Abstract
Background
The genomic heterogeneity of HIV-1 impedes the ability of consensus sequences in vaccines to elicit effective antiviral immune responses. AGS-004 amplifies translation-competent RNA molecules encoding for Gag, Rev, Vpr, and Nef from the patient’s autologous virus and loads them into dendritic cells (DC).
Methods
This phase IIB, multicenter, 2:1 randomized, double blind, placebo-controlled study enrolled 54 HIV-1-infected patients on antiretroviral therapy (ART) with viral loads (VL) <50 copies/mL, current CD4 T-cell counts >450 cells/mm3, and nadir counts >200 cells/mm3, to receive intradermal injections of study product into the axillary lymph node region every 4 weeks. At week 16, a 12-week analytical treatment interruption (ATI) was undertaken.
Results
There was no difference in the end-of-ATI VL (average of values from weeks 11 and 12) between the two arms of the study (4.39 [4.17, 4.69] vs. 4.47 [3.76, 4.64] log10 HIV-1 RNA; P = 0.73). Between arms no change between pre-ART VL and the end-of-ATI VL (−0.06 [0.24, −0.32] vs. −0.17 [0.17, −0.32] log10 HIV-1 RNA; P = 0.43) was observed. When IFN-γ, IL-2, TNF-α, CD107a, and granzyme b expression was measured by multicolor flow cytometry, a greater percentage of AGS-004 than of placebo recipients had multifunctional CTL responses induced in the CD28+/CD45RA-CD8 effector/memory T-cell population to DCs electroporated with autologous antigens. Adverse events consisted of transient, mild (grade 1) local injection site reactions.
Conclusions
Despite the induction of HIV-specific effector/memory CD8 T-cell responses, no antiviral effect was seen after the administration of AGS-004 when compared to placebo.
Keywords: HIV, therapeutic immunization, dendritic cell therapy, autologous antigen, functional cure
INTRODUCTION
Therapeutic immunization has gained renewed attention as an important component of HIV eradication strategies in patients with established infection.1 Cells induced to express latent virus need to be exposed to activated HIV-specific immunity in order to be killed.2 In addition, therapeutic vaccination by itself could effect a “functional” cure by prompting the immune system to fully suppressive control of viral replication in the absence of antiretroviral chemotherapy.3
Challenges to the development of an effective immunotherapy against HIV include the wide inter- and intrapatient HIV genetic diversity and the compromised immune environment in an infected person. In addressing the first limitation, pulses of exposure to the patient’s own virus via treatment interruptions were shown to have a modest, but significant, effect on controlling viral replication in an analytical treatment interruption (ATI) period.4
Another approach to an autologous antigen immunotherapy is to use as the antigen source RNA encoding relevant HIV antigens from the patient’s own virus. AGS-004 is an immunotherapy that loads RNA encoding HIV Gag, Nef, Rev, and Vpr amplified from the patient’s virus into the patient’s dendritic cells (DCs).
Antigen presentation and CD4 T-cell help are impaired in HIV-infected persons. The ex vivo maturation of antigen-presenting DCs, with CD40L providing the signal normally provided by CD4 T cells, attempts to circumvent these weaknesses in the natural immune response.
In previous studies in HIV-infected persons, AGS-004 induced strong central and effector memory CD8 T-cell responses against autologous HIV antigens.5 We studied the ability of AGS-004 to induce HIV-specific immune responses that would translate into improved control of HIV replication during a subsequent ATI in persons with HIV-1 infection on effective ART.
METHODS
Study Design
We conducted a phase IIB, multicenter, 2:1 randomized, double-blind, placebo-controlled study. Eligible study participants were HIV-1-infected men and women between the ages of 18 and 60 years on ART with plasma HIV-1 RNA levels ≤200 copies/mL for at least 3 months prior to study entry and <50 copies/mL at screening; current CD4+ T-cell counts ≥450 cells/mm3 and nadir CD4+ T-cell counts ≥200 cells/mm3; and available pre-ART (within 3 months) plasma for virus isolation.
Participants underwent leukapheresis to obtain monocytes to be transformed into DCs ex vivo to manufacture AGS-004. After a mean time of 4 to 6 weeks, they received 4 intradermal injections of the autologous AGS-004 product into the axillary lymph node region every 4 weeks while on ART. At week 16, a 12-week ATI began, during which the injections every 4 weeks continued. Any study participant with a plasma HIV-1 RNA level >200 copies/mL or a CD4+ T-cell count <450 cells/mm3 at week 14 did not undergo the ATI. Participants receiving non-nucleoside reverse transcriptase inhibitors in their ART regimen were switched to protease inhibitors for at least 1 week before the discontinuation of ART to avoid the development of drug resistance from the prolonged clearance and low genetic barrier to resistance of the non-nucleoside reverse transcriptase inhibitors.
The protocol was approved by each study site’s institutional review board. Written informed consent was obtained from all participants. The study was conducted in the United States and Canada according to human experimentation guidelines of the US Department of Health and Human Services and was monitored by a Data and Safety Monitoring Board.
