Abstract
Nearly 50% of patients with oral squamous cell carcinoma (OSCC) die of metastases or locoregional recurrence. Metastasis is mediated by cancer cell adhesion, migration and invasion. Osteoactivin (OA) overexpression plays a role in metastases in several malignancies.
Objectives
To determine how integrin interactions modulate OA-induced OSCC cell migration; and to investigate OA effects on cell survival and proliferation.
Materials and Methods
We confirmed OA mRNA and protein overexpression in OSCC cell lines. We assessed OA’s interactions with integrins using adhesion inhibition assays, fluorescent immunocytochemistry and co-immunoprecipitation. We investigated OA-mediated activation of mitogen-activated protein kinases (MAPKs) and cell survival. Integrin inhibition effects on OA-mediated cell migration were determined. We assessed effects of OA knock-down on cell migration and proliferation.
Results
OA is overexpressed in OSCC cell lines, and serves as a migration-promoting adhesion molecule. OA co-localized with integrin subunits, and co-immunoprecipitated with the subunits. Integrin blocking antibodies, especially those directed against the β1 subunit, inhibited cell adhesion (p=0.03 for SCC15 cells). Adhesion to OA activated MAPKs in UMSCC14a cells and OA treatment promoted survival of SCC15 cells. Integrin-neutralizing antibodies enhanced cell migration with OA in the extracellular matrix. OA knock-down resulted in decreased proliferation of SCC15 and SCC25 cells, but did not inhibit cell migration.
Conclusion
OA in the extracellular matrix promotes OSCC cell adhesion and migration, and may be a novel target in the prevention of HNSCC spread.
Keywords: Blocking antibodies, Western blotting, Squamous cell carcinoma, Oral cancer, Cell adhesion, Cell line, Cell movement, Cell survival, Mitogen-activated protein kinases, Immunohistochemistry, Integrins
Introduction
Oral cancer is the 14th most common malignancy worldwide1 and oral squamous cell carcinomas (OSCCs) comprise 80–90% of oral malignancies2. OSCC overall survival has remained at approximately 50% for decades. Nearly 25% of patients with OSCC die of distant metastases, while 40–60% of patients succumb to locoregional recurrence.2 Cancer cell adhesion, migration and invasion mediate metastatic spread.
The most studied cell adhesion molecules are the integrins. The integrins are a family of heterodimeric glycoproteins that serve as extracellular matrix (ECM) receptors, and play a role in cell-to-cell adhesion.3 The family consists of 18 α- and 8 β-subunits that form at least 25 recognized distinct pairings with varying affinities for numerous ligands.4 Integrins are involved in a number of physiological and pathological processes including development, differentiation, growth, tissue homeostasis, ECM assembly, immune responses, wound healing, cell survival, proliferation, apoptosis and neoplasia.3, 5, 6
Osteoactivin (OA), also known as gpnmb7–13, dendritic cell heparin sulfate proteoglycan integrin dependent ligand14, 15, and human hematopoietic growth factor inducible neurokinin16, was first identified as a gene differentially expressed in melanoma cells with high metastatic potential12. OA overexpression contributes to metastasis in breast carcinomas17–20, melanoma cells11, 12, hepatic carcinoma12, squamous cell lung carcinoma12, prostate carcinoma21 and an experimental glioma22. OA messenger ribonucleic acid (mRNA) overexpression in head and neck squamous cell carcinoma (HNSCC) relative to normal mucosa has been demonstrated in several studies.23–29 OA exists as a 65 kDa transmembrane protein and a 115 kDa excreted isoform.30 The OA arginine-glycine-aspartic acid (RGD) domain31 serves as a recognition sequence for integrins32, and interacts with endothelial cells15, melanoma cells33 and osteoblasts34.
In this study, we confirm that OA is overexpressed in HNSCC cell lines, and that it serves as a migration-promoting adhesion molecule. We identify OA-integrin interactions in these cells, examining integrins commonly expressed by oral keratinocytes that bind the RGD domain35 and that are associated with migration in malignant cells36, 37. We also assess the effects of OA on cell survival, as well as the effects of OA knock-down on OA-mediated cell proliferation and migration.
Materials and Methods
This study was granted exempt status from Temple University Institutional Review Board (protocol #13653) because commercially-available cells lines were used.
Cell culture
UMSCC-12, -14a, -49 and -69 cells were obtained from the Head and Neck Cancer Biology Laboratory at the University of Michigan (Ann Arbor). UMSCC12 cells were obtained from a patient with T2N1M0 laryngeal cancer. UMSCC14a cells were isolated from a patient with early stage oral squamous cell carcinoma (OSCC, stage T1N0M0). UMSCC-49 cells were isolated from a patient with OSCC that did not invade bone, but was metastatic (stage T2N1M0). UMSCC-69 cells were isolated from a patient with extensive local invasion, but no metastases (stage T4N0M0). SCC-15 and -25 cells were obtained from the American Type Culture Collection (ATCC, Manassas, Virginia). SCC15 cells were isolated from a patient with extensive local invasion and cervical metastases (stage T4N1M0). SCC25 cells were obtained from a patient with stage T2N1 OSCC.38 OKF6/TERT1 immortalized oral keratinocyte cells39 were obtained from the Harvard Skin Disease Research Center and maintained in Invitrogen (Grand Island, New York) keratinocyte serum-free medium supplemented with 25 μg/ml bovine pituitary extract, 0.2 ng/ml epidermal growth factor, 0.3 mM CaCl2, 100 U/ml penicillin and 100 μg/ml streptomycin in a 5% CO2 atmosphere at 37°C. UMSCC-12, -14a, -49 and -69 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with penicillin/streptomycin and 10% fetal bovine serum (FBS). SCC-15 and -25 cells were maintained in DMEM with Hank’s F12 medium supplemented with 400 ng/ml hydrocortisone, penicillin/streptomycin and 10% FBS. UMSCC14a, SCC15 and SCC25 cells were used for adhesion and migration assays because of their ability to adhere in and tolerate serum-free conditions.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
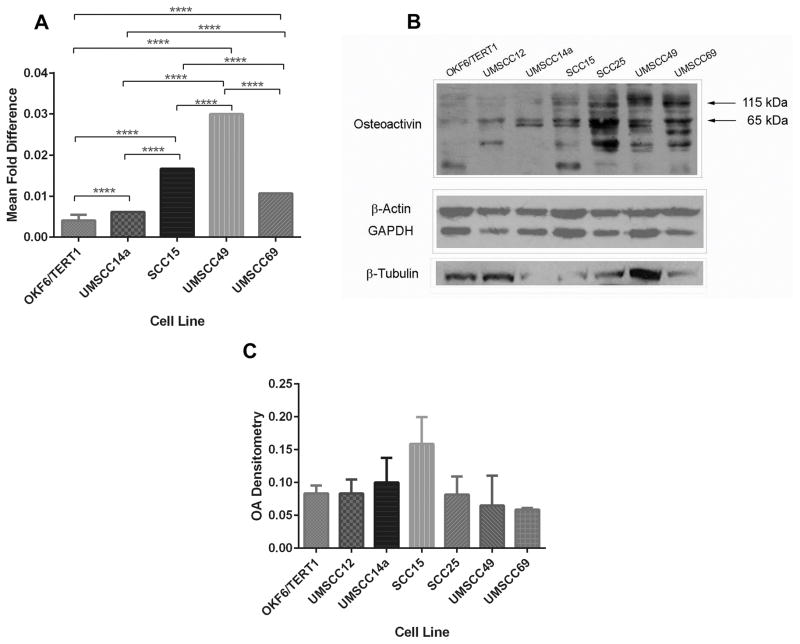
RNA harvest and reverse transcription were performed as previously described.40 qRT-PCR was performed twice with six replicates using the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, California) according to the manufacturer’s recommendations. OA primer sequences were: forward – 5′-TCCGTGAGAATTCAGCATGG-3′, reverse – 5′-AGCACATCATGAAATCGTTTGG-3′. β-actin primer sequences were: forward – 5′-AGGTCATCACCATTGGCAAT-3′, reverse – 5′-ACTCGTCATACTCCTGCTTG-3′. Threshold cycles were used to normalize samples to β-actin, the endogenous control, because it was the most consistently expressed “housekeeping” protein between the cell lines (Figure 1B).
