Abstract
Measurements of thermal stability by circular dichroism (CD) spectroscopy have been widely used to assess the binding of peptides to MHC proteins, particularly within the structural immunology community. Although thermal stability assays offer advantages over other approaches such as IC50 measurements, CD-based stability measurements are hindered by large sample requirements and low throughput. Here we demonstrate that an alternative approach based on differential scanning fluorimetry (DSF) yields results comparable to those based on CD for both class I and class II complexes. As they require much less sample, DSF-based measurements reduce demands on protein production strategies and are amenable for high throughput studies. DSF can thus not only replace CD as a means to assess peptide/MHC thermal stability, but can complement other peptide-MHC binding assays used in screening, epitope discovery, and vaccine design. Due to the physical process probed, DSF can also uncover complexities not observed with other techniques. Lastly, we show that DSF can also be used to assess peptide/MHC kinetic stability, allowing a single experimental setup to probe both binding equilibria and kinetics.
Keywords: MHC protein, binding affinity, thermal stability, kinetic stability, differential scanning fluorimetry
1. Introduction
Quantifying the strength of the interactions between peptides and major histocompatibility complex (MHC) proteins is important in the identification of T cell epitopes and evaluating the consequences of naturally occurring or engineered peptide modifications. In many cases though, the instability of peptide-free “empty” MHC proteins (particularly for class I) has hindered direct measurements of MHC-peptide binding equilibria. To circumvent this complexity, a variety of experimental approaches have been developed that yield proxies for MHC-peptide binding affinities. Perhaps the most common is the use of competition experiments to report IC50 values relative to a labeled competitor (Ruppert et al., 1993). Other assays have been developed, including the use of pre-oxidized protein to reduce instability issues (Harndahl et al., 2009) and indirect assays that rely on antibody or T cell receptor detection of peptide/MHC complexes (Miles et al., 2011).
In addition to the approaches above, measurements of peptide/MHC thermal stability are commonly used to assess how well peptides interact with MHC proteins. Folded proteins exist in an equilibrium with their unfolded states, and due to the principle of coupled equilibria, ligand binding to a folded protein will increase protein thermodynamic stability. Due to this link, the presence of a bound ligand increases protein thermal stability, and the increase in a protein’s Tm (formally defined as the temperature at which 50% of the protein is unfolded) correlates with binding affinity (Crothers, 1971; Brandts and Lin, 1990). Measurements of peptide/MHC thermal stability have been widely adopted by the structural immunology community, with circular dichroism (CD) spectroscopy being the most frequent mode of detection. In this use, CD monitors the loss of α-helical character in the peptide binding domain as the complex dissociates and unfolds, and early studies demonstrated close agreement between peptide/MHC thermal stability and robust affinity determinations by equilibrium dialysis (Morgan et al., 1997). Thermal stability measurements, whether by CD or other approaches (Schlundt et al., 2009; Schlundt et al., 2012) are advantageous in that they are not dependent on the identity or concentration of a competitor peptide. They can also be easily implemented, as they do not require peptide or protein modifications and use relatively simple instrumentation.
A drawback of CD-monitored thermal unfolding of peptide/MHC complexes is the limited signal-to-noise ratio and the large amount of sample required (typically hundreds of microliters of protein at concentrations of tens of micromolar). Although small relative to the significant amount of protein typically needed for structural studies, these requirements nevertheless reduce throughput and limit the use of thermal stability measurements in broader studies such as epitope discovery. Although small volume CD systems can reduce sample requirements, these suffer from low signal-to-noise, and medium-throughput CD instrumentation requiring complex liquid handling robotics has only recently been described (Fiedler et al., 2012).
Differential scanning fluorimetry (DSF; also referred to as ThermoFluor) provides an alternative to CD spectroscopy as a method to assess protein stability and the impact of ligand binding (Pantoliano et al., 2001; Matulis et al., 2005; Niesen et al., 2007). DSF takes advantage of small, environmentally-sensitive fluorescent molecules whose fluorescence is enhanced when specifically bound to exposed hydrophobic surfaces such as those created by protein unfolding. Due to the high sensitivity of modern fluorimeters, DSF is well suited to low volume, rapid measurements on multiple samples. DSF has found numerous uses in characterizing protein stability and ligand binding, and has been recently used to assess the binding of peptides to class I MHC proteins (Gras et al., 2012; Koch et al., 2013a; Koch et al., 2013b; Hassan et al., 2015), as well as the impact of peptides and other molecules binding to class II proteins (Günther et al., 2010; Rupp et al., 2011; Clayton et al., 2014), including HLA-DM (Álvaro-Benito et al., 2015). Although early applications of DSF required customized fluorimeters, the technique is easily adaptable to fluorescence-equipped RT-PCR instruments common to many molecular biology laboratories. DSF is thus highly scalable, with one experimental run capable of assaying dozens of small-volume samples in plate format. Here we performed a comparison of DSF and CD spectroscopy for measuring the thermal stability of both class I and class II peptide/MHC complexes. We found DSF a straightforward, easily implementable approach that yields results equivalent to those obtained from CD spectroscopy while considerably reducing sample requirements.
Lastly, interest in peptide/MHC kinetic as opposed to thermodynamic stability has been growing. In some instances, kinetic stability may be a more biologically relevant parameter, as it more accurately reflects the situation of a MHC-presented peptide on a cell surface. Indeed, some reports indicate that kinetic stability is an improved indicator of antigenicity and immunodominance than binding affinity (Lazarski et al., 2005; Baumgartner et al., 2010; Harndahl et al., 2012). Various approaches have been used to measure peptide-MHC kinetic stability, including using fluorescent or radioactive peptides, surface plasmon resonance, and a recently developed scintillation proximity assay (Gakamsky et al., 1996; Gakamsky et al., 2000; Binz et al., 2003; Baxter et al., 2004; Harndahl et al., 2011; Miles et al., 2011). We show here that for class I peptide/MHC complexes, DSF readily lends itself to direct measurements of peptide dissociation without the need for protein or peptide labeling.
2. Materials and Methods
2.1 Peptides and proteins
Peptides were synthesized commercially (CHI Scientific or 21st Century Biochemicals). For class I protein, recombinant HLA-A*0201 heavy chain and β2-microglobulin were expressed as inclusion bodies in Escherichia coli (Garboczi et al., 1992). MHC folding and assembly from inclusion bodies was performed according to standard procedures (Pierce et al., 2014) Protein was purified using ion exchange followed by size-exclusion chromatography. For class II protein, soluble extracellular domains of HLA-DR1 (DRA*0101/DRB*010101) were expressed in Drosophila S2 cells and purified by immunoaffinity chromatography as previously described (Sloan et al., 1995).