Study Product
RNA encoding HIV Gag, Nef, Rev, and Vpr amplified from the patient’s pre-ART plasma was delivered into the patient’s DCs. The DCs had been matured by sequential exposure to general inflammatory cytokines (TNF-α, IFN-γ) and PGE2 followed by an adaptive signal (CD40L).
HIV protein expression levels were characterized during AGS-004 pre-clinical development.6 However, the patient to patient protein sequence variability makes the detection of protein expression levels unreliable using commercially available anti-HIV antibodies to Gag, Vpr, Rev and Nef proteins. Therefore, detection of protein expression for each subject was not conducted in this cohort. Final concentration of DCs was 1.2 × 107 cells/mL to deliver at least 1 × 107 viable DC in each injection.
The placebo consisted only of the excipient used with AGS-003. AGS-003 contains mature dendritic cells (DC) formulated in heat-treated autologous plasma, dextrose for injection (50%), and dimethylsulfoxide (DMSO) in an 8:1:1 ratio. Placebo was formulated using autologous plasma with the same excipient ratio without autologous DCs.
Study Evaluations
Study participants had interval medical histories, physical examinations, and laboratory safety monitoring at protocol-defined time points. During the ATI, plasma HIV-1 RNA and CD4+ T-cell counts were monitored every 2 weeks and weekly at weeks 10, 11, and 12 of the ATI. Plasma HIV-1 RNA levels obtained by RT-PCR and CD4+ T-cell counts were measured at Covance Laboratory (Conshohocken, PA/USA). Antiretroviral drug levels were measured at LabCorp (Burlington, NC/USA).
Integrated HIV-1 DNA
Whole blood PBMCs were separated by Ficoll gradient. CD4+ T cell enrichment was performed using negative selection with human CD4+ T Cell Isolation kit (Miltenyi Biotec). Genomic DNA was isolated using the Gentra Puregene kit (Qiagen). The levels of integrated HIV DNA was measured according to method described by Liszewski et.al.7, with changes in PCR probes and primers were re-designed according to D’Aquila et al. for better sensitivity.8
Immunologic Assays
Multicolor Flow Cytometry
Frozen PBMCs processed by Ficoll density gradient separation from whole-blood draws collected at the indicated clinical visits were thawed and cultured overnight in X-Vivo 15 supplemented with 10% human AB serum. After overnight culture, PBMCs were labeled with bromodeoxyuridine (BRDU) to track T-cell proliferation and stimulated with autologous DC targets. DC targets for in vitro stimulation were prepared from frozen DCs co-electroporated with CD40L RNA and the HIV antigens Gag, Nef, Vpr, and Rev. To determine background nonspecific activation of in vitro cytotoxic T-lymphocyte (CTL) cultures, PBMCs at each time point were stimulated with CD40L DCs co-electroporated with green fluorescent protein (GFP) RNA. GFP RNA is not included in the product payload. Co-cultures were incubated at 37°C for 6 days. On day 6, cultures were restimulated with antigen-matched DCs. Monensin, GolgiPlug (brefeldin A), and anti-CD107a antibody were added to each tube, and the tubes were incubated at 37°C for 5 hours. After incubation, the cells were stained for viability using LIVE/DEAD Fixable Dye (Invitrogen, Waltham, MA/USA) followed by staining with specific antibodies to detect surface expression of CCR7, CD45RA, CD28, CD27, CD3, and CD8 (Fig. 1A). After surface staining, the cells were fixed with 4% BD Cytofix and stored overnight in bovine serum albumin staining buffer at 4°C. Next day the cells were permeabilized and treated with DNase for 1 hour at 37°C using reagents included in the BRDU staining kit (BD Biosciences, Franklin Lakes, NJ/USA). Intracellular staining for IFN-γ, TNF-α, GrB, IL-2, and BRDU was performed (Fig. 1B). After staining, the cells were washed and diluted in bovine serum albumin buffer and transferred to BD TruCount Tubes for acquisition on a BD LSRII cytometer. Approximately 400,000 to 600,000 events were collected per sample. The number of cells/ml was calculated using the following formula: (number of cellular events collected/number of beads collected) × (bead concentration/microliter) × 1000. PBMCs were stimulated with DC electroporated with GFP (negative control) or with Gag, Nef, Vpr, and Rev total RNA payload in triplicate. Background values were determined using the negative control DCs (GFP) plus 3 times standard deviations and were subtracted from each test value.
FIGURE 1.