Figure 1. OA is differentially expressed in OSCC cell lines.
(A) Total RNA was obtained from OSCC cell lines and immortalized oral keratinocytes, and reverse transcribed. qRT-PCR was used to compare expression of OA mRNA in the cell lines, normalized to β-actin. Graph depicts mean fold differences ± standard deviations. All OSCC cell lines expressed OA mRNA to a greater extent that OKF6/TERT1 immortalized oral keratinocytes (p<0.0001). OA mRNA expression also differed significantly between the cell lines tested (p<0.0001 for all comparisons). (B) OKF6/TERT1 immortalized oral keratinocytes and HNSCC cell lines were grown to subconfluence. Lysates (45 μg/lane) were resolved by SDS-PAGE, and total protein measured with infrared imaging after gel staining with GelCode® Blue (top left). Similarly loaded gels were used for protein transfer to membranes, and stained after transfer (top right). Membranes were probed with OA antibody (bottom left) and OA expression was normalized to total protein (bottom right, means ± standard errors).
Western blot analyses
OA
HNSCC cells and immortalized keratinocytes were were grown to subconfluence and lysed in RIPA buffer. Protein concentrations were measured with a Pierce bicinchonic acid kit (Thermo Fisher Scientific, Waltham, Massachusetts). Protein extracts (45 μg) were resolved by SDS-PAGE. Gels were stained with GelCode® Blue Stain Reagent (Sigma) and infrared imaging was used to quantitate protein density. Similarly loaded gels was used for protein transfer to PVDF membranes, and the membranes were probed with antibodies to OA (Santa Cruz Biotechnology, Santa Cruz, California). Blots were developed using horse radish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, Pennsylvania). Endogenous controls tested were β-actin (Sigma Chemical Company, St. Louis, Missouri), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Santa Cruz Biotechnology) and β-tubulin (Santa Cruz Biotechnology). OA expression was normalized to total protein.41
Integrins
To confirm integrin expression in malignant oral keratinocytes, protein extracts were resolved by SDS-PAGE, transferred to PVDF membranes and probed with antibodies to individual integrin subunits (Santa Cruz Biotechnology).
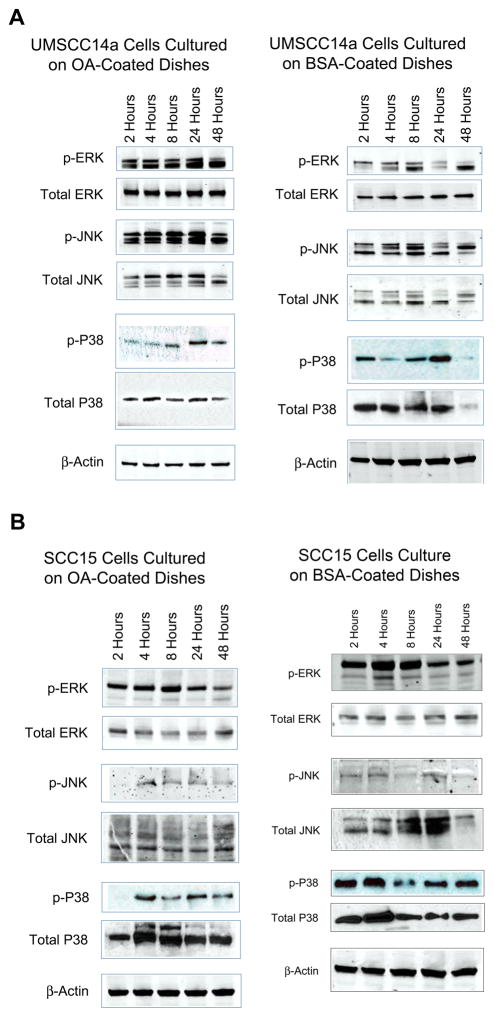
Mitogen-activated protein kinases (MAPKs)
To assess OA effects on intracellular signaling through MAPKs, UMSCC14a and SCC15 were used as examples of early- and late-stage OSCC, respectively, since relative integrin expression and affinity for ligands is known to change with tumor stage.4, 42 These cell lines were also compared because SCC15 cells express OA to a greater extent than UMSCC14a cells (Figure 1). Cells in serum-free medium (SFM) were allowed to adhere to tissue culture dishes coated with phosphate-buffer saline (PBS) with 10 μg/mL recombinant human OA (rhOA, Speed Biosystems, Rockville, Maryland), or with 0.1% bovine serum albumin (BSA) in PBS. Cells were treated for 30 minutes with pervanadate solution prior to harvest and harvested in RIPA buffer at 2, 4, 8, 24 and 48 hours. Protein extracts (50 μg) were resolved by SDS-PAGE, transferred to PVDF or nitrocellulose membranes, and probed with antibodies to p-ERK, non-p-ERK, p-JNK, non-p-JNK, p-p38, non-p-p38 (Cell Signaling Technology, Danvers Massachusetts) and β-actin. Blots were developed by enhanced chemiluminescence or infrared fluorescence imaging.
At least three independent experiments were performed for Western blot analyses and a representative experiment is presented.
Co-immunoprecipitation
UMSCC14a and SCC15 cell lysates were prepared using a Pierce immunoprecipitation kit and 500 μg of total protein each was incubated with integrin antibodies of hamster or rat derivation, or nonspecific hamster or rat IgG (BioLegend, San Diego, California). Immune complexes were captured on protein A/G agarose beads and eluted from columns. The complexes were denatured in SDS-PAGE sample buffer and 10 μL of each precipitate was resolved on 4–20% SDS-PAGE gels, transferred to nitrocellulose membranes, and probed with a rabbit antibody to OA (Bioss). At least three independent experiments were performed for each cell line and a representative experiment presented.
Integrin and OA subcellular localization
UMSCC14a and SCC15 cells were seeded onto 8-well glass culture slides. After 24 hours, cells were fixed with 4% paraformaldehyde and permeabilized with PBS with 0.1% Triton X-100, and treated with antibodies to individual integrin subunits (Santa Cruz Biotechnology). Integrin-OA co-localization was assessed by labeling cells concurrently with integrin antibodies (Santa Cruz Biotechnology) and antibodies to OA (Bioss, Woburn, Massachusetts or custom OA antibody43). Visualization of primary antibody binding was performed with fluorescent secondary antibodies (Jackson ImmunoResearch Laboratories). This experiment was performed five times.
Adhesion assays
Ninety-six well plates were coated with rhOA in Dulbecco’s PBS at various concentrations (5, 10, 20 and 40 μg/mL) or with PBS with 1% BSA. UMSCC14a and SCC15 cells were detached from culture dishes with Accutase (Sigma), suspended in SFM, and allowed to adhere in the coated plates for 20 minutes.44 The wells were washed five times and the numbers of adhered cells/well were quantified using an MTT colorimetric assay (Promega, Madison, Wisconsin). OA adhesion titration assays were performed twice, each time with six replicates.
To assess individual integrin subunit effects on cellular adhesion to OA, UMSCC14a and SCC15 cells were pretreated for 1 hour with function-blocking antibodies to α2, α5, αV, β1, β3, and β5 integrin subunits (BioLegend) in SFM with 1% BSA in a 5% CO2 atmosphere at 37°C with periodic agitation. Control cells were treated with nonspecific hamster or rat IgG, or resuspended in SFM with 1% BSA. Cells were then allowed to adhere to plates coated with 10 μg/mL rhOA. Untreated cells were also allowed to adhere to plates coated with 10 μg/mL fibronectin or with PBS with 1% BSA. Adhesion blocking assays were performed twice, each time with six replicates.
