Abstract
Objective
We investigated whether dietary sodium intake from respondents of a national cross‐sectional nutritional study differed by history of migraine or severe headaches.
Background
Several lines of evidence support a disruption of sodium homeostasis in migraine.
Design
Our analysis population was 8819 adults in the 1999–2004 National Health and Nutrition Examination Survey (NHANES) with reliable data on diet and headache history. We classified respondents who reported a history of migraine or severe headaches as having probable history of migraine. To reduce the diagnostic conflict from medication overuse headache, we excluded respondents who reported taking analgesic medications. Dietary sodium intake was measured using validated estimates of self‐reported total grams of daily sodium consumption and was analyzed as the residual value from the linear regression of total grams of sodium on total calories. Multivariable logistic regression that accounted for the stratified, multistage probability cluster sampling design of NHANES was used to analyze the relationship between migraine and dietary sodium.
Results
Odds of probable migraine history decreased with increasing dietary sodium intake (odds ratio = 0.93, 95% confidence interval = 0.87, 1.00, P = .0455). This relationship was maintained after adjusting for age, sex, and body mass index (BMI) with slightly reduced significance (P = .0505). In women, this inverse relationship was limited to those with lower BMI (P = .007), while in men the relationship did not differ by BMI. We likely excluded some migraineurs by omitting frequent analgesic users; however, a sensitivity analysis suggested little effect from this exclusion.
Conclusions
This study is the first evidence of an inverse relationship between migraine and dietary sodium intake. These results are consistent with altered sodium homeostasis in migraine and our hypothesis that dietary sodium may affect brain extracellular fluid sodium concentrations and neuronal excitability.
Keywords: dietary sodium, analgesic medication, body mass index
Abbreviations
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- CS
central sensitization
- CSF
cerebrospinal fluid
- FIAS
University of Texas Food Intake Analysis System
- NHANES
National Health and Nutrition Examination Survey
- NTG
nitroglycerin
Introduction
Migraine is the most common neurological disorder, affecting 18% of females and 6% of males, with prevalence peaking at age 30–40 years, but its pathophysiology is not fully understood.1 This gap in knowledge is especially true in regard to mechanisms for the increased sensory perception for light, sound, and motion in migraine.
Of the ionic constituents that directly participate in neuronal excitability, evidence is accumulating that sodium homeostasis plays a central role in migraine from both human and animal studies: cerebrospinal fluid (CSF) sodium (but not calcium, magnesium, or potassium) increases during migraine in the absence of any change of sodium in blood plasma2; the diurnal rhythm of CSF sodium is characterized by peaks in early morning and late afternoon,3 corresponding to the most frequent onset times of episodic migraine4, 5, 6, 7, 8; intracranial sodium increases in a rat migraine model of nitroglycerin‐triggered central sensitization9; and computer‐generated simulation and electrophysiology studies indicate higher extracellular sodium contributes to neuronal hyperexcitability.9 An especially compelling reason to explore dietary sodium is the evidence that higher extracellular sodium has been shown to increase the electrical activity of giant squid axons,10 an effect that we replicated in primary neuronal cultures.11 These results collectively point to a sodium disturbance in migraine.
Studies have shown that higher dietary sodium increases ventricular CSF sodium concentration in rats,12 but sensory evaluation was not reported in these experiments. Despite the knowledge that human choroid plexus has an almost identical distribution pattern of water channels and sodium transporters as in the rat,13 we are not aware of prospective human studies focused on the relationship between dietary sodium intake and sodium dysregulation in migraine. The regulation of sodium throughout the body depends on the requirements of different tissues, ranging from the kidneys, skin, and salivary glands to the choroid plexus, but the source of sodium results as a redistribution from elsewhere in the body or from dietary sodium. As sodium has profound effects on neuronal activity, we hypothesized that there would be an association between migraine status (a binary variable) and dietary sodium and used the NHANES database to investigate this possible relationship. We also explored whether an association between migraine status and dietary sodium would vary based on sex and/or body mass index (BMI).
Methods
Study Population
NHANES is an ongoing survey conducted by the National Center for Health Statistics at the Centers for Disease Control and Prevention (CDC) that collects data on the health and nutritional status of U.S. adults and children. Detailed information on survey design and methodology is available at http://www.cdc.gov/nchs/nhanes.htm.14, 15 NHANES includes a nationally representative sample of approximately 5000 subjects per year. The interview component of NHANES ascertains information on demographic, socioeconomic, and health‐related factors and includes a 24‐hour dietary recall assessment. We limited our analysis to adults (ages 20 years and older) who participated in the dietary assessment, whose dietary data were deemed reliable (per the NHANES definition), and who did not take analgesic medications so that respondents who possibly suffered from analgesic overuse headache would be excluded.
