matrix metalloproteinase (MMPs) that degrade extracellular matrix proteins such as gelatin (MMP-2 and MMP-9), collagen (MMP-1, MMP-8 and MMP-13), elastin (MMP-12) and fibrin (MMP-3 and MMP-10). Chronic inflammation has been attributed to the vulnerability of atherosclerotic plaques [27–29]. The inflammatory cells in atherosclerotic plaques have been shown to produce various cytokines including TNF-α, proteases (MMPs), and pro-thrombotic molecules and affect plaque inflammation and vascular function [25]. We hypothesize that TREM-1 is involved in the regulation of various cytokines and MMPs, which in turn promote the vulnerability of atherosclerotic plaques. Though the molecular mechanism of plaque rupture is not clearly understood, it is suggested that increased matrix with more number of SMCs are necessary to form a firm fibrous cap associated with plaque stability [8, 30]. On the other hand the S plaques are characterized by decreased matrix and lower VSMCs. The increased TREM-1 and decreased VSMCs isolated from S plaques with carotid stenosis observed in this study demonstrates the critical role of VSMCs on the stability of carotid plaques. To our knowledge, this is the first report demonstrating the expression of TREM-1 in VSMCs which may suggest a role of TREM-1 in the pathogenesis of atherosclerosis. Inflammation is a major component of both AS and S carotid plaques [1, 28]. It is suggested that pro-inflammatory cells such as macrophages, lymphocytes and mast cells may affect the growth, function and death of intimal VSMCs that are critical for atherosclerotic plaque stability [25, 28, 31].
Macrophages play a key role in the initiation and progression of atherosclerosis as well as in plaque destabilization. The cytokines and growth factors secreted by atherosclerotic plaques play a role in several diseases [32, 33]. TREM-1 is expressed mainly on monocytes and neutrophils and is an amplifier of inflammatory response in acute and chronic inflammatory conditions [12, 13, 34]. However, the role of TREM-1 in the atherosclerotic plaque is not clear. In our studies, TREM-1 blocking experiments with either TREM-1 antibody or TREM-1 fusion protein (rTREM-1/Fc) significantly decreased the expression of both MMP-1 and MMP-9 and increased collagen genes. Recently, we reported [8] increased expression of MMPs and decreased expression of collagen genes in VSMCs isolated from S plaques supporting their role in plaque stability [35]. Other studies have suggested the involvement of collagen in the stability of atherosclerotic plaques [8, 36, 37].
The increased expression of TREM-1 in unstable plaques may suggest that TREM-1 is able to produce pro-inflammatory cytokines, chemokines and may contribute to the inflammatory responses in atherosclerosis. Treatment of VSMCs with TNF-α, a pro-inflammatory cytokine, further increased TREM-1 in both AS and S groups. These data strongly suggest that TREM-1 is involved in the progression of atherosclerosis. Blocking experiments with either TREM-1 antibody or TREM-1 fusion protein (rTREM-1/Fc) demonstrated a significant attenuation of the changes induced by TNF-α in MMPs or the collagen genes. These findings were supported by the reduced serum TNF-α and IL-1β [19] in TREM-1 fusion protein (rTREM-1/Fc) treated in sepsis. Recently we reported increased expression of MMP-1 and MMP-9 and decreased expression of collagen genes (COL1A1 and COL3A1) in VSMCs isolated from S plaques supporting their role in plaque stability. Recent studies using autofluorescence and MMPSense signals have demonstrated that MMP-9 is a major MMP present in advanced atherosclerotic plaques [38]. Other studies have suggested that MMPs are involved in the degradation of interstitial and basement collagens and destabilize the atherosclerotic plaques [37, 39].
The ability TREM-1 to stimulate MMPs depends on the specific signal transduction pathways. Though TREM-1 regulates the expression of MMPs and collagen genes, the molecular mechanism of TREM-1 activation in S plaques is not known. Therefore, we investigated molecular mechanisms of signaling pathways involved in TNF-α mediated activation of TREM-1, MMP-1 and MMP-9 in S plaques.
