Abstract
The high prevalence/incidence of hearing loss (HL) in humans makes it the most common sensory defect. The majority of the cases are of genetic origin. Non-syndromic hereditary HL is extremely heterogeneous. Genetic approaches have been instrumental in deciphering genes that are crucial for auditory function. In this study, we first used NADf chip to exclude the implication of known North-African mutations in HL in a large consanguineous Tunisian family (FT13) affected by autosomal recessive non-syndromic HL (ARNSHL). We then performed genome-wide linkage analysis and assigned the deafness gene locus to ch:5q23.2-31.1, corresponding to DFNB60 ARNSHL locus. Moreover, we performed whole-exome sequencing on FT13 patient DNA and uncovered aminoacid substitution p.Cys113Tyr in SLC22A4, a transporter of organic cations, cosegregating with HL in FT13 and therefore the cause of ARNSHL DFNB60. We also screened a cohort of small Tunisian HL families and uncovered an additional deaf proband of consanguineous parents that is homozygous for p.Cys113Tyr carried by the same microsatellite marker haplotype as in FT13, indicating that this mutation is ancestral. Using immunofluorescence, we found that Slc22a4 is expressed in stria vascularis (SV) endothelial cells of rodent cochlea and targets their apical plasma membrane. We also found Slc22a4 transcripts in our RNA-seq library from purified primary culture of mouse SV endothelial cells. Interestingly, p.Cys113Tyr mutation affects the trafficking of the transporter and severely alters Ergothioneine uptake. We conclude that SLC22A4 is an organic cation transporter of the SV endothelium that is essential for hearing, and its mutation causes DFNB60 form of HL.
Keywords: Deafness, DFNB60, SLC22A4 Mutation, Ear Stria vascularis Endothelial Cells, Organic cation transport
INTRODUCTION
Hearing impairment is the most common sensory defect. It affects one in nearly 500 newborns and the majority of cases are of genetic origin. Hereditary hearing loss (HL) that is associated with no other symptoms or findings, classified as non-syndromic deafness, is extremely heterogeneous. Non-syndromic genetic hearing loss (HL) is inherited in an autosomal recessive mode (ARNSHL) in ~77% of the cases, autosomal dominant HL (ADNSHL) accounts for about 22%, and the remaining ~1% is composed of X-linked and mitochondrial forms. Over 100 ARNSHL gene loci have been localized through genome-wide linkage analysis of large pedigrees of consanguineous families, and over 60 ARNSHL genes have been so far identified by positional cloning (http://hereditaryhearingloss.org/).
In this study, we have mapped autosomal recessive non-syndromic HL-causing gene to DFNB60 locus, within a 12.2-Mb critical region on human chromosome 5q23.2–31.1 in a large consanguineous Tunisian family. We then identified by whole exome sequencing a missense variant in SLC22A4 (also known as OCTN1), the solute career family 22 (organic cation transporter), member 4 (SLC22A4; OMIM#604190), that co-segregated with HL within the family. We present evidence that Slc22a4 is expressed at the apical surface of mammalian adult cochlea stria vascularis (SV) endothelial cells, that the novel uncovered mutation in SLC22A4 affects its apical targeting and nearly abolishes its transport function, and therefore causative of DFNB60 form of HL.
Materials and Methods
Subjects and clinical evaluations
The institutional review boards (IRB) of the University of Miami and the ethical committee of the University Hospital of Sfax (Tunisia) approved this study. All adult subjects and the parents of minor subjects signed informed consents. Clinical history interviews and physical examinations of members of FT13 and FT20 families ruled out environmental factors as causing the hearing loss and the presence of a syndrome. Because SNPs in the novel deafness causative gene, SLC22A4, that we identified in this study has been associated to increase susceptibility to rheumatoid arthritis or Crohn’s disease, we emphasize that there were no clinical symptoms or signs in members of FT13 and FT20 families medical history that could suggest a possibility of an association to rheumatoid arthritis or Crohn’s disease. Indeed, no patient reported abdominal pain, diarrhea or weight loss for suspecting Crohn’s disease, and no patient reported joint pain or limitation of joint mobility for suspecting rheumatoid arthritis. In the absence of any clinical symptoms for these diseases, no additional tests have been performed, and deafness has been concluded to be non-syndromic. In order to assess the degree of hearing impairment, pure audiometry evaluation was performed to test air conduction and bone conduction at frequencies ranging from 0.5 to 8 kHz. Genomic DNA was extracted from peripheral blood samples following a standard phenol-chloroform method. All clinical investigation have been conducted according to the principles expressed in the Declaration of Helsinki.
Known mutations and microsatellite genotyping
Known North African mutations associated with hearing loss have been excluded with a cost-effective North African Deafness chip (NADf) using multiplex PCR coupled with dual-color arrayed primer extension (Chakchouk et al. 2015). A genome-wide scan was performed using 400 fluorescent dye-labeled microsatellites with an average spacing of 10 cM (ABIPRISM_Linkage Mapping Set version 2.5 MD10, Applied Biosystems, Foster City, CA). We used the True Allele PCR Premix for PCR reactions. Fluorescently-labeled alleles were analyzed on an ABI Prism 3100-Avant DNA Analyzer (Applied Biosystems, Foster City, CA).
Whole exome sequencing
Genomic DNA from one affected individual in the family underwent whole exome sequencing as described (Grati et al. 2015).
The average read depth was 47.6-fold. The average variant quality was 982.21. Before filtering, 237,526 SNVs and 23,573 indels were found. We filtered the variants using the Genomes Management Application (GEMapp), University of Miami Miller School of Medicine (https://secureforms.med.miami.edu/hihg/gem-app) according to the inheritance mode (homozygous autosomal recessive), to the function class including missense, nonsense, splice sites, in-frame indels and frame-shift indels, presence and frequency at the dbSNP137, NHLBI (http://evs.gs.washington.edu/EVS/) databases (minor allele frequency of less than 0.5% was used), and in ExAC (http://exac.broadinstitute.org/). We also filtered the variants for their absence in more than 5 families in our internal database that includes over 3,000 exomes. GATK quality score (QUAL) was set to 100 and genotype quality (GQ) was set to 75.
Mutation analysis by Sanger sequencing or by PCR-RFLP on genomic DNA
Exon 1 of SLC22A4 was amplified on 40 ng genomic DNA using forward primer 5′-ACTACGACGAGGTGATCGCCTTC -3′ and reverse primer 5′-TGCTGGAAGTATGAACAGCAG-3′, then either Sanger-sequenced, or digested with BglI restriction enzyme to yield either a 185 + 335 bp fragments (wild type allele) or 520 bp (mutant allele). DNA fragments were separated and visualized on a 2 % agarose gel.
GenBank accession numbers
Human SLC22A4 protein: NP_003050.2; Human SLC22A4 mRNA: NM_003059.2, zebrafish slc22a4 mRNA: NM_200849.1.