Beginning in 1999, NHANES data have been released in 2‐year cycles. Although data are available from 1971 through the present, our analysis includes only the 1999–2004 cycles due to availability of variables that were of interest to our investigation. The CDC Institutional Review Board approved NHANES and all participants provided written informed consent. The Huntington Medical Research Institute Institutional Review Board granted exemption from local review under 45 CFR 46.101(b)(4).
Headache Classification
Data on migraine status were available in the Miscellaneous Pain section of the NHANES interview. We categorized respondents who answered “yes” to the survey question: “During the past 3 months, did you have severe headaches or migraines?” as probable migraineurs. These self‐reports are without the additional parameters necessary for the IHC classification of migraine,16 and alternative diagnoses such as tension‐type or cluster headaches may be erroneously grouped as migraine. Nevertheless, it is reasonable to assume most of these respondents had migraine as it is the most common diagnosis for these complaints, an assumption also made by other expert researchers in this field.17 Because of this limitation, we excluded respondents who may have had severe headaches due to medication overuse (“analgesic users”).
Dietary Sodium
Total grams of daily dietary sodium was an NHANES‐derived variable from the Total Nutrient Intakes File, produced from respondents' reporting of individual foods (including drinks) they consumed during the midnight‐to‐midnight 24‐hour period prior to the in‐person dietary interview. In the 2003–2004 NHANES cycle, a second dietary interview was conducted 3–10 days later; to maintain consistency with the 1999–2002 data, we used data from only the first interview for 2003–2004. Coding of interview data and conversion to total nutrient intake were done by NHANES using the Food Intake Analysis System (FIAS, University of Texas Health Science Center at Houston, Houston, TX, USA) along with the USDA 1994–1998 Survey Nutrient Database in NHANES cycle 1999–2000,18 FIAS in 2001, the USDA's Survey Net in 2002,19 and the USDA's Food and Nutrient Database for Dietary Studies in NHANES cycle 2003–2004.20 Final processing of dietary data by NHANES included modifying sodium content for foods for which respondents indicated that they did not use the usual amount of salt in preparation.
Analgesics
Two sections of the NHANES interview were used to determine use of analgesic medications: Analgesic Medications, which included a question on whether or not the respondent had ever taken over‐the‐counter or prescription pain medications every day for as long as a month (chronic); and Prescription Medications, which included questions on whether the respondent had taken any prescription medications during the past month and, if so, which medications they reported taking. Prescription medications reported by respondents were categorized by NHANES using the Multum Lexicon Therapeutic Classification Scheme.21 In this scheme, medications are classified by 3 nested levels of therapeutic category, with the first level being the most broad. Among medications with first‐level classification “central nervous system agents,” analgesics were selected as those classified as “analgesics” according to the second‐level category name; these included third‐level classifications of miscellaneous analgesics, narcotic analgesics, nonsteroidal anti‐inflammatory agents, salicylates, analgesic combinations, narcotic analgesic combinations, antimigraine agents, and COX‐2 inhibitors. Analgesic users (excluded from our analysis) were defined as respondents who were in either category of analgesic use (chronic or “in the last month”). This definition did not exclude migraineurs who did not treat their migraines at all, ie, migraineurs who used over‐the‐counter medications were included, as were migraineurs who used prescription medications to treat but not in the most recent month.
Statistical Analysis
The primary objective of the analysis was to test for an association between dietary sodium intake and migraine status (a binary variable). Secondary objectives were to explore the sodium‐migraine relationship within subgroups defined by sex and BMI and to estimate the probability of reporting history of migraine as a function of absolute dietary sodium intake.
