Abstract
Background
Insomnia is increasingly recognized as a public health concern in modern society. Insomnia diagnoses appear to be increasing and are associated with poor health outcomes. They may cost $100 billion annually in health services.
Objective
Given the adverse consequences of insomnia such as cardiovascular disease, diabetes, and depression, the present study was designed to examine the relationship of the trajectories of earlier cigarette smoking and later insomnia. The ultimate goal is to reduce the prevalence of insomnia.
Methods
674 participants (53% African Americans, 47% Puerto Ricans, 60% females) were surveyed at 6 points in time. We employed the growth mixture model to obtain the trajectories of cigarette smoking from age 14 to 32. We used logistic regression analyses to examine the associations between the trajectories of smoking and insomnia.
Results
Males were less likely to have insomnia than females (Adjusted odds ratio: AOR=0.34, p<.05). A higher Bayesian posterior probability (BPP) for the chronic smoking trajectory group (AOR=2.69, p<.05) and for the moderate smoking trajectory group (AOR=5.33, p<.01) was associated with an increased likelihood of having insomnia at age 36 compared with the BPP of the no or low smoking trajectory group.
Conclusions
Prevention and treatment programs for individuals who suffer from insomnia should be implemented in parallel with programs for smoking cessation. From a public health perspective, our longitudinal study that examined the association between earlier smoking trajectories and later insomnia suggests that treatments designed to reduce or cease smoking may lessen the occurrence of symptoms of insomnia.
Keywords: Longitudinal study, insomnia, trajectory analysis, cigarette use, ethnic minority youth
INTRODUCTION
Insomnia is characterized by a spectrum of complaints reflecting dissatisfaction with the quality, duration, or continuity of sleep. The predominant nocturnal symptoms include difficulties falling asleep at bedtime, waking up in the middle of the night and having difficulty going back to sleep, or waking up too early in the morning with an inability to return to sleep. In addition to the above criteria, a diagnosis of insomnia, based on the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5), requires the presence of symptoms that do not result from another disorder (American Psychiatric Association, 2013). Insomnia is increasingly recognized as a public health concern in modern society. In the United States, insomnia diagnoses appear to be increasing and are associated with poor health outcomes (Bains, 2006; Namen et al., 2002; Taylor et al., 2007). A recent study conducted in the United Kingdom reported modest increases in the prevalence of insomnia symptoms and diagnoses over their survey periods from 35.0% and 3.1% in 1993 to 38.6% and 5.8% in 2007, respectively (Calem et al., 2012).
Insomnia is related to a wide range of adverse outcomes such as cardiovascular disease, increased body mass index (BMI), diabetes, anxiety, depression, poor interpersonal relationships, car accidents, frequent absenteeism and disability at work (Brenes et al., 2009; Chien et al., 2010; Lee et al., 2009; Leger, Massuel, Metlaine, & Group, 2006; Léger et al., 2010; Ohayon & Roth, 2003; Overland et al., 2008; Vgontzas, Liao, Bixler, Chrousos, & Vela-Bueno, 2009). In addition, a longitudinal study from the National Longitudinal Study of Adolescent Health found that sleep difficulties at earlier waves significantly predicted the use of substances as well as substance-related problems at later waves (Wong, Robertson, & Dyson, 2015). Another longitudinal study found bidirectional relationships between sleep problems and substance use in young adolescents (Pieters et al., 2015). Given the adverse consequences of insomnia, the present study was designed to examine the predictors of insomnia to increase understanding of its genesis. Our ultimate goal is to contribute to a reduction in the prevalence of insomnia.
A number of studies based on self-report data suggest that cigarette smoking plays a role in arousal, sleep disturbance or difficulty, and insomnia (D. W. Brook, Rubenstone, Zhang, & Brook, 2012; Soldatos, Kales, Scharf, Bixler, & Kales, 1980; Wetter & Young, 1994). Most studies (Sahlin, Franklin, Stenlund, & Lindberg, 2009; Soldatos et al., 1980; Wetter & Young, 1994) have used a cross-sectional design. However, Brook and colleagues (D. W. Brook et al., 2012) using data from a 20 year longitudinal study of women in mid-life, found that women with a longitudinal pattern of chronic heavy smoking as compared to non-smokers had an increased likelihood of insomnia.
In addition, physiological research has supported the evidence for the effects of cigarette smoking on sleep by explaining the mechanisms involved. Research has shown that nicotine affects the neurotransmitter systems which may lead to arousal as well as to inhibition of sleep-promoting neurons (Htoo, Talwar, Feinsilver, & Greenberg, 2004; Saint-Mleux et al., 2004). Brain waves also appeared to be different in smokers and non-smokers during sleep. Specifically, increases in alpha frequency waves were found among smokers as compared to non-smokers, suggesting greater arousal (Domino et al., 2009; Zhang, Samet, Caffo, Bankman, & Punjabi, 2008). In the Sleep Heart Health Study consisting of 6,400 participants, sleep architecture was characterized. Compared with never smokers, current smokers had a longer initial sleep latency, less total sleep time, and more stage 1 sleep (Zhang, Samet, Caffo, & Punjabi, 2006).
