Abstract
Compensatory activation in dorsal premotor cortex (PMd) during movement execution has often been reported after stroke. However, the role of PMd in the planning of skilled movement after stroke has not been well studied. The current study investigated the behavioral and neural response to the addition of action selection (AS) demands, a motor planning process that engages PMd in controls, to movement after stroke. Ten individuals with chronic, left hemisphere stroke and 16 age‐matched controls made a joystick movement with the right hand under two conditions. In the AS condition, participants moved right or left based on an abstract, visual rule; in the execution only condition, participants moved in the same direction on every trial. Despite a similar behavioral response to the AS condition (increase in reaction time), brain activation differed between the two groups: the control group showed increased activation in left inferior parietal lobule (IPL) while the stroke group showed increased activation in several right/contralesional regions including right IPL. Variability in behavioral performance between participants was significantly related to variability in brain activation. Individuals post‐stroke with relatively poorer AS task performance showed greater magnitude of activation in left PMd and dorsolateral prefrontal cortex (DLPFC), increased left primary motor cortex‐PMd connectivity, and decreased left PMd‐DLPFC connectivity. Changes in the premotor‐prefrontal component of the motor network during complex movement conditions may negatively impact the performance and learning of skilled movement and may be a prime target for rehabilitation protocols aimed at improving the function of residual brain circuits after stroke. Hum Brain Mapp 37:1816–1830, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: motor planning, upper extremity, imaging, functional connectivity
Abbreviations
- ANOVA
Analysis of variance
- AS
Action selection
- DLPFC
Dorsolateral prefrontal cortex
- DTI
Diffusion tensor images
- EO
Execution only
- FA
Fractional anisotropy
- IPL
Inferior parietal lobule
- MRI
Magnetic resonance imaging
- MT
Movement time
- PPI
Psychophysiological interaction
- ROI
Region of interest
- RT
Reaction time
INTRODUCTION
The performance of skilled actions recruits a number of nodes in a distributed neural network that is behavior specific. For skilled movement, this network often includes cortical regions in both hemispheres, especially for complex tasks [Catalan et al., 1998; Verstynen et al., 2005]. A commonly reported compensatory activation pattern in older adults and in individuals with neurologic diagnoses is an increase in the number and magnitude of nodes recruited to support behavioral performance [Rehme et al., 2012; Seidler et al., 2010; Spreng et al., 2010]. This increase in activation is often bilateral and has been found across a variety of cognitive and motor tasks.
After stroke, persistent motor deficits are common and often associated with changes in activation in the motor network. Compensatory brain activation during movement within both ipsilesional and contralesional brain regions has been frequently reported [Carey et al., 2002; Lotze et al., 2006; Schaechter and Perdue, 2008; Schaechter et al., 2008; Ward et al., 2003]. One often cited region of compensatory activation is bilateral dorsal premotor cortex (PMd) [Bestmann et al., 2010; Fridman et al., 2004; Johansen‐Berg et al., 2002; Ward et al., 2006], although the precise role of PMd in the control of movement after stroke is not fully known. Studies to date have reported an important role for PMd in movement execution after stroke that is increased in individuals with greater motor impairment [Ward et al., 2006, 2007]. However, PMd plays a variety of roles in the control of skilled movement beyond simple execution, especially in movement planning [Kantak et al., 2012]. It is not currently known how the stroke damaged brain responds to task conditions that target the PMd node of the motor network.
One well‐studied role for PMd in movement planning in nondisabled individuals is in the process of action selection (AS). AS, or selection of a movement response, is a critical feature of voluntary action. Task conditions that relatively increase AS demands for movement result in longer planning times than simple motor execution tasks, with a corresponding increase in bilateral PMd and bilateral parietal cortex activation [Grafton et al., 1998; Grol et al., 2006; O'Shea et al., 2007; Toni et al., 2002]. Selection of a movement response based on external, visual cues that are abstract in nature and require some degree of visuomotor transformation have been shown to engage PMd to a greater extent [Hanakawa et al., 2006]. However, the neural correlates of AS and the role of PMd for AS after stroke have not been reported.
Response to behavioral interventions aimed at improving arm motor function varies greatly after stroke [Cramer, 2008; Prabhakaran et al., 2008; Stinear, 2010]. Both degree of motor impairment and residual corticospinal tract integrity have been shown to be significant predictors of response to treatment [Chen and Winstein, 2009; Riley et al., 2011; Stinear, 2010; Stinear et al., 2007]. However, continued difficulty incorporating the affected arm and hand into everyday life can persist, even in individuals deemed to have “good” motor recovery [Stewart and Cramer, 2013], suggesting additional factors play a role. One important factor may be the ability to effectively engage a node or set of nodes of the motor network based on selected changes in task demands. Structuring practice during rehabilitation to engage specific neural resources may serve as an adjunct to repetitive movement training after stroke [Cramer et al., 2011; Dodakian et al., 2013]. Given the role PMd is hypothesized to play in motor recovery after stroke, this region is a prime candidate for a targeted rehabilitation protocol aimed at improving the function of residual brain circuits.
The purpose of this study was to determine the behavioral and neural correlates of motor AS in individuals with motor system damage due to stroke. We hypothesized that individuals post‐stroke would show a significant increase in planning time that corresponded to an increase in bilateral PMd and parietal cortex activation for AS compared to simple movement execution. We also hypothesized that variability in AS task performance between individuals would correlate with variability in brain activation (magnitude and functional connectivity), specifically in relation to PMd. Since AS task performance could have had a positive or negative correlation with PMd activation, this behavior‐brain function hypothesis was two‐tailed.
MATERIALS AND METHODS
Participants
Ten individuals in the chronic phase of stroke recovery and sixteen age‐matched nondisabled controls were recruited from the local community. Potential participants had to be at least 18 years old and right‐hand dominant [Oldfield, 1971] (determined by pre‐stroke handedness). Participants with stroke were included if they had a history of stroke at least 6 months prior to enrollment and mild to moderate motor impairment in the right arm as demonstrated by a score between 30 and 60 on the upper extremity Fugl‐Meyer (UE FM) motor section [Fugl‐Meyer et al., 1975]. Potential participants were excluded if they had a Mini‐Mental State Exam [Folstein et al., 1975] score less than 26, history of any non‐stroke neurologic diagnosis in participants with stroke and any neurologic diagnosis in controls that affected movement of the arms, or contraindication to magnetic resonance imaging (MRI) [Kleim et al., 2007]. Participants with stroke were excluded if they had significant sensory loss (<50% of normal on the Nottingham sensory scale [Lincoln et al., 1998]) or severe ideomotor apraxia (score <65 on the Test of Upper Limb Apraxia (TULA) [Vanbellingen et al., 2010]. All participants provided informed consent on a form approved by the university institutional review board.