2.2 Differential scanning fluorimetry
Differential scanning fluorimetry was performed using Applied Biosystems StepOnePlus (for class I complexes) or Bio-Rad C1000 Thermal Cycler (for class II complexes) RT-PCR instruments with the excitation and emission wavelengths set to 587 and 607 nm, respectively. For class I complexes, solution volumes were 20 µL in 96-well plates. Assay buffer was 10 mM HEPES (pH 7.4), 150 mM NaCl, 3 mM EDTA, with 0.005% surfactant P20 added to prevent protein adherence to the plate. For class II complexes, solution volumes were 25 µL in 96-well plates. Assay buffer was 100 mM sodium citrate (pH 5.5), 50 mM NaCl, 5 mM EDTA, 0.1% NaN3, 1 mM DTT, 1 mM PMSF, and 0.1% octylglucoside. The different solution conditions for class I and class II complexes were based on optimal conditions for preparation and storage of the different complexes, and are conventional buffers for the two systems (Khan et al., 2000; Yin et al., 2015). For class II complexes, the low pH is believed to approximate conditions for endosomal loading. Pilot studies of class I complexes under the conditions used to assess class II resulted in substantial reductions in apparent Tm.
For thermal stability measurements, the temperature scan rate was fixed at 1 °C/min unless otherwise indicated. Protein and SYPRO orange (Invitrogen) concentrations were varied as described. The temperature range spanned 20 °C to 95 °C. Data analysis was performed in OriginPro 7 or 9. Apparent Tm values were first determined by identifying the point at which the transition was 50% complete. For a more rigorous analysis, the temperature derivative of the melting curve was computed. The resulting derivative curve was processed with the peak fitting algorithm in OriginPro, applying a sigmoidal baseline and fitting the peak to determine the Tm and its standard error. A bi-Gaussian function, commonly used in spectroscopy and chromatography, was used for peak fitting as the peaks were usually noticeably skewed, presumably due contributions from irreversible aggregation that occurs co-incident with unfolding. When multiple transitions were observed, the data were subject to the automated multiple peak fitting process in OriginPro, using default options except that bi-Gaussian functions were used. This proved successful in identifying the major transitions and their associated Tm and standard error as shown in Figs. 1C and 3A. For kinetic measurements, 10 μM of protein was incubated in the instrument at a constant temperature of 37 °C with 10X SYPRO orange, with fluorescence measured every five minutes. Kinetic data were fit to a biexponential function. As performed previously (Binz et al., 2003), the slowest rate constant was attributed to peptide dissociation from the class I MHC heterotrimer and used to determine half-lives.
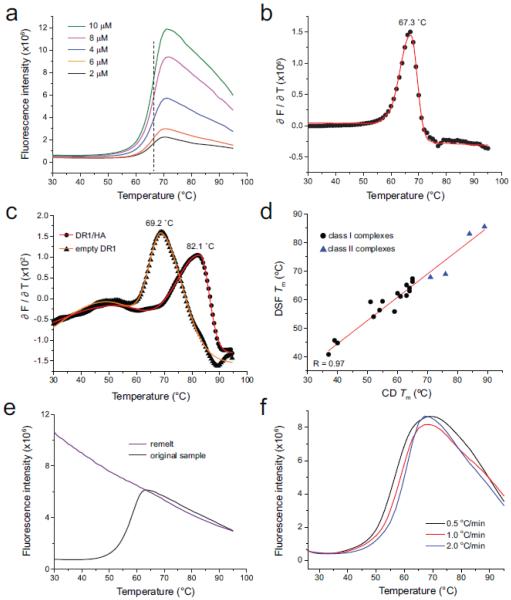
Figure 1.
Quantitative analysis of differential scanning fluorimetry in assessing class I and class II MHC/peptide stability. A) Progress curves for different concentrations of the class I TYR/A2 complex. Overall fluorescent intensity varied with concentration, but the midpoint of the transition was a constant 67 °C, as indicated by the vertical dashed line. B) The first derivative of the progress curves provides a means to robustly determine Tm values, as shown for the TYR/A2 complex at 2 µM. Black dots indicate the first derivative data; the red line is a baseline-corrected bi-Gaussian peak fit. C) First-derivative analysis and associated fit of the data for the class II HA/DR1 complex (circles) and ‘empty’ DR1 (triangles). D) peptide/MHC Tm values determined by DSF correlate with those determined by CD spectroscopy. Black circles are class I complexes; blue triangles are class II. The correlation coefficient (R) for the linear fit to the data (red line) is 0.98. E) peptide/A2 denaturation remains irreversible when studied by DSF. The black curve shows the DSF scan of a fresh sample of the SL9 peptide bound to A2 with a Tm of 59 °C. The purple line shows a rescan of the same sample after cooling to 25 °C. F) Thermal denaturation of peptide/A2 complexes show a scan rate dependence, as indicated here for the MART126(2L)-35 peptide bound to A2.
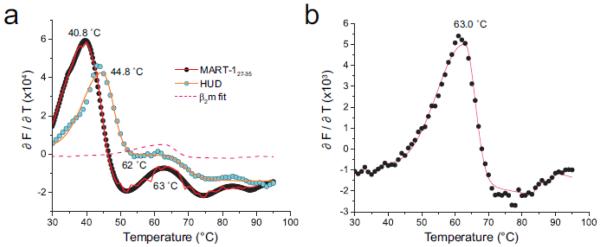
Figure 3.
DSF detects separate β2m unfolding in low stability class I MHC complexes. A) Derivative analyses of the progress curves for the MART-127-35 and HUD complexes with A2. A separate peak is seen for both with a Tm at or near 63 °C. The pink dashed line shows the fit from an analysis of β2m alone superimposed on the MART-1 and HUD data. B) DSF analysis of β2m alone shows a single transition with a Tm of 63 °C. The fitted curve from this analysis is shown as the pink dashed line in panel A. The reduced intensity of the β2m alone sample in panel B compared to the pMHC samples in panel A is attributable to a lower concentration and the smaller size of β2m, resulting in less binding SYPRO orange.