Gender, ethnicity, depressive symptoms, age, educational level, partner status, and BMI are also related to insomnia. Women report more frequent symptoms of insomnia than men (Jaussent et al., 2011; Yoshioka et al., 2012). As regards ethnicity, Blacks as compared to Hispanics reported higher odds of insomnia using the diagnostic criteria from the International Classification of Diseases (Roth et al., 2011). In clinical samples, about three quarters of all depressed patients complain of difficulty either in initiation or in maintaining sleep (Tsuno, Besset, & Ritchie, 2005; Yates et al., 2007). Age also has some effects on sleep. For example, linear regression analysis revealed that increasing age is associated with shorter sleep latencies and greater insomnia (Xian et al., 2014). Lower education (Kim et al., 2013; Talala, Martelin, Haukkala, Härkänen, & Prättälä, 2012) and obesity (Roth et al., 2011) were related to insomnia. Studies of impaired sleep show differences in partner status. Married individuals are less likely to report frequent insufficient sleep than never married individuals (Chapman et al., 2012). Therefore, we use these variables as controls in the current study.
Although the adverse effects of cigarette smoking on sleep are clearly documented, there is little research about long-term patterns of smoking and insomnia. The current longitudinal study is uniquely positioned to examine the effects of smoking trajectories beginning in adolescence into the mid thirties on symptoms of insomnia; we identify the trajectories of cigarette smoking as predictors of insomnia over several developmental stages spanning a 22 year period. We also assess the predictors of insomnia among relatively understudied ethnic groups of African Americans and Puerto Ricans living in an urban area. A better understanding of the effects of long-term patterns of smoking on insomnia may help to guide the treatment of smoking cessation programs and treatment programs focused on insomnia.
We hypothesize that: 1) Trajectory groups with higher levels of smoking (e.g., the chronic smoking trajectory group) as compared to the no or low smoking trajectory group will be associated with an increased likelihood of insomnia; and 2) The associations between the patterns of cigarette smoking and insomnia will be maintained after controlling for gender, ethnicity, depressive symptoms, age, educational level, partner status, and BMI.
METHODS
Participants
Sample of the current research consisted of ethnic minority groups of African Americans and Puerto Ricans living in an urban area. Questionnaires were completed by 674 participants (53% African Americans, 47% Puerto Ricans) for the sixth wave (time 6; T6) of this study. Sixty percent were females (n=405). Data on the participants were first collected in 1990 (time 1; T1) when the participants were students attending schools in the East Harlem area of New York City. At T1, the questionnaires were administered in classrooms under the supervision of the study research staff with no teachers present. At time 2 (T2), the National Opinion Research Center located and interviewed the participants in person or by phone. At time 3 (T3), the Survey Research Center of the University of Michigan collected the data. At Time 4 (T4), Time 5 (T5), and T6, the data were collected by our research group. Table 1 shows time points of data collection, the years, the number of participants, and ages of the participants at each time point. More information regarding the sample description can be found in previous research (Blinded for review).
TABLE 1.
Description of the original sample
| Wave | Year | Number of participants | Mean age (Standard deviation) | Inter-quartile range of age |
|---|---|---|---|---|
| Time 1 | 1990 | 1,332 | 14.1 (1.3) | 13–15 |
| Time 2 | 1994–1996 | 1,190 | 19.2 (1.5) | 18–20 |
| Time 3 | 2000–2001 | 662 | 24.4 (1.3) | 23–25 |
| Time 4 | 2004–2006 | 838 | 29.2 (1.4) | 28–30 |
| Time 5 | 2007–2010 | 816 | 32.3 (1.3) | 31–34 |
| Time 6 | 2010–2013 | 674 | 35.9 (1.4) | 35–37 |
Note: The reduction in funding at T3 is the reason for the somewhat smaller sample at T3.
The Institutional Review Board (IRB) of the New York University School of Medicine approved the study for T4, T5, and T6. The IRBs of both the Mount Sinai School of Medicine and New York Medical College approved the study’s procedures for data collections in the earlier waves. A Certificate of Confidentiality was obtained from the National Institute on Drug Abuse for T1-T4 and from the National Cancer Institute at T5 and at T6. At T1 and T2, passive consent was obtained from the parents of minors. At each time wave, we obtained informed assent or consent from each participant.
At T6, we attempted to follow-up all those who participated at T1. We compared the demographic variables for the 674 adults who participated at both T1 and T6 with the 658 who participated at T1 but not at T6. There were no significant differences between the T6 non-participants and the T6 participants in the proportion of African Americans and Puerto Ricans (χ21 = 3.48, p=0.06) and mean level of depressive symptoms at T1 (t1=0.81, p=0.42). However, the percentage of males at T1 among T6 non-participants (53%) was higher than the percentage of males who participated at T6 (40%) (χ21 = 26.06, p <.001). The mean age at T1 among T6 non-participants (14.5) was higher than the mean age at T1 among T6 participants (14.0) (t1=7.14, p<.001). The frequency of cigarette use at T1 among T6 non-participants (0.36) was higher than the frequency of cigarette use at T1 among T6 participants (0.20) (t1=4.26, p<.001).