Constructs encoding for SLC22A4 and immunocytochemistry
We purchased from OriGene a plasmid construct encoding for C-terminally GFP-tagged human SLC22A4 variant 1 under the control of CMV promoter (catalog number: RG206575). Plasmid construct encoding for Interleukin Receptor α2 subunit (TAC) has been previously described (Grati et al. 2006). Immunocytochemistry was performed on COS7 cells expressing the wild type SLC22A4-GFP as described (Grati & Kachar 2011). The expression and targeting study of wild type and mutant SLC22A4-GFP in polarized LLC-PK1 cells has been performed as described (Grati et al. 2006).
Primary culture of strial endothelial cells and next–generation RNA sequencing
Cochleae from 10 or 15 day old C57BL/6J mice were harvested under sterile conditions. The stria vascularis was gently pulled away from the spiral ligament, placed in ice-cold Hank’s calcium and magnesium-free balanced salt solution, and minced into small pieces (~0.15 – 0.20 mm3) with ophthalmic tweezers. To produce endothelial cells, the minced stria vascularis was cultured on collagen-coated dishes in medium 254 (Invitrogen, Eugene, OR, USA). The detailed protocol for generating the primary endothelial cell line was previously reported (Neng et al. 2013). For gene sequencing, the purified endothelial cell culture at passage 3 was washed with PBS (3 times for 5 min), followed by incubation with trypsin-EDTA for 5 min at 37 C and 5% CO2. The cell suspension was pipetted up and down a few times to detach the cell colony. After adjusting the concentration of the cell suspension to 1×107/ml with the endothelial cell media, a 1ml cell suspension was transferred to an RNase-free polypropylene centrifuge tube. The suspension was centrifuged at 300 × g for 5 min, and supernatant discarded. RNAlater® (Life technologies) was added to the tube and the cells kept on ice. Specimens were submitted to Otogenetics Corporation (Norcross, GA USA) for RNA-Seq assays. Briefly, total RNA was extracted from cell pellets using the E.Z.N.A. Total RNA Kit II (Omega Biotek, Norcross, GA USA, catalog #R6934) and the integrity and purity of total RNA were assessed using Agilent Bioanalyzer and OD260/280. 1–2 µg of cDNA was generated using Clontech SMARTer cDNA kit (Clontech Laboratories, Inc., Mountain View, CA USA, catalog# 634925) from 100ng of total RNA, and adaptors were removed by digestion with RsaI. The resulting cDNA was fragmented using Covaris (Covaris, Inc., Woburn, MA USA) or Bioruptor, profiled using Agilent Bioanalyzer, and subjected to Illumina library preparation using NEBNext reagents (New England Biolabs, Ipswich, MA USA, catalog# E6040). The quality and quantity and the size distribution of the Illumina libraries were determined using an Agilent Bioanalyzer 2100. The libraries were then submitted for Illumina HiSeq2000 sequencing according to the standard operation. Paired-end 90 or 100 nucleotide (nt) reads were generated and checked for data quality using FASTQC (Babraham Institute, Cambridge, UK), and subjected to data analysis using the platform provided by DNAnexus (DNAnexus, Inc, Mountain View, CA USA) or the platform provided by Center for Biotechnology and Computational Biology (University of Maryland, College Park, MD USA) as previously described (Trapnell et al. 2012).
Animal use
Male and female wild type Sprague-Dawley rats were used in this study. C57BL/6J male and female mice were used in this study. Rats and mice have been humanely narcotized/asphyxiated with CO2 in a transparent CO2 chamber, followed by decapitation. All procedures were approved by the University of Miami Institutional Animal Care and Use Committee following the National Institutes of Health guidelines on “Using Animals in Intramural Research (http://oacu.od.nih.gov/training/PI/main_menu.htm)”.
Antibody validation and immunofluorescence preparations
H00006583-A01 SLC22A4 mouse polyclonal antibody directed against amino acids 43–142 of NP_003050 was obtained from Novus Biologicals. This antibody was tested for specificity by immunocytochemistry on COS7 cells expressing SLC22A4-GFP and western blot of proteins from several adult rat tissues and from COS7 cells, as previously described (Grati et al. 2015). H00006583-A01 showed high specificity on western blot (dilution: 1/2,000) and in immunocytochemistry on COS7 cells expressing SLC22A4-GFP (dilution: 1/700). H00006583-A01 was used for immunofluorescence on wholemount inner ear preparations (dilution 1/400). Wholemount immunofluorescence preparations on postnatal day 2–3 rat pup and on adult rats were performed as described (Grati et al. 2015). Immunoctochemistry of sections of cryo-embedded P2–3 rat inner ears have been performed as described (Yasunaga et al. 1999). Immuno-purified rabbit polyclonal Myosin 7a antibody PB206 has been described (Grati and Kachar 2011). Images were taken on a LSM710 confocal microscope equipped with a 63× 1.4 numerical aperture (N.A.) objective (Zeiss Microimaging). Confocal images were processed using Adobe photoshop. Relative fluorescence intensity quantifications have been performed using NIH ImageJ software.
Uptake of [3H]ERGO in HEK293 cells expressing human wild-type or mutant SLC22A4
Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum was supplied by Biowest (Nuaillé, France). Lipofectamine® 3000 Reagent was purchased from Thermo Fisher Scientific (Waltham, MA, USA). [3H]ERGO (170 Ci/mol) was obtained from Moravek Biochemicals (Brea, CA). Clearsol I was obtained from Nacalai Tesque (Kyoto, Japan). All other chemicals and reagents were of the highest purity available and were purchased from commercial sources.
HEK293 cells were cultured in DMEM supplemented with 10% FBS and passaged at least three times. Cultures were maintained in a humidified atmosphere of 5% CO2/ 95% air at 37°C. HEK293 Cells cultured for 3 days were dispersed with 0.1% trypsin in PBS containing 0.03% EDTA at 37°C for 1 min, and then transiently transfected with Plasmid DNA encoding human wild-type or mutant SLC22A4 by using Lipofectamine® 3000 Reagent in Opti-MEM according to the manufacturer’s instructions. The cells were seeded at a density of 5 × 104 cells/well on 24-well dishes. After 42 hours from the transfection, the cells were used for microscopic observation and uptake study.
In the microscopic observation, the cells were rinsed with PBS and observed under a LSM710 confocal laser scanning microscope, followed by calculation of the percentage of GFP-positive cells versus the total number of cells.
After checking the transfection efficiency by microscopic observation, HEK293 cells transfected with wild-type or mutant SLC22A4 were washed with transport buffer (125 mM NaCl, 4.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KaH2PO4, 5.6 mM glucose and 25 mM HEPES, pH 7.4) and incubated with 0.23 µM [3H]ERGO in transport buffer at 37°C for various periods in the presence or absence of 5, 50 and 500 µM unlabeled ERGO. Cells were then solubilized with 0.2 M NaOH at 25°C for 6 hours. The solubilized solution was neutralized with 5 M HCl, followed by addition of Clearsol I for liquid scintillation spectrometry. Protein concentration was determined with a Bio-Rad Protein Assay Kit. Km and Vmax values were calculated by fitting the data on dose-dependent inhibition by unlabeled ERGO to the Michaelis-Menten kinetics and Eadie–Hofstee plot.