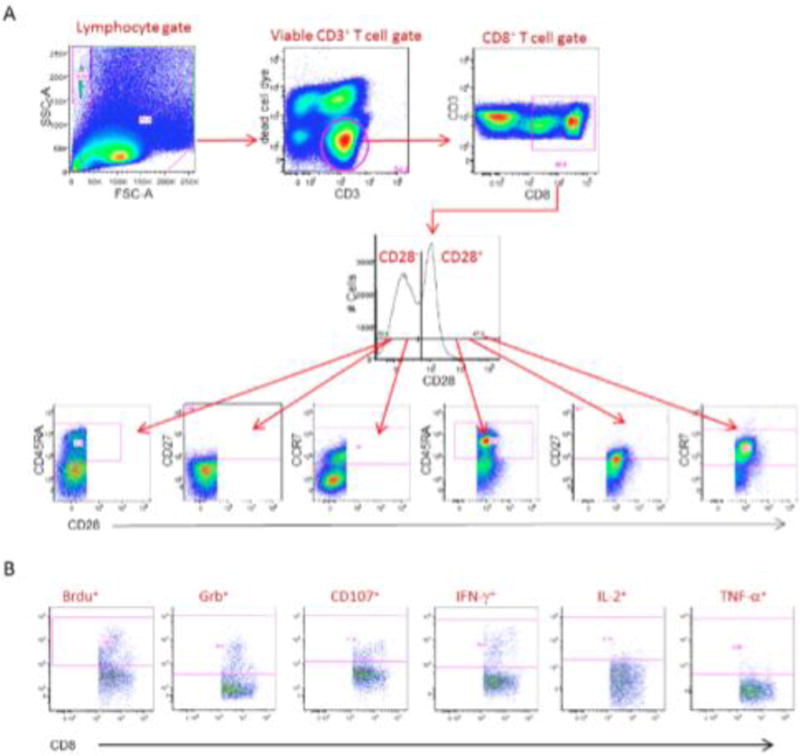
Cell surface Boolean gating of immune monitoring samples. A, Representative immune monitoring sample depicting gate sets to identify CD8+ T-cell subsets. CD28 positive and negative cells identified in the CD28 histogram are further subgated on the basis of the expression of CD45RA, CD27, and CCR7. The CD28+/CD45RA− effector memory subset is determined by the expression of CD28 and the absense of CD45RA expression. B, Representative immune monitoring sample depiciting gate sets to identify the six functional markers within the CD28+/CD45RA− effector/memory phenotype.
Assays of Inflammatory Markers
Soluble CD14 (sCD14) and D-dimer levels were measured at Covance by ELISA; CRP levels, at Covance by immunonephelometry; TNF-Rll levels, at Argos Therapeutics (Durham, NC/USA)by immunoassay; and the levels of 24 other cytokines, at Argos by multiplexed microscale ELISA.
Statistical Analysis
Sample Size Estimate
Sample size was determined by assuming that > 1.0 log viral load difference between study arms would be clinically meaningful. For 80% power to detect a beneficial treatment effect with a Type–I error probability of 0.05, if the distribution of the median plasma HIV-1 RNA in the population receiving AGS-004 is shifted lower by at least 1.1 log10 copies/mL, and the between-subject standard deviation of the endpoint in this study population is 1.19 log10 copies/mL (based on data from ACTG A50684), we determined that we needed to enroll a total sample size of 42 subjects, approximately 28 subjects in Arm A and 14 subjects in Arm B, allowing for an approximate 10% dropout rate. Since the statistical test will be a one-sided 5.0% level Wilcoxon Rank Sum test, the efficiency of the test relative to a t-test is 0.864 (this is the worst-case asymptotic relative efficiency of the Wilcoxon Rank Sum test).
Baseline Demographics and HIV Disease Indicators
Descriptive statistics were utilized for basic demographics and for pre-ART values of CD4 cell counts and plasma HIV-1 viral loads. For continuous variables, median values with associated 25% to 75% interquartile ranges are presented. Tests for differences of demographics or for pre-ART CD4 cell count or viral loads utilized Pearson’s chi-square or the Fisher exact test (for categorical variables) and Wilcoxon rank-sum tests (for continuous variables); median values or percentages are reported by arm, with associated P values. P values ≤ 0.05 for differences between treatment arms were considered to be statistically significant.
Primary and Secondary End Points and Comparisons of CD4 Cell Counts or Viral Loads by Analytical Treatment Interruption Week
The primary end point for the study was the end of ATI viral load, calculated as the geometric mean titer of plasma HIV-1 viral loads (log 10) at weeks 11 and 12 of the ATI period. The secondary end point was evaluated as the change in plasma HIV-1 viral load from pre-ART values to end-point viral loads (log 10; at weeks 11 and 12). Additionally, pairwise comparisons of CD4 cell counts and plasma HIV-1 RNA viral loads were made between study arms. Comparisons were made between patients receiving AGS 004 and individuals in the placebo control arm. Differences in the distributions of primary and secondary end points were evaluated using Wilcoxon rank-sum tests as described above; results are presented with median values per arm, with associated 25% to 75% interquartile ranges. For intention-to-treat analyses, worst ranks were assigned to patients without end-point data; Wilcoxon rank sum tests were performed including those patients who were assigned with worst rank data.