Measures
Control variables
Gender (1=female, 2=male)
Ethnicity (1=African American, 2=Puerto Rican)
Depressive symptoms (Derogatis, Lipman, Rickels, Uhlenhuth, & Covi, 1974) at T1 were assessed with a 2 item scale, i.e. “Do you sometimes feel unhappy, sad, or depressed?” and “Do you sometimes feel hopeless about the future?” using a 4-point Likert scale that ranged from “not at all” to “extremely.” The inter-correlation between the 2 items was 0.47 (p<.001).
Age at T6
Educational level at T6 (0=11th grade or below, 1=12th grade or GED, 2=Business or technical school, 3=College freshman, 4=College sophomore or Associate’s Degree, 5=College junior, 6=College senior or Bachelor’s Degree, 7=Postgraduate business, law, medical, masters, or doctoral program)
Partner status at T6 (0=Single, separated, divorced, or widowed, 1=Cohabiting or married)
Body mass index at T6 [BMI= (Weight in pounds/Height in inches2)×703] (A. Brook, 2006)
Cigarette use (T1-T5)
The participants reported on their cigarette smoking at each wave between T1 and T6. Participants were asked “How many cigarettes do you smoke?” at T1 and “How many cigarettes did you smoke in the past 5 years?” at T2 through T5. The answer options were 0=none, 1=a few cigarettes or less a week, 2=1–5 cigarettes a day, 3=about half a pack a day, 4=about 1 pack a day, 5=about 1 and half packs a day, and 6=more than 1 and half packs a day.
Insomnia at T6
Insomnia was assessed at T6 by using an adaptation of the Insomnia Severity Index measure (Bastien, Vallières, & Morin, 2001; Morin, 1993). Insomnia in this study included five of the seven Insomnia Severity Index criteria: 1) difficulty falling asleep, 2) difficulty staying asleep, 3) waking up too early in the morning, 4) distress caused by sleep difficulties, and 5) interference of sleep difficulties with daytime functioning. A 5-point Likert scale was used for each item (0=none, 1=mild, 2=moderate, 3=severe, 4=very severe), yielding a total score ranging from 0 to 20. Based on the guidelines for scoring (Morin, 1993), the total score with the percentages is categorized as follows: 0–5=no clinically significant insomnia (62.2%); 6–10=subthreshold insomnia (20.9%); 11–15=moderate insomnia (10.8%); and 16–20=severe insomnia (6.1%). The prevalence of severe insomnia, 6.1%, is consistent with the prevalence reported in the DSM-5 discussion of insomnia, which ranged from 6–10% (American Psychiatric Association, 2013). The internal consistency of the measure as estimated by Cronbach’s alpha was 0.93. In this study, a score of 1 was assigned to a participant with insomnia (i.e., a total score of 16 or higher indicating severe insomnia). Otherwise a participant was scored 0.
Analytic Procedure
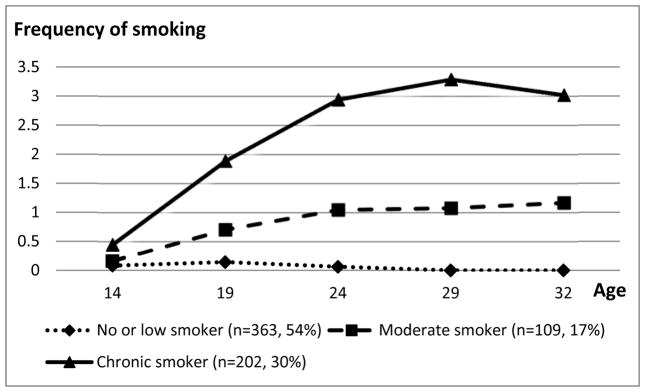
We employed the growth mixture model method using Mplus software (Muthén & Muthén, 2010) to obtain the trajectories of cigarette smoking from T1 to T6. Cigarette smoking at each point in time was treated as a censored normal variable. We applied the full information maximum likelihood approach for missing data (Muthén & Muthén, 2010). We used the optimal Bayesian Information Criterion (BIC; the smallest absolute value indicates the most parsimonious model) (Raftery, 1985; Schwarz, 1978), the entropy measure (values closer to 1 indicate better fit) (McLachlan & Peel, 2000), and Lo-Mendell-Rubin adjusted likelihood ratio test (LMR-LRT) (Lo, Mendell, & Rubin, 2001) to estimate the number of trajectory groups provided that each trajectory group had a prevalence of 5% or more. The LMR-LRT has a null hypothesis of the model with one less class. We also considered the accuracy of group classification indicated by the average Bayesian posterior probability (BPP). Average BPP of 0.70 or higher are considered sufficient to avoid classification error (Nagin, 2005). The observed trajectory for a group consisted of the average frequency of cigarette smoking at each point in time when participants were assigned to the group with the largest BPP (see Figure 1).
FIGURE 1.