RESULTS
Mutation screening on NADf chip and genomic mapping of HL-causing gene in Tunisian consanguineous family FT13
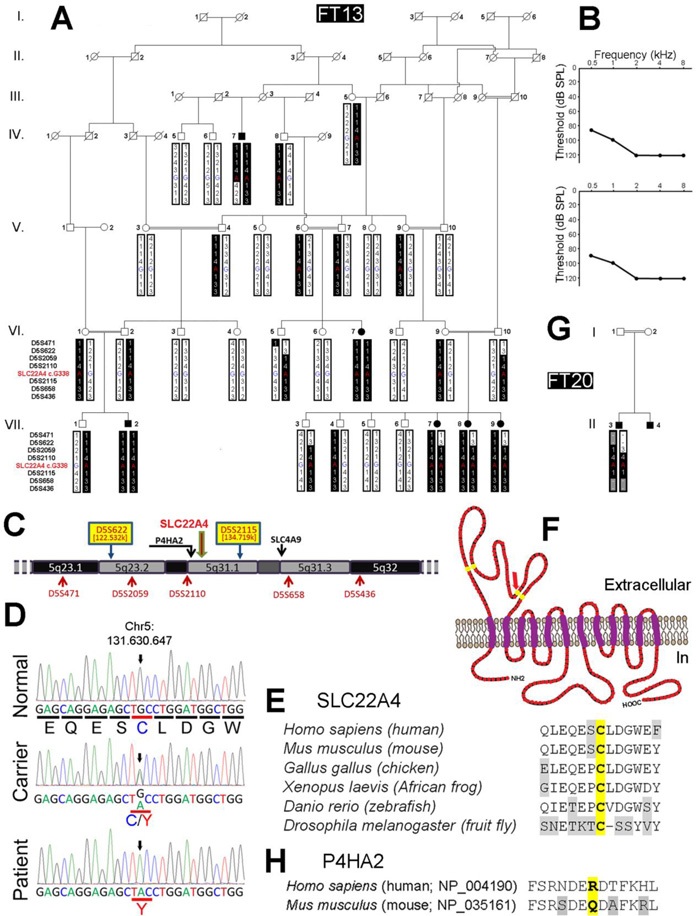
Tunisia is among North African countries with high consanguinity rate, and therefore increased autosomal recessive deafness frequency. Besides, several features, such as history and social behavior that are common to this population, promote the spreading of founder mutations (Ben Arab et al. 2004). Therefore, a home-built North African Deafness chip (NADf) that allows the screening of 58 known deafness-causing mutations in 21 distinct genes (Chakchouk et al. 2015), has first been used to screen for the presence of a known variant in family FT13 in which affected members had non-syndromic bilateral prelingual onset profound deafness (figure 1A, B). HL in family FT13 was determined to be autosomal recessive. After excluding common mutations using NADf chip, we performed a genome-wide linkage analysis on selected individuals from the FT13 family using 400 fluorescently-labeled informative microsatellite markers spaced in average of 10 cM/Mbp covering 22 autosomes and X chromosome (ABI PRISM® Linkage Mapping Set version 2.5 MD10, Applied Biosystems, Foster City, CA). Possible linkage was found with the marker D5S2115. In order to confirm linkage and determine the critical interval, additional closely spaced markers (D5S622, D5S2059, D5S2110 and D5S658; figure 1A, C) were genotyped. A maximum two-point LOD score of 4.13 was obtained for the most informative marker D5S658. Haplotype analysis disclosed key recombination event between D5S2110 and D5S2115 in affected individual IV:7, defining the telomeric boundary of the disease interval (figure 1A). As affected individuals VII:7, VII:8, VII:9 were heterozygous for marker D5S622, we were able to limit the centromeric boundary of the disease interval (figure 1A). Therefore, this locus lies within a 12.18-Mb region delimited by D5S622 (122,532,459–122,532,846) and D5S2115 (134,719,248–134,719,548) markers (http://www.genome.ucsc.edu) on chromosome 5q23-q31, corresponding to the DFNB60 locus (HGNC:18300 at http://www.genenames.org/).
Figure 1.
Molecular genetic and structural analysis of SLC22A4 mutation. (A) Homozygosity mapping of recessive isolated HL-causing gene in consanguineous family FT13. Family FT13 pedigree showing the co-segregation of HL with a haplotype reconstructed between microsatellite markers D5S471 and D5S436, including the genotype of SLC22A4 c.G338 position within chromosomal region 5q23.1–q32. Key recombination events leading to heterozygosity in deaf individual IV:7 telomeric to marker D5S2115, and in deaf individuals VII:7, VII:8 and VII:9 centromeric to marker D5S622 allowed us to map the causative gene between these two markers within a 12.2 cM region. (B) Audiograms of deaf individuals VII:7 (upper panel) and IV:7 (lower panel). Audiograms of other deaf individuals in the family were not significantly different. Hearing loss is bilateral and profound, with a very similar pattern for both ears. (C) Physical map of chromosomal region 5q23.1–q32 showing the location of candidate genes SLC22A4, P4HA2 and SLC4A9 where homozygous aminoacid variations were found by whole exome sequencing on patient VII:7. (D) Electrofluorograms of hearing wild type individual (VII:6), hearing carrier individual (VI:9) and deaf individual (VII:7) showing the identification of c.338G>A nucleic substitution (ch5:131,630,647) causing SLC22A4 p.Cys113Tyr amino acid substitution in FT13 family. Sanger sequencing on genomic DNA samples from all available FT13 family deaf and hearing members confirmed the segregation of c.338G>A with HL. (E) Interspecies alignment of amino acid sequences around residue p.Cys113 of SLC22A4 showing its high conservation. (F) Predicted dodeca-transmembrane protein SLC22A4 secondary structure. Yellow residues are highly conserved Cysteine residues predicted to be essential for the formation of the large extracellular loop located between TM1 and TM2; red arrow pinpoint to residue p.Cys113. (G) Pedigree of consanguineous family FT20 showing the haplotype and the homozygous OCTN1 homozygous c.338G>A mutation in the deaf child. (H) Alignment of amino acid sequences of human P4HA2 and mouse P4ha2 around residue p.Arg439 of P4HA2 (NP_004190) showing no conservation in mouse orthologous sequence (NP_035161) corresponding to residue p.Gln441 that is identical to substitution pArg439Gln found in FT13, therefore concluded as a non-pathogenic polymorphism.