Analyses were done using SAS procedures SURVEYFREQ, SURVEYREG and SURVEYLOGISTIC (SAS v9.2, SAS Institute, Cary, NC, USA) to account for the stratified, multistage probability cluster sampling design of NHANES. The NHANES stratification variable (SDMVSTRA) and Primary Sampling Unit (SDMVPSU) were used as the strata and sampling unit variables, respectively. NHANES provides sampling weights to be used in analyses that account for oversampling of certain subgroups, differences between the sample and the population due to nonresponse, and population sizes, ie, the weights allow each NHANES respondent to represent multiple people from the population from which they were sampled. NHANES provides sampling weights to be used specifically for dietary analyses, which also account for the fact that not all participants completed the dietary interview and that different days of the week were represented in the 24‐hour periods for which dietary intake was assessed. In general, the NHANES weights are calculated for 2‐year intervals, but a 4‐year weight is available for the 1999–2002 time period. A 2‐year weight is smaller than a 4‐year weight because less of the population is included in a sample collected over 2 years compared to a sample collected over 4 years (so each respondent in a 2‐year sample has to represent more people in the population compared to a 4‐year sample). Because we combined data from three 2‐year sampling cycles (which included the 1999–2002 period), per NHANES guidelines we derived a 6‐year weight equal to 2/3 of the 1999–2002 weight for respondents sampled during 1999–2002 or 1/3 of the 2003–2004 weight for respondents sampled during 2003–2004. This is possible because the 2003–2004 2‐year weights are comparable to the 1999–2002 4‐year weights as both sets of weights were based on 2000 Census counts.20
Total grams of dietary sodium for each respondent was expressed as the respondent's residual value from the linear regression of total grams of sodium on total calories, ie, the difference between the respondent's actual grams of dietary sodium and that predicted by his or her total caloric intake. This approach isolates the effect of grams of dietary sodium from factors closely associated with total caloric intake that may be related to migraine (eg, body size, metabolic efficiency) without directly modeling total caloric intake, which is highly correlated with total sodium intake.22 Residuals were analyzed both as continuous and categorical variables, with categories based on quartiles of the distribution of residuals.
Multivariable logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) in the analysis of the association between dietary sodium (the predictor) and migraine status (the outcome). Trend tests were done by analyzing sodium residuals as a continuous variable. Age, sex, and BMI were included as covariates as these are well‐known to be related to both dietary intake and migraine. An analysis stratified by sex with age and BMI as covariates was also performed. Interactions were tested between the predictor in its original form and each covariate in their original forms. For stratified analyses, BMI was dichotomized at the median reported value within sex. Correlations were assessed for each pair of independent variables to check for collinearity. A sensitivity analysis that included analgesic medication users was done to evaluate the effect of omitting these respondents. A second sensitivity analysis was done to compare results between the two dietary interviews done on separate days among respondents from the 2003–2004 NHANES cycle. Subgroup analyses were accomplished using the DOMAIN statement in the SAS procedures SURVEYFREQ, SURVEYREG, and SURVEYLOGISTIC. Only respondents for whom all analysis variables were non‐missing were included. All statistical tests were two‐sided with .05 significance levels.
Results
Characteristics of the study population are shown in Table 1. Of the 15,332 adults who participated in the NHANES 1999–2004 cycles, 13,424 (88%) had reliable data from the 24‐hour dietary assessment, and 13,033 of these (97%) had non‐missing dietary total grams of sodium and migraine status. Given the relatively low rate of unreliable and/or missing data, per the NHANES guidelines,23 we deemed it unnecessary to account for missing data in the analysis, ie, through imputation or additional weighting. The number of respondents who were not analgesic users was 8819 (68%); of these, 4551 (52%) were women. Of the 4214 analgesic users, 3098 were because of chronic use (2383 of 10,346 (23%) non‐migraineurs, 715 of 2687 (27%) migraineurs), ie, they took analgesics every day for at least a month at some point in their life. The remaining 1116 exclusions were due to prescription analgesic use during the most recent month (780 of 10,346 (8%) non‐migraineurs, 336 of 2687 (13%) migraineurs). A flow diagram describes the participants:
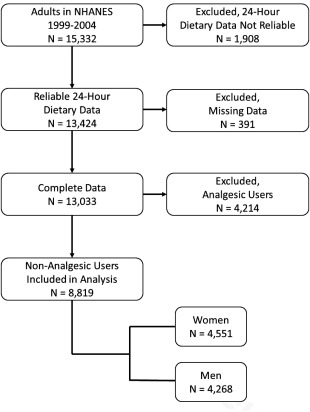
Table 1.
Demographic Characteristics of Analysis Population by Headache Status (NHANES 1999–2004)
| Headache YES | Headache NO | |||
|---|---|---|---|---|
| N | %† | N | %† | |
| Adult respondents with reliable interview data | 2742 | 10,682 | ||
| All relevant data‡ non‐missing | 2687 | 98.1 | 10,346 | 97.6 |
| Did not take analgesic medication§ | 1636 | 58.2 | 7183 | 68.8 |
| Female# | 1100 | 63.4 | 3451 | 46.2 |
| Age <50 years# | 1250 | 80.6 | 4345 | 67.6 |
| BMI < sex‐specific median# | 754 | 48.7 | 3654 | 53.8 |
†Based on weighted frequencies.
‡Dietary sodium, total caloric intake, analgesic use, age, sex, BMI.