Smoking trajectories from the mid adolescence to the early thirties
Note. The answer options for smoking were 0=none, 1=a few cigarettes or less a week, 2=1–5 cigarettes a day, 3=about half a pack a day, 4=about 1 pack a day, 5=about 1 and half packs a day, and 6=more than 1 and half packs a day.
To examine the associations of membership in a trajectory group, we used logistic regression analyses (Cody & Smith, 2005) designating the insomnia indicator variable (i.e., insomnia=1; otherwise=0) as the dependent variable and the BPPs of membership in the trajectory groups as the independent variables. The BPPs of the no or low cigarette smoking trajectory group were used as the reference probability. Gender, ethnicity, depressive symptoms at T1, age at T6, educational level at T6, partner status at T6, and BMI at T6 were used as control variables.
RESULTS
Among the 674 participants, the mean and standard deviation (SD) of cigarette smoking at each point in time were 0.2 (0.6), 0.8 (1.3), 1.2 (1.6), 1.1 (1.6), and 1.1 (1.5) for T1-T5, respectively. We computed solutions for 2 through 4 trajectory groups. The BICs, the entropy measures, and p values for LMR-LRT were 6493, 0.95, <0.0001 for the 2 group model, 6411, 0.87, <0.0001 for the 3 group model, and 6405, 0.77, 0.1679 for the 4 group model. We chose the 3 trajectory group model because the 4 trajectory group model had a small difference in BIC as compared to the 3 trajectory group model, the lower entropy measure than the 3 trajectory group model, and non-significant LMR-LRT result. The mean BPP of the participants who were assigned to the groups ranged from 88% to 98%, which indicated an adequate classification.
As shown in Figure 1, we labeled the three cigarette smoking trajectory groups as follows. The no or low cigarette smoking trajectory group had an estimated prevalence of 54% and included participants who reported no or low use of cigarette at each wave. The moderate cigarette smoking trajectory group (17%) included participants who reported no use of cigarettes at age 14, use of a few cigarettes or less a day (i.e., on average use of 0.7 cigarettes) at age 19, about a few cigarettes a day at ages 24, 29, and 32 (i.e., on average use of 1.0–1.2 cigarettes). The chronic cigarette smoking trajectory group (30%) included participants who reported use of less than a few cigarettes a day at age 14, less than 1–5 cigarettes a day at age 19, and about half a pack a day at age 24, and more than half a pack a day at ages 29 and 32. Table 2 contains the means with SDs or percentages in each trajectory group for the variables in the study.
TABLE 2.
Means with standard deviations or percentages of sleep problems and other variables by the smoking trajectory groups
| No or low smoker (54%, n=363) | Moderate smoker (17%, n=109) | Chronic smoker (30%, n=202) | Whole sample (N=674) | |
|---|---|---|---|---|
| Sleep measures | ||||
| Insomnia (T6) | 3.0% | 11.9% | 8.4% | 6.1% |
| Difficulty falling asleep (T6) | 0.9 (1.1) | 1.2 (1.2) | 1.1 (1.2) | 1.0 (1.2) |
| Difficulty staying asleep (T6) | 0.9 (1.0) | 1.2 (1.3) | 1.0 (1.2) | 1.0 (1.1) |
| Problems waking up too early (T6) | 1.0 (1.1) | 1.4 (1.3) | 1.2 (1.2) | 1.1 (1.2) |
| Distress caused by sleep difficulties (T6) | 1.0 (1.2) | 1.3 (1.4) | 1.2 (1.3) | 1.0 (1.3) |
| Interference of sleep difficulties with daytime functioning (T6) | 0.9 (1.2) | 1.2 (1.4) | 1.2 (1.4) | 1.1 (1.3) |
| Demographics | ||||
| Gender-Female | 68.3% | 56.9% | 47.0% | 60.1% |
| Ethnicity-African American | 58.9% | 39.5% | 49.0% | 52.8% |
| Depressive symptoms (T1) | 2.5 (0.9) | 2.6 (0.8) | 2.7 (0.9) | 2.6 (0.9) |
| Age (T6) | 36.0 (1.5) | 35.8 (1.3) | 35.8 (1.4) | 35.9 (1.4) |
| Educational level (T6) | 3.6 (2.3) | 3.0 (2.3) | 2.1 (2.0) | 3.1 (2.3) |
| Partner status-Cohabiting or married (T6) | 52.9% | 46.8% | 48.5% | 50.1% |
| BMI (T6) | 30.2 (6.7) | 28.6 (7.5) | 29.8 (8.7) | 29.8 (7.5) |
Notes: T1=Time1 (Mean age 14), T6=Time 6 (Mean age 36)
Answer options for sleep problems at T6; 0=none, 1=mild, 2=moderate, 3=severe, 4=very severe
Answer options for depressive symptoms at T1; 0=not at all, 1= a little, 2=somewhat, 3=extremely
Answer options for educational level at T6; 0=11th grade or below, 1=12th grade or GED, 2=Business or technical school, 3=College freshman, 4=College sophomore or Associate’s Degree, 5=College junior, 6=College senior or Bachelor’s Degree, 7=Postgraduate business, law, medical, masters, or doctoral program
Table 3 presents: a) the odds ratios (OR) without the control variables and b) the adjusted odds ratios (AOR) of each variable after adjustment for the other variables in the model. With regard to the control variables, males were less likely to have insomnia than females (AOR=0.34, p<.05). Greater depressive symptoms at T1 (OR=1.69, p<.01) and lower educational level at T6 (OR=0.82, p<.05) were associated with a greater likelihood of insomnia. Compared to the BPP of the no or low smoking trajectory group, a higher BPP for the chronic smoking trajectory group (OR=3.01, p<.01; AOR=2.69, p<.05) and for the moderate smoking trajectory group (OR=4.58, p<.01; AOR=5.33, p<.01) were associated with an increased likelihood of having insomnia at T6 after adjustment for the control variables.