Cell survival assays
UMSCC14a and SCC15 cells were seeded in 96-well dishes (105 cells/well). After overnight adherence, the media were aspirated and cells were treated with SFM containing rhOA (1, 10, 50 and 100 ng/mL). Controls were treated with SFM. Media were changed day 3 in culture, and cells were assessed for survival using MTT assays at 3 and 5 days.
OA knock down
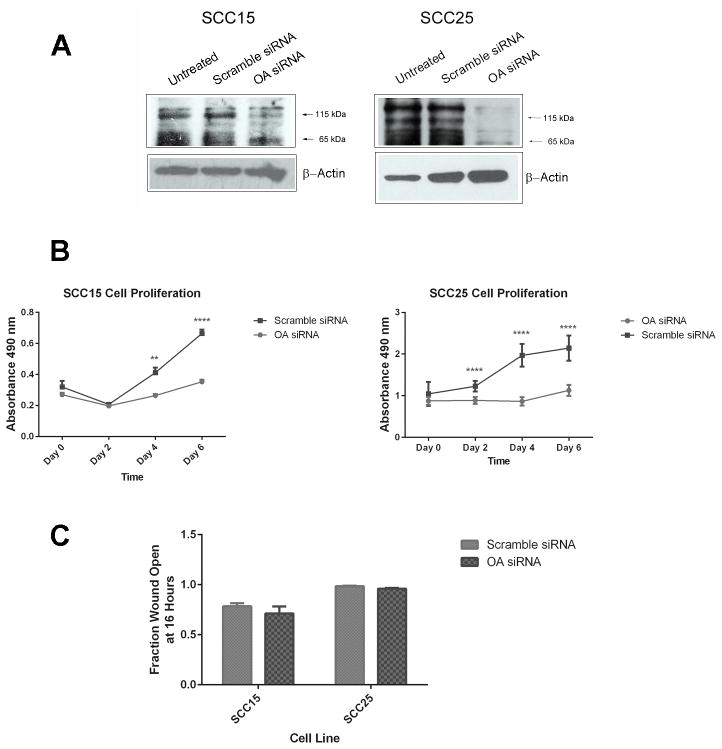
SCC15 and SCC25 cells were used for knock down experiments because they demonstrated increased OA expression relative to immortalized oral keratinocytes (Figure 1). Cells were transfected with pooled OA siRNA or scramble siRNA (100 μM, Santa Cruz Biotechnology) using LipoJet Transfection Reagent (SignaGen Laboratories, Gaithersburg, Maryland) according to manufacturer instructions. OA knock-down was confirmed with Western blot analyses. Time-course experiments revealed that cells began to recover from OA knock-down within 72 hours (data not shown).
Proliferation assays
Cells were harvested 24 hours after treatment with OA or scramble siRNA and seeded in 96-well plates at a density of 500 cells/well. Cell densities were measured with an MTT assay at 6 hours, and at days 2, 4 and 6 after seeding. Proliferation assays were performed twice, each time with 12 replicates.
Migration assays
Monolayer wound healing assays were performed with UMSCC14a and SCC15 cells seeded at confluence on tissue culture dishes coated with 10 μg/mL rhOA or with PBS with 0.1% BSA.45 To determine if integrins played a role in cell migration on rhOA, cultures were treated with integrin-neutralizing antibodies (BioLegend) at a 1:200 dilution in SFM with 1% BSA after wounding. Control cultures were treated with nonspecific hamster or rat IgG, or with SFM with 1% BSA.
SCC15 and SCC25 cells transduced with OA or scramble siRNAs were lifted 24 hours after transduction and seeded in uncoated dishes at confluence for migration assays. Transduced cells were allowed to adhere and spread for 4–6 hours before the migration assay was commenced.
Statistical analysis
IBM SPSS statistical software was used for statistical analyses (Armonk, New York). Analyses of variance were used to compare sample means for adhesion and migration inhibition assays. A Bonferroni multiple comparisons test was used to identify significant pairwise differences (adhesion assays, migration inhibition assays and survival assays). A P value <0.05 was considered statistically significant.
Results
OA is overexpressed in HNSCC cell lines
All OSCC cell lines expressed OA mRNA to a greater extent than OKF6/TERT1 immortalized oral keratinocytes (p<0.0001, Figure 1A) and OA mRNA expression also differed significantly between the individual OSCC cell lines tested (p<0.0001 for all comparisons). OA immunoblots confirmed differing expression levels between the HNSCC cell lines with greatest expression detected in SCC15 cells, and that all malignant cell lines overexpressed OA relative to OKF6/TERT1 cells (Figure 1B and 1C). OA protein expression did not correlate with mRNA expression and this may reflect differences in mRNA and protein degradation rates between cell lines46, 47, as well as mRNA translation repression by microRNAs48, 49.
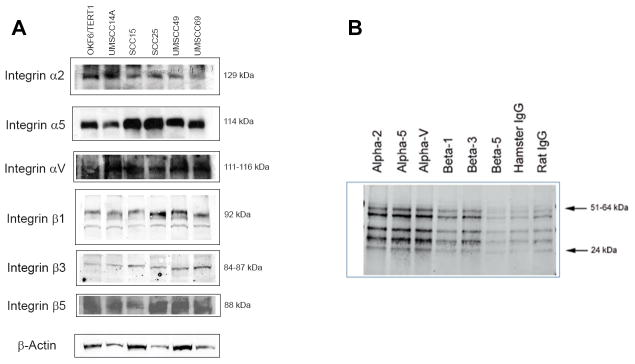
OA co-immunoprecipitates with integrins
OSCC cellular expression of the α2, α5, αV, β1, β3 and β5 integrin subunits was confirmed with Western blots (Figure 2A). To confirm the physical association of integrins with OA, OSCC cellular lysates were immunoprecipitated with integrin antibodies. OA co-immunoprecipitated with the α2, α5, αV and β3 integrin subunits. There was less co-immunoprecipitation with the β1 subunit and little co-immunoprecipitation with the β5 subunit. (Figure 2B).
Figure 2. OA co-immunoprecipitates with integrins.
OSCC cellular lysates were immunoprecipitated with integrin antibodies of hamster and rat origin, as well as nonspecific hamster and rat IgG. Immune complexes were probed with an OA antibody of rabbit origin. (A) Western blot confirmed expression of integrins in oral keratinocytes. (B) In UMSCC14a cells OA co-immunoprecipitated with the α2, α5, αV and β3 integrin subunits. There was less co-immunoprecipitation with the β1 subunit and little co-immunoprecipitation with the β5 subunit.