§Denominator for percentage = respondents with all relevant data non‐missing.
#Denominator for percentage = respondents with all relevant data non‐missing who did not take analgesics.
Results from both crude and covariate‐adjusted analyses of the relationship between dietary sodium and migraine status are shown in Table 2. Compared to respondents with the lowest dietary sodium residuals, there was a significant trend of decreasing odds of migraine history with increasing levels of dietary sodium residuals (OR = 0.93, 95% CI = 0.87, 1.00, P = .0455); this relationship remained after covariate adjustment (OR = 0.93, 95% CI = 0.87, 1.00, P = .0503). Including analgesic users in the analysis had little effect (OR = 0.95, 95% CI = 0.89, 1.01, P = .08), and results from the two separate days of dietary interviews among respondents from the NHANES 2003–2004 cycle were comparable (Day 1: OR = 0.88, 95% CI = 0.78, 0.99, P = .04; Day 2: OR = 0.90, 95% CI = 0.81, 1.01, P = .08).
Table 2.
Effect of Dietary Sodium on Headache Status (Respondents Who Did Not Use Chronic Analgesics or Prescription Analgesics in Past Month, NHANES 1999–2004)
| Population distribution† | Crude (N = 8819) | Adjusted‡ (N = 8819) | ||||||
|---|---|---|---|---|---|---|---|---|
| Exposure category | % Headache YES | % Headache NO | Odds ratio | (95% CI) | P trend | Odds ratio | (95% CI) | P trend |
| Dietary sodium residuals | ||||||||
| 1st quartile | 23.6 | 21.4 | 1.00 | — | .0455 | 1.00 | — | .0503 |
| 2nd quartile | 22.1 | 21.1 | 0.95 | (0.78, 1.16) | 0.91 | (0.74, 1.10) | ||
| 3rd quartile | 24.8 | 24.4 | 0.92 | (0.74, 1.15) | 0.87 | (0.70, 1.08) | ||
| 4th quartile | 29.5 | 33.2 | 0.81 | (0.66, 0.99) | 0.81 | (0.66, 0.99) | ||
†Based on weighted frequencies.
‡Adjusted for age, sex, and BMI. Degree of correlation among independent variables was negligible.
We designed our analysis to study those not taking analgesics to avoid the confounding effect of medications; when we included analgesic users in a post hoc study, a sensitivity analysis suggested little effect from this exclusion.
To investigate the effect of sexual dimorphism, we analyzed the relationship between dietary sodium and migraine for men and women separately and observed the same inverse association by sex as was observed for both sexes combined (P for interaction = .99, Table 3); however, the trend was significant only for women (OR = 0.93, 95% CI = 0.86, 1.00, P = .049). In women but not men, BMI was a significant effect modifier of the sodium/migraine relationship (P = .025); results by BMI for both sexes are shown in Table 4. In women, there was a significant effect of decreasing odds of migraine history with increasing dietary sodium residuals in respondents with lower BMI (OR = 0.87, 95% CI = 0.78, 0.96, P = .007). In women with higher BMI, there was no relationship between dietary sodium and migraine history.
Table 3.
Effect of Dietary Sodium on Headache Status by Sex† (Respondents Who Did Not Use Chronic Analgesics or Prescription Analgesics in Past Month, NHANES 1999–2004)
| Men (N = 4268) | Women (N = 4551) | |||||
|---|---|---|---|---|---|---|
| Exposure category | Odds ratio | (95% CI) | P trend | Odds ratio | (95% CI) | P trend |
| Dietary sodium residuals | ||||||
| 1st quartile | 1.00 | — | .24 | 1.00 | — | .049 |
| 2nd quartile | 0.92 | (0.73, 1.14) | 0.93 | (0.76, 1.13) | ||
| 3rd quartile | 0.92 | (0.69, 1.22) | 0.82 | (0.65, 1.04) | ||
| 4th quartile | 0.80 | (0.57, 1.13) | 0.81 | (0.65, 1.00) | ||
†Adjusted for age and BMI.
Table 4.