Motor Task
All participants performed the motor task with the dominant/paretic, right hand. The task involved right or left movement of a standard joystick based on a visual cue in two different conditions. In the AS condition, the individual moved right or left based on an abstract rule (Fig. 1). When a small square or large circle was shown, a joystick movement to the right was made; when a large square or small circle was shown, a joystick movement to the left was made. Small cues were 50 × 50 pixels in size while large cues were 200 × 200 pixels. In the execution only (EO) condition, the visual cues were the same, however, the participant made a joystick movement in the same direction on every trial irrespective of the size/shape of the cue. Movement direction for EO was counterbalanced across participants. In both conditions, a single cue was presented for 2 sec in a pseudorandom order such that each cue was presented six times in each block (36 trials per block). The inter‐trial interval varied between 2.0 and 3.25 sec to minimize anticipatory responses prior to the cue.
Figure 1.

Participants moved a joystick under two experimental conditions. During action selection, movement direction was dictated by an abstract rule (large square or small circle = move left; small square or large circle = move right). During execution only, movement direction was the same on every trial regardless of visual cue. Movement direction (right/left) for execution only was counterbalanced across participants.
Prior to MRI, a training session in the laboratory was completed to ensure understanding of both task conditions. First, verbal and visual instruction on the AS condition was provided followed by a practice block of the AS condition. Three blocks of each condition were then completed in alternating order; the condition completed in the first block (AS/EO) was counterbalanced across participants. After completion of the training blocks, the participant practiced the MRI version of the task. This version alternated periods of movement (cues were green) with periods of view only (cues were red) in a block design (see below) and included a total of 10 movement trials (five trials per movement epoch).
Brain Imaging
All brain imaging sessions were performed on a 3T Achieva MRI scanner (Phillips Medical System, Best, Netherlands). Functional MRI (fMRI) data were acquired using a block design while the participants performed the AS and EO tasks with an MRI compatible joystick (Current Designs, Philadelphia, PA). Periods of movement (Move, 24 sec) alternated with periods of view only (View, 24 sec) with a fixation period (red cross, 8 sec) between each epoch. Cue duration (2 sec) and the inter‐trial interval (varied between 2.0 and 3.5 sec) were the same as during practice in the laboratory. Just prior to entering the scanner, the movement rule for the AS condition was reviewed; no additional reminders of the rule were provided during scanning. Each participant completed four fMRI runs, two in the AS condition and two in the EO condition in alternating order; the condition completed in the first run (AS/EO) was counterbalanced across participants. Functional runs lasted for 2 minutes 10 seconds during which 65 brain volumes were acquired (TR = 2000 ms, TE = 30 ms); each volume included 31 slices that were 4 mm thick with a slice gap of 1 mm (acquisition voxel size 2.5 mm × 2.5 mm × 4 mm). Next, a high resolution structural MPRAGE image was acquired (TR = 8.4 ms, TE = 3.9 ms) which included 150, 1 mm thick slices with no interslice gap (acquisition voxel size 1 mm × 1 mm × 1 mm). Diffusion tensor images (DTI) were acquired using echo planar imaging (TR = 11,190 ms, TE = 69 ms) and included 60, 2 mm axial slices with no interslice gap, 32 directions, and a b value of 800 s/mm2. Total scan time for each session was approximately 45 minutes.
Data Analysis
Behavioral data
Data from the joystick were used to determine task accuracy, reaction time (RT), and movement time (MT) using a custom script in Matlab (Matworks, Inc., Natick, MA). Position data (x,y) were recorded throughout each trial (60 Hz in the laboratory, 30 Hz in the MRI) and used to derive movement velocity [Winter, 2005]. RT, the primary behavioral outcome measure, was the time between cue presentation and movement onset. Movement onset was determined by searching backward in time from initial peak velocity until velocity dropped below 5°/sec for two consecutive samples or the change in velocity dropped below 1°/sec for two consecutive samples, whichever was identified first. Movement offset was determined by searching forward in time from peak velocity until velocity dropped below 10°/sec for two consecutive samples. AS RT was normalized to EO RT to determine RT cost (AS RT – EO RT), a measure of the relative increase in planning time for the AS condition for each participant. MT was the time between movement onset and movement offset. All movement data were analyzed with a mixed model analysis of variance (ANOVA) that included two within group factors (condition, trial block) and one between group factor (control, stroke). Data collected during fMRI were analyzed separately with an ANOVA to determine differences between conditions and groups during scanning. Significant level was set at P < 0.05 for all statistical tests. JMP 8 (SAS, Cary, NC) statistical software was used for analyses.
Corticospinal tract integrity
Corticospinal tract integrity was quantified using the DTI images. Analysis was completed in FSL (FMRIB Center, Oxford, UK) using the FDT toolbox [Behrens et al., 2003]. Diffusion images were corrected for eddy currents and head motion followed by removal of the skull and dura [Smith, 2002]. A voxelwise map of fractional anisotropy (FA) was then created. FA is a measure of the structural integrity of white matter with values ranging between 0 and 1 with higher values indicating greater structural integrity [Mori and Zhang, 2006]. To determine corticospinal tract integrity, a region of interest (ROI) mask was manually drawn on the axial slice that showed the largest cross‐sectional area of the cerebral peduncle [Burke et al., 2014; Mark et al., 2008]. Mean FA was extracted from each ROI using a threshold of FA > 0.2 and used to determine FA asymmetry between the lesioned and nonlesioned side (FAcontralesional‐FAipsilesional/FAcontralesional + FAipsilesional) [Stinear et al., 2007].
Functional imaging data
All functional imaging data were analyzed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK). First, volumes from each run were realigned to the first volume and resliced to account for motion artifact. The mean image for each participant was then normalized to the standard Montreal Neurological Institute EPI template in SPM. The normalization parameters were then applied to all of the functional volumes for that participant, and the normalized images were resampled to 2 mm × 2 mm × 2 mm voxels. Images were then spatially smoothed with an isotropic Gaussian filter (FWHM = 8 mm) and a temporal filter was applied (1/128 Hz) to remove low frequency confounds. Data from each functional run were inspected for outliers due to excessive head motion (>1mm translation or >0.2 radians rotation between each volume) or signal noise (Z > 3 from the mean image intensity) using the Artifact Detection Tool toolbox (http://www.nitrc.org/projects/artifact_detect); outliers were de‐weighted during statistical analysis. A single run from two participants in the stroke group were excluded from data analysis due to excessive noise (both for the EO conditions); all remaining runs for all participants were included in analyses.
First‐level statistical analysis was performed separately for each participant using a general linear model [Friston et al., 1995a, 1995b]. For each run, Move and View epochs were modeled separately against fixation for later contrast. To determine the regions active during each condition (EO, AS), Move was contrasted with View (Move > View); both runs for each condition were weighted equally in all contrasts. For first‐level analyses, the first derivative of head motion for all six directions, which was uncorrelated with stimulus presentation, was added as a regressor of no interest to account for the effect of head motion in the data.