2.3 Circular dichroism spectroscopy
CD spectroscopy was performed as previously described (Khan et al., 2000; Borbulevych et al., 2010; Borbulevych et al., 2011; Ekeruche-Makinde et al., 2012), using a Jasco J815 instrument. Temperature was increased from 10 °C to 100 °C at an increment of 1 °C/min, monitoring a wavelength of 218 nm. Protein concentrations were between 5 µM and 10 µM in 20 mM phosphate (pH 7.4), 75 mM NaCl. Data analysis was performed in Kaleidagraph or OriginPro. Data in the transition region were differentiated or fit to a polynomial, and apparent Tm values were determined as the point in which the transition was 50% complete.
2.4 IC50 measurements
IC50 measurements for peptide binding to HLA-DR1 were determined using a fluorescence polarization assay, using Alexa488-labeled HA306-318 as the indicator peptide as previously described (Yin et al., 2012; Yin and Stern, 2014).
3. Results
3.1 Implementation and optimization of DSF for peptide/MHC stability
Differential scanning fluorimetry was implemented utilizing the environmentally-sensitive fluorescent molecule SYPRO orange. SYPRO orange is minimally fluorescent in solution, but experiences a >400% enhancement of fluorescence in the presence of unfolded protein by binding exposed hydrophobic regions (Niesen et al., 2007). For class I complexes, we utilized a StepOnePlus RT-PCR instrument, using a solution volume of 20 µL in 96 well plates. We first explored the thermal stability of the tyrosinase peptide (TYR, sequence YMDGTMSQV) bound to the class I MHC protein HLA-A*0201 (A2). With a protein concentration of 10 µM, 10X SYPRO orange (SYPRO orange is provided as a 5000X stock solution; the actual concentration, chemical composition, and molecular weight are withheld by manufacturer), and a scan rate of 1 °C/min we observed a clear fluorescence response similar to that seen in DSF studies of other protein-ligand interactions, including peptide binding to MHC (e.g., (Matulis et al., 2005; Niesen et al., 2007; Clayton et al., 2014; Hassan et al., 2015). The progress curve was sigmoidal over the temperature range of 30 °C to 70 °C, demonstrative of cooperative thermal unfolding (Fig. 1A). Unlike thermal denaturation by CD, there was virtually no scatter in the data, eliminating the need to fit the data to a polynomial in order to determine the midpoint of the curve. For the TYR/A2 complex, the midpoint occurred at 67 °C. We refer to this midpoint as the “apparent Tm” (sometimes simplified to just Tm; we use the word “apparent” as the complexity of peptide/MHC systems hinders rigorous thermodynamic analyses as discussed below).
To explore sensitivity, we assessed unfolding of the TYR/A2 complex as a function of concentration, reducing concentration in 2 µM steps with SYPRO orange concentration held constant at 10X. Although fluorescence intensity dropped with concentration, signal-to-noise remained high, with a clear sigmoidal curve visible at a concentration as low as 2 µM (Fig. 1A). As judged by the midpoint of the curve, the apparent Tm showed no variation with concentration over this range. We also varied the SYPRO orange concentration from 5X to 20X with no systematic variation in Tm, but obtained the clearest signal with 10X.
3.2 Tm values determined by DSF and CD spectroscopy are comparable for both class I and class II complexes
We next examined a large number of different peptide/A2 complexes. These complexes included peptides with a wide range of A2 binding affinities, from weak (MART-127-35/A2) to strong (HTLV-1 Tax11-19/A2). As anticipated, we observed a wide range of apparent Tm values, detecting not only large differences between peptides and significant consequences of anchor residue modification, but also subtler effects resulting from more conservative peptide modifications (Table 1).
Table 1.
Comparison of peptide/MHC apparent Tm values determined by differential scanning fluorimetry and circular dichroism.
| Peptide name | Sequence | Tm by DSF (°C) | Tm by CD (°C) |
|---|---|---|---|
| Class I peptide/MHC complexes | |||
| MART-127-35 | AAGIGILTV | 40.8 (± 0.1) | 37 |
| MART-127(2L)-35 | ALGIGILTV | 59.2 (± 0.2) | 51 |
| MART-126-35 | EAAGIGILTV | 45.7 (± 0.3) | 39 |
| MART-126(2L)-35 | ELAGIGILTV | 59.4 (± 0.3) | 55 |
| TAX | LLFGYPVYV | 66.3 (± 0.1) | 65 |
| LLE | LLEEMFLTV | 54.0 (± 0.6) | 52 |
| gp100209(2M)-217 | IMDQVPFSV | 62.2 (± 0.5) | 60 |
| HUD | LGYGFVNYI | 44.8 (± 0.3) | 40 |
| HUD87(2L,9V)-95 | LLYGFVNYV | 65.1 (± 0.5) | 63 |
| NS31406-1415 | KLVALGINAV | 64.0 (± 0.6) | 64 |
| NS31406(4T)-1415 | KLVTLGINAV | 63.0 (± 0.1) | 64 |
| NS31406(7N)-1415 | KLVALGNNAV | 55.8 (± 0.2) | 59 |
| NS31406(3S,4G,7L)-1415 | KLSGLGLNAV | 61.1 (± 0.1) | 61 |
| WT1 | RMFPNAPYL | 56.3 (± 0.3) | 54 |
| TYR | YMDGTMSQV | 67.3 (± 0.1) | 65 |
| TYR (T5F) | YMDGFMSQV | 61.4 (± 0.3) | 63 |
| Class II peptide/MHC complexes | |||
| HA | PKYVKQNTLKLAT | 82.1 (± 0.1) | 84 |
| FRR-HA-F308A | PRAVKQNTLRLAT | 68.9 (± 0.1) | 76 |
| Min4 | YRAL | 67.8 (± 0.1) | 71 |
| Yak | AAYAAAAAAKAAA | 85.5 (± 0.1) | 89 |
We also used an alternative approach for determining Tm values from the data. Quantitative analyses of sigmoidal transitions can be performed (Niesen et al., 2007), but accuracy requires accounting for pre- and post-transition baselines (Pace, 1990). We took a simpler approach derived from analysis of differential scanning calorimetry data and utilized the maximum of the first derivative of the sigmoidal curve rather than the midpoint of the transition, determined by subjecting the transition to a quantitative peak analysis (Fig. 1B). This approach is advantageous as it can be more easily implemented and provides an objective means of determining error in the Tm. In all cases, the Tm values determined via assessing 50% of the sigmoidal transition and the midpoint of the first derivative were nearly identical. To assess reproducibility, we repeated the measurement of the MART-126(2L)-35/HLA-A2 complex three times using separate samples. The data were in very close agreement: the standard deviation of the Tm was ±0.2 °C, less than the fitting error determined from the quantitative peak analysis.