Effect of Dietary Sodium on Headache Status by Sex and BMI† (Respondents Who Did Not Use Chronic Analgesics or Prescription Analgesics in Past Month, NHANES 1999–2004)
| Men, Lower BMI (N = 2133) | Men, Higher BMI (N = 2135) | Women, Lower BMI (N = 2275) | Women, Higher BMI (N = 2276) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure category | Odds ratio | (95% CI) | P trend | Odds ratio | (95% CI) | P trend | Odds ratio | (95% CI) | P trend | Odds ratio | (95% CI) | P trend |
| Dietary sodium residuals | ||||||||||||
| 1st quartile | 1.00 | — | .36 | 1.00 | — | .40 | 1.00 | — | .007 | 1.00 | — | .95 |
| 2nd quartile | 1.02 | (0.67, 1.55) | 0.86 | (0.59, 1.25) | 0.91 | (0.68, 1.22) | 0.93 | (0.72, 1.20) | ||||
| 3rd quartile | 0.99 | (0.67, 1.46) | 0.89 | (0.59, 1.33) | 0.78 | (0.56, 1.09) | 0.87 | (0.64, 1.17) | ||||
| 4th quartile | 0.82 | (0.52, 1.27) | 0.80 | (0.50, 1.28) | 0.66 | (0.49, 0.88) | 0.99 | (0.71, 1.37) | ||||
†Adjusted for age. In women, interaction between sodium residuals and BMI was significant (P = .025).
Discussion
Our analysis of nutritional assessment data from NHANES 1999–2004 provides the first evidence to suggest an inverse relationship between migraine and dietary sodium intake levels independent of age, sex, and BMI; in women, this observation was limited to those with lower BMI. A comprehensive literature exists that describes the impact that diet can have on migraine occurrence,24 however, few studies have attempted to assess the direct relationship between dietary sodium intake patterns and migraine. The NHANES 1999–2004 analysis by Evans et al, limited to women, reported that sodium dietary intake was descriptively (but not significantly) lower in migraineurs compared to non‐migraineurs, and in women of normal weight the sodium component of dietary quality indicated less sodium intake in migraineurs compared to non‐migraineurs (P = .04 without adjustment for multiple comparisons).17 Thus, their observations are consistent with our analysis of women with lower BMI. We analyzed the NHANES 1999–2004 data from a strategically different approach; for example, we included men and women of all ages vs women aged 20–50 years, and we analyzed sodium residuals rather than a transformed version of the original sodium variable. This limits a direct comparison between the two studies but, alternatively, lends support to our findings as the two different approaches led to similar conclusions regarding sodium and migraine in women.
It is well‐known that higher dietary sodium has been associated with negative outcomes in other fields of medicine and is considered a major health problem worldwide,25 contributing to hypertension and its consequences of stroke and cardiac and renal disease.26 Hypertension and hypotension, as well as obesity, are comorbid with migraine,27, 28 and the cardiovascular risk profile is higher in migraineurs.29, 30 Sodium intake is also recognized as a sexually dimorphic behavior, controlled by perinatal and adult androgen in rats,31 and includes greater intake by females during acute sodium depletion.32 From these two established correlations for dietary sodium, we expected to find: (1) a higher sodium intake in migraineurs to match their increased cardiovascular risk, and (2) a sexually dimorphic relationship.
We found a migraine relationship with dietary sodium and it had sexual dimorphic influences, but the direction of sodium intake lower in migraineurs. This finding was unexpected for several reasons. First, the inverse relationship between migraine and dietary sodium intake in women was restricted to those with lower BMI, whereas the relationship between hypertension when sodium sensitivity has been reported to be limited to those with higher BMI.33 Second, we had previously reported higher CSF sodium during migraine2 and Kawano et al found higher dietary sodium increases human CSF sodium independent of salt sensitivity/insensitivity34; thus it was a surprise that migraineurs would report less dietary sodium intake. Third, a post hoc analysis of dietary intervention study of “low,” “medium,” and “high” sodium intake reported a lower risk of headache on a “low” sodium diet.35 However, the authors suspected that most of the headaches were tension type. Further, the controlled “high” sodium intake in the trial corresponded to the average American Intake, whereas the NHANES database used for our analysis was representative of the population distribution of dietary sodium and thus included much higher levels of exposure. Extensive studies on the regulation of dietary sodium intake based on salt appetite36, 37 demonstrate the limited understanding of this behavior, and further study is necessary to explain these apparent paradoxes.
We previously proposed that brain sodium homeostasis is altered in migraine,38 largely mediated by disturbance of the Na, K, ATPase, but many other mechanisms have been invoked for migraine. A compelling question is whether disruption of a common activation pathway or component can cause migraine. Candidate pathways or components include: cortical spreading depression39, 40, 41; neuropeptides42; sterile meningeal neuroinflammation with triggering of dural mast cells43, 44, 45; central excitatory/inhibitory homeostasis (glutamate/gamma‐aminobutyric acid)46, 47; cortical neuromodulation (serotoninergic, noradrenergic, cholinergic, or dopaminergic)47, 48, 49, 50; or channelopathy.49, 51 It will be important to identify if sodium dysregulation is the underlying mechanism for these other pathways and/or is a common mechanism for migraine.