The contrast maps for each participant and each condition (Move > View contrasts for EO and AS) were moved to a second‐level random effects analysis. Group analyses were performed using a factorial design that included a factor for group (control, stroke) and a factor for condition (EO, AS). This design allowed for the analysis of main effects (group, condition) as well as group × condition interactions. Additionally, a t contrast between conditions (EO > AS, AS > EO) was run for each group separately. MT (mean during all fMRI trials) was included as a regressor of no interest to account for differences in movement response unrelated to RT. To examine the relationship between variability in behavioral performance and variability in brain activation between individuals, regression analysis was performed between RT cost and the AS contrast map. RT cost represents the relative increase in RT for each individual participant for the AS condition after controlling for EO RT. Age and MT were included as regressors of no interest. For group comparisons, statistical significance was set at P < 0.001 uncorrected for multiple comparisons.
Functional connectivity analysis
Psychophysiological interaction (PPI) analysis was performed to identify brain regions in which the connectivity with M1 and PMd changed as a function of task condition [Friston et al., 1997; Gitelman et al., 2003]. PPI is a measure of functional connectivity that identifies changes in correlation between the seed region and other regions based on a psychological variable (e.g., task condition: EO, AS); analyses were carried out separately using M1 and PMd as seed regions. For each subject, after carrying out a GLM statistical analysis, the time series of the mean corrected, high‐pass filtered BOLD signal was extracted from M1 and PMd (4 mm radius sphere centered on each participants peak of activation determined using previously defined masks of M1 and PMd [Mayka et al., 2006]). This time series and the psychological vector of interest (action selection minus execution only) were used to create the PPI interaction term. The interaction term as well as the seed region time series and psychological vector were entered into a first‐level model of connectivity. Brain areas showing an increase in connectivity with the seed region in the AS condition were determined by testing for significant positive changes in slope of the PPI regressor (t‐contrast with +1 for the PPI regressor). Regions showing a decrease in connectivity with the seed regions in the AS condition were determined by testing for significant negative changes in slope of the PPI regressor (t‐contrast −1 for the PPI regressor). Contrast images from both comparisons (increased connectivity in AS, decreased connectivity in AS) were then entered into separate second‐level random effects analyses (one‐sample t‐test) to determine regions whose connectivity with M1 or PMd changed between task conditions. Statistical significance was set at P < 0.001 without correction for multiple comparisons.
To further explore the relationship between variability in behavior and variability in brain activation, we examined the correlation between brain connectivity and RT cost. PPI analysis is designed to test for differences in the regression slope between the seed region and other brain areas based on task condition. Therefore, the time series data from one ROI was plotted against the time series data of another ROI and the slope of the regression line was extracted separately for each of the two conditions (EO, AS) [Lin et al., 2013]. The change in slope from EO to AS was the measure used to define the effect of task condition on functional connectivity. If the slope increased, the activity in the two brain regions was more correlated in the AS condition than the EO condition. If the slope decreased, the activity between the two brain regions was less correlated in the AS condition. To determine if change in connectivity related to variability in task performance between participants, the change in slope between left M1 (centered on the group peak derived from average activation across conditions) and each region identified in the regression analysis was correlated with RT cost. Significance level was set at P < 0.05 and the strength of correlations were interpreted based on the value of the correlation coefficient: r < 0.25 = little or no relationship; r of 0.25 to 0.50 = fair; r of 0.50 to 0.75 = moderate; r > 0.75 = strong [Portney and Watkins, 2009].
RESULTS
Participants
Participant demographics are presented in Table 1. Overall, the stroke group presented with mild to moderate motor impairment (mean UE FM motor score = 52.3), reported moderate difficulty in using the paretic hand to perform functional activities (mean Stroke Impact Scale hand domain score = 3.6), and did not have apraxia (mean TULA score = 230.8). Stroke lesions were subcortical except for a single participant who had injury into temporoparietal cortex (Fig. 2). M1 and PMd were not injured by stroke in any participant. The stroke and control groups did not differ in age or cognitive status (total digit span score) but did differ in motor status (Box & Blocks ratio) and FA asymmetry in the corticospinal tract.
Table 1.
Participant demographics
| Stroke | Control | |
|---|---|---|
| N | 10 | 16 |
| Age | 66.3 ± 5.8 | 65.0 ± 9.0 |
| Gender | 4F/6M | 10F/6M |
| Corticospinal FA Asymmetry | 0.11 ± 0.08 | 0.00 ± 0.03a |
| Box & Blocks Ratio (R/L) | 0.64 ± 0.31 | 1.03 ± 0.05a |
| Digit Span Total | 13.9 ± 4.8 | 17.0 ± 2.8 |
| Months post‐stroke | 42.5 ± 24.7 | |
| UE FM Motor | 52.3 ± 9.1 | |
| Stroke Impact Scale Hand Domain | 3.6 ± 1.1 | |
| TULA Total | 230.8 ± 6.8 |
Values represent group mean ± standard deviation.
P < 0.05 between groups.
Figure 2.
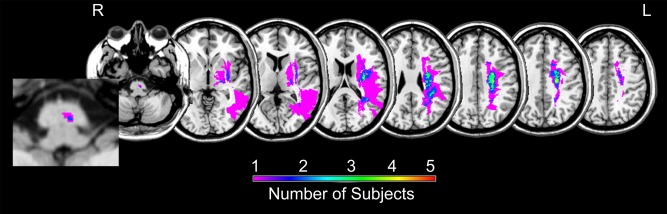
Summary mask of stroke lesions created using MRIcron (http://www.mccauslandcenter.sc.edu/micro/mricron/). Color represents number of participants with a stroke in that region.
Motor Task Performance
Accuracy, RT, MT, and RT cost in both conditions across blocks are shown in Figure 3. As expected, participants in both groups were very accurate during performance of the EO task. Accuracy on the AS task was overall lower in the stroke group compared to the control group during pre‐scan behavioral testing (P = 0.007), however, both groups showed an improvement in accuracy over successive blocks (P = 0.006) such that by the time of fMRI scanning, task accuracy for the AS condition averaged >90% and did not differ between groups (P = 0.4). Across groups, RT for the AS task was significantly longer than for the EO task (P < 0.0001). Action selection RT significantly decreased over practice blocks (P < 0.0001) while EO RT did not change (P = 0.1002). Therefore, changes in RT cost over practice blocks (P < 0.0001) were driven by faster RT during AS. There was no significant difference between groups for RT or RT cost (P > 0.311), however, MT was significantly longer in the stroke group (P = 0.005).
Figure 3.

Behavioral performance for both experimental conditions and groups shown for task accuracy (A), RT (B), MT (C), and RT cost (D). Each data point represents the group mean with standard error bars. Practice through Block 3 was completed in the laboratory prior to MRI.