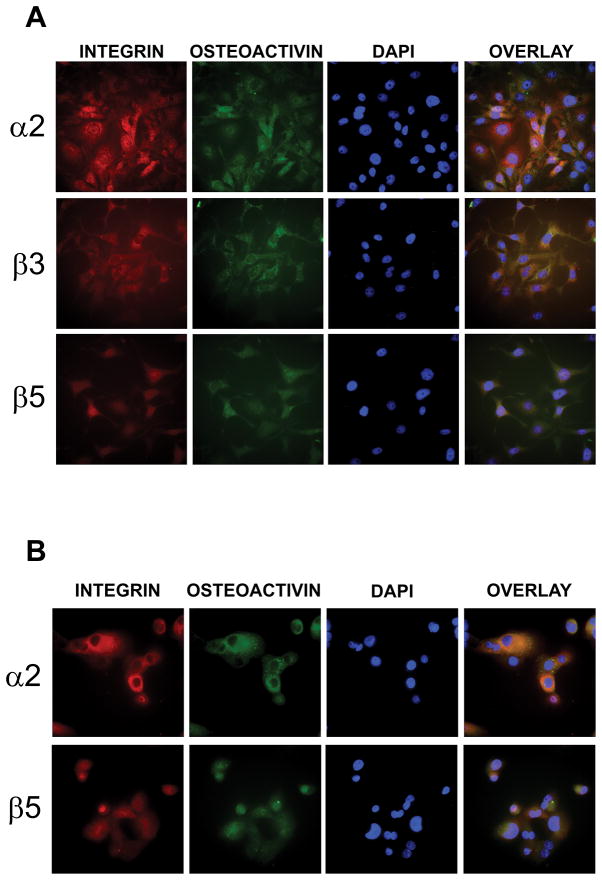
Integrin and OA localization in UMSCC14a and SCC15 cells
To evaluate OA co-localization with integrins in cultured OSCC cells, cells were seeded onto culture slides and treated with antibodies to the integrin subunits and OA. In UMSCC14a cells OA co-localized with α2, β3 and β5 integrin subunits (Figure 3A), while in SCC15 cells OA co-localized with the α2 and β5 subunits, but co-localization with the β3 subunit was not detected (Figure 3B).
Figure 3. OA co-localizes with integrins.
OSCC cells were seeded onto tissue culture slides and treated with antibodies to OA and integrin subunits. (A) In UMSCC14a cells endogenous OA co-localized with the α2, β3 and β5 integrin subunits. (B) In SCC15 cells OA co-localized with the α2 and β5 integrin subunits. 40x magnification.
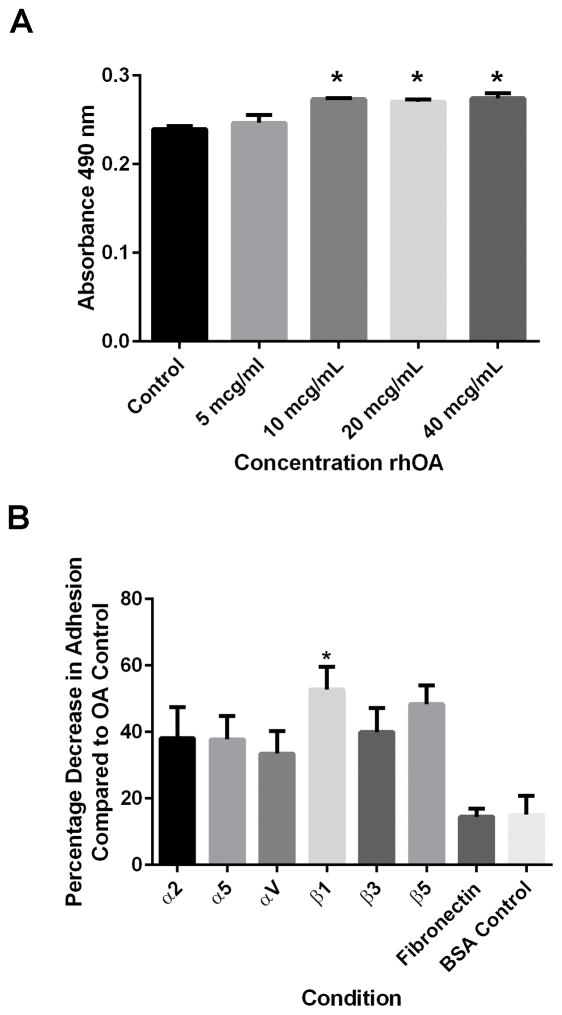
OA facilitates SCC15 integrin-mediated cell adhesion
To evaluate OA’s role in OSCC cell adhesion, cells were allowed to adhere to rhOA-coated plates or BSA-coated plates. UMSCC14a cell adhesion to substrate did not differ significantly at any OA concentration tested, or between adhesion to OA and BSA (p=0.6754, data not shown), indicating a nonspecific effect on adhesion of these cells. However, cell adhesion for SCC15 cells was enhanced by higher OA concentrations (greater than 5 μg/mL, p=0.0133, Figure 4A). Because maximal OSCC cell adhesion was observed at 10 μg/mL, this concentration was used for subsequent assays.
Figure 4. Adhesion of SCC15 cells to OA and effects of integrin inhibition.
(A) SCC15 cells were allowed to adhere to rhOA at various concentrations to determine an optimum adhesion concentration range. Cells adhered to rhOA in a concentration-dependent manner (10, 20 and 40 μg/mL, p=0.0133). (B) SCC15 cells were treated with function-blocking antibodies to integrin subunits prior to adhesion to rhOA to determine if specific integrin subunits participated in rhOA cell adhesion. Blocking of β1 integrin function inhibited SCC15 cell adhesion to rhOA (p=0.0307). Data represents means ± standard errors.
Antibodies to the integrin subunits and nonspecific IgG inhibited UMSCC14a adhesion to rhOA (p<0.0001). In addition, UMSCC14a cells adhered better to rhOA than to fibronectin (p<0.0001) and the BSA control (p<0.0001). Only β1 integrin subunit inhibition negatively affected SCC15 adhesion to rhOA (p=0.0307, Figure 4B).
OSCC adhesion to OA has differential effects on MAPK activation
Integrin signaling was further studied by assessing MAPK activation by cellular adhesion to rhOA. UMSCC14a cell adhesion to rhOA resulted in more pronounced ERK and JNK MAPK activation, with a peaking response between 8 and 24 hours (Figure 5A). In contrast, SCC15 cells adhered to rhOA showed delayed and decreased MAPK activation as compared to controls. SCC15 cells demonstrated constitutive activation of ERK and p38, and to a lesser extent JNK (Figure 5B).
Figure 5. OA activates MAPKs in OSCC.
OSCC cells were allowed to adhere to tissue culture dishes coated with rhOA or BSA. Cells were treated with pervanadate 30 minutes before cellular lysates were harvested after 2, 4, 8, 24 and 48 hours. Lysates were probed with antibodies to MAPKs. (A) As compared to control, UMSCC14a cells adhered to rhOA demonstrated significant activation of ERK and JNK MAPKs that peaked between 8 and 24 hours. (B) As compared to control, SCC15 cells adhered to rhOA demonstrated delayed and less MAPK activation.
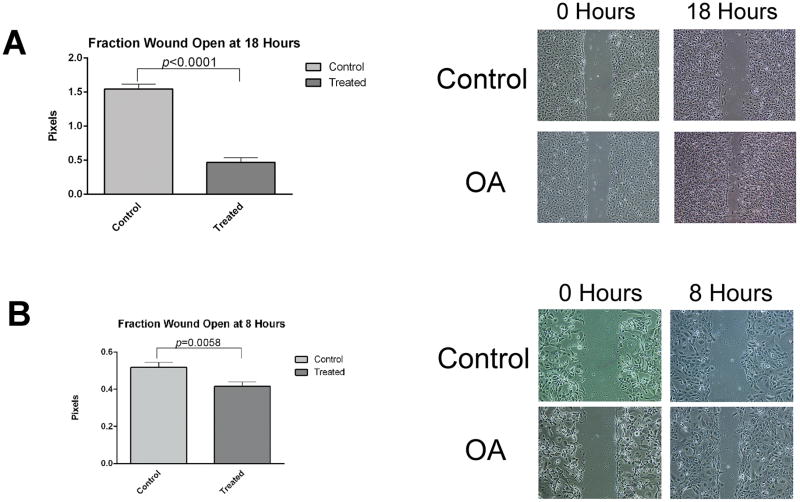
OA in the ECM promotes OSCC cell migration
Wound healing assays were performed with OSCC cells adhered to tissue culture dishes coated with rhOA or BSA (controls) to determine whether OA in the ECM promotes cell migration. OSCC cells adhered to rhOA demonstrated significantly accelerated wound healing (p<0.0001 for UMSCC14a cells and p=0.0058 for SCC15 cells, Figure 6A–D). UMSCC14a cells exhibited collective and individual cell migration, while wound healing in SCC15 cells was primarily by collective migration.