It is noteworthy that low or high blood pressures are known comorbidities of migraine,27 and that altered sodium homeostasis and the endogenous Na, K, ATPase inhibitor, ouabain, have been identified in the regulation of blood pressure28 and depressive disorders.52 We predict that regulators that have already been found for salt‐sensitive hypertension and depression disorders,52 such as endogenous ouabain‐like compounds,28, 53 are worthy of study as modulators of the dietary sodium/migraine relationship.
Our analysis reported here adds support for the involvement of sodium dysregulation in migraine but is limited due to the retrospective, self‐reported, cross‐sectional nature of NHANES data. This type of data cannot demonstrate cause and effect; ie, one explanation for our observation of lower sodium intake among migraineurs is that respondents who suffered from migraine intentionally lowered their sodium intake as a possible preventive measure. Also, the 3‐month prevalence of headaches captured by NHANES does not allow for an analysis of the effect of dietary sodium on headache frequency. To investigate whether brain sodium alterations and dietary sodium in particular represent a culprit in migraine, it will be necessary to pursue prospective dietary studies with more detailed and valid migraine data, correlated with biochemical parameters.
There were other limitations to our analysis. Migraine classification was not based on the International Headache Classification criteria16 and was nonspecific to migraine. Another national database, the Women's Health Initiative, has more conventional migraine classification24 but does not have dietary data comparable to the quality of NHANES 1999–2004 data. We likely excluded some migraineurs by omitting respondents who had ever used analgesics chronically or who had taken prescription analgesics during the month prior to interview. This latter group, in particular, was more likely to have in included some true migraineurs who were, therefore, not in our “migraineur” group. However, our goal was to have the most valid “migraineur” group possible, given the limitations of the data in terms of migraine classification. Therefore, we felt it was more important to exclude some true migraineurs than to include as “migraineurs” those suffering from medication‐overuse headache. Ultimately, a sensitivity analysis suggested there was little effect from this exclusion. Nutritional assessment was based on 24‐hour recall, a method with inherent limitations.54 Importantly, due to lack of 2 days of dietary recall in 2/3 of our data, we were unable to use more sophisticated methods to account for episodically consumed foods and beverages. Despite these limitations, the NHANES 1999–2004 database and its associated analytical methodology represents best available, state‐of‐the‐art practice for survey studies.
Conclusion
Our analysis of NHANES 1999–2004 data provides the first evidence of an inverse association between migraine and dietary sodium intake, independent of common migraine covariates. We propose that this dietary sodium intake may impact migraine by modulating the regulation of brain extracellular sodium. Our results may stimulate prospective, controlled studies of dietary and body sodium to evaluate whether altered sodium homeostasis is casual in, or an effect of, migraine.
Statement of Authorship
Category 1
-
(a) Conception and Design
Janice M. Pogoda & Michael G. Harrington
-
(b) Acquisition of data
Janice M. Pogoda
-
(c) Analysis an interpretation of data
Janice M. Pogoda, Noah B. Gross, Xianghong Arakaki, Alfred N. Fonteh, Robert P. Cowen, & Michael G. Harrington
Category 2
-
(a) Drafting the manuscript
Janice M. Pogoda & Michael G. Harrington
-
(b) Revising it for intellectual content
Janice M. Pogoda, Noah B. Gross, Xianghong Arakaki, Alfred N. Fonteh, Robert P. Cowen, & Michael G. Harrington
Category 3
-
(a) Final approval of the completed manuscript
Janice M. Pogoda, Noah B. Gross, Xianghong Arakaki, Alfred N. Fonteh, Robert P. Cowen, & Michael G. Harrington
Conflict of Interest: None.
Funding: MGH, NG, XA, and ANF received support from NIH 5RO1NS072497 and HMRI.