We next assessed the applicability of DSF for determining the stability of class II peptide/MHC complexes using the well-characterized complex of HLA-DR1 (DR1) bound to the influenza HA peptide (PKYVKQNTLKLAT) (Sato et al., 2000a). We observed a clear transition with a high Tm of 83 °C, as shown in Fig. 1C. As DR1 can be obtained by expression in insect cells as empty protein (free of associated peptides) (Yin et al., 2015), we were also able to assess empty DR1, which unfolded with a Tm of 69 °C. As with the class I complexes, the stability of peptide/DR1 complexes varied with peptide (Table 1).
The thermal stabilities of six of the peptide/A2 complexes and all four of the peptide/DR1 complexes in Table 1 have been previously determined using CD spectroscopy (Khan et al., 2000; Sato et al., 2000b; Borbulevych et al., 2010; Borbulevych et al., 2011; Ekeruche-Makinde et al., 2012). We utilized CD to determine the stabilities of the remainder, using our previously established methods. The values determined by fluorimetry were in excellent agreement with those determined by CD: the CD and DSF values were highly correlated (correlation coefficient (R) of 0.97), and there were no systematic differences between the DSF and CD data (Fig. 1D). The close agreement between the DSF and CD is all the more impressive given that the CD data were collected for class I and class II systems in different laboratories over a period of several years.
3.3 Irreversibility leads to a scan rate dependence of peptide/A2 thermal unfolding
A complexity of peptide/MHC thermal stability measurements is that, as assessed by CD spectroscopy, thermal unfolding is irreversible due to aggregation (although the extent varies with class and allele). Irreversibility limits more detailed interpretation of Tm values beyond serving as a proxy for affinity and hinders determination of thermodynamic parameters. To examine if the greater sensitivity of DSF could better detect reversibility, we cooled peptide/A2 samples to 25 °C after monitoring unfolding and repeated the measurement after equilibration at 25 °C for 10 minutes. Despite the greater sensitivity of fluorimetry compared to CD, no clear indications of reversibility were observed, as shown in Fig. 1E for the SL9/HLA-A2 complex.
We next assessed the influence of scan rate on the apparent Tm. Scan rate is a key parameter in thermal unfolding experiments as heat transfer can be rate limiting. In cases where irreversible thermal unfolding occurs, scan rate can also influence side reactions such as aggregation. A scan rate dependence is another tool for demonstrating irreversible thermal unfolding (Sanchez-Ruiz et al., 1988; Ibarra-Molero et al., 2016). Using CD, a slight scan rate dependence has been shown for thermal denaturation of DR1 (Zarutskie et al., 1999).
Exploring different peptide/A2 complexes and altering scan rate from 0.5 °C/min to 2 °C/min, we observed regular variations in Tm, with lower scan rates yielding lower Tm values, as shown in Fig. 1F for the anchor-modified MART-126(2L)-35/HLA-A2 complex. Given this variation, we maintained the 1°C/min scan rate for the remainder of the study as it maintains consistency with prior CD measurements.
3.4 Tm values determined by DSF correlate with predicted and measured IC50 values
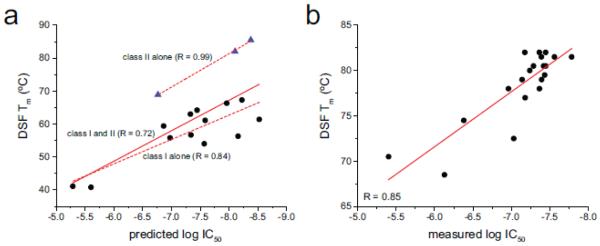
We next examined the agreement between DSF-determined Tm values and predicted and measured peptide binding affinities (IC50 values). Using the NetMHC 3.4 and NetMHCII 2.2 servers (Lundegaard et al., 2008; Nielsen and Lund, 2009), we predicted the affinities of each of the peptide/MHC complexes in Table 1 (excluding the YRAL peptide, which at four amino acids is beneath the length threshold for NetMHCII). In aggregate the results were reasonably well correlated (R = 0.72; Fig. 2A). Evaluating the class I and class II data separately yielded improved correlations: 0.84 for class I and 0.99 for class II (although the agreement for the class II data seems remarkable, the significance of this is debatable given the small number of data points).
Figure 2.
Tm values determined by DSF correlate with predicted and measured IC50 values. A) Comparison of DSF-determined Tm values and IC50 values predicted by NetMHC and NetMHCII. Black circles are class I complexes; blue triangles are class II. The solid red line is a linear fit to the class I and class II data together. The upper dashed line is a linear fit to the class II data alone, and the lower dashed line is a linear fit to the class I data alone. Correlation coefficients for all three fits are indicated. B) Comparison of DSF-determined Tm values and experimental IC50 values for peptide/DR1 complexes. The red line is a linear fit to the data. The correlation coefficient is 0.85.
We then compared the DSF-determined Tm values with experimentally-determined IC50 values. As peptide exchange with class I MHC proteins is complicated by slow kinetics and protein aggregation, we restricted this comparison to a class II system. We recently published IC50 values for the HLA-A2104-117 peptide and five variants binding to DR1 (Yin et al., 2014). Values for an additional 14 peptides were determined here using our previously established methods (Supplemental Table 1). As shown in Fig. 2B, the IC50 data were well correlated with the Tm values determined by DSF (R = 0.85), confirming the relationship between Tm and peptide-MHC binding affinity.
3.5 DSF detects β2-microglobulin dissociation from low stability complexes
In the analysis of some class I complexes, we observed multiple transitions in the thermal melting curves. This was most reproducible in the lowest stability complexes we studied, those with the HUD (sequence LGYGFVNYI) and MART-127-35 (sequence AAGIGILTV) nonamer peptides bound to HLA-A2 (Fig. 3A). Although the DSF-determined Tm values for the major transitions in these cases were close to Tm values determined by CD (Table 1), the presence of a second transition was surprising, particularly as multiple transitions have not been seen by CD for these peptides. The Tm of the second transition was 62 - 63 °C.