Activation of TREM-1 by TNF-α depends on specific signal transduction signaling pathways. MMP-1 and MMP-9 were regulated by different signaling pathways in various cell types. In the present investigation, we demonstrate that inhibitors p38 MAPK and JNK MAPK attenuated the changes in TREM-1, MMP-1 and MMP-9 induced by TNF-α in the atherosclerotic plaques. Involvement of Erk1/2, Akt and STAT3 in TREM-1 signaling pathways is also reported [40, 41].
Number of reports suggest the involvement of NF-kB in the pathogenesis of atherosclerosis. Expression of NF-kB and CD68 in unstable plaques [42] was higher than stable plaques. NF-kB is required for cytokine up-regulation of MMPs [1, 3, 9] in VSMCs which may suggest that NF-kB inhibition may promote plaque stabilization [43, 44]. It was demonstrated that targeting NF-kB nuclear translocation hampers inflammation and atherosclerotic development. In the present investigation, we found that the NF-kB inhibitor attenuated the expression of TREM-1, MMP-1 and MMP-9 in TNF-α treated VSMCs isolated from S plaques demonstrating the role of NF-kB in the stability of plaques (Fig. 7).
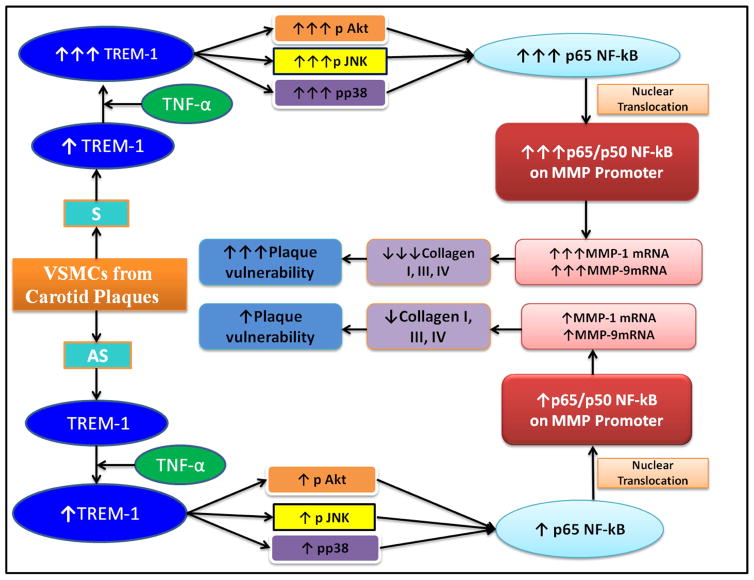
Fig. 7.
Schematic diagram showing the degree of increased or decreased expression of various molecules and potential sequence of events in VSMCs of carotid plaques from both symptomatic (S) and asymptomatic (AS) patients with carotid stenosis.
5. Conclusion
In summary, our results demonstrate that the TREM-1 expression is upregulated, most likely under the influence of atheromatous cytokines, including TNF-α, in vulnerable atherosclerotic plaques from patient with carotid stenosis. The upregulated TREM-1 in turn activates MMP-1 and MMP-9 leading to collagen degradation resulting in plaque instability. The TREM-1 upregulation by TNF-α involves MAPK and NF-kB signaling pathways. This study also provides the molecular and biochemical evidence that selective blockade of TREM-1 may be a novel strategy and promising target for stabilizing unstable atherosclerotic plaques.