References
- 1. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–391. [DOI] [PubMed] [Google Scholar]
- 2. Harrington MG, Fonteh AN, Cowan RP, et al. Cerebrospinal fluid sodium increases in migraine. Headache. 2006;46:1128–1135. [DOI] [PubMed] [Google Scholar]
- 3. Harrington MG, Salomon RM, Pogoda JM, et al. Cerebrospinal fluid sodium rhythms. Cerebrospinal Fluid Res. 2010;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alstadhaug KB, Bekkelund S, Salvesen R. Circannual periodicity of migraine? Eur J Neurol. 2007;14:983–988. [DOI] [PubMed] [Google Scholar]
- 5. Fox AW, Davis RL. Migraine chronobiology. Headache. 1998;38:436–441. [DOI] [PubMed] [Google Scholar]
- 6. Kelman L. Pain characteristics of the acute migraine attack. Headache. 2006;46:942–953. [DOI] [PubMed] [Google Scholar]
- 7. Solomon GD. Circadian rhythms and migraine. Cleve Clin J Med. 1992;59:326–329. [DOI] [PubMed] [Google Scholar]
- 8. Spierings EL, Sorbi M, Maassen GH, Honkoop PC. Psychophysical precedents of migraine in relation to the time of onset of the headache: The migraine time line. Headache. 1997;37:217–220. [DOI] [PubMed] [Google Scholar]
- 9. Harrington MG, Chekmenev EY, Schepkin V, Fonteh AN, Arakaki X. Sodium MRI in a rat migraine model and a NEURON simulation study support a role for sodium in migraine. Cephalalgia. 2011;31:1254–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949;108:37–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arakaki X, Foster H, Su L, et al. Extracellular sodium modulates the excitability of cultured hippocampal pyramidal cells. Brain Res. 2011;1401:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bayorh MA, Socci RR, Eatman D, Wang M, Thierry‐Palmer M. The role of gender in salt‐induced hypertension. Clin Exp Hypertens. 2001;23:241–255. [DOI] [PubMed] [Google Scholar]
- 13. Praetorius J, Nielsen S. Distribution of sodium transporters and aquaporin‐1 in the human choroid plexus. Am J Physiol Cell Physiol. 2006;291:C59–67. [DOI] [PubMed] [Google Scholar]
- 14. Briefel RR. Assessment of the US diet in national nutrition surveys: National collaborative efforts and NHANES. Am J Clin Nutr. 1994;59:164S–167S. [DOI] [PubMed] [Google Scholar]
- 15. NHANES Analytic Guidelines , June 2004 Edition. Available from http://www.cdc.gov/nchs/data/nhanes/nhanes_general_guidelines_june_04.pdf (accessed July 9, 2010).
- 16. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 17. Evans EW, Lipton RB, Peterlin BL, et al. Dietary intake patterns and diet quality in a nationally representative sample of women with and without severe headache or migraine. Headache. 2015;55:550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.USDA Agricultural Research Service, CSFII 1994‐1996, 1998 and DHKS 1994‐1996. Available from http://www.ars.usda.gov/Services/docs.htm?docid=14531 (accessed February 26, 2016).
- 19. Raper N, Perloff B, Ingwersen L, Steinfeldt L, Anand J. An overview of USDA's dietary intake system. J Food Comp Analysis 2004;17:545–55. [Google Scholar]
- 20.USDA Agricultural Research Service, Food and Nutrient Database for Dietary Studies. Available from https://www.ars.usda.gov/services/docs.htm?docID=12089 (accessed February 26, 2016).
- 21.Cerner Multum Lexicon Therapeutic Classification Scheme. Available from http://www.multum.com/ (accessed February 26, 2016).
- 22. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S; discussion 9S‐31S. [DOI] [PubMed] [Google Scholar]
- 23.NHANES Dietary Web Tutorial. Available from http://www.cdc.gov/nchs/tutorials/dietary/index.htm (accessed July 9, 2010).