A limitation of CD-based thermal measurements of class I peptide/MHC stability is the inability to detect unfolding of β2m due to its lack of α-helical character. As DSF probes a different physical process than CD (exposure of hydrophobic surfaces vs. helix content), we asked whether the second peak observed in the sample with the HUD and MART-127-35 peptides reflected melting of free β2m. DSF of β2m alone showed a peak with a Tm of 63 °C (Fig. 3A,B). As all samples were carefully purified, the β2m transition is not likely attributable to excess β2m in the sample. We thus attribute the additional transition in the HUD and MART-127-35 samples to the presence of dissociated β2m, resulting from low temperature-induced breakdown of the complex. As multiple transitions are not detected in the more stable complexes, we presume that with higher affinity peptides dissociation and unfolding proceeds in a more cooperative manner (further support for this interpretation is found in Fig. 3A, in which the β2m peak is more clearly defined in the lowest stability complex). This conclusion is consistent with studies of the denaturation of mouse H-2Db and H-2Kb complexes, which followed different paths for low and high affinity peptides (Saini et al., 2013).
3.6 SF-based measurements of class I peptide/MHC kinetic stability
We last explored the ability of DSF to measure the kinetic stability of class I peptide/MHC complexes, taking advantage of the rapid aggregation of class I heavy chains that occurs after peptide dissociation (Gakamsky et al., 1996; Gakamsky et al., 1999). We examined two MART-1 peptides: the 27-35 nonamer (AAGIGILTV) and the 26-35 decamer (EAAGIGILTV), in addition to the corresponding anchor-modified variants in which the alanines at peptide position two are replaced with leucine (ALGIGILTV and ELAGIGILTV). Each sample was incubated in the RT-PCR instrument at a constant temperature of 37 °C for approximately 10 hours at a protein concentration of 10 μM with 10X SYPRO orange. Fluorescence intensity was read every five minutes. All four samples showed exponential increases in fluorescence intensity (Fig. 4), indicative of peptide dissociation and subsequent protein aggregation. Supporting this interpretation, the progress curves for both anchor-modified peptides were slower.
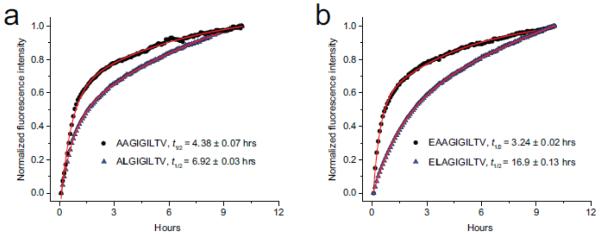
Figure 4.
Differential scanning fluorimetry can be used to assess peptide dissociation kinetics and thus the kinetic stability of class I MHC complexes. A) Dissociation of the native MART-127-35 nonamer (circles) and anchor-modified variant (triangles) from HLA-A2 at 37 °C. B) Dissociation of the native MART-126-35 decamer (circles) and anchor-modified variant (triangles) from HLA-A2 at 37 °C. For both panels, red lines indicate biexponential fits to the data. The slowest time constant was used to report complex half-lives, indicated in the inset as t1/2. Half-lives for the faster time constants in hours are 0.4 for AAGIGILTV, 0.5 hours for ALGIGILTV, 0.3 hours for EAAGIGILTV, and 1.1 hours for ELAGIGILTV.
Consistent with our previous studies of peptide dissociation from HLA-A2 using fluorescence anisotropy (Binz et al., 2003; Baxter et al., 2004), the dissociation data were fit best by a biphasic exponential function. As in our previous work we attribute the slowest phase to peptide dissociation from the class I heterotrimer. Accordingly, anchor-modification of the MART-127-35 nonamer increases the half-life of the complex from 4.4 to 6.9 hours. Anchor-modification of the MART-126-35 decamer increases the half-life from 3.2 to 16.9 hours. We note that for the MART-126-35 decamer peptides, the half-lives are nearly 2-fold shorter than those determined previously using a surface plasmon resonance-based assay (Cole et al., 2010). The most likely reason for this is the previous studies were performed at a lower temperature, which yields slower kinetics (25 °C vs. 37 °C; our use of the higher, more physiologically relevant temperature was necessitated by the need to promote the aggregation required for SYPRO orange binding and fluorescence). However, the 5.3-fold change in half-life for the anchor-modified MART-1 decamer reported here is exactly the same as that reported previously, indicating that DSF provides a convenient alternative for assessing class I peptide/MHC kinetic stability.
4. Discussion and Conclusions
Over the past two and a half decades, multiple approaches for quantifying the interactions of peptides with MHC proteins have been described. Measurements of thermal stability by circular dichroism spectroscopy have been widely adopted for this purpose by the structural immunology community, as well as investigators studying the physical properties of peptide/MHC complexes. Downsides to CD-based thermal stability measurements are the large sample requirements and single-sample throughput. Here we demonstrate that an alternative approach based on differential scanning fluorimetry yields results comparable to those based on CD. We emphasize that, because of the complexity of peptide/MHC complexes and their tendency to denature irreversibly, the analysis of thermal stability measurements, whether by CD or DSF, does not allow for straightforward extraction of rigorous thermodynamic parameters. A consequence is that comparative experiments must be collected under well controlled experimental conditions maintained across samples. In this way, and despite their underlying complexity, the apparent Tm values allow for a rapid and straightforward assessment of how strongly peptides interact with MHC proteins.
Additionally, the same physical process that allows for Tm determinations also readily lends itself to measurements of kinetic stability. Both types of measurements can be performed in widely available RT-PCR instruments, permitting the simultaneous analysis of multiple samples. The greater sensitivity of fluorescence translates into low sample requirements, illuminating the potential for DSF to aid not only physical and structural studies of select antigens, but also experiments that depend on the rapid assessment of many samples, such as studies in epitope discovery, vaccine design, and immune evasion. In principle, protein production strategies for both class I and class II MHC could be scaled down to facilitate DSF’s integration into a true high throughput pipeline.