Whole exome sequencing and identification of HL-causing gene in family FT13
Using whole exome sequencing on one affected individual DNA, and applying selection criteria given in methods (Grati et al. 2015), we identified two homozygous chromosomal variations at positions Chr5:131,533,954 and Chr5:131,630,647 within the candidate genetic interval, and a third one at position Chr5:139,740,434 right downstream of the D5S2115 telomeric bordering marker, causing p.Arg439Gln (NP_004190.1), p.Cys113Tyr (NP_003050.2) and p.Gly90Ser (NP_001245355.1) substitutions in P4HA2 (c.1316C>T in NM_004199.2 corresponding to rs192638050; encoding for Prolyl 4-Hydroxylase, Alpha-2 subunit; OMIM#600608), SLC22A4 (c.338G>A in NM_003059; encoding for Organic Cation Transporter-1; OMIM#604190) and SLC4A9 (c.268G>A in NM_001258426.1; encoding Anion Exchanger Protein 4; OMIM#310207), respectively. We used prediction software Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/) and SIFT (http://sift.bii.a-star.edu.sg/www/SIFT_related_seqs_submit.html) to evaluate the likely pathogenicity of each variant. Variant p.Arg439Gln (rs192638050; allele frequency on ExAC server = 9.891e-05; allele count = 12 / 121,322) in isoform-1 of P4HA2 (NP_004190) has been predicted “tolerated” or non-pathogenic (SIFT score= 0.61, Polyphen-2 score=0). Precisely, the human P4HA2 p.Arg439 residue is not conserved in the mouse P4ha2 orthologous isoform (NP_035161) that contains instead residue p.Gln441 that is identical to the substitution found in FT13 family (figure 1H), and therefore this variant has been excluded to be the cause of HL in family FT13.
Variant p.Gly90Ser in SLC4A9 (allele frequency on ExAC server = 6.301e-05; allele count = 7 / 111,090) has been predicted “damaging” (SIFT score= 0.02, Polyphen-2 score=0.969) and has been excluded to be the cause of HL since it did not co-segregate with HL within family FT13. Variant p.Cys113Tyr in SLC22A4 (c.338G>A in NM_003059; allele frequency on ExAC server = 4.545e-05; allele count = 3 / 66,008), however, occurs in a highly conserved residue within the first extracellular loop of the protein (figure 1F), has been predicted “damaging/deleterious” (SIFT score= 0, Polyphen-2 score=1) and found to co-segregate with HL within family FT13 (figure 1A). Besides, screening of a cohort of 71 Tunisian HL patients led to uncover homozygous p.Cys113Tyr substitution in a deaf child of consanguineous parents (Family FT20; figure 1G). This patient carried a homozygous microsatellite marker haplotype bordering SLC22A4 gene locus that was identical to that comprising p.Cys113Tyr variant that was transmitted within FT13 family, indicating that this mutation is ancestral. Moreover, we screened 122 Tunisian control individuals and found one carrier for the mutation within the same haplotype.
Targeting properties of wild type and p.Cys113Tyr mutant SLC22A4 in LLC-PK1 polarized epithelial cells
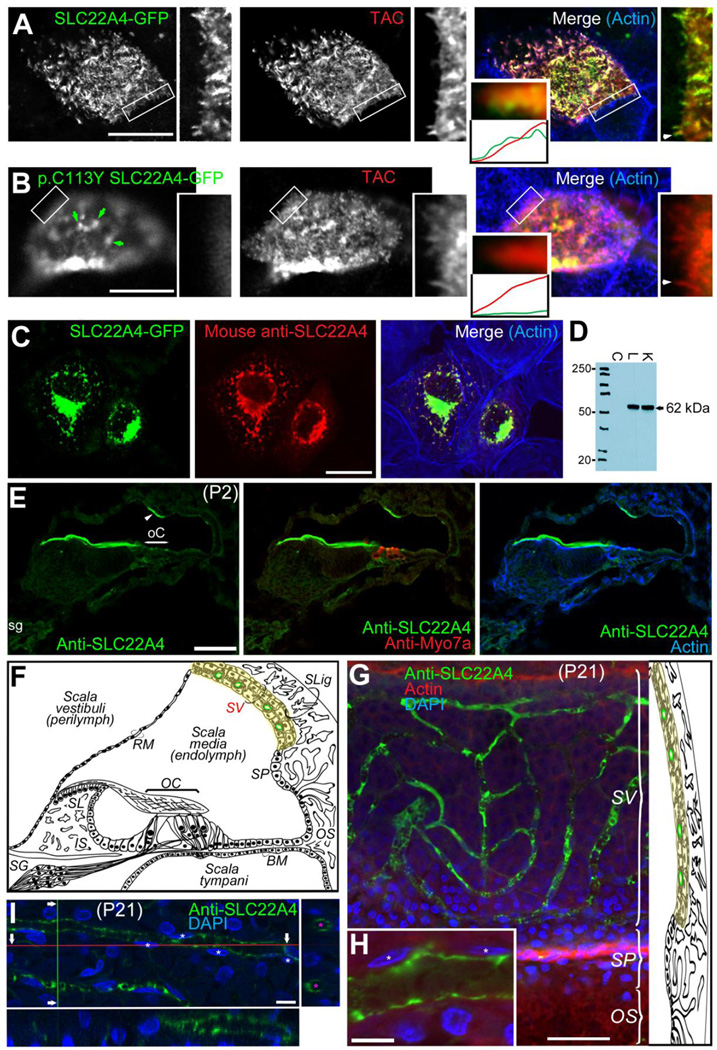
We studied the targeting properties of COOH-terminally GFP-tagged wild type and p.Cys113Tyr mutant SLC22A4 in polarized LLC-PK1 cells. We coexpressed each of these forms together with Interleukin Receptor α2 subunit (TAC) that we used as a marker to visualize the apical plasma membrane (PM) in polarized LLC-PK1 cells, as previously described (Grati et al. 2006). Wild type SLC22A4 was found to be abundantly targeted to the apical PM (green in figure 2A) and perfectly co-localized with TAC (red in figure 2A). The targeting to the apical PM of LLC-PK1 polarized cells of p.Cys113Tyr mutant SLC22A4, however, appeared to be hindered, reflected by the very low abundance of GFP fluorescence at the apical PM (green in figure 2B). Moreover, fluorescence at the apical PM related to mutant SLC22A4-GFP (green in figure 2B) did not co-localize (merged channels in figure 2B) with that collected for TAC (red channel in figure 2B), although few green fluorescent aggregates that would correspond to abnormally folded molecules, could have been able to reach the apical PM (green arrows in figure 2B).
Figure 2.