- 24. Rist PM, Buring JE, Kurth T. Dietary patterns according to headache and migraine status: A cross‐sectional study. Cephalalgia. 2015;35:767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38:791–813. [DOI] [PubMed] [Google Scholar]
- 26. Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. [DOI] [PubMed] [Google Scholar]
- 27. Tietjen GE, Herial NA, Hardgrove J, Utley C, White L. Migraine comorbidity constellations. Headache. 2007;47:857–865. [DOI] [PubMed] [Google Scholar]
- 28. Blaustein MP, Leenen FH, Chen L, et al. How NaCl raises blood pressure: A new paradigm for the pathogenesis of salt‐dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1031–H1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mancia G, Rosei EA, Ambrosioni E, et al. Hypertension and migraine comorbidity: Prevalence and risk of cerebrovascular events: Evidence from a large, multicenter, cross‐sectional survey in Italy (MIRACLES study). J Hypertens. 2011;29:309–318. [DOI] [PubMed] [Google Scholar]
- 30. Scher AI, Terwindt GM, Picavet HS, Verschuren WM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: The GEM population‐based study. Neurology. 2005;64:614–620. [DOI] [PubMed] [Google Scholar]
- 31. Chow SY, Sakai RR, Witcher JA, Adler NT, Epstein AN. Sex and sodium intake in the rat. Behav Neurosci. 1992;106:172–180. [DOI] [PubMed] [Google Scholar]
- 32. Sakai RR, Frankmann SP, Fine WB, Epstein AN. Prior episodes of sodium depletion increase the need‐free sodium intake of the rat. Behav Neurosci. 1989;103:186–192. [DOI] [PubMed] [Google Scholar]
- 33. Rocchini AP, Key J, Bondie D, et al. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989;321:580–585. [DOI] [PubMed] [Google Scholar]
- 34. Kawano Y, Yoshida K, Kawamura M, et al. Sodium and noradrenaline in cerebrospinal fluid and blood in salt‐sensitive and non‐salt‐sensitive essential hypertension. Clin Exp Pharmacol Physiol. 1992;19:235–241. [DOI] [PubMed] [Google Scholar]
- 35. Amer M, Woodward M, Appel LJ. Effects of dietary sodium and the DASH diet on the occurrence of headaches: Results from randomised multicentre DASH‐Sodium clinical trial. BMJ Open. 2014;4:e006671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp Physiol. 2008;93:177–209. [DOI] [PubMed] [Google Scholar]
- 37. Vivas L, Godino A, Dalmasso C, Caeiro XE, Macchione AF, Cambiasso MJ. Neurochemical circuits subserving fluid balance and baroreflex: A role for serotonin, oxytocin, and gonadal steroids In: De Luca LA. Jr, Menani JV, Johnson AK, eds. Neurobiology of Body Fluid Homeostasis: Transduction and Integration. Boca Raton, FL; CRC Press, 2014. [PubMed] [Google Scholar]
- 38. Harrington MG, Fonteh AN, Arakaki X, et al. Capillary endothelial Na(+), K(+), ATPase transporter homeostasis and a new theory for migraine pathophysiology. Headache. 2010;50:459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Charles A. Does cortical spreading depression initiate a migraine attack? Maybe not. Headache. 2010;50:731–733. [DOI] [PubMed] [Google Scholar]
- 40. Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011;69:855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: Implications for migraine with aura. J Neurosci. 2010;30:8807–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Messlinger K, Lennerz JK, Eberhardt M, Fischer MJ. CGRP and NO in the trigeminal system: Mechanisms and role in headache generation. Headache. 2012;52:1411–1427. [DOI] [PubMed] [Google Scholar]
- 43. Buzzi MG, Moskowitz MA. The trigemino‐vascular system and migraine. Pathol Biol (Paris). 1992;40:313–317. [PubMed] [Google Scholar]
- 44. Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Markowitz S, Saito K, Buzzi MG, Moskowitz MA. The development of neurogenic plasma extravasation in the rat dura mater does not depend upon the degranulation of mast cells. Brain Res. 1989;477:157–165. [DOI] [PubMed] [Google Scholar]
- 46. Robert C, Bourgeais L, Arreto CD, et al. Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J Neurosci. 2013;33:8827–8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Siniatchkin M, Sendacki M, Moeller F, et al. Abnormal changes of synaptic excitability in migraine with aura. Cereb Cortex. 2012;22:2207–2216. [DOI] [PubMed] [Google Scholar]
- 48. Antal A, Lang N, Boros K, Nitsche M, Siebner HR, Paulus W. Homeostatic metaplasticity of the motor cortex is altered during headache‐free intervals in migraine with aura. Cereb Cortex. 2008;18:2701–2705. [DOI] [PubMed] [Google Scholar]
- 49. Charbit AR, Akerman S, Goadsby PJ. Trigeminocervical complex responses after lesioning dopaminergic A11 nucleus are modified by dopamine and serotonin mechanisms. Pain. 2011;152:2365–2376. [DOI] [PubMed] [Google Scholar]
- 50. Sun YG, Pita‐Almenar JD, Wu CS, et al. Biphasic cholinergic synaptic transmission controls action potential activity in thalamic reticular nucleus neurons. J Neurosci. 2013;33:2048–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cregg R, Momin A, Rugiero F, Wood JN, Zhao J. Pain channelopathies. J Physiol. 2010;588:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goldstein I, Levy T, Galili D, et al. Involvement of Na(+), K(+)‐ATPase and endogenous digitalis‐like compounds in depressive disorders. Biol Psychiatry. 2006;60:491–499. [DOI] [PubMed] [Google Scholar]
- 53. Lichtstein D, Samuelov S. Endogenous 'ouabain like' activity in rat brain. Biochem Biophys Res Commun. 1980;96:1518–1523. [DOI] [PubMed] [Google Scholar]
- 54. Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;36:e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]