DSF also has potential to identify characteristics of MHC-peptide interactions not visible via other techniques. An example here is the detection of dissociated β2m from low-stability class I complexes. In principle, the analysis could detect additional complexities, such as partial/multi-step dissociation, register-shifting, or multi-mode binding. Partial dissociation has been implicated in peptide immunogenicity (Duan et al., 2014) and in facilitating optimal T cell receptor binding (Madura et al., 2015). Register shifting or multi-mode binding has been seen in peptides that bind both class I and class II MHC proteins (Borbulevych et al., 2007; Landais et al., 2009; Günther et al., 2010). The binding of ANS, which like SYPRO orange interacts with exposed hydrophobic surfaces, has been used to examine pH dependent conformational changes in class II MHC proteins (Boniface et al., 1996; Runnels et al., 1996). Insight into these complexities would not be available from other methods to study MHC-peptide binding such as IC50 measurements or CD spectroscopy. This potential to uncover complexities in how peptides interact with MHC proteins, combined with its enhanced sensitivity, lower sample requirements, and capacity for high throughput assessments of both thermal and kinetic stability make DSF an appealing tool for assessing the interactions of peptides and other ligands with MHC proteins.
Supplementary Material
Highlights.
The thermal stability of peptide/MHC complexes correlates with peptide affinity
Differential scanning fluorimetry permits rapid assessment of peptide/MHC stability
Substantially lower volume requirements of DSF provides for high throughput
DSF also allows measurements of peptide/MHC kinetic stability / dissociation rates
Complexities not detectable with other binding assays can be observed with DSF
Acknowledgements
Supported by NIH grants GM067079 and GM103773 (to BMB), AI038996 (to LJS), CA154778 and CA153789 (to MIN), TR001108 (to TPR), and CA180731 (to TTS).
Abbreviations
- DSF
differential scanning fluorimetry
- Tm
melting temperature
- CD
circular dichroism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Álvaro-Benito M, Wieczorek M, Sticht J, Kipar C, Freund C. HLA-DMA Polymorphisms Differentially Affect MHC Class II Peptide Loading. The Journal of Immunology. 2015;194:803–816. doi: 10.4049/jimmunol.1401389. [DOI] [PubMed] [Google Scholar]
- Baumgartner CK, Ferrante A, Nagaoka M, Gorski J, Malherbe LP. Peptide-MHC Class II Complex Stability Governs CD4 T Cell Clonal Selection. The Journal of Immunology. 2010;184:573–581. doi: 10.4049/jimmunol.0902107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter TK, Gagnon SJ, Davis-Harrison RL, Beck JC, Binz A-K, Turner RV, Biddison WE, Baker BM. Strategic mutations in the class I MHC HLA-A2 independently affect both peptide binding and T cell receptor recognition. J. Biol. Chem. 2004;279:29175–29184. doi: 10.1074/jbc.M403372200. [DOI] [PubMed] [Google Scholar]
- Binz AK, Rodriguez RC, Biddison WE, Baker BM. Thermodynamic and kinetic analysis of a peptide-class I MHC interaction highlights the noncovalent nature and conformational dynamics of the class I heterotrimer. Biochemistry. 2003;42:4954–61. doi: 10.1021/bi034077m. [DOI] [PubMed] [Google Scholar]
- Boniface JJ, Lyons DS, Wettstein DA, Allbritton NL, Davis MM. Evidence for a conformational change in a class II major histocompatibility complex molecule occurring in the same pH range where antigen binding is enhanced. The Journal of Experimental Medicine. 1996;183:119–126. doi: 10.1084/jem.183.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbulevych OY, Do P, Baker BM. Structures of native and affinity-enhanced WT1 epitopes bound to HLA-A*0201: Implications for WT1-based cancer therapeutics. Molecular Immunology. 2010;47:2519–2524. doi: 10.1016/j.molimm.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbulevych OY, Insaidoo FK, Baxter TK, Powell DJ, Jr., Johnson LA, Restifo NP, Baker BM. Structures of MART-1(26/27-35) Peptide/HLA-A2 Complexes Reveal a Remarkable Disconnect between Antigen Structural Homology and T Cell Recognition. J Mol Biol. 2007;372:1123–36. doi: 10.1016/j.jmb.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbulevych OY, Piepenbrink KH, Baker BM. Conformational Melding Permits a Conserved Binding Geometry in TCR Recognition of Foreign and Self Molecular Mimics. J Immunol. 2011;186:2950–8. doi: 10.4049/jimmunol.1003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandts JF, Lin LN. Study of strong to ultratight protein interactions using differential scanning calorimetry. Biochemistry. 1990;29:6927–6940. doi: 10.1021/bi00481a024. [DOI] [PubMed] [Google Scholar]
- Clayton Gina M., Wang Y, Crawford F, Novikov A, Wimberly Brian T., Kieft Jeffrey S., Falta Michael T., Bowerman Natalie A., Marrack P, Fontenot Andrew P., Dai S, Kappler John W. Structural Basis of Chronic Beryllium Disease: Linking Allergic Hypersensitivity and Autoimmunity. Cell. 2014;158:132–142. doi: 10.1016/j.cell.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DK, Edwards ES, Wynn KK, Clement M, Miles JJ, Ladell K, Ekeruche J, Gostick E, Adams KJ, Skowera A, Peakman M, Wooldridge L, Price DA, Sewell AK. Modification of MHC anchor residues generates heteroclitic peptides that alter TCR binding and T cell recognition. J Immunol. 2010;185:2600–10. doi: 10.4049/jimmunol.1000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers DM. Statistical thermodynamics of nucleic acid melting transitions with coupled binding equilibria. Biopolymers. 1971;10:2147–2160. doi: 10.1002/bip.360101110. [DOI] [PubMed] [Google Scholar]