Supplementary Material
Supplemental Figure 1: Anti-TREM-1 attenuates the TNF-α-mediated increase in the expression of MMP-1, MMP-9, COL1A1 and COL3A1. VSMCs isolated from AS and S carotid plaques were treated with either TREM-1 antibody (12 μg/ml) or isotype matched, non-immune IgG for 24 hours in the presence or absence of TNF- α. The mRNA isolated from the treated cells was subjected to qPCR and the results are shown in Supplemental Figure 1 (Panel A, MMP-1; Panel B, MMP-9; Panel C, Col I α1; and Panel D, Col III α1). Data were normalized to GAPDH and are expressed as fold change compared to AS group (assigned as one fold). Data are shown as mean ± SD (N=3); *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Supplemental Figure 2: Immunofluorescence microscopy. Co-localization of TREM-1 with pp38 (Panels A–H), pJNK (Panels I–P) and p65 NF-kB (Panels Q–X) are shown in Supplemental Fig. 2. Representative images are shown for TREM-1 (red) with pp38 (green) in sections of AS (Panels, A–D) and S (Panels, E–F,). Co-localization of TREM-1 (red) with pJNK (green) is shown in Panels I–L (AS) and Panels M–P (S) and p65 NF-kB (green) in Panels Q–T for AS and Panels U–X for S. The immunofluorescence intensity of pp38 (Panel a), pJNK-1 (Panel b) and p65 NF-kB (Panel c) was quantified using Image-pro software. The immunofluorescence intensity of p-p38, p-JNK and p65 NF-kB was greater in S carotid tissue sections (Panel E, M and U) compared to AS carotid plaque (Panels A, I and Q). Data are shown as mean ± SD (N=5); **p<0.01, ***p<0.001, ****p<0.0001; Scale bar = 200 μm for all images.
Highlights.
We report, for the first time, increased expression of TREM-1 in the carotid plaques of symptomatic compared to asymptomatic patients.
There is a positive correlation between increased TREM-1 and both MMP-1 and MMP-9 in carotid plaques of symptomatic patients with carotid stenosis.
Atherogenic cytokines, including TNF-α, increases TREM-1 expression in vascular smooth muscle cells.
Selective blockade of TREM-1 could be a novel therapeutic approach to stabilize vulnerable atherosclerotic plaques.
Acknowledgments
This work was supported by research grant R01HL073349 to DK Agrawal from the National Heart, Lung and Blood Institute, NIH USA.
Appendix A. Supplementary data
Supplementary data related to this article can be found on the journal website.
Footnotes
Financial & competing interests disclosure: All authors have read the journal’s authorship agreement. The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Dzau VJ. Transcription factor decoy. Circ Res. 2002;90(12):1234–6. doi: 10.1161/01.res.0000025209.24283.73. [DOI] [PubMed] [Google Scholar]
- 4.Dhume AS, Agrawal DK. Inability of vascular smooth muscle cells to proceed beyond S phase of cell cycle, and increased apoptosis in symptomatic carotid artery disease. J Vasc Surg. 2003;38(1):155–161. doi: 10.1016/s0741-5214(02)75463-3. [DOI] [PubMed] [Google Scholar]
- 5.Spagnoli LG, et al. Role of inflammation in atherosclerosis. J Nucl Med. 2007;48(11):1800–15. doi: 10.2967/jnumed.107.038661. [DOI] [PubMed] [Google Scholar]
- 6.Molloy KJ, et al. Unstable carotid plaques exhibit raised matrix metalloproteinase-8 activity. Circulation. 2004;110(3):337–43. doi: 10.1161/01.CIR.0000135588.65188.14. [DOI] [PubMed] [Google Scholar]
- 7.Adiguzel E, et al. Collagens in the progression and complications of atherosclerosis. Vasc Med. 2009;14(1):73–89. doi: 10.1177/1358863X08094801. [DOI] [PubMed] [Google Scholar]
- 8.Rao VH, et al. MMP-1 and MMP-9 regulate epidermal growth factor-dependent collagen loss in human carotid plaque smooth muscle cells. Physiol Rep. 2014;2(2):e00224. doi: 10.1002/phy2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branen L, et al. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24(11):2137–42. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 10.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86(2):515–81. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 11.Derive M, Massin F, Gibot S. Triggering receptor expressed on myeloid cells-1 as a new therapeutic target during inflammatory diseases. Self Nonself. 