Abstract
Adherence to treatment is one of the most consistent factors associated with a favorable addiction treatment outcome. Little is known about factors associated with treatment adherence in individuals affected with comorbid Attention-Deficit/Hyperactivity Disorder (ADHD) and Substance Use Disorders (SUD). This study aimed to explore whether treatment-associated factors, such as the prescribing physician’s (sub)specialty and methylphenidate (MPH) dose, or patient-related factors, such as sex, age, SUD subtype and psychiatric comorbidity, were associated with adherence to MPH treatment. Swedish national registers were used to identify adult individuals with prescriptions of MPH and medications specifically used in the treatment of SUD or a diagnosis of SUD and/or coexisting psychiatric diagnoses. Primary outcome measure was days in active MPH treatment in stratified dose groups (≤ 36mg, ≥37mg – ≤54mg, ≥55mg – ≤72mg, ≥73mg – ≤90mg, ≥91mg – ≤108mg and ≥109mg). Lower MPH doses (i.e. ≤36 mg day 100) were associated with treatment discontinuation between day 101 and 830 (HR≤36 mg 1.67; HR37-54mg 1.37; HR55-72mg 1.36; HR73-90mg 1.19; HR≥108mg 1.09). The results showed a linear trend (p<0.0001) towards decreased risk of treatment discontinuation along with increase of MPH doses. In conclusion this study shows that higher MPH doses were associated with long-term treatment adherence in individuals with ADHD and SUD.
Keywords: MPH dose treatment adherence in SUD
INTRODUCTION
Attention Deficit/Hyperactivity Disorder (ADHD) and Substance Use Disorders (SUD) frequently coexist (1) creating substantial functional impairments for the affected individuals and their families, while causing a great economic burden on society (2). Despite the well-established overlap, evidence of common genetic etiology (3) and signs of similar pathophysiological mechanisms (4), little is known about the effectiveness and safety of pharmacological treatment in individuals affected with both ADHD and SUD (5). Methylphenidate (MPH) is advocated as first line stimulant medication in adults with ADHD only (6, 7), but previous literature is inconclusive regarding its effectiveness in individuals with comorbid ADHD and SUD (5). Also, central stimulant medications are controlled substances that increase dopamine in parts of the brain involved in the development of addictive behaviors (8), and MPH can be recreationally used and abused (9). Just recently however, two randomized controlled trials (10, 11) showed that individuals with ADHD and SUD experience positive treatment effects in regard to both ADHD and SUD-related outcomes. Due to drug-induced changes in the brain and similarities in mechanism of action between stimulant medication and substances of abuse (12), individuals with a history of drug use might experience less effect of MPH doses recommended for individuals with ADHD only. In addition, these studies showed that high stimulant doses were well tolerated and that serious adverse events were rare (10, 11).
Adherence to treatment is one of the most consistent factors associated with a favorable addiction treatment outcome (13) and is sometimes used as a proxy for successful treatment outcome (e.g., reduction or cession of drug intake) (14, 15). Treatment discontinuation is a costly and medically significant problem (16) associated with worse treatment outcome and course of both somatic and psychiatric disorders (17–19). In light of a recent meta-analysis showing only limited effect on ADHD symptoms and no effect on SUD-related outcomes for stimulant treatment in individuals affected with dual conditions paired with inadequate guidance in current treatment guidelines (20–23), clinicians might be reluctant to use adequate MPH doses in the presence of comorbid SUD. The conception that MPH treatment is (a) ineffective, (b) harmful, and/or (c) could put susceptible individuals at risk for relapse or worsening of ongoing SUD, may result in the withholding of pharmacological treatment in these affected individuals.
Previous research is inconsistent regarding factors that might influence treatment adherence. Patient age, and in particular the time when individuals transition from child/adolescent to adult healthcare services, has consistently been a strong predictor for treatment discontinuation for both ADHD and SUD (13, 24–26). Consistent with studies investigating treatment discontinuation in individuals with ADHD only, a large meta-analysis exploring adherence to addiction treatment in individuals with SUD suggests that even though young age is an important demographic factor for treatment discontinuation, other patient-related factors might be of limited importance (13). As such, the authors suggested that future research should focus primarily on treatment-associated factors (13).
In the present nationwide population-based longitudinal cohort study, we chose to explore how patient-related factors (sex, age, SUD subtype, and psychiatric comorbidity) and treatment-associated factors such as the prescribing physician’s (sub)specialty (i.e. psychiatry, addiction medicine or other) or MPH dose are associated with adherence to pharmacological treatment. To this end we identified 4870 individuals with one or more dispensed prescriptions of MPH combined with either a dispensed prescription of a medication used uniquely in the treatment of SUD or a diagnosis of SUD through a nationwide register linkage.
METHODS
Study design and patients
We utilized data from a record linkage of four population-based registries in Sweden; personal identification numbers enabled accurate linkage. The Swedish Prescribed Drug register (PDR) includes information on drug identity using Anatomical Therapeutic Chemical (ATC) codes and dates of all prescribed drugs dispensed at pharmacies in Sweden since July 2005. Every prescription has a specific dose text describing quantity and dosage filled out by the prescribing physician. The National Patient Register (NPR) provides data on the main discharge diagnosis and secondary diagnoses of hospital admissions and outpatient care in Sweden, categorized using coding by the eighth, ninth and tenth revision of the International Classification of Diseases (ICD-8, ICD-9 and ICD-10). Psychiatric in-patient care is covered since 1973 (complete coverage since 1987) (ICD-8 to ICD-10) and outpatient care (ICD-10) since 2001. The Cause of Death Register (CDR) provides mandatorily reported information from death certificates, including dates and direct and contributory causes of death. The Migration Register (MR) includes information on dates of all registered migrations into or out of Sweden since 1969.