- Duan F, Duitama J, Al Seesi S, Ayres CM, Corcelli SA, Pawashe AP, Blanchard T, McMahon D, Sidney J, Sette A, Baker BM, Mandoiu II, Srivastava PK. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. The Journal of Experimental Medicine. 2014;211:2231–2248. doi: 10.1084/jem.20141308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekeruche-Makinde J, Clement M, Cole DK, Edwards ESJ, Ladell K, Miles JJ, Matthews KK, Fuller A, Lloyd KA, Madura F, Dolton GM, Pentier J, Lissina A, Gostick E, Baxter TK, Baker BM, Rizkallah PJ, Price DA, Wooldridge L, Sewell AK. T-cell Receptor-optimized Peptide Skewing of the T-cell Repertoire Can Enhance Antigen Targeting. Journal of Biological Chemistry. 2012;287:37269–37281. doi: 10.1074/jbc.M112.386409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler S, Cole L, Keller S. Automated Circular Dichroism Spectroscopy for Medium-Throughput Analysis of Protein Conformation. Analytical Chemistry. 2012;85:1868–1872. doi: 10.1021/ac303244g. [DOI] [PubMed] [Google Scholar]
- Gakamsky DM, Bjorkman PJ, Pecht I. Peptide interaction with a class I major histocompatibility complex-encoded molecule: allosteric control of the ternary complex stability. Biochemistry. 1996;35:14841–8. doi: 10.1021/bi961707u. [DOI] [PubMed] [Google Scholar]
- Gakamsky DM, Boyd LF, Margulies DH, Davis DM, Strominger JL, Pecht I. An allosteric mechanism controls antigen presentation by the H-2K(b) complex. Biochemistry. 1999;38:12165–73. doi: 10.1021/bi9905821. [DOI] [PubMed] [Google Scholar]
- Gakamsky DM, Davis DM, Strominger JL, Pecht I. Assembly and dissociation of human leukocyte antigen (HLA)-A2 studied by real-time fluorescence resonance energy transfer. Biochemistry. 2000;39:11163–9. doi: 10.1021/bi000763z. [DOI] [PubMed] [Google Scholar]
- Garboczi DN, Hung DT, Wiley DC. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci U S A. 1992;89:3429–33. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras S, Wilmann PG, Chen Z, Halim H, Liu YC, Kjer-Nielsen L, Purcell AW, Burrows SR, McCluskey J, Rossjohn J. A Structural Basis for Varied αβ TCR Usage against an Immunodominant EBV Antigen Restricted to a HLA-B8 Molecule. The Journal of Immunology. 2012;188:311–321. doi: 10.4049/jimmunol.1102686. [DOI] [PubMed] [Google Scholar]
- Günther S, Schlundt A, Sticht J, Roske Y, Heinemann U, Wiesmüller K-H, Jung G, Falk K, Rötzschke O, Freund C. Bidirectional binding of invariant chain peptides to an MHC class II molecule. Proceedings of the National Academy of Sciences. 2010;107:22219–22224. doi: 10.1073/pnas.1014708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harndahl M, Justesen S, Lamberth K, Røder G, Nielsen M, Buus S. Peptide Binding to HLA Class I Molecules: Homogenous, High-Throughput Screening, and Affinity Assays. Journal of Biomolecular Screening. 2009;14:173–180. doi: 10.1177/1087057108329453. [DOI] [PubMed] [Google Scholar]
- Harndahl M, Rasmussen M, Roder G, Buus S. Real-time, high-throughput measurements of peptide–MHC-I dissociation using a scintillation proximity assay. Journal of Immunological Methods. 2011;374:5–12. doi: 10.1016/j.jim.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harndahl M, Rasmussen M, Roder G, Dalgaard Pedersen I, Sørensen M, Nielsen M, Buus S. Peptide-MHC class I stability is a better predictor than peptide affinity of CTL immunogenicity. European Journal of Immunology. 2012;42:1405–1416. doi: 10.1002/eji.201141774. [DOI] [PubMed] [Google Scholar]
- Hassan C, Chabrol E, Jahn L, Kester MGD, de Ru AH, Drijfhout JW, Rossjohn J, Falkenburg JHF, Heemskerk MHM, Gras S, van Veelen PA. Naturally Processed Non-canonical HLA-A*02:01 Presented Peptides. Journal of Biological Chemistry. 2015;290:2593–2603. doi: 10.1074/jbc.M114.607028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Molero B, Naganathan AN, Sanchez-Ruiz JM, Muñoz V. Chapter Twelve - Modern Analysis of Protein Folding by Differential Scanning Calorimetry. In: Andrew LF, editor. Methods in Enzymology. Vol. 567. Academic Press; 2016. pp. 281–318. [DOI] [PubMed] [Google Scholar]
- Khan AR, Baker BM, Ghosh P, Biddison WE, Wiley DC. The structure and stability of an HLA-A*0201/octameric tax peptide complex with an empty conserved peptide-N-terminal binding site. J Immunol. 2000;164:6398–405. doi: 10.4049/jimmunol.164.12.6398. [DOI] [PubMed] [Google Scholar]
- Koch CP, Perna AM, Pillong M, Todoroff NK, Wrede P, Folkers G, Hiss JA, Schneider G. Scrutinizing MHC-I Binding Peptides and Their Limits of Variation. PLoS Comput Biol. 2013a;9:e1003088. doi: 10.1371/journal.pcbi.1003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch CP, Perna AM, Weissmüller S, Bauer S, Pillong M, Baleeiro RB, Reutlinger M, Folkers G, Walden P, Wrede P, Hiss JA, Waibler Z, Schneider G. Exhaustive Proteome Mining for Functional MHC-I Ligands. ACS Chemical Biology. 2013b;8:1876–1881. doi: 10.1021/cb400252t. [DOI] [PubMed] [Google Scholar]
- Landais E, Romagnoli PA, Corper AL, Shires J, Altman JD, Wilson IA, Garcia KC, Teyton L. New Design of MHC Class II Tetramers to Accommodate Fundamental Principles of Antigen Presentation. The Journal of Immunology. 2009;183:7949–7957. doi: 10.4049/jimmunol.0902493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarski CA, Chaves FA, Jenks SA, Wu S, Richards KA, Weaver JM, Sant AJ. The Kinetic Stability of MHC Class II:Peptide Complexes Is a Key Parameter that Dictates Immunodominance. Immunity. 2005;23:29–40. doi: 10.1016/j.immuni.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Lundegaard C, Lund O, Nielsen M. Accurate approximation method for prediction of class I MHC affinities for peptides of length 8, 10 and 11 using prediction tools trained on 9mers. Bioinformatics. 2008;24:1397–1398. doi: 10.1093/bioinformatics/btn128. [DOI] [PubMed] [Google Scholar]
- Madura F, Rizkallah PJ, Holland CJ, Fuller A, Bulek A, Godkin AJ, Schauenburg AJ, Cole DK, Sewell AK. Structural basis for ineffective T-cell responses to MHC anchor residue-improved “heteroclitic” peptides. European Journal of Immunology. 2015;45:584–591. doi: 10.1002/eji.201445114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulis D, Kranz JK, Salemme FR, Todd MJ. Thermodynamic Stability of Carbonic Anhydrase: Measurements of Binding Affinity and Stoichiometry Using ThermoFluor. Biochemistry. 2005;44:5258–5266. doi: 10.1021/bi048135v. [DOI] [PubMed] [Google Scholar]