During fMRI, motor performance was stable and similar to performance in the laboratory (Fig. 3). In both groups, RT for AS was significantly longer than for EO (P < 0.0001) while MT did not differ between conditions (P = 0.535). While RT for both conditions was longer during MRI testing compared with in the laboratory, RT cost during fMRI was consistent with the end of practice in the laboratory across groups (P = 0.927). MT was longer in the stroke group compared to controls during fMRI (P = 0.023), but RT and RT cost did not differ between groups (P > 0.119). For the control group, age had a moderate, positive correlation with AS RT (r = 0.508, P = 0.044) and RT cost (r = 0.494, P = 0.052) but not EO RT (r = 0.205, P = 0.447) during fMRI. In the stroke group, EO RT, AS RT and RT cost did not significantly correlate with age, UE FM motor score or Box & Blocks ratio (r < 0.31, P > 0.417 for all comparisons), or corticospinal tract integrity as measured by FA asymmetry (r < 0.482, P > 0.158).
Brain Activation during Task Performance
Brain activation during the EO and AS task conditions is shown in Figure 4. Performance of the EO task (repetitive movement in the same direction) activated the expected motor network including left M1, PMd, supplemental motor area, thalamus, and cerebellum (Table 2). Overall, across control and stroke groups, activation increased during performance of the AS task in both hemispheres. However, for comparisons of activation between the two conditions (AS > EO; EO > AS), no significant clusters were found at the predefined significance level (P < 0.001). When a more liberal threshold was applied (P < 0.005), differences between groups were found in how brain activation changed from the EO to the AS task (Fig. 4; Table 3). In the control group, performance of the AS task corresponded to increased activation in left inferior parietal lobule (IPL). In the stroke group, performance of the AS task corresponded to increased activation in several regions in the right, contralesional hemisphere including inferior frontal gyrus, fusiform gyrus, anterior cingulate cortex, and IPL along with the left cerebellum. A group by condition interaction revealed three significant clusters where change in activation from the EO to the AS condition differed by group: right anterior cingulate cortex, right visual association cortex, and right fusiform gyrus. For all three clusters, this difference between groups was driven by no change in activation between conditions in the control group and an increase in activation from the EO to the AS condition in the stroke group.
Figure 4.
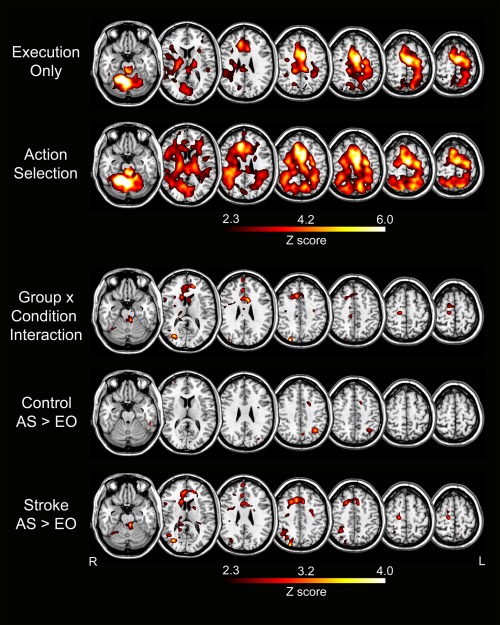
Group analysis of brain activation including main effect of condition (execution only, action selection) for the control and stroke groups combined, group × condition interaction, and a t‐contrast of conditions (AS > EO) for each group separately (Control, Stroke). MT was included as a regressor of no interest in all comparisons.
Table 2.
Location of significant clusters for each condition
| Condition | Brain region | Volume | Peak Z | MNI coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Execution only | R cerebellum | 2630 | Inf | 10 | −54 | −18 |
| L supplementary motor area | 2553 | 7.43 | −4 | −8 | 54 | |
| R supplementary motor area | 10 | 4 | 50 | |||
| R anterior cingulate cortex | 6 | 12 | 40 | |||
| L dorsal premotor cortex | −24 | −12 | 56 | |||
| L cerebellum | 185 | 6.16 | −32 | −50 | −36 | |
| R midbrain | 192 | 5.61 | 5 | −25 | −14 | |
| L thalamus | 129 | 5.41 | −16 | −18 | 4 | |
| R thalamus | 40 | 4.96 | 16 | −12 | 8 | |
| R anterior cingulate cortex | 31 | 4.90 | 10 | 30 | 28 | |
| L primary motor cortex | 23 | 4.78 | −30 | −28 | 52 | |
| Action selection | R cerebellum | 6483 | Inf | 8 | −54 | −16 |
| L cerebellum | −32 | −50 | −34 | |||
| R midbrain | 4 | −30 | −16 | |||
| L midbrain | −2 | −30 | −20 | |||
| L supplementary motor area | 4204 | 7.05 | −6 | 0 | 48 | |
| R supplementary motor area | 4 | 16 | 66 | |||
| L dorsal premotor cortex | −28 | −16 | 58 | |||
| L anterior cingulate cortex | 10 | 30 | 28 | |||
| R anterior cingulate cortex | −8 | 22 | 36 | |||
| L precuneus | 420 | 5.63 | −12 | −62 | 50 | |
| L inferior frontal gyrus | 169 | 5.42 | −30 | 22 | −6 | |
| R inferior parietal lobule | 531 | 5.25 | 38 | −48 | 36 | |
| R precuneus | 73 | 5.08 | 10 | −66 | 60 | |
| L inferior parietal lobule | 64 | 4.94 | −30 | −46 | 42 | |
| R dorsal premotor cortex | 30 | 4.85 | 32 | −8 | 42 | |
All clusters were significant at P < 0.05 with familywise error correction for analysis of each condition with control and stroke groups combined. Volume = number of 8 mm3 voxels in cluster; Peak Z = peak Z value within the cluster; L = Left; R = Right; Inf = Infinity. For large clusters, the locations of several local maxima within the cluster are listed.
Table 3.
Location of significant clusters for group analyses
| Comparison | Brain region | Volume | Peak Z | MNI coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Control group AS>EO | L inferior parietal lobule | 147 | 3.52 | −34 | −56 | 44 |
| Stroke group AS>EO | R inferior frontal gyrus | 789 | 4.06 | 24 | 30 | 0 |
| R fusiform gyrus | 1121 | 4.02 | 26 | −50 | −12 | |
| L cerebellum | 448 | 3.66 | −4 | −48 | −18 | |
| R dorsal anterior cingulate | 623 | 3.57 | 10 | 24 | 44 | |
| R inferior parietal lobule | 253 | 3.35 | 42 | −56 | 38 | |
| Group × condition interaction | R dorsal anterior cingulate | 429 | 4.02 | 8 | 20 | 44 |
| 665 | 3.46 | 4 | 44 | 8 | ||
| R visual association cortex | 290 | 3.84 | 30 | −76 | 12 | |
| R fusiform gyrus | 488 | 3.41 | 30 | −54 | −14 | |
All clusters were significant at P < 0.005 uncorrected for multiple comparisons. Volume = number of 8 mm3 voxels in cluster; Peak Z = peak Z value within the cluster; R = Right; L = Left; AS = Action Selection; EO = Execution Only.