Figure 6. OA promotes migration of OSCC cells.
OSCC cells were plated at confluence on rhOA- or BSA-coated dishes. Wound healing migration assays were used to determine OA’s effect on cell migration. Graphs indicate means ± standard errors. OA accelerated migration of OSCC cells in monolayer wound healing assays. (A) UMSCC14a cells exhibited migration of individual cells as well as groups of cells. Die-back of UMSCC14a accounted for larger wounds at 18 hours in plates coated with BSA. (B) SCC15 cells exhibited primarily group migration.
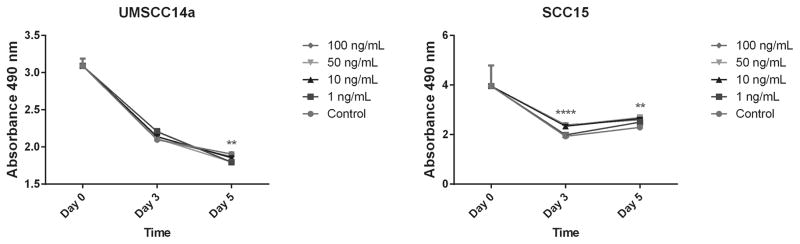
OA promotes SCC15 cell survival
OSCC cells in SFM were treated with varying rhOA concentrations. MTT assays at day 3 for UMSCC14a cells revealed no difference between experimental groups (p=0.119). By day 5, control UMSCC14a cells had greater survival than OA-treated cells (p=0.001), particularly for 1 ng/mL and 50 ng/mL concentrations (p=0.004 and 0.005, respectively (Figure 7). In contrast, OA promoted SCC15 cell survival relative to control in a concentration-dependent manner. This difference was present at days 3 and 5 for all rhOA concentrations tested (p<0.0001 for day 3 and 5).
Figure 7. Effects of OA on OSCC cell survival.
OSCC cell were treated with various concentrations of rhOA in SFM. Graphs represent mean absorbances ± standard errors. (Left) OA concentration had no effect on UMSCC14a cell survival (p=0.119). By day 5, controls outlived OA-treated cells and there was a significant decrease in cell survival regardless of OA concentration (p=0.0012). (Right) OA promoted survival of SCC15 cells on days 3 and 5 in a dose-dependent manner (p<0.0001 for both time points).
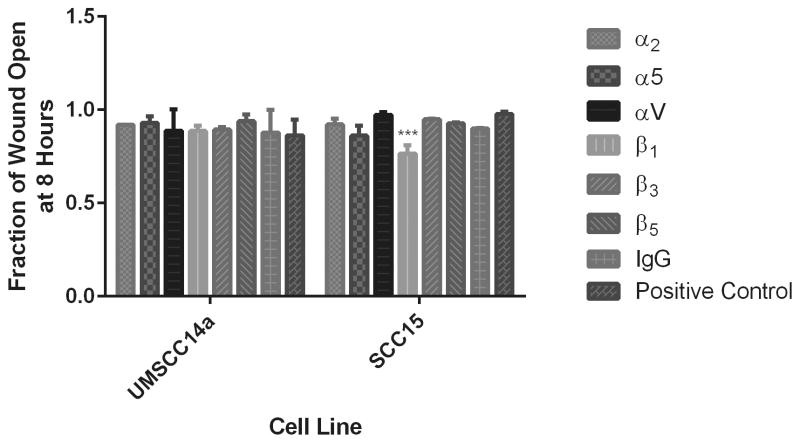
Integrin inhibition effects on cell migration
To investigate which integrins where involved in OSCC OA-mediated migration, cells were treated with integrin function-blocking antibodies after monolayer wounding. Treatment of UMSCC14a cells with α5, αv and β5 neutralizing antibodies tended to inhibit migration, while treatment with β1 and β3 function-blocking antibodies tended to promote migration. However, these effects were not significantly different from control (p=0.9352). Treatment with all integrin-neutralizing antibodies as well as nonspecific IgG enhanced SCC15 cell migration (Figure 8). This was statistically significant for β1 integrin subunit inhibition (p=0.003).
Figure 8. Effects of integrin inhibition on OSCC cell migration.
OSCC cells were seeded at confluence on tissue culture plates coated with rhOA or BSA. After monolayer wounding, cells were treated with integrin function-blocking antibodies or nonspecific IgG, and allowed to migrate for 8 hours. The fraction of wound closure for each condition was calculated relative to control. (A) Treatment of UMSCC14a cells with α5, αv and β5 neutralizing antibodies inhibited migration, while treatment with β1 and β3 function-blocking antibodies as well as nonspecific IgG tended to promote migration. However, these effects were not significantly different from control (p=0.9352). (B) Treatment with all integrin-neutralizing antibodies as well as nonspecific IgG enhanced SCC15 cell migration. This was statistically significant for β1 integrin inhibition (p=0.003). Treatment of OSCC cells with nonspecific IgG also tended to promote migration.
OA knock down limits OSCC cell proliferation
OA knock down in SCC15 and SCC25 cells was confirmed with Western blot (Figure 9A). Cells treated with OA siRNA demonstrated decreased proliferation as compared to those treated with scramble siRNA (p<0.0001 for both cell lines, Figure 9B). In SCC15 cells, proliferation of scramble siRNA-transduced cells exceeded that of OA siRNA-transduced cells by day 4 (p=0.008) and continued into day 6 (p<0.0001). For SCC25 cells proliferation of scramble siRNA-transduced cells exceeded that of OA siRNA-transduced cells on days 2–6 (p<0.0001 for the three time points). In contrast, OA knock-down did not affect the migration of SCC15 and SCC25 cells (p=0.25, Figure 9C).
Figure 9. Effects of OA knock-down on OSCC cell proliferation and migration.
OSCC cells were treated with OA- or scramble siRNAs to assess effects of OA RNA interference on cell proliferation and migration. (A) OA knock-down was confirmed with Western blot analysis and was more pronounced for SCC25 cells than for SCC15 cells. (B) OA knock-down significantly inhibited proliferation in both cell lines. In SCC15 cells, proliferation of scramble siRNA-transduced cells exceeded that of OA siRNA-transduced cells by day 4 (p=0.008) and continued into day 6 (p<0.0001). For SCC25 cells proliferation of scramble siRNA-transduced cells exceeded that of OA siRNA-transduced cells on days 2–6 (p<0.0001 for the three time points). (C) OA knock-down did not inhibit cell migration in uncoated dishes.
Discussion
Cancer cell migration is a complex process involving adhesion to and detachment from ECM proteins; ECM remodeling including controlled degradation and new ECM protein deposition; and modifications in integrin expression as malignant transformation proceeds.4 ECM and tumor microenvironment contributions to cancer progression are active investigational areas. The glycoprotein OA/gpnmb has been shown to promote endothelial15, melanoma33 and osteoblast34 adhesion, and this study demonstrated that its presence in the ECM can also facilitate malignant oral keratinocyte adhesion (Figure 4). UMSCC14a cells adhered to rhOA more avidly than to fibronectin (data not shown), a recognized mucosal ECM component35, suggesting that these cells express receptors for OA.