TABLE 3.
Odds ratios and adjusted odds ratios with 95% confidence intervals of insomnia at age 36 as predicted by BPPs of smoking trajectories and control variables.
| Odds ratio (95% confidence interval) | Adjusted odds ratio (95% confidence interval) | |
|---|---|---|
| Gender | 0.53 (0.26, 1.08) | 0.34 (0.15, 0.81) * |
| Ethnicity | 1.81 (0.95, 3.46) | 1.42 (0.68, 2.99) |
| Depressive symptoms at T1 | 1.69 (1.16, 2.48) ** | 1.36 (0.88, 2.12) |
| Age at T6 | 0.90 (0.71, 1.14) | 0.94 (0.71, 1.24) |
| Educational level at T6 | 0.82 (0.70, 0.96) * | 0.84 (0.71, 1.01) |
| Partner status at T6 | 0.68 (0.36, 1.28) | 0.86 (0.42, 1.76) |
| BMI at T6 | 1.03 (0.99, 1.07) | 1.03 (0.99, 1.07) |
| Chronic smokers vs. No or low smokers | 3.01 (1.34, 6.78) ** | 2.69 (1.06, 6.82) * |
| Moderate smokers vs. No or low smokers | 4.58 (1.85, 11.34) ** | 5.33 (1.94, 14.64) ** |
Notes.
p<.05,
p<.01;
T1=Time1 (Mean age 14), T6=Time 6 (Mean age 36);
Male gender coded with a higher score; Puerto Rican ethnicity coded with a higher score.
DISCUSSION
As hypothesized, the findings indicated that 1) the higher level of smoking trajectory groups (i.e., the chronic and the moderate smoking trajectory groups) compared to the no or low smoking trajectory group were associated with an increased likelihood of insomnia; and 2) the associations between the trajectories of cigarette smoking and insomnia were maintained after controlling for gender, ethnicity, depressive symptoms, age, educational level, partner status, and BMI.
Our findings are consistent with the results from other investigators who found, for instance, that current smokers had a longer initial sleep latency and less total sleep time than never smokers (Sahlin et al., 2009; Zhang et al., 2006). Our findings are also consistent with the findings that smokers had more difficulty initiating sleep (Wetter & Young, 1994).
The linkage between smoking and insomnia can be explained by psychosocial and physiological mechanisms. Smoking has been found to be related to depressive symptoms (Almeida & Pfaff, 2005; Holma, Holma, Melartin, Ketokivi, & Isometsä, 2013; Pratt & Brody, 2010). Depressive symptoms often co-occur with insomnia, separated, divorced, or widowed marital status, and higher BMI (Foley, Ancoli-Israel, Britz, & Walsh, 2004; Ohayon & Roth, 2003; Roth, 2007; Stewart et al., 2006). However, our analysis controlled for depressive symptoms, partner status, and BMI as well as demographic variables such as gender, ethnicity, age, and educational level, and our findings were maintained.
Biological effects can also explain the association of smoking with insomnia. Increased alpha frequencies and the release of neurotransmitters are associated with arousal involved in sleep (Saint-Mleux et al., 2004; Zhang et al., 2008). Heavy smoking during the day may cause nicotine withdrawal at night time, leading to sleep disturbances and awakening (Wetter & Young, 1994). Indeed, about 20% of heavy smokers awoke from sleep due to nighttime nicotine withdrawal (Rieder, Kunze, Groman, Kiefer, & Schoberberger, 2001). One possibility is that there are changes in both reward and sleep-related systems that occur during adolescence, and smoking (particularly chronic levels) may impair normal development of these resulting in increased rates of insomnia.
Limitations and Strengths
We did not examine several other factors (e.g., physical or mental health conditions, social support) that may contribute to insomnia among cigarette smokers. Indeed, heart disease, bodily pain, diabetes, lung disease, stroke, sleep apnea, and osteoporosis have been found to be associated with sleep-related problems such as breathing pauses, snoring, daytime sleepiness, restless leg syndrome, or insufficient sleep (Foley et al., 2004). According to Gosling and colleagues, having an insecure job and job skills that are not marketable, rarely taking time to relax, having less support from friends or family, and poor physical or mental health increased the odds of sleep disturbances (Gosling, Batterham, Glozier, & Christensen, 2014). Future studies should include these factors as well as symptoms of insomnia at earlier time points in order to obtain a clearer association between smoking and insomnia. Also, future study should include the shorter intervals between smoking measurements in order to deal with bias and/or more complex trajectories of smoking.