SLC22A4 targeting, antibody specificity validation and wholemount immunofluorescence on rat inner ear tissues. (A) A representative confocal micrograph of the apical surface of polarized LLC-PK1 cells co-expressing GFP-tagged wild type SLC22A4 (green channel) together with Interleukin Receptor α2 subunit (TAC, red channel), showing the targeting and high degree of colocalization (Merge) of both SLC22A4-GFP (green) and TAC (red) at the apical plasma membrane (PM); actin is stained in blue in merged channels panel. Number of scanned cells with similar pattern = 22. A zoom-in view of an inset in separate channels and in the merged view (rectangle) is also represented. The relative fluorescence intensity plots in green and red channels at the plasma membrane of a chosen microvillus (arrowhead) shows the abundance of both TAC (Red) and wild type SLC22A4-GFP. (B) A representative confocal micrograph of the apical surface of polarized LLC-PK1 cells co-expressing GFP-tagged p.Cys113Tyr mutant SLC22A4 (green channel) together with TAC (red channel), showing a low abundance of p.Cys113Tyr mutant SLC22A4-GFP (green) compared to TAC (red) at the apical PM, and also compared to wild type SLC22A4-GFP (green in A). The merged channels panel shows the low degree of colocalization of both proteins at the apical PM; actin is stained in blue. Number of scanned cells with similar pattern = 25. A zoom-in view of an inset in separate channels and in the merged view (rectangle) is also represented. The relative fluorescence intensity plots in green and red channels at the plasma membrane of a chosen microvillus (arrowhead) shows the abundance of TAC (Red) and a very low level of p.Cys113Tyr SLC22A4-GFP. Arrows show abnormal aggregates of p.Cys113Tyr SLC22A4-GFP at the apical PM. (C) SLC22A4 mouse polyclonal antibody (red panel) validation by immunocytochemistry in COS7 cells expressing SLC22A4-GFP (green) showing colocalization (Merge panel); actin is in blue. SLC22A4 is not endogenously expressed in COS7 cells shown by the absence of immunoreactivity in non-transfected COS7 cells (counterstained for actin in blue in the Merge panel). SLC22A4 does not seem to efficiently target the plasma membrane in COS7 cells and remained predominantly within intracellular protein synthesis and trafficking compartments. (D) Western blot of protein extracts from COS7 cells (C) that do not endogenously express SLC22A4 and that were not transfected with construct expressing SLC22A4-GFP (negative control), adult rat liver (L) and kidney (K) using mouse polyclonal antibody (Novus Biologicals; dilution: 1/2000) showing the absence of immunoreactivity in COS7 cells (as seen by immunocytochemistry in C), and the revelation of a unique band around the 62 kDa predicted SLC22A4 size in both liver and kidney. (E) Immunofluorescence using SLC22A4 antibody (green) on P2 rat inner ear cryosections, counterstained for Myosin VIIa to visualize hair cells (red) and with DAPI to visualize the nuclei (blue). The overall immunofluorescence signal is weak and diffuse across the tissue, with higher fluorescence at spiral ganglion neurons (sg) and a much higher apical enrichment of the signal in a subset of marginal cells (arrowhead). The antibody cross-reacts with tectorial membrane. (F) Schematic representation of adult mammalian cochlea cross-section showing its different fluidic and cellular compartments highlighting in yellow the stria vascularis (SV) compartment and in green the blood capillaries of the SV. OC, organ of Corti; SLig, spiral ligament; SV, stria vascularis; SP, spiral prominence; RM, Reissner’s membrane; BM, basilar membrane; OS, outer sulcus; IS, inner sulcus; SL, spiral limbus; SG, spiral ganglion neurons. (G) Confocal micrograph of a wholemount immunofluorescence preparation of rat cochlea lateral wall using SLC22A4 antibody (green); Actin is red and nuclei are revealed with DAPI (Blue). (H) A closer look at a capillary segment showing immunofluorescence (green) at the apical surface of the endothelial cells; starts pinpoint to the nuclei of endothelial cells. (I) An image of a 3D-reconstruction of a confocal image stack through a wholemount immunofluorescence preparation of adult rat stria vascularis and orthogonal cross-sections following red (lower panel) and green (right panel) axes, showing SLC22A4 (green) distribution all around the apical surface of blood capillary endothelial cells; pink stars show the lumen of the capillaries and white stars shows the nuclei of endothelial cells counterstained with DAPI. Scale bars: 5 µm in (A, B); 10 µm in (C); 5 µm in (H) and (I); 50 µm in (E) and (G).
Immunolocalization of Slc22a4 in mammalian cochlea
In order to immunolocalize Slc22a4 in early postnatal and adult rat inner ears, we used an immuno-purified antibody for which we characterized the specificity to Slc22a4 by immunocytochemistry (figure 2C) and by Western blotting (figure 2D). At early postnatal stage (postnatal day 2 in figure 2E), Slc22a4 expression was diffuse across all rat inner ear epithelia (figure 2E), including the hair cells, and in spiral ganglion neurons. These observations were relatively consistent with the data reported in SHIELD database (Shen et al. 2015) on the detection of Slc22a4 mRNA in hair cells and in spiral ganglion neurons. At this stage, we observed a higher fluorescence at spiral ganglion neurons and at the stria vascularis (SV). We also consistently noticed a high expression of the protein by a subset of marginal cells which appeared to be enriched at their apical plasma membrane. At around the onset of hearing in mature rats and thereafter (3 week postnatal in figure 2G–I), Slc22a4 was only found highly expressed in the endothelial cells of the SV, precisely at the apical surface plasma membrane of the endothelial cells (figure 2E–G).
Expression of Slc22a4 in mouse strial endothelial cell line
In order to further confirm our immunofluorescence observations on the mammalian inner ear tissues, we searched for the expression of Slc22a4 in a purified primary culture of P10–15 mouse cochlea endothelial cells (EC) that we have established (Neng et al. 2013). We therefore generated a library of massive mRNA next generation sequences prepared from this primary culture and looked for the presence of Slc22a4 mRNA. Slc22a4 RNA expression was determined for three replicates of one purified primary strial EC sample (Neng et al. 2013). The specific EC marker gene, vWf, was also identified in the same sample. Slc22a4 had a read count of 22, which is higher than the count for the EC marker gene, Vwf (read count of 9). However, both Slc22a4 and vWf were expressed at a low level compared to the housekeeping gene Gapdh, which had a read count of 1.95E+04. In a control experiment the primary strial pericyte cell line was shown to not express Slc22a4.