Whole brain PPI analysis was carried out separately with left M1 (mean peak coordinates: −32 −20 64) and left PMd (mean peak coordinates: −24 −7 69) used as the seed region. This analysis was performed to determine changes in the correlation between M1/PMd and other brain regions based on task condition (EO > AS; AS > EO). There were no significant clusters that changed their relationship with M1 or PMd between the EO and AS conditions across participants in the whole brain analysis, both when the groups were analyzed separately and when the groups were combined.
Neural Correlates of Interindividual Variability in Task Performance
Whole brain regression analysis was run in order to determine if variability in task performance between participants was related to variability in brain activation. Since RT cost did not significantly differ between the control and stroke groups during fMRI, all participants from both groups were included in a single regression analysis. Three clusters were found where activation significantly correlated with RT cost: left dorsolateral prefrontal cortex (DLPFC), left PMd, and left visual association cortex (Fig. 5; Table 4). All three clusters showed a positive correlation with RT cost, i.e. individuals who took relatively longer to prepare a response in the AS condition had greater activation in each of these regions. A post‐hoc analysis determined that the relationship between brain activation and RT cost was similar in the stroke and control groups (no group by activation interaction for explaining RT cost; see Fig. 5). Additionally, within the stroke group, variability in brain activation between participants was not significantly related to clinical measures of arm function (no relationship between percent signal change and UE FM motor score or Box & Blocks ratio; P > 0.1 for all comparisons) or corticospinal tract integrity as measured by FA asymmetry (P > 0.82).
Figure 5.
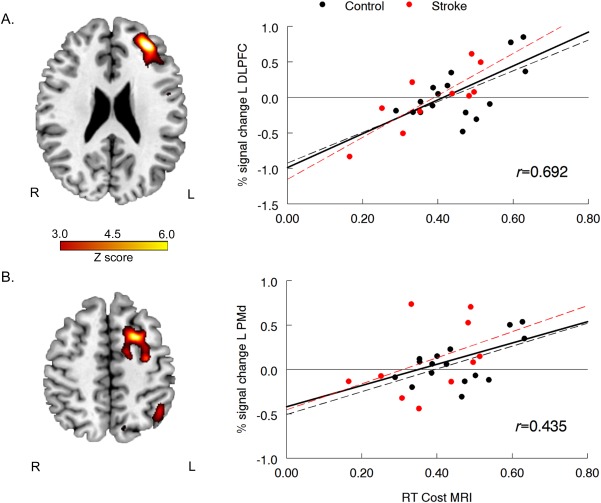
Results of whole brain regression analysis between RT cost during MRI and brain activation during the action selection condition with age and MT included as regressors of no interest: (A) left dorsolateral prefrontal cortex (DLPFC), and (B) left dorsal premotor cortex (PMd). For scatter plots, percent signal change was extracted from the significant cluster (P < 0.001 uncorrected for multiple comparisons) during action selection; each data point represents performance for a single participant. Solid regression line and r value = all participants combined; black dashed regression = Control group only; red dashed regression line = Stroke group only.
Table 4.
Location of significant clusters for regression analyses
| MNI Coordinates | ||||||
|---|---|---|---|---|---|---|
| Brain Region | Volume | Peak Z | x | y | z | |
| AS with RT Cost (+) | L dorsolateral prefrontal cortex | 956 | 5.06 | −32 | 44 | 24 |
| L dorsal premotor | 301 | 4.49 | −32 | −10 | 54 | |
| L visual association cortex | 154 | 3.93 | −48 | −70 | −16 |
All clusters were significant at P < 0.001 uncorrected for multiple comparisons. Volume = number of 8 mm3 voxels in cluster; Peak Z = peak Z value within the cluster; L = Left; AS = Action Selection; RT = Reaction Time; (+)=positive relationship.
To further explore the relationship between variability in behavioral performance and variability in brain activation, the correlation between brain connectivity and RT cost was examined. This analysis focused on changes in connectivity from the EO to the AS task condition between left M1, PMd, and DLPFC (Fig. 6A,B) based on the results of the regression analysis above. For controls, no significant correlation between change in connectivity and RT was found for any comparison. In the analysis of PMd‐M1 connectivity, two groups of control participants emerged (increase in connectivity, decrease in connectivity) that was explored in a previous analysis [Stewart et al., 2014]. For the stroke group, a relationship between change in connectivity and RT cost was found. Change in PMd‐DLPFC connectivity showed a significant negative correlation with RT cost (r = −0.640, P = 0.046); individuals with longer RT cost tended to show a decrease in connectivity between these two brain regions during AS (Fig. 6D). Change in PMd‐M1 connectivity showed a relationship with RT cost in the opposite direction; individuals with longer RT tended to show an increase in PMd‐M1 connectivity (Fig. 6C). While the relationship between PMd‐M1 connectivity and behavior was fair in strength, the correlation was not statistically significant. However, when an outlier was removed (stroke subject with the lowest RT cost across all participants), the relationship between PMd‐M1 connectivity and RT cost was strong and significant (r = 0.842, P = 0.004). Therefore, overall, individuals who had relatively lower RT cost (better performance), showed a decrease in PMd‐M1 connectivity and an increase in PMd‐DLPFC connectivity; individuals who had relatively higher RT cost (worse performance) showed an increase in PMd‐M1 connectivity and a decrease in PMd‐DLPFC connectivity. These relationships were not related to clinical presentation; connectivity between regions did not correlate with UE FM motor or Box & Blocks ratio (P > 0.62 for all comparisons) or with corticospinal tract integrity as measured by FA asymmetry (P > 0.381).
Figure 6.

Results of PPI analysis. (A) Exemplary data from an individual participant who had a decrease in left primary motor (M1) ‐ left dorsal premotor cortex (PMd) connectivity during the action selection (AS) condition compared to the execution only (EO) condition (each data point represents activity for a single brain volume). Slope and correlation coefficient (r) were extracted for each participant and condition. (B) The same participant showed an increase in PMd – left dorsolateral prefrontal (DLPFC) connectivity during AS compared to EO. (C) Change in M1‐PMd connectivity (AS slope – EO slope) plotted against RT cost separately for each group. A positive change equates to an increase in connectivity while a negative change equates to a decrease in connectivity from the EO to the AS conditions. (D) Change in PMd‐DLPFC connectivity (AS slope – EO slope) plotted against RT cost separately for each group. *significant correlation at P < 0.05.