Additional Analyses
For longitudinal analyses of repeated measures of CD4 cell counts and log10 plasma HIV-1 RNA copies/ml, we ran linear mixed effects models in R (“lme” function in the “nmle” package); these were random intercept models, with time (weeks), treatment, and time*treatment (interaction terms) as fixed effects.
Analyses of Adverse Events
Given the relatively low number of adverse events, we listed adverse events by study arm; within each arm, adverse events were listed along with the number of patients experiencing the adverse events. Events were further classified as having been judged to be definitely related to, probably related to, possibly related to, or unlikely to be related to the study drug AGS 004. The proportion of patients experiencing adverse events was compared between arms using Pearson’s chi-square or the Fisher exact test (when the number of incident events was rare). Results of these comparisons are reported with associated P values.
RESULTS
Study Population
Between July 2010 and November 2012, 54 study participants were enrolled. A total of 37 participants were randomized to AGS-004; 17 participants were randomized to placebo. Two participants randomized to the AGS-004 arm never received AGS-004 because of production issues and were excluded from the analyses. The baseline characteristics of participants in each study arm were similar (Table 1). The median entry CD4+ T-cell count was 647 in the active AGS-004 arm and 580 in the placebo arm. All participants maintained virological suppression (VL <50 copies/mL) during the pre-ATI study treatment period.
Table 1.
Baseline Characteristics of Participants, by Treatment Arm
| Characteristic | Treatment arm
|
|||
|---|---|---|---|---|
| Total (n = 52) | Vaccine (n = 35) | Placebo (n = 17) | P Value | |
| Median age, years (25%, 75% IQR) | 39 (31,48) | 38 (30,50) | 40 (33, 47) | 0.92 |
| Sex, no. (%) | 1.00 | |||
| Male | 47 (90.4) | 31 (88.6) | 16 (94.1) | |
| Female | 5 (9.6) | 4 (11.4) | 1 (5.9) | |
| Race, no. (%) | 0.44 | |||
| White | 34 (65.4) | 23 (65.7) | 11 (64.7) | |
| Black | 15 (28.9) | 11 (31.4) | 4 (23.5) | |
| Asian | 1 (1.9) | 0 | 1 (5.9) | |
| Multiple | 2 (3.8) | 1 (2.9) | 1 (5.9) | |
| Baseline CD4 count/mla (25%,75% IQR) | 632 (513, 765) |
647 (526, 793) |
580 (505, 682) |
0.24 |
| Median log10 Baseline HIV-1 RNA copies/ml* |
1.70 (1.70, 1.70) |
1.70 (1.70, 1.70) |
1.70 (1.70, 1.70) |
0.17 |
| Median log10 Pre-ART HIV-1 RNA copies/ml† |
4.74 (4.39,5.05) |
4.69 (4.36, 5.03) |
4.85 (4.51, 5.20) |
1.00 |
| Median log10 Pre-ART HIV-1 RNA copies/ml‡ |
4.77 (4.37, 5.18) |
4.85 (4.51, 5.20) |
4.81 (4.42, 5.32) |
0.20 |
Values for each patient are means of screening and eligibility values at time of enrollment.
Values are averages of between one and three pre-ART VL determinations available for each patient.
Values are from single determinations of pre-ART plasmas collected within 90 days of screening/enrollment.
ART, antiretroviral therapy; IQR, interquartile range; VL, viral load.
Analytical Treatment Interruption Viral Measures
There was no difference between treatment arms with respect to plasma viral kinetics during the ATI (Fig. 2A). The median plasma HIV-1 levels were similar over all 12 weeks of the ATI (as treated analysis). No statistically significant differences were observed at each ATI time point, including the primary end point—the average of weeks 11 and 12 plasma HIV-1 RNA levels (as treated analysis). There was no difference between treatment arms in the secondary end point, with changes in plasma HIV-1 levels between pre-ART levels and the end of ATI (average of weeks 11 and 12) HIV-1 levels being similar in both arms. This finding remained true whether the pre-ART value was considered to be the measure of the single pre-ART sample obtained within 90 days of starting ART and used to produce the study product, or the mean of all samples obtained within 6 months before starting ART. Including worst rank plasma HIV-1 RNA values for patients who dropped out of the study prior to the end of ATI (ITT analysis) did not alter any of these results.
FIGURE 2.