Uptake of [3H]ERGO in HEK293 cells expressing exogenous wild-type or mutant SLC22A4-GFP
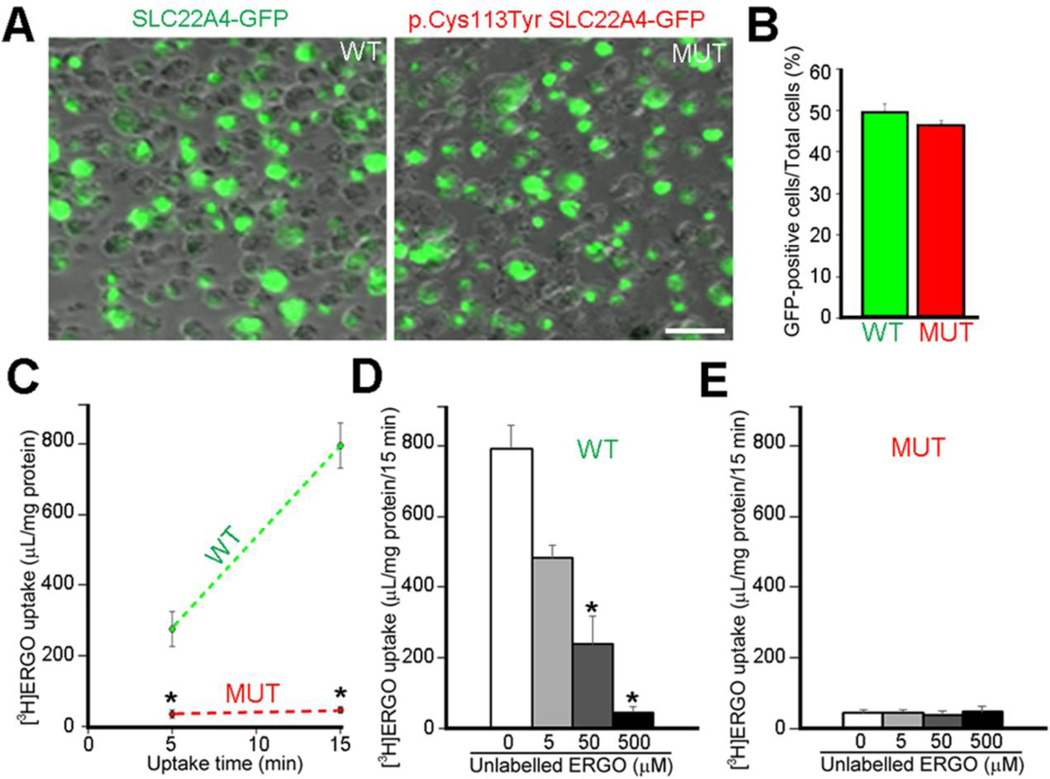
In order to evaluate the ERGO uptake efficiency of both wild type (WT) and p.Cys113Tyr mutant (MUT) SLC22A4, we transfected HEK293 with plasmids expressing either of these forms. Transfection efficiencies of WT and MUT SLC22A4 plasmids were very similar since HEK293 transfection rates were evaluated to 49.7% and 46.4%, respectively (figure 3A, B). Uptake of [3H]ERGO into HEK293 cells transfected with wild-type SLC22A4 (figure 3A, B) increased markedly in a time-dependent manner (figure 3C), and the [3H]ERGO uptake was inhibited by simultaneous addition of unlabeled ERGO in a dose-dependent manner over the concentration range of 5 to 500 µM (figure 3D). The Km value of 23 and 29 µM obtained from the results of Michaelis-Menten plot and Eadie-Hofstee plot respectively is close to the value of 21 µM obtained in HEK293 cells transfected with human OCTN1 (Gründemann et al. 2005). On the other hand, [3H]ERGO uptake into the cells transfected with mutant SLC22A4 (figure 3A, B) was much lower than that of wild-type SLC22A4 (figure 3C), and did not significantly fluctuate by the addition of unlabeled ERGO (figure 3E).
Figure 3.
Transport activity of wild-type and p.Cys113Tyr mutant SLC22A4. (A) Plasmid DNA encoding C-terminally GFP-tagged wild-type (WT) or p.Cys113Tyr (MUT) SLC22A4 were transiently transfected into HEK293 cells. After 42 hours from the transfection, transfection efficiency was examined by microscopic observation of GFP-positive cells. Scale bar: 50 µm. (B) The result of quantitative analysis showing the percentage of GFP-positive cells versus the total number of cells. Each value represents the means ± SEM (n = 4). (C) Time-dependent uptake of [3H]ERGO which is a typical substrate of SLC22A4. The vertical axis unit means cell to medium ratio (µL/mg protein) = ([3H] dpm in the cell/amount (mg) of cell protein)/([3H] dpm in the transport buffer/amount (µL) of transport buffer). Student's t-test was performed to determine the significance of differences. Each value represents the means ± SEM (n = 3), significance: *, P < 0.05. (D, E) After 42 hours from the transfection of WT (D) or MUT (E) SLC22A4, the cells were incubated with [3H]ERGO for 15 min in the absence or presence of 5, 50 or 500 µM of unlabeled ERGO, followed by determination of [3H]ERGO uptake. Bonferroni correction was used as multiple comparison technique. Each value represents the means ± SEM (n = 3), significance: *, P < 0.05.
DISCUSSION
In this study, we discovered that p.Cys113Tyr mutation in SLC22A4, the solute career family 22 (organic cation transporter), member 4, causes isolated recessive HL in humans. This mutation was found to cause HL in a large consanguineous Tunisian family (FT13) and in a child of a small unrelated Tunisian consanguineous family (FT20); in both cases, the mutation was embedded within the same microsatellite marker haplotype, indicating a founder effect within Tunisian/North African population. This hypothesis was corroborated by the finding of one carrier of the same mutation among 122 screened Tunisian healthy individuals.
SLC22 family of transporters together with MATE (multidrug and toxin extrusion) H+/drug antiporters constitute the family of polyspecific organic cation transporters. Members of the SLC22 family are divided into two distinct groups: the organic cation transporter sub-group made of OCT1 (SLC22A1), OCT2 (SLC22A2) and OCT3 (SL22A3), and the so-called “Novel” organic cation/carnitine transporter sub-group to which OCTN1 (SLC22A4) belongs, together with OCTN2 (SLC22A5) and OCT6 (SLC22A16) (Tamai 2013; Farthing & Sweet 2014; Pelis & Wright 2014). The SLC22 family members have a predicted membrane topology that comprises 12 α-helical transmembrane domains (TM), an intracellular NH2 and COOH termini, a large glycosylated extracellular loop between TM1 and TM2, and a large intracellular loop with predicted phosphorylation sites located between TM6 and TM7. Mutation p.Cys113Tyr occurs in a highly conserved Cysteine residue of the first large extracellular loop of SLC22A4 and is predicted to form disulfide bonds and strongly contribute to the formation and stability of the tertiary structure of the protein. We show in vitro evidence that p.Cys113 residue is crucial for the proper targeting of the transporter to the apical plasma membrane of polarized epithelial cells, and for its solute uptake function.