DISCUSSION
This study examined the behavioral and neural correlates of motor AS after stroke. Similar to controls, there was a significant increase in movement preparation time to perform the AS task compared to simple movement execution (EO) in the stroke group. However, despite a similar increase in RT in the stroke and control groups, the neural resources utilized to perform the AS task differed between groups. In the control group, the increase in planning time for the AS condition corresponded to increased activation in the IPL contralateral to the moving hand similar to previous studies in young controls [Grafton et al., 1998; Grol et al., 2006; O'Shea et al., 2007; Toni et al., 2002] and discussed in a previous paper [Stewart et al., 2014]. In the stroke group, the increase in planning time for the AS condition corresponded to increased activation in several contralesional brain regions that were ipsilateral to the moving hand. Additionally, variability in behavioral performance on the AS task between participants with stroke correlated with brain activation in ipsilesional premotor and prefrontal cortices. Specifically, relatively better performers (lower RT cost) showed decreased activation magnitude in left PMd and DLPFC, decreased left PMd‐M1 connectivity, and increased left PMd‐DLPFC connectivity. On the other hand, relatively worse performers (higher RT cost) showed increased activation magnitude in left PMd and DLPFC, increased left PMd‐M1 connectivity, and decreased left PMd‐DLPFC connectivity. Overall, the findings of the current study suggest that the behavioral response to the addition of AS demands to movement after stroke relates to the pattern of engagement of ipsilesional premotor and prefrontal brain regions.
As a group, performance of the AS task with paretic, right hand corresponded to increased activation in several right, contralesional brain regions and left cerebellum using a threshold of P < 0.005. Since behavioral response to the AS task did not differ between the stroke and control groups (no difference in RT cost), the engagement of contralesional brain regions after stroke likely reflects compensatory activation to maintain task performance [Lotze et al., 2006; Schaechter and Perdue, 2008]. This increase in contralesional activation in the AS condition is consistent with a previous study that showed increased contralesional activation after stroke in response to an increase in task difficulty of a hand movement [Schaechter and Perdue, 2008]. Additional research is needed to fully understand modulation of brain activity based on changes in task conditions after stroke and whether this modulation changes with targeted practice. Increased neural resource utilization in the contralesional hemisphere during AS after stroke may impact motor learning under training conditions that relatively increase AS demands.
The contralesional brain regions activated in the AS condition included the right IPL, fusiform gyrus, inferior frontal gyrus, anterior cingulate gyrus, and visual association cortex. These brain regions have been previously reported be active during the performance of a motor AS task [Grafton et al., 1998; Grol et al., 2006; O'Shea et al., 2007; Toni et al., 2002], performance of a motor task that has increased planning demands [Lin et al., 2012; Lin et al., 2011], and completion of a complex motor task in older adults [Heuninckx et al., 2005]. The task in the current study was visually cued. The increase in visual cortex and IPL activation during the AS condition may represent an increase in visual attention [Cross et al., 2007; Kahlbrock et al., 2012] or visuomotor processing [Fogassi and Luppino, 2005; Grefkes and Fink, 2005] in response to the increase in planning demands. Activation in the fusiform gyrus, inferior frontal gyrus, and anterior cingulate cortex suggest an increase in the cognitive control of movement during motor AS after stroke through increased attention and response inhibition [Hampshire et al., 2010; Picton et al., 2007; Sebastian et al., in press] or processing of feedback [Amiez et al., 2012; Holroyd et al., 2004]. Overall, the increase in activation in these regions on the contralesional side may indicate slower learning such that participants with stroke remained at an earlier phase of learning for the AS task despite the same number of practice trials as controls prior to scanning [Patel et al., 2013].
Magnitude of activation in ipsilesional PMd and DLPFC varied between individuals post‐stroke and this variability in activation correlated with task performance. Better performance (lower RT cost) was related to lower magnitude of activation in PMd and DLFPC while worse performance (higher RT cost) was related to higher activation magnitude in these regions. The increase in activation magnitude in PMd and DLPFC in poorer performers was not related to age (age was included as a covariate in regression analyses), level of motor impairment, or corticospinal tract integrity, and was similar in the control and stroke groups. This relationship suggests a similar compensatory increase in activation magnitude to maintain task performance in older adults with and without stroke. Increased activation to maintain behavioral performance during movement has previously been reported in older adults [Heuninckx et al., 2008; Mattay et al., 2002; Noble et al., 2011; Ward and Frackowiak, 2003] and after stroke [Carey et al., 2002; Lotze et al., 2006; Schaechter and Perdue, 2008; Schaechter et al., 2008; Ward et al., 2003]. The current study extends this finding to a task that has increased planning demands through the addition of AS demands to movement.
Variability in AS task performance between individuals in the stroke group also correlated with variability in the functional connections in the premotor‐prefrontal component of the motor network. PMd connectivity with M1 and DLPFC varied between participants in a manner that related to task performance. In individuals with better performance, left PMd showed increased connectivity with left DLPFC and decreased connectivity with left M1 in the AS condition compared to the EO condition. In individuals with worse performance, however, the relationship was opposite: left PMd showed decreased connectivity with left DLPFC and increased connectivity with left M1. Overall, these results suggest that PMd may have played a greater role in movement execution during the AS task in some individuals (increased connectivity with M1) and a greater role in movement planning in other individuals (increased connectivity with DLPFC). In individuals who are using PMd to support movement execution [Fridman et al., 2004], this region may be limited in its ability to support other movement related processes such as AS. It has been suggested that PMd may serve as a region of interaction between the motor and cognitive systems [Abe and Hanakawa, 2009; Hanakawa, 2011]. The current study suggests that the ability to effectively allocate PMd resources between the motor and cognitive systems may vary after stroke and that this ability may have an effect on the performance of skilled motor tasks that require both systems. The role of PMd in motor planning after stroke and how this role changes with practice warrants further investigation.
Increased utilization of the cognitive system during movement has been shown in older, nondisabled adults [Heuninckx et al., 2005; Li and Lindenberger, 2002]. Changes in motor performance or neural activation during movement may be a better indicator of aging than chronological age [Schaefer, 2015; Talelli et al., 2008]. The current study suggests a similar relationship in older adults with motor impairment after stroke. Altered patterns of ipsilesional PMd and DLPFC activity during motor AS may indicate changes in the attention and cognitive resources needed to complete a motor planning task that has clinical implications. If the performance of a motor task under conditions of increased complexity requires increased premotor and prefrontal neural resources, less resource may be available to perform additional cognitive tasks (e.g. dual task), to monitor task performance, or to anticipate and plan for the next motor task. Such changes in neural resource utilization may help explain why some individuals post‐stroke do not use their arm for the performance of everyday, functional activities despite having minimal to no motor impairment [Han et al., 2013; Stewart and Cramer, 2013]. With the current study design, we cannot determine whether this increased in premotor‐prefrontal activation changes with targeted practice; future studies can investigate this question.