Adhesion to OA has been found to be in part dependent on its RGD moiety15, 33, indicating that some of its receptors are integrins32, 34. Our adhesion blocking assays indicate that OA interacts with integrins that combine with the β1 subunit in UMSCC14a and SCC15 cells (Figure 4B). β1 integrin family members are the most commonly expressed integrins in oral epithelium.3 The relationship between expression of various integrins and cancer progression is by no means simple and in some instances contradictory. While some studies have shown that loss of β1 integrin expression correlates with malignant transformation3, 50, 51, others have shown that increased β1 expression correlates with motility and invasion5, 52. The β1 integrin has been proposed as an OSCC stem cell marker and has been suggested as a clinical target.5
Decreased expression of the α2 integrin subunit relative to the immortalized oral keratinocytes was demonstrated in several OSCC cell lines (Figure 2A). The α2β1 integrin is a laminin and collagen receptor.3, 35, 53 Loss of this integrin has been associated with poor OSCC differentiation.3, 51
Upregulation of the α2, α5, αV, β3 and β5 integrin subunits has been associated with increased cell motility and tumor progression in two studies36, 52, while another study found reduced αVβ5 integrin expression with malignant transformation3. Differences in integrin expression and affinity for ligands has been demonstrated between malignant cells and their premalignant counterparts for various tumors, and during tumor progression.4, 42 Dissimilar integrin expression between UMSCC14a and SCC15 cells may reflect differences in parent tumor stages (stage I for UMSCC14a and stage IV for SCC15).38 Disparities in tumorigenic potential between UMSCC14a and SCC15 have been documented in animal xenograft models, with UMSCC14a cells rarely being able to form tumors in immunodeficient animals.54–56
Co-immunoprecipitation revealed that several integrin subunits can interact with OA. All of the integrin subunits tested co-immunoprecipitated with OA in SCC15 cells, while the β5 subunit did not significantly co-immunoprecipitate with OA in UMSCC14a cells (Figure 2B). Immunofluorescence also indicated that several integrin subunits are involved in OSCC cell adhesion to rhOA (Figure 3). Nonspecific IgGs to some extent inhibited cell adhesion and migration, and immunoprecipitated with OA (Figures 2B, 4B, and 8). This may be due to the fact that OA contains an immunoreceptor tyrosine-based activation motif that may bind IgG.14, 57
Integrin contacts provide cells with positional and molecular signals that activate MAPK signaling. ERK and JNK activation is known to promote cancer cell migration.4, 36 Integrin-mediated phosphatidylinositol 3-kinase pathway activation has also been linked to breast carcinoma cell line invasion and survival.58, 59 Integrins co-localize with growth factor receptors on cell surfaces, modulating their activity and providing pro-survival signals through MAPKs.4 Endogenous OA co-localization with α2, β3 and β5 integrins demonstrated in this study may indicate that OA plays a role in modulating integrin signaling (Figure 3). OA treatment activated MAPKs in UMSCC14a (Figure 5A), and this is consistent with the finding of Furochi et al who found that exogenous OA activated the ERK and p38 pathways in fibroblasts.60 This effect was attenuated in SCC15 cells possibly due to constitutive MAPK activation in this cell line (Figure 5B). OA promoted SCC15 cell survival and this is consistent with findings in breast and prostate carcinoma cells (Figure 7).18, 21 That OA’s effects OA on cell survival in this cell line were not dependent on adhesion is consistent with the findings of Furochi et al.60
The migratory phenotype is thought to be regulated by the balance of integrin-mediated signals.4 OA in the ECM accelerated migration of UMSCC14a and SCC15 cells in monolayer wounding assays (Figure 6). This is consistent with findings in breast and prostate carcinoma cells in which OA promoted cell invasion.20, 21 Integrin inhibition did not significantly affect UMSCC14a cell migration, though it promoted SCC15 cell migration (Figure 8). This is consistent with the findings of Lin et al who found that β1 integrin inhibition promoted SCC15 cell migration.5 Similarly, β1 integrin inhibition was found to promote metastasis and epithelial-mesenchymal transformation in breast carcinoma cells.61 These results indicate that integrin inhibition may have unexpected effects on tumor progression. We did not assess the function of other integrins that are known to exhibit de novo expression in OSCC such as the αVβ6 integrin.3, 62
OA knock-down was more effective in SCC25 cells and had a more marked impact on proliferation in this cell line (Figure 9). However, OA knock-down did not impact migration in these cells. This may be due to the fact that the migration assays in the siRNA-treated cells were performed in uncoated tissue-culture dishes and suggests that the presence of OA in the ECM promotes cell migration.
In conclusion, OA interacts with various integrins in OSCC cells and promotes integrin-dependent adhesion. OA treatment resulted in MAPK activation in UMSCC14a and promoted SCC15 cell survival. OA in the ECM accelerated migration in both cell lines. Integrin inhibition stimulated cell migration in SCC15 cells. OA knock-down adversely effected proliferation in SCC15 and SCC25 cells, but did not affect two-dimensional migration of these cells. These findings confirm previous reports of OA’s role in tumor progression in other malignancies. While we demonstrated that OA is overexpressed in some OSCC cell lines, future studies will determine the prognostic significance of this overexpression in patients with OSCC.
Supplementary Material
Acknowledgments
Contract grant sponsor: American Academy of Facial Plastic Reconstructive Surgery Foundation; Contract grant number: 2008 Leslie Bernstein Award (Dr. Arosarena)
Contract grant sponsor: National Institutes of Health; Contract grant number: NIH-NCI 1R21CA167126-01A1 (Dr. Arosarena)
Contract grant sponsor: National Institutes of Health: Contract grant number: NIH-NIAMS AR0556001-01A1 (Dr. Safadi)
Contract grant sponsor: National Institutes of Health; Contract grant number: NIH-NHLBI 1 R25 HL096331 (Dr. Dela Cadena)
This work was supported by the Eugene N. Myers, MD Head and Neck Cancer Research Fund, Department of Otolaryngology – Head and Neck Surgery, Temple University School of Medicine; in part by an American Academy of Facial Plastic Reconstructive Surgery Leslie Bernstein Award to Dr. Arosarena; and in part by a Temple University Junior Faculty Seed Money Grant to Dr. Arosarena.