2010;1(3):225–230. doi: 10.4161/self.1.3.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klesney-Tait J, I, Turnbull R, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7(12):1266–73. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 13.Tessarz AS, Cerwenka A. The TREM-1/DAP12 pathway. Immunol Lett. 2008;116(2):111–6. doi: 10.1016/j.imlet.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Kuai J, et al. TREM-1 expression is increased in the synovium of rheumatoid arthritis patients and induces the expression of pro-inflammatory cytokines. Rheumatology (Oxford) 2009;48(11):1352–8. doi: 10.1093/rheumatology/kep235. [DOI] [PubMed] [Google Scholar]
- 15.Collins CE, et al. Elevated synovial expression of triggering receptor expressed on myeloid cells 1 in patients with septic arthritis or rheumatoid arthritis. Ann Rheum Dis. 2009;68(11):1768–74. doi: 10.1136/ard.2008.089557. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Roldan N, et al. Expression of triggering receptor on myeloid cell 1 and histocompatibility complex molecules in sepsis and major abdominal surgery. World J Gastroenterol. 2005;11(47):7473–9. doi: 10.3748/wjg.v11.i47.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koussoulas V, et al. Soluble triggering receptor expressed on myeloid cells (sTREM-1): a new mediator involved in the pathogenesis of peptic ulcer disease. Eur J Gastroenterol Hepatol. 2006;18(4):375–9. doi: 10.1097/00042737-200604000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Wang DY, et al. Expression of TREM-1 mRNA in acute pancreatitis. World J Gastroenterol. 2004;10(18):2744–2746. doi: 10.3748/wjg.v10.i18.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchon A, Dietrich J, Colonna M. Cutting edge:inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164(10):4991–5. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 20.Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3(6):445–53. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- 21.Chen JJ, et al. Global analysis of gene expression in invasion by a lung cancer model. Cancer Res. 2001;61(13):5223–30. [PubMed] [Google Scholar]
- 22.Matesanz-Isabel J, et al. New B-cell CD molecules. Immunology letts. 2011;134(2):104–112. doi: 10.1016/j.imlet.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Carr S, et al. Atherosclerotic plaque rupture in symptomatic carotid artery stenosis. J Vasc Surg. 1996;23(5):755–65. doi: 10.1016/s0741-5214(96)70237-9. discussion 765–6. [DOI] [PubMed] [Google Scholar]
- 24.Jia G, et al. Tumor necrosis factor-α regulates p27 kip expression and apoptosis in smooth muscle cells of human carotid plaques via forkhead transcription factor O1. Exp Mol Pathol. 2011;90(1):1–8. doi: 10.1016/j.yexmp.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouchon A, et al. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410(6832):1103–7. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 27.Schenk M, et al. TREM-1–expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest. 2007;117(10):3097–3106. doi: 10.1172/JCI30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 29.Barnett HJ, et al. The appropriate use of carotid endarterectomy. CMAJ. 2002;166(9):1169–79. [PMC free article] [PubMed] [Google Scholar]
- 30.Loftus IM, et al. Matrix metalloproteinases and atherosclerotic plaque instability. Br J Surg. 2002;89(6):680–94. doi: 10.1046/j.1365-2168.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- 31.Leskinen MJ, Kovanen PT, Lindstedt KA. Regulation of smooth muscle cell growth, function and death in vitro by activated mast cells—a potential mechanism for the weakening and rupture of atherosclerotic plaques. Biochem Pharmcol. 2003;66(8):1493–1498. doi: 10.1016/s0006-2952(03)00503-3. [DOI] [PubMed] [Google Scholar]
- 32.Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003;23(5):769–75. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- 33.Newby AC, et al. Vulnerable atherosclerotic plaque metalloproteinases and foam cell phenotypes. Thromb Haemost. 2009;101(6):1006–11. [PMC free article] [PubMed] [Google Scholar]