The sample consisted of African American and Puerto Rican inner city adolescents studied until the mid 30’s. Consequently, the association between the trajectories of cigarette smoking and insomnia may not apply to the general population. Further studies should include other ethnic groups. The individuals in our current sample smoked less at T1 than the individuals who dropped out. This may have some impacts on our results. Our data are also based on self-reports. However, studies have shown that the use of this type of self-report data yields reliable results (Ledgerwood, Goldberger, Risk, Lewis, & Price, 2008).
Despite these limitations, the study supports and adds to the literature on this topic in a number of ways. First, unlike most research studies in this area that focus on one point in time, we assess cigarette smoking at five points in time over a span of up to 18 years. The prospective nature of the data allowed us to go beyond a cross-sectional analysis and to consider the temporal sequencing of variables. Second, a major contribution of the study is a unique set of findings associated with different trajectories of cigarette smoking beginning in adolescence as related to adult insomnia in a sample of African American and Puerto Rican living in an urban area of New York City.
Conclusions and Clinical Implications
The clinical implications of our findings shed light on the importance of quitting smoking in order to reduce insomnia among African American and Puerto Rican adults living in an urban area. As a result, we conclude that treatment programs for insomnia should be implemented in parallel with treatment programs focused on smoking. This could form a component or module of existing smoking treatment programs and also may lead to a reduction in insomnia. Indeed, Luo reported that never quitters who continue to smoke as compared to ever quitters who made successful or unsuccessful attempts to quit were more likely to have insomnia (Luo, 2013).
From a public health perspective, our longitudinal study to examine the association between earlier smoking trajectories and later insomnia suggests that treatments designed to reduce or cease smoking may lessen the occurrence of symptoms of insomnia. In sum, a reduction in smoking may be accompanied by a decrease in insomnia.
Acknowledgments
This research was supported by the following grants from the National Institutes of Health: research grant CA084063 from the National Cancer Institute and research grant DA05702 from the National Institute on Drug Abuse.
GLOSSARY
- Trajectory analysis
An approach using trajectory analysis enables us to examine the magnitude, length of time, and the starting point of the use of substance
Footnotes
DECLARATION OF INTERESTS
None declared.
References
- Almeida Osvaldo P, Pfaff Jon J. Depression and smoking amongst older general practice patients. J Affect Disorders. 2005;86(2):317–321. doi: 10.1016/j.jad.2005.02.014. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-V) American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- Bains Oneil S. Insomnia: difficulty falling and staying asleep. In: Watson BBNF, editor. Clinician’s Guide to Sleep Disorders. New York, NY: Informa Healthcare; 2006. pp. 83–109. [Google Scholar]
- Bastien Célyne H, Vallières Annie, Morin Charles M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Brenes Gretchen A, Miller Michael E, Stanley Melinda A, Williamson Jeff D, Knudson Mark, McCall W Vaughn. Insomnia in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry. 2009;17(6):465–472. doi: 10.1097/jgp.0b013e3181987747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook Adam. The Golden Gate Diet: How to Lose Weight And Maintain Your Health, A Scientific Method for Weight Loss. Los Angeles, CA: Midsummer Press; 2006. [Google Scholar]
- Brook David W, Rubenstone Elizabeth, Zhang Chenshu, Brook Judith S. Trajectories of cigarette smoking in adulthood predict insomnia among women in late mid-life. Sleep Med. 2012;13(9):1130–1137. doi: 10.1016/j.sleep.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calem Maria, Bisla Jatinder, Begum Aysha, Dewey Michael, Bebbington Paul E, Brugha Traolach, McManus Sally. Increased prevalence of insomnia and changes in hypnotics use in England over 15 years: analysis of the 1993, 2000, and 2007 National Psychiatric Morbidity Surveys. Sleep. 2012;35(3):377–384. doi: 10.5665/sleep.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman Daniel P, Wheaton Anne G, Perry Geraldine S, Sturgis Stephanie L, Strine Tara W, Croft Janet B. Household demographics and perceived insufficient sleep among US adults. J Commun Health. 2012;37(2):344–349. doi: 10.1007/s10900-011-9451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Kuo-Liong, Chen Pei-Chung, Hsu Hsiu-Ching, Su Ta-Chen, Sung Fung-Chang, Chen Ming-Fong, Lee Yuan-Teh. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33(2):177–184. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody RP, Smith JK. Applied Statistics and the SAS Programing Language. 5. Upper Saddle River, NJ: Prentice Hall; 2005. [Google Scholar]