References
- 1.Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 2.Wikner J, Grobe A, Pantel K, Riethdorf S. Squamous cell carcinoma of the oral cavity and circulating tumour cells. World J Clin Oncol. 2014;5(2):114–124. doi: 10.5306/wjco.v5.i2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas GJ, Jones J, Speight PM. Integrins and oral cancer. Oral Oncol. 1997;33(6):381–388. doi: 10.1016/s0964-1955(97)00021-3. [DOI] [PubMed] [Google Scholar]
- 4.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2(2):91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 5.Lin HC, Wu CL, Chen YL, Huang JS, Wong TY, Yuan K. High-level beta1-integrin expression in a subpopulation of highly tumorigenic oral cancer cells. Clin Oral Investig. 2014;18(4):1277–1284. doi: 10.1007/s00784-013-1088-y. [DOI] [PubMed] [Google Scholar]
- 6.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305(3):285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 7.Anderson MG, Libby RT, Mao M, Cosma IM, Wilson LA, Smith RS, John SW. Genetic context determines susceptibility to intraocular pressure elevation in a mouse pigmentary glaucoma. BMC Biol. 2006;4:20. doi: 10.1186/1741-7007-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30(1):81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- 9.Kuan CT, Wakiya K, Dowell JM, Herndon JE, 2nd, Reardon DA, Graner MW, Riggins GJ, Wikstrand CJ, Bigner DD. Glycoprotein nonmetastatic melanoma protein B, a potential molecular therapeutic target in patients with glioblastoma multiforme. Clin Cancer Res. 2006;12(7 Pt 1):1970–1982. doi: 10.1158/1078-0432.CCR-05-2797. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto I, Pirker C, Bilban M, Berger W, Losert D, Marosi C, Haas OA, Wolff K, Pehamberger H. Seven novel and stable translocations associated with oncogenic gene expression in malignant melanoma. Neoplasia. 2005;7(4):303–311. doi: 10.1593/neo.04514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollack VA, Alvarez E, Tse KF, Torgov MY, Xie S, Shenoy SG, MacDougall JR, Arrol S, Zhong H, Gerwien RW, Hahne WF, Senter PD, Jeffers ME, Lichenstein HS, LaRochelle WJ. Treatment parameters modulating regression of human melanoma xenografts by an antibody-drug conjugate (CR011-vcMMAE) targeting GPNMB. Cancer Chemother Pharmacol. 2007;60(3):423–435. doi: 10.1007/s00280-007-0490-z. [DOI] [PubMed] [Google Scholar]
- 12.Tse KF, Jeffers M, Pollack VA, McCabe DA, Shadish ML, Khramtsov NV, Hackett CS, Shenoy SG, Kuang B, Boldog FL, MacDougall JR, Rastelli L, Herrmann J, Gallo M, Gazit-Bornstein G, Senter PD, Meyer DL, Lichenstein HS, LaRochelle WJ. CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma. Clin Cancer Res. 2006;12(4):1373–1382. doi: 10.1158/1078-0432.CCR-05-2018. [DOI] [PubMed] [Google Scholar]
- 13.Weterman MA, Ajubi N, van Dinter IM, Degen WG, van Muijen GN, Ruitter DJ, Bloemers HP. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int J Cancer. 1995;60(1):73–81. doi: 10.1002/ijc.2910600111. [DOI] [PubMed] [Google Scholar]
- 14.Chung JS, Sato K, Dougherty II, Cruz PD, Jr, Ariizumi K. DC-HIL is a negative regulator of T lymphocyte activation. Blood. 2007;109(10):4320–4327. doi: 10.1182/blood-2006-11-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shikano S, Bonkobara M, Zukas PK, Ariizumi K. Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J Biol Chem. 2001;276(11):8125–8134. doi: 10.1074/jbc.M008539200. [DOI] [PubMed] [Google Scholar]
- 16.Bandari PS, Qian J, Yehia G, Joshi DD, Maloof PB, Potian J, Oh HS, Gascon P, Harrison JS, Rameshwar P. Hematopoietic growth factor inducible neurokinin-1 type: a transmembrane protein that is similar to neurokinin 1 interacts with substance P. Regul Pept. 2003;111(1–3):169–178. doi: 10.1016/s0167-0115(02)00288-4. [DOI] [PubMed] [Google Scholar]
- 17.Metz RL, Patel PS, Hameed M, Bryan M, Rameshwar P. Role of human HGFIN/nmb in breast cancer. Breast Cancer Res. 2007;9(5):R58. doi: 10.1186/bcr1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose AA, Annis MG, Dong Z, Pepin F, Hallett M, Park M, Siegel PM. ADAM10 releases a soluble form of the GPNMB/Osteoactivin extracellular domain with angiogenic properties. PLoS One. 2010;5(8):e12093. doi: 10.1371/journal.pone.0012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose AA, Grosset AA, Dong Z, Russo C, Macdonald PA, Bertos NR, St-Pierre Y, Simantov R, Hallett M, Park M, Gaboury L, Siegel PM. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res. 2010;16(7):2147–2156. doi: 10.1158/1078-0432.CCR-09-1611. [DOI] [PubMed] [Google Scholar]
- 20.Rose AA, Pepin F, Russo C, Abou Khalil JE, Hallett M, Siegel PM. Osteoactivin promotes breast cancer metastasis to bone. Mol Cancer Res. 2007;5(10):1001–1014. doi: 10.1158/1541-7786.MCR-07-0119. [DOI] [PubMed] [Google Scholar]
- 21.Fiorentini C, Bodei S, Bedussi F, Fragni M, Bonini SA, Simeone C, Zani D, Berruti A, Missale C, Memo M, Spano P, Sigala S. GPNMB/OA protein increases the invasiveness of human metastatic prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9 activity. Exp Cell Res. 2014;323(1):100–111. doi: 10.1016/j.yexcr.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Rich JN, Shi Q, Hjelmeland M, Cummings TJ, Kuan CT, Bigner DD, Counter CM, Wang XF. Bone-related genes expressed in advanced malignancies induce invasion and metastasis in a genetically defined human cancer model. J Biol Chem. 2003;278(18):15951–15957. doi: 10.1074/jbc.M211498200. [DOI] [PubMed] [Google Scholar]
- 23.Cromer A, Carles A, Millon R, Ganguli G, Chalmel F, Lemaire F, Young J, Dembele D, Thibault C, Muller D, Poch O, Abecassis J, Wasylyk B. Identification of genes associated with tumorigenesis and metastatic potential of hypopharyngeal cancer by microarray analysis. Oncogene. 2004;23(14):2484–2498. doi: 10.1038/sj.onc.1207345. [DOI] [PubMed] [Google Scholar]
- 24.Ginos MA, Page GP, Michalowicz BS, Patel KJ, Volker SE, Pambuccian SE, Ondrey FG, Adams GL, Gaffney PM. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res. 2004;64(1):55–63. doi: 10.1158/0008-5472.can-03-2144. [DOI] [PubMed] [Google Scholar]
- 25.Toruner GA, Ulger C, Alkan M, Galante AT, Rinaggio J, Wilk R, Tian B, Soteropoulos P, Hameed MR, Schwalb MN, Dermody JJ. Association between gene expression profile and tumor invasion in oral squamous cell carcinoma. Cancer Genet Cytogenet. 2004;154(1):27–35. doi: 10.1016/j.cancergencyto.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Talbot SG, Estilo C, Maghami E, Sarkaria IS, Pham DK, Oc P, Socci ND, Ngai I, Carlson D, Ghossein R, Viale A, Park BJ, Rusch VW, Singh B. Gene expression profiling allows distinction between primary and metastatic squamous cell carcinomas in the lung. Cancer Res. 2005;65(8):3063–3071. doi: 10.1158/0008-5472.CAN-04-1985. [DOI] [PubMed] [Google Scholar]
- 27.Pyeon D, Newton MA, Lambert PF, den Boon JA, Sengupta S, Marsit CJ, Woodworth CD, Connor JP, Haugen TH, Smith EM, Kelsey KT, Turek LP, Ahlquist P. Fundamental differences in cell cycle deregulation in human papillomavirus-positive and human papillomavirus-negative head/neck and cervical cancers. Cancer Res. 2007;67(10):4605–4619. doi: 10.1158/0008-5472.CAN-06-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye H, Yu T, Temam S, Ziober BL, Wang J, Schwartz JL, Mao L, Wong DT, Zhou X. Transcriptomic dissection of tongue squamous cell carcinoma. BMC Genomics. 2008;9:69. doi: 10.1186/1471-2164-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estilo CL, Oc P, Talbot S, Socci ND, Carlson DL, Ghossein R, Williams T, Yonekawa Y, Ramanathan Y, Boyle JO, Kraus DH, Patel S, Shaha AR, Wong RJ, Huryn JM, Shah JP, Singh B. Oral tongue cancer gene expression profiling: Identification of novel potential prognosticators by oligonucleotide microarray analysis. BMC Cancer. 2009;9:11. doi: 10.1186/1471-2407-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdelmagid SM, Barbe MF, Rico MC, Salihoglu S, Arango-Hisijara I, Selim AH, Anderson MG, Owen TA, Popoff SN, Safadi FF. Osteoactivin, an anabolic factor that regulates osteoblast differentiation and function. Exp Cell Res. 2008;314(13):2334–2351. doi: 10.1016/j.yexcr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Safadi FF, Xu J, Smock SL, Rico MC, Owen TA, Popoff SN. Cloning and characterization of osteoactivin, a novel cDNA expressed in osteoblasts. J Cell Biochem. 2001;84(1):12–26. doi: 10.1002/jcb.1259. [DOI] [PubMed] [Google Scholar]