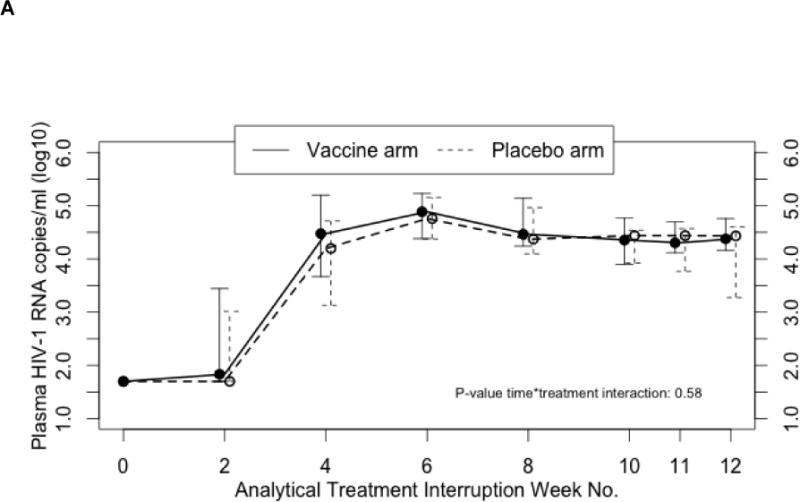
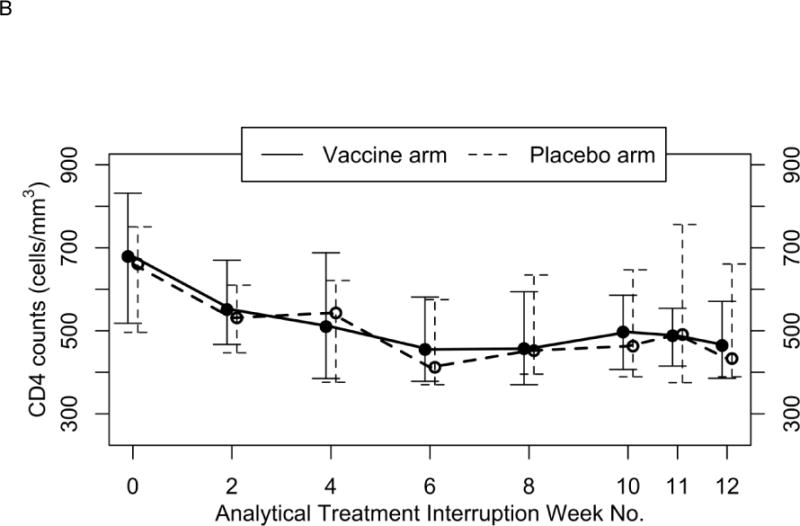
(A) Median plasma HIV-1 RNA copies/ml and (B) median CD4 cell counts over 12-week analytical treatment interruption period. Median plasma HIV-1 values are presented with associated 25% to 75% interquartile ranges. Treatment effect over time was not significant (P =0.58). Median CD4 cell counts are presented with associated 25% to 75% interquartile ranges. Treatment effect over time was was not significant (P >0.05).
One study participant receiving placebo maintained undetectable plasma HIV-1 RNA levels during the ATI for 2 years. This participant reported that she was not taking any antiretroviral medication but antiretroviral drugs were detected in her plasma throughout the ATI period. Measurements of drug levels found evidence of ART exposure not explained by pharmacokinetic clearance during the ATI in one other study participant who received placebo. The inclusion or exclusion of the data from these two participants did not affect the results of the analyses of the ATI viral kinetics.
The plasma HIV-1 RNA level went below the level of detection in all participants who restarted ART after an ATI. There were no significant differences between study arms in the change in integrated cell HIV-1 DNA from baseline to weeks 8, 18, and 28.
CD4+ T-Cell Counts
Similar to what was observed for plasma HIV-1 RNA levels, CD4+ T-cell count trajectories did not differ by treatment arm (Fig. 2B). Median CD4+ T-cell counts and percentages were similar during the 12 weeks of the ATI (as treated analysis). CD4+ T-cell counts and percentages decreased steadily over the ATI period, decreasing 200 cells/mm3 and approximately 12%, respectively, during this period. No statistically significant differences were observed at each ATI time point, including at the week 11/12 end point (as treated analysis). Including worst rank CD4+ T-cell count or percentage values for patients who dropped out of the study prior to the end of ATI did not alter the results (ITT analysis).
Other Immunologic Data
Previously published data from in vitro modeling of CTL responses to a defined tumor antigen revealed increased expansion of multifunctional CD28+/CD45RA− effector memory CTL after repeated stimulation with DCs produced under methods similar to those used with AGS-004 DC products.9 On the basis of these results, we surmised that the mechanism of action of AGS-004 in vivo would be predicated on the expansion of CD28+/CD45RA− multifunctional CTL after repetitive administrations. Therefore, we focused our analysis on measuring the magnitude of the induced HIV-specific CTL response based on increases in the number of CD28+/CD45RA− effector memory CTL with functionality. Increases in the number of functional CD28+/CD45RA− effector memory CTL in both arms were measured at weeks 8, 18, and 26 over baseline (week 0) and are reported in Fig. 3.
FIGURE 3.