- Derogatis Leonard R, Lipman Ronald S, Rickels Karl, Uhlenhuth Eberhard H, Covi Lino. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behavioral Science. 1974;19(1):1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- Domino Edward F, Ni Lisong, Thompson Michael, Zhang Huilei, Shikata Hiroki, Fukai Hiromi, Ohya Ippei. Tobacco smoking produces widespread dominant brain wave alpha frequency increases. Int J Psychophysiol. 2009;74(3):192–198. doi: 10.1016/j.ijpsycho.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley Daniel, Ancoli-Israel Sonia, Britz Patricia, Walsh James. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Gosling John A, Batterham Philip J, Glozier Nick, Christensen Helen. The influence of job stress, social support and health status on intermittent and chronic sleep disturbance: an 8-year longitudinal analysis. Sleep Med. 2014;15(8):979–985. doi: 10.1016/j.sleep.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Holma Irina AK, Holma K Mikael, Melartin Tarja K, Ketokivi Mikko, Isometsä Erkki T. Depression and smoking: A 5-year prospective study of patients with major depressive disorder. Depress Anxiety. 2013;30(6):580–588. doi: 10.1002/da.22108. [DOI] [PubMed] [Google Scholar]
- Htoo Aung, Talwar Arunabh, Feinsilver Steven H, Greenberg Harly. Smoking and sleep disorders. Med Clin N Am. 2004;88(6):1575–1591. doi: 10.1016/j.mcna.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Jaussent Isabelle, Dauvilliers Yves, Ancelin Marie-Laure, Dartigues Jean-François, Tavernier Béatrice, Touchon Jacques, Besset Alain. Insomnia symptoms in older adults: associated factors and gender differences. Am J Geriatr Psychiatry. 2011;19(1):88–97. doi: 10.1097/JGP.0b013e3181e049b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Won-Hyoung, Kim Byung-Soo, Kim Shyn-Kyum, Chang Sung-Man, Lee Dong-Woo, Cho Maeng-Je, Bae Jae-Nam. Prevalence of insomnia and associated factors in a community sample of elderly individuals in South Korea. Int Psychogeriatr. 2013;25(10):1729–1737. doi: 10.1017/S1041610213000677. [DOI] [PubMed] [Google Scholar]
- Ledgerwood David M, Goldberger Bruce A, Risk Nathan K, Lewis Collins E, Price Rumi Kato. Comparison between self-report and hair analysis of illicit drug use in a community sample of middle-aged men. Addictive Behaviors. 2008;33(9):1131–1139. doi: 10.1016/j.addbeh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Choh AC, Demerath EW, Knutson KL, Duren DL, Sherwood RJ, Siervogel RM. Sleep disturbance in relation to health-related quality of life in adults: the Fels Longitudinal Study. J Nutr Health Aging. 2009;13(6):576–583. doi: 10.1007/s12603-009-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger Damien, Massuel M, Metlaine Arnaud Group, SISYPHE Study. Professional correlates of insomnia. Sleep. 2006;29(2):171–178. [PubMed] [Google Scholar]
- Léger Damien, Partinen Markku, Hirshkowitz Max, Chokroverty Sudhansu, Touchette Evelyne, Hedner Jan. Daytime consequences of insomnia symptoms among outpatients in primary care practice: Equinox international survey. Sleep Med. 2010;11(10):999–1009. doi: 10.1016/j.sleep.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Lo Yungtai, Mendell Nancy R, Rubin Donald B. Testing the number of components in a normal mixture. Biometrika. 2001;88(3):767–778. [Google Scholar]
- Luo Sheng. Joint analysis of stochastic processes with application to smoking patterns and insomnia. Stat Med. 2013;32(29):5133–5144. doi: 10.1002/sim.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan Geoffrey, Peel David. Finite mixture models. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- Morin CM. Insomnia: psychological assessment and management. Guilford; New York: 1993. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- Nagin Daniel. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Namen Andrew M, Dunagan Donnie P, Fleischer Alan, Tillett Janine, Barnett Molly, McCall W Vaughn, Haponik Edward F. Increased Physician-Reported Sleep Apnea The National Ambulatory Medical Care Survey. Chest. 2002;121(6):1741–1747. doi: 10.1378/chest.121.6.1741. [DOI] [PubMed] [Google Scholar]
- Ohayon Maurice M, Roth Thomas. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- Overland Simon, Glozier Nicholas, Sivertsen Børge, Stewart Robert, Neckelmann Dag, Krokstad Steinar, Mykletun Arnstein. A comparison of insomnia and depression as predictors of disability pension: the HUNT Study. Sleep. 2008;31(6):875–880. doi: 10.1093/sleep/31.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters Sara, Burk William J, Van der Vorst Haske, Dahl Ronald E, Wiers Reinout W, Engels Rutger CME. Prospective relationships between sleep problems and substance use, internalizing and externalizing problems. Journal of Youth and Adolescence. 2015;44(2):379–388. doi: 10.1007/s10964-014-0213-9. [DOI] [PubMed] [Google Scholar]