We did not find an increase in the magnitude of PMd activation when moving from the EO to the AS condition, ipsilesionally or contralesionally, for the stroke group as hypothesized. There are several possible reasons for this finding. First, PMd was significantly active during the EO condition consistent with previous work showing increased PMd activation with movement execution tasks in older adults [Mattay et al., 2002; Ward and Frackowiak, 2003] and after stroke [Ward et al., 2003; Ward et al., 2006]. Increased PMd activation during the EO condition in participants post‐stroke may have made it more difficult to find statistically significant increases in activation during performance of the AS task. Second, modulation of brain activity to meet a change in task demands may be altered after stroke. The control participants in the current study showed limited changes in brain activation between task conditions consistent with previous work showing a decrease in the modulation of brain activation to meet changes in motor task demands in older adults [Heuninckx et al., 2010; Ward et al., 2008]. This decrease in modulation may also be present in older adults who have had a stroke. Third, level of PMd activation during AS in the current study may reflect the stage of learning. Brain activation changes with practice in nondisabled adults [Floyer‐Lea and Matthews, 2004; Karni et al., 1995; Lin et al., 2011; Wu et al., 2004] and after stroke [Boyd et al., 2010; Carey et al., 2002; Meehan et al., 2011]. Additional practice may be needed in individuals post‐stroke to achieve a level of task performance that corresponds to PMd activation that is similar to previous studies in young adults. Finally, this study may have not have had enough power to detect a significant difference between conditions given the sample size of the stroke group.
This study was cross‐sectional by design and cannot provide insight into how brain activation during motor AS changes with practice. Future research might examine whether contralesional brain activation changes with practice and determine whether the brain‐behavior relationships between individuals found here persist or are altered over time. The measure of connectivity used in this analysis provides information on functional connections between brain regions but does not give an indication of the direction of these connections. Future analyses may be able to determine whether the PMd connections with M1 and DLPFC found during AS are facilitatory or inhibitory. The sample size in the current study was small and only included individuals in the chronic stage of stroke recovery with a mild to moderate degree of motor impairment. Therefore, inferences of the current results to the larger population of individuals with motor impairment due to stroke and analyses of possible covariates such as lesion location and extent are limited. The results of the current study suggest additional studies that address such variables are warranted.
In conclusion, individuals post‐stroke showed an increase in planning time and engaged several contralesional brain regions to successfully perform a motor AS task. Individuals who took longer to respond (poorer performance) showed increased magnitude of activation in PMd and DLPFC and decreased functional connectivity between these two regions. The ability to efficiently engage neural resources during motor planning tasks may have implications for motor training after stroke. Structuring practice during rehabilitation to engage specific behavioral processes and neural resources (e.g., motor AS) may serve as an adjunct to repetitive movement execution [Cramer et al., 2011; Dodakian et al., 2013]. The premotor‐prefrontal component of the motor circuit may be a prime candidate for a targeted rehabilitation protocol aimed at improving the function of residual brain circuits after stroke.
ACKNOWLEDGMENTS
The authors thank Vu Le for his assistance with experimental task development. Steven C. Cramer is a consultant for GlaxoSmithKline, MicroTransponder, Dart Neuroscience, RAND Corporation, and Roche. He is also cofounder of personalRN.
REFERENCES
- Abe M, Hanakawa T (2009): Functional coupling underlying motor and cognitive functions of the dorsal premotor cortex. Behav Brain Res 198:13–23. [DOI] [PubMed] [Google Scholar]
- Amiez C, Hadj‐Bouziane F, Petrides M (2012): Response selection versus feedback analysis in conditional visuo‐motor learning. Neuroimage 59:3723–3735. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen‐Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM (2003): Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50:1077–1088. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Teo J, Weiskopf N, Driver J, Rothwell JC, Ward NS (2010): The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS‐fMRI. J Neurosci 30:11926–11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Vidoni ED, Wessel BD (2010): Motor learning after stroke: Is skill acquisition a prerequisite for contralesional neuroplastic change? Neurosci Lett 482:21–25. [DOI] [PubMed] [Google Scholar]
- Burke E, Dodakian L, See J, McKenzie A, Riley JD, Le V, Cramer SC (2014): A multimodal approach to understanding motor impairment and disability after stroke. J Neurol 261:1178–1186. [DOI] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K (2002): Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain 125:773–788. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M (1998): The functional neuroanatomy of simple and complex sequential finger movements: A PET study. Brain 121(Pt 2):253–264. [DOI] [PubMed] [Google Scholar]
- Chen SY, Winstein CJ (2009): A systematic review of voluntary arm recovery in hemiparetic stroke: Critical predictors for meaningful outcomes using the international classification of functioning, disability, and health. J Neurol Phys Ther 33:2–13. [DOI] [PubMed] [Google Scholar]
- Cramer SC (2008): Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol 63:272–287. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, Rumsey JM, Hicks R, Cameron J, Chen D and others. (2011): Harnessing neuroplasticity for clinical applications. Brain 134(Pt 6):1591–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross ES, Schmitt PJ, Grafton ST (2007): Neural substrates of contextual interference during motor learning support a model of active preparation. J Cogn Neurosci 19:1854–1871. [DOI] [PubMed] [Google Scholar]
- Dodakian L, Sharp KG, See J, Abidi NS, Mai K, Fling BW, Le VH, Cramer SC (2013): Targeted engagement of a dorsal premotor circuit in the treatment of post‐stroke paresis. NeuroRehabilitation 33:13–24. [DOI] [PubMed] [Google Scholar]
- Floyer‐Lea A, Matthews PM (2004): Changing brain networks for visuomotor control with increased movement automaticity. J Neurophysiol 92:2405–2412. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Luppino G (2005): Motor functions of the parietal lobe. Curr Opin Neurobiol 15:626–631. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG (2004): Reorganization of the human ipsilesional premotor cortex after stroke. Brain 127:747–758. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R (1995a): Analysis of fMRI time‐series revisited. Neuroimage 2:45–53. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ (1995b): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2:189–210. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Fugl‐Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S (1975): The post‐stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 7:13–31. [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ (2003): Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. Neuroimage 19:200–207. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Arbib MA (1998): Dorsal premotor cortex and conditional movement selection: A PET functional mapping study. J Neurophysiol 79:1092–1097. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR (2005): The functional organization of the intraparietal sulcus in humans and monkeys. J Anat 207:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grol MJ, de Lange FP, Verstraten FA, Passingham RE, Toni I (2006): Cerebral changes during performance of overlearned arbitrary visuomotor associations. J Neurosci 26:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM (2010): The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage 50:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CE, Kim S, Chen S, Lai YH, Lee JY, Osu R, Winstein CJ, Schweighofer N (2013): Quantifying arm nonuse in individuals poststroke. Neurorehabil Neural Repair 27:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T (2011): Rostral premotor cortex as a gateway between motor and cognitive networks. Neurosci Res 70:144–154. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Zito G, Dimyan MA, Hallett M (2006): Brain activity during visuomotor behavior triggered by arbitrary and spatially constrained cues: An fMRI study in humans. Exp Brain Res 172:275–282. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP (2005): Neural basis of aging: The penetration of cognition into action control. J Neurosci 25:6787–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP (2008): Systems neuroplasticity in the aging brain: Recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci 28:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP (2010): Age‐related reduction in the differential pathways involved in internal and external movement generation. Neurobiol Aging 31:301–314. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MG, Cohen JD (2004): Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat Neurosci 7:497–498. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM (2002): The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA 99:14518–14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlbrock N, Butz M, May ES, Schnitzler A (2012): Sustained gamma band synchronization in early visual areas reflects the level of selective attention. Neuroimage 59:673–681. [DOI] [PubMed] [Google Scholar]