- Miles KM, Miles JJ, Madura F, Sewell AK, Cole DK. Real time detection of peptide–MHC dissociation reveals that improvement of primary MHC-binding residues can have a minimal, or no, effect on stability. Molecular Immunology. 2011;48:728–732. doi: 10.1016/j.molimm.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CS, Holton JM, Olafson BD, Bjorkman PJ, Mayo SL. Circular dichroism determination of class I MHC-peptide equilibrium dissociation constants. Protein Sci. 1997;6:1771–3. doi: 10.1002/pro.5560060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M, Lund O. NN-align. An artificial neural network-based alignment algorithm for MHC class II peptide binding prediction. BMC bioinformatics. 2009;10:296. doi: 10.1186/1471-2105-10-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protocols. 2007;2:2212–2221. doi: 10.1038/nprot.2007.321. [DOI] [PubMed] [Google Scholar]
- Pace CN. Measuring and increasing protein stability. Trends in Biotechnology. 1990;8:93–98. doi: 10.1016/0167-7799(90)90146-o. [DOI] [PubMed] [Google Scholar]
- Pantoliano MW, Petrella EC, Kwasnoski JD, Lobanov VS, Myslik J, Graf E, Carver T, Asel E, Springer BA, Lane P, Salemme FR. High-Density Miniaturized Thermal Shift Assays as a General Strategy for Drug Discovery. Journal of Biomolecular Screening. 2001;6:429–440. doi: 10.1177/108705710100600609. [DOI] [PubMed] [Google Scholar]
- Pierce BG, Hellman LM, Hossain M, Singh NK, Vander Kooi CW, Weng Z, Baker BM. Computational Design of the Affinity and Specificity of a Therapeutic T Cell Receptor. PLoS Comput Biol. 2014;10:e1003478. doi: 10.1371/journal.pcbi.1003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels HA, Moore JC, Jensen PE. A structural transition in class II major histocompatibility complex proteins at mildly acidic pH. The Journal of Experimental Medicine. 1996;183:127–136. doi: 10.1084/jem.183.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp B, Günther S, Makhmoor T, Schlundt A, Dickhaut K, Gupta S, Choudhary I, Wiesmüller K-H, Jung G, Freund C, Falk K, Rötzschke O, Kühne R. Characterization of Structural Features Controlling the Receptiveness of Empty Class II MHC Molecules. PLoS ONE. 2011;6:e18662. doi: 10.1371/journal.pone.0018662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert J, Sidney J, Celis E, Kubo RT, Grey HM, Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993;74:929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- Saini SK, Abualrous ET, Tigan A-S, Covella K, Wellbrock U, Springer S. Not all empty MHC class I molecules are molten globules: Tryptophan fluorescence reveals a two-step mechanism of thermal denaturation. Molecular Immunology. 2013;54:386–396. doi: 10.1016/j.molimm.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ruiz JM, Lopez-Lacomba JL, Cortijo M, Mateo PL. Differential scanning calorimetry of the irreversible thermal denaturation of thermolysin. Biochemistry. 1988;27:1648–1652. doi: 10.1021/bi00405a039. [DOI] [PubMed] [Google Scholar]
- Sato AK, Zarutskie JA, Rushe MM, Lomakin A, Natarajan SK, Sadegh-Nasseri S, Benedek GB, Stern LJ. Determinants of the Peptide-induced Conformational Change in the Human Class II Major Histocompatibility Complex Protein HLA-DR1. Journal of Biological Chemistry. 2000a;275:2165–2173. doi: 10.1074/jbc.275.3.2165. [DOI] [PubMed] [Google Scholar]
- Sato AK, Zarutskie JA, Rushe MM, Lomakin A, Natarajan SK, Sadegh-Nasseri S, Benedek GB, Stern LJ. Determinants of the peptide-induced conformational change in the human class II major histocompatibility complex protein HLA-DR1. J Biol Chem. 2000b;275:2165–73. doi: 10.1074/jbc.275.3.2165. [DOI] [PubMed] [Google Scholar]
- Schlundt A, Günther S, Sticht J, Wieczorek M, Roske Y, Heinemann U, Freund C. Peptide Linkage to the α-Subunit of MHCII Creates a Stably Inverted Antigen Presentation Complex. Journal of Molecular Biology. 2012;423:294–302. doi: 10.1016/j.jmb.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Schlundt A, Kilian W, Beyermann M, Sticht J, Günther S, Höpner S, Falk K, Roetzschke O, Mitschang L, Freund C. A Xenon-129 Biosensor for Monitoring MHC–Peptide Interactions. Angewandte Chemie International Edition. 2009;48:4142–4145. doi: 10.1002/anie.200806149. [DOI] [PubMed] [Google Scholar]
- Sloan VS, Cameron P, Porter G, Gammon M, Amaya M, Mellins E, Zaller DM. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- Yin L, Calvo-Calle JM, Dominguez-Amorocho O, Stern LJ. HLA-DM Constrains Epitope Selection in the Human CD4 T Cell Response to Vaccinia Virus by Favoring the Presentation of Peptides with Longer HLA-DM–Mediated Half-Lives. The Journal of Immunology. 2012;189:3983–3994. doi: 10.4049/jimmunol.1200626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Maben ZJ, Becerra A, Stern LJ. Evaluating the Role of HLA-DM in MHC Class II–Peptide Association Reactions. The Journal of Immunology. 2015;195:706–716. doi: 10.4049/jimmunol.1403190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Stern LJ. A novel method to measure HLA-DM-susceptibility of peptides bound to MHC class II molecules based on peptide binding competition assay and differential IC50 determination. Journal of Immunological Methods. 2014;406:21–33. doi: 10.1016/j.jim.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Trenh P, Guce A, Wieczorek M, Lange S, Sticht J, Jiang W, Bylsma M, Mellins ED, Freund C, Stern LJ. Susceptibility to HLA-DM is determined by a dynamic conformation of major histocompatibility complex class II molecule bound with peptide. Journal of Biological Chemistry. 2014 doi: 10.1074/jbc.M114.585539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarutskie JA, Sato AK, Rushe MM, Chan IC, Lomakin A, Benedek GB, Stern LJ. A Conformational Change in the Human Major Histocompatibility Complex Protein HLA-DR1 Induced by Peptide Binding†. Biochemistry. 1999;38:5878–5887. doi: 10.1021/bi983048m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.