Slc22a4 is ubiquitously expressed in the body and transports across the cellular plasma membrane various compounds including organic cations (Yabuuchi et al. 1999), such as physiological acetylcholine and carnitine, as well as naturally-occurring antioxidant ergothioneine (ERGO) (Gründemann et al. 2005; Taubert and al. 2009). Within the cell, carnitine facilitates the transit of long carbon-chain fatty acids through mitochondia inner membrane as acylcarnitine esters for their β-oxydation and the generation of metabolic energy, although Slc22a5 was found to be much more carnitine-selective (Tamai et al. 1998). Defects in carnitine reabsorption at the renal proximal tubules and its transport into heart in a mouse model for juvenile visceral steatosis have been attributed to a point mutations in Slc22a5 (Horiuchi et al. 1994; Hashimoto et al. 1998; Nezu et al. 1999). Mutations in the orthologous gene SLC22A5 leads to systemic primary carnitine deficiency (CDSP) in human that is characterized by various symptoms such as chronic muscle weakness, cardiomyopathy, hypoglycemia and liver dysfunction (and in cases to sudden death), not including hearing loss (Nezu et al. 1999). Polymorphisms in SLC22A4 were associated with the development of rheumatoid arthritis (Tokuhiro et al. 2003; Kawasaki et al. 2004), and Crohn’s disease (Peltekova et al. 2004; Kato et al. 2010). Using microarray analysis, SLC22A4 was found to be expressed at significant levels in kidney, lung and neutrophils and at lower levels in many other tissues (including skeletal muscle, brain and retina; Bleasby et al. 2006). In the inner ear, Slc22a4 transcript was detected in purified mouse SV endothelial cell line, and the encoded protein was found abundant at the apical surface of the blood capillary endothelial cells of the SV, which led us to speculate that Slc22a4 may be indispensable for carnitine transport into the inner ear and for the cellular energy production in the SV.
SLC22A4 represents the fourth member of SLC gene family that have so far been identified to be responsible for a form of human HL. Other members include SLC26A4 (OMIM: 605646) encoding an anion (chloride and iodide) transporter known as pendrin and is the gene mutant in Pendred syndrome (PDS; OMIM: 274600) and in DFNB4 form of deafness with enlarged vestibular aqueduct (EVA; OMIM: 600791), SLC26A5 (OMIM: 604943) encoding for prestin, the motor protein for outer hair cells and mutant in DFNB61 deafness form, and SLC17A8 (OMIM: 607557) that encodes for hair cell synaptic vesicular glutamate transporter 3 (VGLUT3), responsible for dominant deafness form DFNA25.
In Caenorabditis elegans, knockout of OCT-1, the orthologous of mammalian OCTN1, resulted in significant elevation of oxidative damage accompanied by increased susceptibility to reactive oxygen species (ROS), and leading to shortening of lifespan (Cheah et al. 2013). Aging and extracellular insults, such as ototoxic drugs, cause oxidative stress via the accumulation of ROS in the inner ear that lead to cellular damage and hearing loss (Yamane et al. 1995; Clerici et al. 1996); free radicals trigger cell death pathways resulting in apoptosis (Forge & Schacht 2000). A number of experiments argued in favor of the ROS theory causing hair cell loss, via the administration of antioxidant that protected hearing against the effects of external insults, such as aminoglycosides and noise (Lautermann et al. 1997; Dehne et al. 2000). Slc22a4 has been found to specifically facilitate the transport of a powerful antioxidant, ergothioneine (ERGO; Gründemann et al. 2005). Interestingly, we have shown evidence that Slc22A4 is highly expressed in the inner surface of capillary wall of the SV. We speculate that SV dysfunction caused by SLC22A4 p.Cys113Tyr mutation would in turn alter the overall cochlear fatty-acid-based metabolic energy production pathway. SLC22A4 is therefore the first HL-causing protein in cochlear SV described so far with potential important role within this pathway. Further studies will be needed to estimate the frequency of this mutation within North African/Tunisian population, to evaluate the worthiness of its systematic screening for deafness prognosis in newly-wed couples, and to elucidate the mechanisms and molecular environment of the SV related to SLC22A4. Stem cell and gene-based therapeutic approaches applied to the SV capillary blood endothelial cells would be envisaged to overcome the defect in SLC22A4 function in DFNB60 individuals. More generally, as the unique known transporter for potent antioxidant ERGO, SLC22A4 presence in the cochlea makes it an ideal target for delivering ERGO into the cochlea and represent a promising direction for possible intervention in HL related to oxidative stress caused by genetic and environmental factors in the wider population.
Acknowledgments
We gratefully thank all the subjects in this study for their collaboration. This study was supported by the National Institutes of Health [grant numbers R01DC005575, 2P50DC000422-Sub-Project 6432 and R01DC012115] to X.Z.L., the National Institutes of Health [grant number R21DC009879], the University of Miami Provost Research Award, and the College of Arts and Sciences Gabelli Fellowship to Z.L., the National Institutes of Health [grant numbers R21DC012398 and R01DC010844] to X.R.S., and the National Institutes of Health [grant number R01DC009645] to M.T. This work was also supported by funds from the ICGEB (International Centre for Genetic Engineering and Biotechnology) and the Ministry of Higher Education and Research of Tunisia to S.M., and by a Grant-in-Aid of Scientific Research (B) from the Japanese Ministry of Education, Science and Culture [grant number 15H04664] and a grant from Hoansha Foundation (3-1-8 Dosho-machi, Chuo-ku, Osaka 541-0045, Japan) to Y.K.
Footnotes
Contributors - M.G., M.B.S., X.R.S., T.I, N.N., Y.K., S.M., Z.L., M.H. and X.L. designed research; M.G., M.B.S., I.C., B.Z., Q.M., T.I, Q.Y., B.H., R.M., N.N, A.G., L.N., X.R.S., Z.L. and M.H. performed research; M.G., M.B.S., B.Z., B.H., D.Y., A.G., T.I, N.N., Y.K., L.N., M.T., X.R.S., S.M., Z.L., M.H. and X.L. analyzed the data; M.G., M.B.S. and T.I. wrote the paper; M.G., M.B.S., I.C., D.Y., T.I, N.N., Y.K., M.T., X.R.S., S.M., Z.L., M.H. and X.L. edited the paper.
Competing financial interests - The authors have declared that no competing financial interests exist.
Ethics approval - The institutional review boards (IRB) of the University of Miami, and by the ethical committee of the University Hospital of Sfax, Tunisia.
Patient consent - Parental consents obtained.