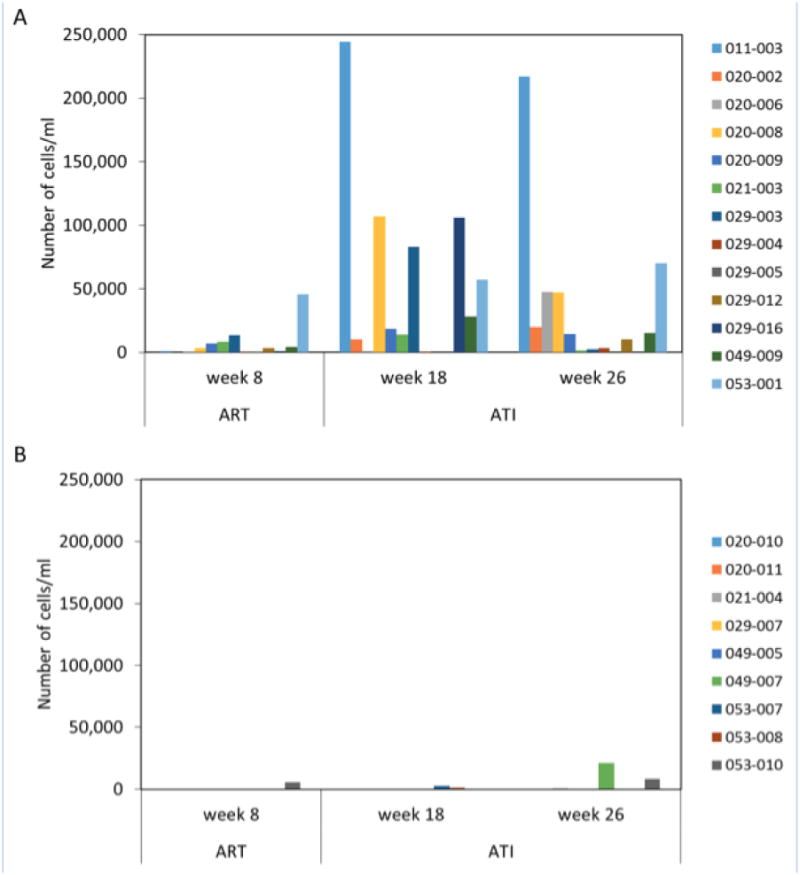
Increases in functional CD28+/CD45RA− CTL after in vitro restimulation with AGS-004. The total number of cells/ml expressing any functional marker was determined at each time point. After background subtraction, the value determined at baseline (week 0) was subtracted from week 8, week 18, and week 26 values. Each bar represents a separate subject either receiving AGS-004 (A) or placebo (B).
To determine which subjects had increases in HIV-specific CTL responses, PBMCs collected at the indicated time points were restimulated in vitro with autologous AGS-004 product. Figure 3 plots the increase in the number of CD28+/CD45RA− CTL with effector marker expression over baseline for each subject receiving either AGS-004 (Fig. 3A) or placebo (Fig. 3B) at weeks 8, 18, and 26. The statistical analysis plan did not contemplate analyses related to the immune monitoring exploratory endpoint, but arbitrarily defining a positive immune response post-hoc as >1000 cell increase, at the week 8 time point, 69% of subjects receiving only 2 doses of AGS-004 had a response to DCs encoding the Gag, Nef, Vpr, and Rev antigen payload, compared to only 11% receiving placebo at the same time point. Furthermore, the magnitude of the induced functional response was greater in subjects receiving AGS-004 compared to subjects receiving placebo. AGS-004-specific responses increased in both arms during ATI. However, responses in the AGS-004 treatment arm were 83% and 92% at weeks 18 and 26, respectively, compared to only 25% in those subjects receiving placebo at both weeks 18 and 26 (Table 2).
Table 2.
Percentage of Subjects Who Responded To AGS-004
| Percent responding subjects* > 1000 cells/ml for CD28+/CD45RA− effector memory CTL | |||
|---|---|---|---|
| Arm | Week 8 (ART) | Week 18 (ATI) | Week 26 (ATI) |
|
| |||
| AGS-004 treated | 69.2% (9 of 13) | 83.3% (10 of 12) | 91.7% (11 of 12) |
| Placebo | 11.1% (1 of 9) | 25.0% (2 of 8) | 25.0% (2 of 8) |
A positive immune response to AGS-004 was defined as >1000 cells/ml expressing any functional marker for the CD28+/CD45RA− subset.
ART, antiretroviral therapy; ATI, analytical treatment interruption.
No differences were observed between study arms in levels of sCD14, CRP, D-dimer, TNF-RII, or other cytokines at any time point.
Adverse Events
Severe adverse events (SAEs) were judged as unrelated to treatment with the study product. The numbers of SAEs and AEs were similar between treatment arms (five per arm). SAEs included infections, exacerbation of chronic lung disease, seizures, thrombocytopenia, and neutropenia, the latter occurring during the ATI in a participant who had neutropenia before starting ART. Grade 1 injection site reactions, including erythema, swelling, tenderness, induration, and pruritus, occurred in 25 of 35 (72%) participants in the active vaccine arm and 14 of 17 (82%) participants in the placebo arm. Other adverse events deemed at least possibly related to study treatment included headache, nausea, depression, dizziness, increase in vivid dreams, lymphadenopathy, and skin rash in the active vaccine arm; fatigue, dyspnea, nausea, distended abdomen, chills, night sweats, and skin rash in the placebo arm.