- 32.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 33.Tomihari M, Hwang SH, Chung JS, Cruz PD, Jr, Ariizumi K. Gpnmb is a melanosome-associated glycoprotein that contributes to melanocyte/keratinocyte adhesion in a RGD-dependent fashion. Exp Dermatol. 2009;18(7):586–595. doi: 10.1111/j.1600-0625.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moussa FM, Hisijara IA, Sondag GR, Scott EM, Frara N, Abdelmagid SM, Safadi FF. Osteoactivin promotes osteoblast adhesion through HSPG and alphavbeta1 integrin. J Cell Biochem. 2014;115(7):1243–1253. doi: 10.1002/jcb.24760. [DOI] [PubMed] [Google Scholar]
- 35.Larjava H, Koivisto L, Hakkinen L, Heino J. Epithelial integrins with special reference to oral epithelia. J Dent Res. 2011;90(12):1367–1376. doi: 10.1177/0022034511402207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizqiawan A, Tobiume K, Okui G, Yamamoto K, Shigeishi H, Ono S, Shimasue H, Takechi M, Higashikawa K, Kamata N. Autocrine galectin-1 promotes collective cell migration of squamous cell carcinoma cells through up-regulation of distinct integrins. Biochem Biophys Res Commun. 2013;441(4):904–910. doi: 10.1016/j.bbrc.2013.10.152. [DOI] [PubMed] [Google Scholar]
- 37.Seftor RE, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJ. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci U S A. 1992;89(5):1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin CJ, Grandis JR, Carey TE, Gollin SM, Whiteside TL, Koch WM, Ferris RL, Lai SY. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head Neck. 2007;29(2):163–188. doi: 10.1002/hed.20478. [DOI] [PubMed] [Google Scholar]
- 39.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20(4):1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arosarena OA, Del Carpio-Cano FE, Dela Cadena RA, Rico MC, Nwodim E, Safadi FF. Comparison of bone morphogenetic protein-2 and osteoactivin for mesenchymal cell differentiation: Effects of bolus and continous administration. J Cell Physiol. 2011 doi: 10.1002/jcp.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eaton SL, Roche SL, Llavero Hurtado M, Oldknow KJ, Farquharson C, Gillingwater TH, Wishart TM. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS One. 2013;8(8):e72457. doi: 10.1371/journal.pone.0072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juliano RL, Varner JA. Adhesion molecules in cancer: the role of integrins. Curr Opin Cell Biol. 1993;5(5):812–818. doi: 10.1016/0955-0674(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 43.Abdelmagid SM, Barbe MF, Hadjiargyrou M, Owen TA, Razmpour R, Rehman S, Popoff SN, Safadi FF. Temporal and spatial expression of osteoactivin during fracture repair. J Cell Biochem. 2010;111(2):295–309. doi: 10.1002/jcb.22702. [DOI] [PubMed] [Google Scholar]
- 44.Humphries MJ. Cell adhesion assays. Mol Biotechnol. 2001;18(1):57–61. doi: 10.1385/MB:18:1:57. [DOI] [PubMed] [Google Scholar]
- 45.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 46.Vogel C, de Abreu RS, Ko D, Le SY, Shapiro BA, Burns SC, Sandhu D, Boutz DR, Marcotte EM, Penalva LO. Sequence signatures and mRNA concentration can explain two-thirds of protein abundance variation in a human cell line. Mol Syst Biol. 2010;6:400. doi: 10.1038/msb.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landry JJ, Pyl PT, Rausch T, Zichner T, Tekkedil MM, Stutz AM, Jauch A, Aiyar RS, Pau G, Delhomme N, Gagneur J, Korbel JO, Huber W, Steinmetz LM. The genomic and transcriptomic landscape of a HeLa cell line. G3 (Bethesda) 2013;3(8):1213–1224. doi: 10.1534/g3.113.005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore KJ. microRNAs: small regulators with a big impact on lipid metabolism. J Lipid Res. 2013;54(5):1159–1160. doi: 10.1194/jlr.E036954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohara T, Kawashiri S, Tanaka A, Noguchi N, Kitahara H, Okamune A, Kato K, Hase T, Nakaya H, Yoshizawa K. Integrin expression levels correlate with invasion, metastasis and prognosis of oral squamous cell carcinoma. Pathol Oncol Res. 2009;15(3):429–436. doi: 10.1007/s12253-008-9142-9. [DOI] [PubMed] [Google Scholar]
- 51.Maragou P, Bazopoulou-Kyrkanidou E, Panotopoulou E, Kakarantza-Angelopoulou E, Sklavounou-Andrikopoulou A, Kotaridis S. Alteration of integrin expression in oral squamous cell carcinomas. Oral Dis. 1999;5(1):20–26. doi: 10.1111/j.1601-0825.1999.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 52.Takayama S, Hatori M, Kurihara Y, Kinugasa Y, Shirota T, Shintani S. Inhibition of TGF-beta1 suppresses motility and invasiveness of oral squamous cell carcinoma cell lines via modulation of integrins and down-regulation of matrix-metalloproteinases. Oncol Rep. 2009;21(1):205–210. [PubMed] [Google Scholar]
- 53.Jones JI, Doerr ME, Clemmons DR. Cell migration: interactions among integrins, IGFs and IGFBPs. Prog Growth Factor Res. 1995;6(2–4):319–327. doi: 10.1016/0955-2235(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 54.Henson B, Li F, Coatney DD, Carey TE, Mitra RS, Kirkwood KL, D’Silva NJ. An orthotopic floor-of-mouth model for locoregional growth and spread of human squamous cell carcinoma. J Oral Pathol Med. 2007;36(6):363–370. doi: 10.1111/j.1600-0714.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 55.Liu SC, Bassi DE, Zhang SY, Holoran D, Conti CJ, Klein-Szanto AJ. Overexpression of cyclin D2 is associated with increased in vivo invasiveness of human squamous carcinoma cells. Mol Carcinog. 2002;34(3):131–139. doi: 10.1002/mc.10057. [DOI] [PubMed] [Google Scholar]
- 56.Bassi DE, Mahloogi H, Lopez De Cicco R, Klein-Szanto A. Increased furin activity enhances the malignant phenotype of human head and neck cancer cells. Am J Pathol. 2003;162(2):439–447. doi: 10.1016/s0002-9440(10)63838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomihari M, Chung JS, Akiyoshi H, Cruz PD, Jr, Ariizumi K. DC-HIL/glycoprotein Nmb promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T cells. Cancer Res. 2010;70(14):5778–5787. doi: 10.1158/0008-5472.CAN-09-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16(10):2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91(7):949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 60.Furochi H, Tamura S, Mameoka M, Yamada C, Ogawa T, Hirasaka K, Okumura Y, Imagawa T, Oguri S, Ishidoh K, Kishi K, Higashiyama S, Nikawa T. Osteoactivin fragments produced by ectodomain shedding induce MMP-3 expression via ERK pathway in mouse NIH-3T3 fibroblasts. FEBS Lett. 2007;581(30):5743–5750. doi: 10.1016/j.febslet.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 61.Truong HH, Xiong J, Ghotra VP, Nirmala E, Haazen L, Le Devedec SE, Balcioglu HE, He S, Snaar-Jagalska BE, Vreugdenhil E, Meerman JH, van de Water B, Danen EH. beta1 integrin inhibition elicits a prometastatic switch through the TGFbeta-miR-200-ZEB network in E-cadherin-positive triple-negative breast cancer. Sci Signal. 2014;7(312):ra15. doi: 10.1126/scisignal.2004751. [DOI] [PubMed] [Google Scholar]
- 62.Yap LF, Jenei V, Robinson CM, Moutasim K, Benn TM, Threadgold SP, Lopes V, Wei W, Thomas GJ, Paterson IC. Upregulation of Eps8 in oral squamous cell carcinoma promotes cell migration and invasion through integrin-dependent Rac1 activation. Oncogene. 2009;28(27):2524–2534. doi: 10.1038/onc.2009.105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.