- Pratt Laura A, Brody Debra J. Depression and smoking in the US household population aged 20 and over, 2005–2008. NCHS Data Brief. 2010;(34):1–8. [PubMed] [Google Scholar]
- Raftery Adrian E. A model for high-order Markov chains. Journal of the Royal Statistical Society. Series B (Methodological) 1985;47(3):528–539. [Google Scholar]
- Rieder Anita, Kunze Ursula, Groman E, Kiefer Ingrid, Schoberberger R. Nocturnal Sleep-Disturbing Nicotine Craving: A Newly Described Symptom of Extreme Nicotine Dependence. Acta Med Austriaca. 2001;28(1):21–22. doi: 10.1046/j.1563-2571.2001.01005.x. [DOI] [PubMed] [Google Scholar]
- Roth Thomas. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7. [PMC free article] [PubMed] [Google Scholar]
- Roth Thomas, Coulouvrat Catherine, Hajak Goeran, Lakoma Matthew D, Sampson Nancy A, Shahly Victoria, Kessler Ronald C. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders, criteria: results from the America insomnia survey. Biol Psychiatry. 2011;69(6):592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Sahlin Carin, Franklin Karl A, Stenlund Hans, Lindberg Eva. Sleep in women: normal values for sleep stages and position and the effect of age, obesity, sleep apnea, smoking, alcohol and hypertension. Sleep Medicine. 2009;10(9):1025–1030. doi: 10.1016/j.sleep.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Saint-Mleux Benoît, Eggermann Emmanuel, Bisetti Arnaud, Bayer Laurence, Machard Danièle, Jones Barbara E, Serafin Mauro. Nicotinic enhancement of the noradrenergic inhibition of sleep-promoting neurons in the ventrolateral preoptic area. J Neurosci. 2004;24(1):63–67. doi: 10.1523/JNEUROSCI.0232-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Gideon. Estimating the dimension of a model. The Annals of Statistics. 1978;6(2):461–464. [Google Scholar]
- Soldatos Constantin R, Kales Joyce D, Scharf Martin B, Bixler Edward O, Kales Anthony. Cigarette smoking associated with sleep difficulty. Science. 1980;207(4430):551–553. doi: 10.1126/science.7352268. [DOI] [PubMed] [Google Scholar]
- Stewart Robert, Besset Alain, Bebbington Paul, Brugha Traolach, Lindesay James, Jenkins Rachel, Meltzer Howard. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29(11):1391–1397. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- Talala Kirsi M, Martelin Tuija P, Haukkala Ari H, Härkänen Tommi T, Prättälä Ritva S. Socio-economic differences in self-reported insomnia and stress in Finland from 1979 to 2002: a population-based repeated cross-sectional survey. BMC public health. 2012;12(1):650. doi: 10.1186/1471-2458-12-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor Daniel J, Mallory Laurel J, Lichstein Kenneth L, Durrence H, Riedel Brant W, Bush Andrew J. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213–218. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- Tsuno Norifumi, Besset Alain, Ritchie Karen. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254–1269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- Vgontzas Alexandros N, Liao Duanping, Bixler Edward O, Chrousos George P, Vela-Bueno Antonio. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter David W, Young Terry B. The relation between cigarette smoking and sleep disturbance. Prev Med. 1994;23(3):328–334. doi: 10.1006/pmed.1994.1046. [DOI] [PubMed] [Google Scholar]
- Wong Maria M, Robertson Gail C, Dyson Rachel B. Prospective relationship between poor sleep and substance-related problems in a national sample of adolescents. Alcoholism: Clinical and Experimental Research. 2015;39(2):355–362. doi: 10.1111/acer.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian Hong, Gonzalez Carlos, Deych Elena, Farris Suzan, Ding Jimin, Shannon William, McCall W Vaughn. Age-Related Effects on Circadian Phase in the Sleep of Patients with Depression and Insomnia. Behav Sleep Med. 2014:1–9. doi: 10.1080/15402002.2013.855213. (in press) [DOI] [PubMed] [Google Scholar]
- Yates William R, Mitchell Jeff, Rush A John, Trivedi Madhukar, Wisniewski Stephen R, Warden Diane, Gaynes Bradley N. Clinical features of depression in outpatients with and without co-occurring general medical conditions in STAR* D: confirmatory analysis. Prim Care Companion J Clin Psychiatry. 2007;9(1):7–15. doi: 10.4088/pcc.v09n0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka Eiji, Saijo Yasuaki, Kita Toshiko, Satoh Hiroki, Kawaharada Mariko, Fukui Tomonori, Kishi Reiko. Gender differences in insomnia and the role of paid work and family responsibilities. Soc Psychiatry Psychiatr Epidemiol. 2012;47(4):651–662. doi: 10.1007/s00127-011-0370-z. [DOI] [PubMed] [Google Scholar]
- Zhang Lin, Samet Jonathan, Caffo Brian, Bankman Isaac, Punjabi Naresh M. Power spectral analysis of EEG activity during sleep in cigarette smokers. Chest. 2008;133(2):427–432. doi: 10.1378/chest.07-1190. [DOI] [PubMed] [Google Scholar]
- Zhang Lin, Samet Jonathan, Caffo Brian, Punjabi Naresh M. Cigarette smoking and nocturnal sleep architecture. Am J Epidemio. 2006;164(6):529–537. doi: 10.1093/aje/kwj231. [DOI] [PubMed] [Google Scholar]