DISCUSSION
In this randomized, double-blind, placebo-controlled study in chronically HIV-infected persons on effective ART, autologous DCs loaded with autologous HIV-1 RNA, used to provide a source of autologous antigen for presentation, and RNA encoding CD40L, used to obviate the need for CD4+ T-cell help, an immunotherapeutic product known as AGS-004 did not induce immune responses that were effective in improving host control of viral replication compared to placebo, as measured during an ATI of ART. All aspects of the viral kinetics during the ATI were similar between the active treatment group and the placebo control. This was true of the new end-of-ATI plasma HIV-1 RNA set point, the primary end point of the study; it was also true of the time to reappearance of plasma HIV-1, the upward slope of viral levels, the peak level, and the change in plasma HIV-1 RNA level from the pre-ART value to the new end-of-ATI value. Thus, there was no measurable antiviral effect of this autologous HIV-1 RNA immunotherapy as administered in this study.
However, the experimental therapy did seem to induce CD28+/CD45RA− effector memory CTL with expanded effector function when measured after 2 doses while participants were receiving ART. Furthermore, the induced HIV-specific response was expanded with continued administration of AGS-004 during treatment interruption. Whereas we detected HIV-specific responses in both arms, after ATI, it was only in subjects receiving AGS-004 that we saw a robust expansion in the numbers of HIV-specific CTL in the CD28+/CD45RA− CTL compartment. Given that the response in AGS-004-treated subjects was robust and sustained at both time points during ATI, it is likely that these responses were dependent on the administration of AGS-004 and not just a consequence of the reappearance of virus. Taken together, these data provide evidence that AGS-004 can induce HIV-specific CTL responses during ART and that these responses are augmented during ATI. These measured responses have the characteristic of CD28+/CD45RA− effector memory CTL that has been shown to be induced in vitro in model experiments of the AGS-004 mechanism of action.9 However, the induced HIV-specific CTL response did not correlate with control of viral load at any time point.
It should be noted that one individual enrolled in the study maintained undetectable levels of plasma HIV-1 RNA during the ATI for 2 years. This finding led us to measure the levels of antiretroviral drugs during the ATI in this person and ultimately in the rest of the study participants. This individual had detectable levels of antiretroviral drugs when she reported that she was not taking them during the ATI. By measuring drug levels, we found evidence of continued ART exposure during the ATI in one other study participant. These findings, though they did not affect the results of our study, suggest that ART drug levels should be measured in all study participants during the ATIs of studies that include ATI as an important outcome measurement tool and that reports of “cure” of HIV-1 infection in patients reportedly not taking ART should include measurement of antiretroviral drug levels to verify the absence of ART use.
A randomized, controlled trial in HIV-infected individuals of DC-based vaccination using consensus antigens did not show improvement in HIV-specific immune responses or overall control of viral replication during an ATI compared to standard vaccination.10 DC vaccine studies using autologous HIV-1 antigens have shown more promise. An uncontrolled small trial in patients not treated with ART of Aldrithiol-inactivated autologous HIV-1 found a modest (0.7 log) median decline in the plasma HIV RNA level.11 A preliminary uncontrolled study of AGS-004 in ART-treated persons also reported a decline compared to pre-ART levels.12
A randomized, controlled trial of DCs loaded with heat-inactivated autologous virus noted a significant lowering of median plasma HIV-1 RNA levels from pre-ART levels (−0.65 log copies/mL).13 Pre-ART viral load was defined as the mean of all values obtained “one year before” ART. Thus, the pre-ART viral load was historical, and different numbers of values could have been ascertained at different time points in individual patients. The median viral load values at specific time points in the ATI were not reported and were not compared between study arms but were noted to have rebounded to detectable levels in all patients. The reported decline of viral load from pre-ART levels in the uncontrolled preliminary AGS-004 trial12 was not confirmed in our study, which lends further pause to using change in viral load from pre-ART level as a primary end point in immunotherapy clinical trials.
The AGS-004 immunotherapeutic approach was safe, and the ATI was well tolerated. There was one episode of neutropenia during the ATI, occurring in a patient who had neutropenia historically prior to starting ART. An individual with a history of neutropenia or thrombocytopenia before staring ART should probably be excluded from studies that include antiretroviral treatment interruptions.