- Kantak SS, Stinear JW, Buch ER, Cohen LG (2012): Rewiring the brain: Potential role of the premotor cortex in motor control, learning, and recovery of function following brain injury. Neurorehabil Neural Repair 26:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG (1995): Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377:155–158. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Kleim ED, Cramer SC (2007): Systematic assessment of training‐induced changes in corticospinal output to hand using frameless stereotaxic transcranial magnetic stimulation. Nat Protoc 2:1675–1684. [DOI] [PubMed] [Google Scholar]
- Li KZ, Lindenberger U (2002): Relations between aging sensory/sensorimotor and cognitive functions. Neurosci Biobehav Rev 26:777–783. [DOI] [PubMed] [Google Scholar]
- Lin CH, Knowlton BJ, Chiang MC, Iacoboni M, Udompholkul P, Wu AD (2011): Brain‐behavior correlates of optimizing learning through interleaved practice. Neuroimage 56:1758–1772. [DOI] [PubMed] [Google Scholar]
- Lin CH, Chiang MC, Wu AD, Iacoboni M, Udompholkul P, Yazdanshenas O, Knowlton BJ (2012): Age related differences in the neural substrates of motor sequence learning after interleaved and repetitive practice. Neuroimage 62:2007–2020. [DOI] [PubMed] [Google Scholar]
- Lin CH, Chiang MC, Knowlton BJ, Iacoboni M, Udompholkul P, Wu AD (2013): Interleaved practice enhances skill learning and the functional connectivity of fronto‐parietal networks. Hum Brain Mapp 34:1542–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln NB, Jackson JM, Adams SA (1998): Reliability and revision of the Nottingham sensory assessment for stroke patients. Physiotherapy 84:358–365. [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C (2006): The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci 26:6096–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark VW, Taub E, Perkins C, Gauthier LV, Uswatte G, Ogorek J (2008): Poststroke cerebral peduncular atrophy correlates with a measure of corticospinal tract injury in the cerebral hemisphere. AJNR Am J Neuroradiol 29:354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR (2002): Neurophysiological correlates of age‐related changes in human motor function. Neurology 58:630–635. [DOI] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE (2006): Three‐dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: A meta‐analysis. Neuroimage 31:1453–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan SK, Randhawa B, Wessel B, Boyd LA (2011): Implicit sequence‐specific motor learning after subcortical stroke is associated with increased prefrontal brain activations: An fMRI study. Hum Brain Mapp 32:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Zhang J (2006): Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539. [DOI] [PubMed] [Google Scholar]
- Noble JW, Eng JJ, Kokotilo KJ, Boyd LA (2011): Aging effects on the control of grip force magnitude: An fMRI study. Exp Gerontol 46:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea J, Johansen‐Berg H, Trief D, Gobel S, Rushworth MF (2007): Functionally specific reorganization in human premotor cortex. Neuron 54:479–490. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Turner GR (2013): Functional brain changes following cognitive and motor skills training: A quantitative meta‐analysis. Neurorehabil Neural Repair 27:187–199. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S (2007): Effects of focal frontal lesions on response inhibition. Cereb Cortex 17:826–838. [DOI] [PubMed] [Google Scholar]
- Portney L, Watkins M. 2009. Foundations of Clinical Research: Applications to Practice. Upper Saddle River, NJ: Prentice‐Hall. [Google Scholar]
- Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, Marshall RS, Krakauer JW (2008): Inter‐individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair 22:64–71. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C (2012): Activation likelihood estimation meta‐analysis of motor‐related neural activity after stroke. Neuroimage 59:2771–2782. [DOI] [PubMed] [Google Scholar]
- Riley JD, Le V, Der‐Yeghiaian L, See J, Newton JM, Ward NS, Cramer SC (2011): Anatomy of stroke injury predicts gains from therapy. Stroke 42:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL (2008): Enhanced cortical activation in the contralesional hemisphere of chronic stroke patients in response to motor skill challenge. Cereb Cortex 18:638–647. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL, Wang R (2008): Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage 39:1370–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY (2015): Preserved motor asymmetry in late adulthood: Is measuring chronological age enough? Neuroscience 294:51–59. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Jung P, Neuhoff J, Wibral M, Fox PT, Lieb K, Fries P, Eickhoff SB, Tuscher O, Mobascher A: Dissociable attentional and inhibitory networks of dorsal and ventral areas of the right inferior frontal cortex: A combined task‐specific and coordinate‐based meta‐analytic fMRI study. Brain Struct Funct (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010): Motor control and aging: Links to age‐related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL (2010): Reliable differences in brain activity between young and old adults: A quantitative meta‐analysis across multiple cognitive domains. Neurosci Biobehav Rev 34:1178–1194. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Cramer SC (2013): Patient‐reported measures provide unique insights into motor function after stroke. Stroke 44:1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JC, Tran X, Cramer SC (2014): Age‐related variability in performance of a motor action selection task is related to differences in brain function and structure among older adults. Neuroimage 86:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear C (2010): Prediction of recovery of motor function after stroke. Lancet Neurol 9:1228–1232. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD (2007): Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 130:170–180. [DOI] [PubMed] [Google Scholar]
- Talelli P, Ewas A, Waddingham W, Rothwell JC, Ward NS (2008): Neural correlates of age‐related changes in cortical neurophysiology. Neuroimage 40:1772–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni I, Shah NJ, Fink GR, Thoenissen D, Passingham RE, Zilles K (2002): Multiple movement representations in the human brain: An event‐related fMRI study. J Cogn Neurosci 14:769–784. [DOI] [PubMed] [Google Scholar]
- Vanbellingen T, Kersten B, Van Hemelrijk B, Van de Winckel A, Bertschi M, Muri R, De Weerdt W, Bohlhalter S (2010): Comprehensive assessment of gesture production: A new test of upper limb apraxia (TULIA). Eur J Neurol 17:59–66. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB (2005): Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol 93:1209–1222. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS (2003): Age‐related changes in the neural correlates of motor performance. Brain 126:873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS (2003): Neural correlates of outcome after stroke: A cross‐sectional fMRI study. Brain 126:1430–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS (2006): Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 129(Pt 3):809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Frackowiak RS, Thompson AJ, Greenwood RJ, Rothwell JC (2007): The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci 25:1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Swayne OB, Newton JM (2008): Age‐dependent changes in the neural correlates of force modulation: An fMRI study. Neurobiol Aging 29:1434–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D. 2005. Biomechanics and Motor Control of Human Movement. Hoboken: Wiley. [Google Scholar]
- Wu T, Kansaku K, Hallett M (2004): How self‐initiated memorized movements become automatic: A functional MRI study. J Neurophysiol 91:1690–1698. [DOI] [PubMed] [Google Scholar]