- 34.Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003;41(Suppl 4):15S–22S. doi: 10.1016/s0735-1097(02)02834-6. [DOI] [PubMed] [Google Scholar]
- 35.Rao VH, et al. Blockade of Ets-1 attenuates epidermal growth factor-dependent collagen loss in human carotid plaque smooth muscle cells. Am J Physiol Heart Circ Physiol. 2015;309(6):H1075–86. doi: 10.1152/ajpheart.00378.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sluijter JP, et al. Matrix metalloproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotid atherosclerotic lesions: a study in human endarterectomy specimen pointing to a role for different extracellular matrix metalloproteinase inducer glycosylation forms. Stroke. 2006;37(1):235–9. doi: 10.1161/01.STR.0000196986.50059.e0. [DOI] [PubMed] [Google Scholar]
- 37.Shah PK, Galis ZS. Matrix metalloproteinase hypothesis of plaque rupture: players keep piling up but questions remain. Circulation. 2001;104(16):1878–80. [PubMed] [Google Scholar]
- 38.Jager NA, et al. Distribution of Matrix Metalloproteinases in Human Atherosclerotic Carotid Plaques and Their Production by Smooth Muscle Cells and Macrophage Subsets. Mol Imag Biol. 2015:1–9. doi: 10.1007/s11307-015-0882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arts RJ, et al. TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. J Leukoc Biol. 2013;93(2):209–15. doi: 10.1189/jlb.0312145. [DOI] [PubMed] [Google Scholar]
- 40.Fortin CF, Lesur O, Fulop T., Jr Effects of TREM-1 activation in human neutrophils: activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int Immunol. 2007;19(1):41–50. doi: 10.1093/intimm/dxl119. [DOI] [PubMed] [Google Scholar]
- 41.Lin J, Kakkar V, Lu X. Impact of matrix metalloproteinases on atherosclerosis. Curr Drug Targets. 2014;15(4):442–453. doi: 10.2174/1389450115666140211115805. [DOI] [PubMed] [Google Scholar]
- 42.Bond M, et al. Inhibition of transcription factor NF-κB reduces matrix metalloproteinase-1,-3 and-9 production by vascular smooth muscle cells. Cardiovasc Res. 2001;50(3):556–565. doi: 10.1016/s0008-6363(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 43.Woo CH, Lim JH, Kim JH. Lipopolysaccharide induces matrix metalloproteinase-9 expression via a mitochondrial reactive oxygen species-p38 kinase-activator protein-1 pathway in Raw 264.7 cells. J Immunol. 2004;173(11):6973–6980. doi: 10.4049/jimmunol.173.11.6973. [DOI] [PubMed] [Google Scholar]
- 44.Mallavia B, et al. Peptide inhibitor of NF-κB translocation ameliorates experimental atherosclerosis. Am J Pathol. 2013;182(5):1910–1921. doi: 10.1016/j.ajpath.2013.01.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Anti-TREM-1 attenuates the TNF-α-mediated increase in the expression of MMP-1, MMP-9, COL1A1 and COL3A1. VSMCs isolated from AS and S carotid plaques were treated with either TREM-1 antibody (12 μg/ml) or isotype matched, non-immune IgG for 24 hours in the presence or absence of TNF- α. The mRNA isolated from the treated cells was subjected to qPCR and the results are shown in Supplemental Figure 1 (Panel A, MMP-1; Panel B, MMP-9; Panel C, Col I α1; and Panel D, Col III α1). Data were normalized to GAPDH and are expressed as fold change compared to AS group (assigned as one fold). Data are shown as mean ± SD (N=3); *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Supplemental Figure 2: Immunofluorescence microscopy. Co-localization of TREM-1 with pp38 (Panels A–H), pJNK (Panels I–P) and p65 NF-kB (Panels Q–X) are shown in Supplemental Fig. 2. Representative images are shown for TREM-1 (red) with pp38 (green) in sections of AS (Panels, A–D) and S (Panels, E–F,). Co-localization of TREM-1 (red) with pJNK (green) is shown in Panels I–L (AS) and Panels M–P (S) and p65 NF-kB (green) in Panels Q–T for AS and Panels U–X for S. The immunofluorescence intensity of pp38 (Panel a), pJNK-1 (Panel b) and p65 NF-kB (Panel c) was quantified using Image-pro software. The immunofluorescence intensity of p-p38, p-JNK and p65 NF-kB was greater in S carotid tissue sections (Panel E, M and U) compared to AS carotid plaque (Panels A, I and Q). Data are shown as mean ± SD (N=5); **p<0.01, ***p<0.001, ****p<0.0001; Scale bar = 200 μm for all images.