In sum, we did not observe an improvement in host immune control of viremia in this randomized, double-blind, placebo-controlled trial of an autologous DC–autologous antigen-based immunotherapeutic approach to treating HIV infection. It is conceivable that the boosted HIV-specific CD8+ T-cell responses observed could translate into an important clinical effect in another setting, such as providing the CD8 cell lytic effect (i.e., the kill) in combination cure strategies that induce viral activation and expression of viral antigens in latently infected cell reservoirs in order to make the cells susceptible to immune attack. However, the immune responses induced by this immunotherapy by itself did not lead to reductions in plasma HIV RNA, the source of which is HIV-infected cells. It is possible that adding “checkpoint” inhibitors or other immunoregulatory molecules could enhance the activity of the product.14
Acknowledgments
Other members of the study team: Meghan Martin and Jennifer Capobianchi, Drexel University College of Medicine; Nathalie Paisible, McGill University; Nancy Tremblay and Melissa Bonnetsmueller, The Ottawa Hospital; Tammy Yotter, University of California, Davis; Angelo Seda, Albert Einstein College of Medicine; Debbie McMullen, Duke University; Cynthia Gay and Joann Kuruc, University of North Carolina at Chapel Hill; Ionnis Vertzagias, Clinique Medicale l’Actuel; Esther Villiard, Argostherapeutics, Inc.
In addition, we thank the staff of the research units and blood centers of the participating study sites; the referring physicians; Pamela Fried for editorial assistance; and the study participants for their dedication and time.
Sources of Funding: This work was funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. N01-AI-60019; by the Canadian Institutes of Health Research [grants MOP #103230 and CTN #256]; the Canadian HIV Cure Enterprise Team [Grant HIG-133050] (JPR and JA) from the CIHR and Fonds de la Recherche Quebec Sante (FRQ-S): Réseau SIDA/Maladies infectieuses. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: Mark DeBenedette, Irina Tcherepanova, and Charles Nicolette are employees of Argostherapeutics, Inc. (Durham, North Carolina). Jeffrey M. Jacobson, Jean-Pierre Routy, and David M. Margolis have each received an honorarium from Merck Pharmaceuticals. For the remaining authors no conflicts of interest were declared.
Clinical Trials Registry Number. NCT00672191
References
- 1.Archin NM, Sung JM, Garrido C, et al. Eradicating HIV-1 infection: seeking to clear a persistent pathogen. Nat Rev Microbiol. 2014;12:750–764. doi: 10.1038/nrmicro3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after viral reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kutzler MA, Jacobson JM. Treatment interruption as a tool to measure changes in immunologic response to HIV-1. Curr Opin HIV AIDS. 2008;3:131–135. doi: 10.1097/COH.0b013e3282f54cde. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson JM, Pat Bucy R, Spritzler J, et al. Evidence that intermittent structured treatment interruption, but not immunization with ALVAC-HIV vCP1452, promotes host control of HIV replication: the results of AIDS Clinical Trials Group 5068. J Infect Dis. 2006;194:623–632. doi: 10.1086/506364. [DOI] [PubMed] [Google Scholar]
- 5.Routy JP, Boulassel MR, Yassine-Diab B, et al. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin Immunol. 2010;134:140–147. doi: 10.1016/j.clim.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tcherepanova I, Starr A, Lackford B, et al. The immunosuppressive properties of the HIV Vpr protein are linked to a single highly conserved residue, R90. PLos One. 2009;4:e5853. doi: 10.1371/journal.pone.0005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liszewski MK, Yu JJ, O’Doherty U. Detecting HIV-1 integration by repetitive-sampling Alu-gag PCR. Methods. 2009;47:254–260. doi: 10.1016/j.ymeth.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Aquila RT, Bechtel LJ, Videler JA, et al. Maximizing sensitivity and specificity of PCR by pre-amplification heating. Nucleic Acids Res. 1991;19:3749. doi: 10.1093/nar/19.13.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBenedette MA, Calderhead DM, Ketteringham H, et al. Priming of a novel subset of CD28+ rapidly expanding high-avidity effector memory CTL by post maturation electroporation-CD40L dendritic cells is IL-12 dependent. J Immunol. 2008;181:5296–5305. doi: 10.4049/jimmunol.181.8.5296. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi RT, O’Neill D, Bosch RJ, et al. A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine. 2009;27:6088–6094. doi: 10.1016/j.vaccine.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu W, Arraes L, Ferreira W, et al. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 12.Routy JP, Angel J, Vezina S, et al. Final analysis of a phase 2 study of an autologous DC immunotherapy (AGS-004) showed positive outcomes in primary endpoint of viral load control, and favorable safety and immunogenicity profile, in subjects undergoing structured treatment interruption of ART (abstract 385); 18th Conference on Retroviruses and Opportunistic Infections; Boston. February 27–March 2, 2011. [Google Scholar]
- 13.García F, Climent N, Guardo AC, et al. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci Transl Med. 2013;5:166ra2. doi: 10.1126/scitranslmed.3004682. [DOI] [PubMed] [Google Scholar]
- 14.Routy JP, Nicolette C. Arcelis AGS-004 dendritic cell-based immunotherapy for HIV infection. Immunotherapy. 2010;2:467–476. doi: 10.2217/imt.10.28. [DOI] [PubMed] [Google Scholar]