REFERENCES
- Ben Arab S, Masmoudi S, Beltaief N, Hachicha S, Ayadi H. Consanguinity and endogamy in Northern Tunisia and its impact on non-syndromic deafness. Genet Epidemiol. 2004;27:74–79. doi: 10.1002/gepi.10321. [DOI] [PubMed] [Google Scholar]
- Bleasby K, Castle JC, Roberts CJ, Cheng C, Bailey WJ, Sina JF, Kulkarni AV, Hafey MJ, Evers R, Johnson JM, et al. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica. 2006;36:963–988. doi: 10.1080/00498250600861751. [DOI] [PubMed] [Google Scholar]
- Chakchouk I, Ben Said M, Jbeli F, Benmarzoug R, Loukil S, Smeti I, Chakroun A, Gibriel AA, Ghorbel A, Hadjkacem H, et al. NADf chip, a two-color microarray for simultaneous screening of multigene mutations associated with hearing impairment in North African Mediterranean countries. J Mol Diagn. 2015;17:155–161. doi: 10.1016/j.jmoldx.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Cheah IK, Ong RL, Gruber J, Yew TS, Ng LF, Chen CB, Halliwell B. Knockout of a putative ergothioneine transporter in Caenorhabditis elegans decreases lifespan and increases susceptibility to oxidative damage. Free Radic Res. 2013;47:1036–1045. doi: 10.3109/10715762.2013.848354. [DOI] [PubMed] [Google Scholar]
- Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98:116–124. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- Dehne N, Lautermann J, ten Cate WJ, Rauen U, de Groot H. In vitro effects of hydrogen peroxide on the cochlear neurosensory epithelium of the guinea pig. Hear Res. 2000;143:162–170. doi: 10.1016/s0378-5955(00)00036-8. [DOI] [PubMed] [Google Scholar]
- Farthing CA, Sweet DH. Expression and function of organic cation and anion transporters (SLC22 family) in the CNS. Curr Pharm Des. 2014;20:1472–1486. doi: 10.2174/13816128113199990456. [DOI] [PubMed] [Google Scholar]
- Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000;5:3–22. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- Grati M, Aggarwal N, Strehler EE, Wenthold RJ. Molecular determinants for differential membrane trafficking of PMCA1 and PMCA2 in mammalian hair cells. J Cell Sci. 2006;119:2995–3007. doi: 10.1242/jcs.03030. [DOI] [PubMed] [Google Scholar]
- Grati M, Chakchouk I, Ma Q, Bensaid M, Desmidt A, Turki N, Yan D, Baanannou A, Mittal R, Driss N, et al. A missense mutation in DCDC2 causes human recessive deafness DFNB66, likely by interfering with sensory hair cell and supporting cell cilia length regulation. Hum Mol Genet. 2015;24:2482–2491. doi: 10.1093/hmg/ddv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grati M, Kachar B. Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc Natl Acad Sci USA. 2011;108:11476–11481. doi: 10.1073/pnas.1104161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründemann D, Harlfinger S, Golz S, Geerts A, Lazar A, Berkels R, Jung N, Rubbert A, Schömig E. Discovery of the ergothioneine transporter. Proc Natl Acad Sci USA. 2005;102:5256–5261. doi: 10.1073/pnas.0408624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Suzuki F, Tamai I, Nikaido H, Kuwajima M, Hayakawa J, Tsuji A. Gene-dose effect on carnitine transport activity in embryonic fibroblasts of JVS mice as a model of human carnitine transporter deficiency. Biochem Pharmacol. 1998;55:1729–1732. doi: 10.1016/s0006-2952(97)00670-9. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Kobayashi K, Yamaguchi S, Shimizu N, Koizumi T, Nikaido H, Hayakawa J, Kuwajima M, Saheki T. Primary defect of juvenile visceral steatosis (jvs) mouse with systemic carnitine deficiency is probably in renal carnitine transport system. Biochim Biophys Acta. 1994;1226:25–30. doi: 10.1016/0925-4439(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Kato Y, Kubo Y, Iwata D, Kato S, Sudo T, Sugiura T, Kagaya T, Wakayama T, Hirayama A, Sugimoto M, et al. Gene knockout and metabolome analysis of carnitine/organic cation transporter OCTN1. Pharm Res. 2010;27:832–840. doi: 10.1007/s11095-010-0076-z. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kato Y, Sai Y, Tsuji A. Functional characterization of human organic cation transporter OCTN1 single nucleotide polymorphisms in the Japanese population. J Pharm Sci. 2004;93:2920–2926. doi: 10.1002/jps.20190. [DOI] [PubMed] [Google Scholar]
- Lautermann J, Crann SA, McLaren J, Schacht J. Glutathione-dependent antioxidant systems in the mammalian inner ear: effects of aging, ototoxic drugs and noise. Hear Res. 1997;114:75–82. doi: 10.1016/s0378-5955(97)00154-8. [DOI] [PubMed] [Google Scholar]
- Neng L, Zhang W, Hassan A, Zemla M, Kachelmeier A, Fridberger A, Auer M, Shi X. Isolation and culture of endothelial cells, pericytes and perivascular resident macrophage-like melanocytes from the young mouse ear. Nat Protoc. 2013;8:709–720. doi: 10.1038/nprot.2013.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezu J, Tamai I, Oku A, Ohashi R, Yabuuchi H, Hashimoto N, Nikaido H, Sai Y, Koizumi A, Shoji Y, et al. Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat Genet. 1999;21:91–94. doi: 10.1038/5030. [DOI] [PubMed] [Google Scholar]
- Pelis RM, Wright SH. SLC22, SLC44, and SLC47 transporters--organic anion and cation transporters: molecular and cellular properties. Curr Top Membr. 2014;73:233–261. doi: 10.1016/B978-0-12-800223-0.00006-2. [DOI] [PubMed] [Google Scholar]
- Peltekova VD, Wintle RF, Rubin LA, Amos CI, Huang Q, Gu X, Newman B, Van Oene M, Cescon D, Greenberg G, et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genet. 2004;36:471–475. doi: 10.1038/ng1339. [DOI] [PubMed] [Google Scholar]
- Shen J, Scheffer DI, Kwan KY, Corey DP. SHIELD: an integrative gene expression database for inner ear research. Database. 2015;2015:1–9. doi: 10.1093/database/bav071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21) Biopharm Drug Dispos. 2013;34:29–44. doi: 10.1002/bdd.1816. [DOI] [PubMed] [Google Scholar]
- Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem. 1998;273:20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- Taubert D, Jung N, Goeser T, Schömig E. Increased ergothioneine tissue concentrations in carriers of the Crohn's disease risk-associated 503F variant of the organic cation transporter OCTN1. Gut. 2009;58:312–314. doi: 10.1136/gut.2008.164418. [DOI] [PubMed] [Google Scholar]
- Tokuhiro S, Yamada R, Chang X, Suzuki A, Kochi Y, Sawada T, Suzuki M, Nagasaki M, Ohtsuki M, Ono M, et al. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat Genet. 2003;35:341–348. doi: 10.1038/ng1267. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi H, Tamai I, Nezu J, Sakamoto K, Oku A, Shimane M, Sai Y, Tsuji A. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. J Pharmacol Exp Ther. 1999;289:768–773. [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Takayama M, Iguchi H, Nakagawa T, Kojima A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur Arch Otorhinolaryngol. 1995;252:504–508. doi: 10.1007/BF02114761. [DOI] [PubMed] [Google Scholar]
- Yasunaga S, Grati M, Cohen-Salmon M, El-Amraoui A, Mustapha M, Salem N, El-Zir E, Loiselet J, Petit C. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat Genet. 1999;21:363–369. doi: 10.1038/7693. [DOI] [PubMed] [Google Scholar]