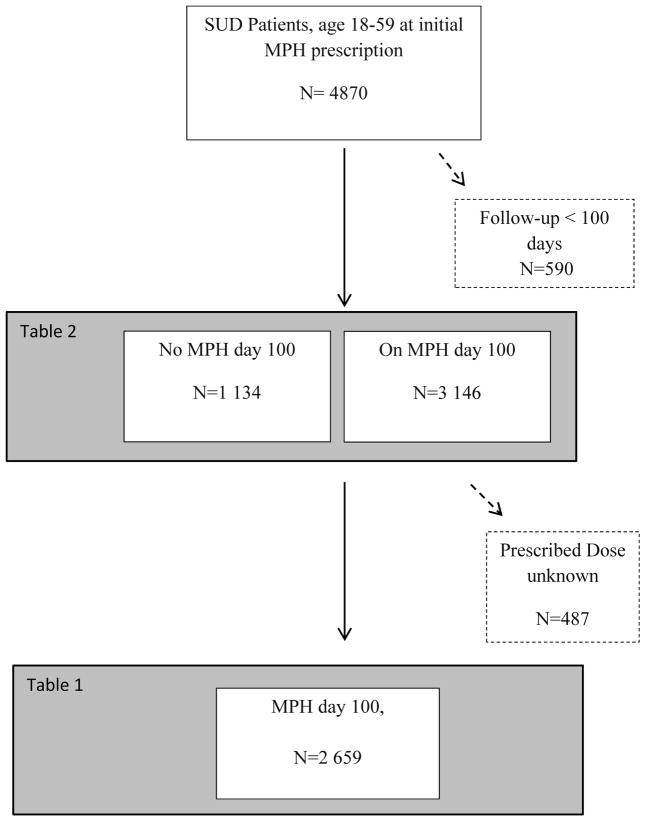
An eligible sample of 4870 adult individuals with SUD diagnosis prescribed MPH treatment between January 1, 2006, and December 31, 2009 was identified from the register linkages. After excluding individuals with inadequate follow-up time (n= 590), treatment discontinuation before day 100 (n=1134), and lack of dose information (n=487) 2659 individuals with a known prescribed MPH dose at day 100 were included in the main analyses. Out of the 4280 individuals with over 100 days follow up 66% had SUD diagnosis before start of PDR. Among the 1474 individuals with a SUD diagnosis recorded after July 2005, 55% were identified from the NPR only, 30% from both NPR and PDR and 15% from PDR only.
The study was approved by the research ethics committee at Karolinska Institutet, Stockholm, Sweden. Protocol Nr 2009/5:10, 2009/939-31/5.
Diagnostic categories
We extracted data about 4870 individuals aged 18–59 years at the first prescription, with one or more dispensed prescriptions of MPH between January 1 2006 and December 31 2009 from the PDR according to the ATC classification system (ATC-code for MPH N06BA04) (Figure 1).
Figure 1.
Flow Chart
Flowchart of the eligible sample of 4870 adult individuals with SUD diagnosis prescribed MPH treatment between January 1, 2006, and December 31, 2009 identified from the register linkages. Individuals with inadequate follow-up time (n= 590), treatment discontinuation before day 100 (n=1134), and lack of dose information (n=487) was excluded. The main analyses included 2659 individuals with a known prescribed MPH dose at day 100.
We used the NPR and the PDR to identify patients diagnosed with alcohol and/or drug use disorders in accordance with the International Classification of Diseases (ICD) eighth, ninth and tenth revision diagnostic guidelines and/or a prescription with an ATC-code for drugs used in the treatment of SUD. Alcohol use disorder was defined using ICD-codes from the NPR (ICD-8: 291 and 303, ICD-9: 291, 303 and 305A and ICD-10: F10.0-F10.9). The alcohol use disorder index from the PDR was based on ATC-codes for prescriptions of drugs used in the treatment of alcoholism (N07BB03 (acamprosate), N07BB04 (naltrexone) and N07BB01 (disulfiram)). Psychoactive drug abuse was measured by ICD-codes from the NPR (ICD-8: 304, ICD-9: 292, 304 and 305X and ICD-10: F11.0-F16.9 and F18.0-F19.9) and ATC-codes from the PDR for prescriptions of drugs used in the treatment of drug abuse (N02AE01 (buprenorphine), N07BC51 (buprenorphine+naltrexone) and N07BC02 (methadone)). After adjustment for overlaps between the two registers 2659 unique individuals with SUD diagnosis and a known prescribed MPH dose were identified.
Patient related factors
Based on previous research (25) measured baseline characteristics included sex, age (18–26, 27–39 and 40–59 years of age), SUD subtype according ICD-8, ICD-9 and ICD-10, and comorbid psychiatric disorders (i.e. schizophrenia (ICD-8: 295.0-295.4, 295.6, 295.8-295.9; ICD-9: 295A-295E, 295G, 295W, 295X; ICD-10: F20), mood disorders (including bipolar disorders (ICD-8: 296.1, 296.3, 296.8; ICD-9: 296A/C/D/E/W; ICD-10: F30-F31)) and affective disorders (ICD8: 296.2, 298.0, 300.4; ICD9: 296B, 300E; ICD10: F32-F39)), anxiety disorders (ICD8, ICD9: 300; ICD10:F40-48)), eating disorders (ICD8: 306.5; ICD9: 307B/F; ICD10: F50), personality disorders (ICD8, ICD9: 301; ICD10: F60 and conduct disorder (ICD9: 312; ICD-10: F91) were identified from the NPR. The rational behind stratifying SUD into different subtypes (alcohol, stimulant or combined) relies on the assumption that drugs of abuse, while having a common action of increasing dopamine transmission in cortical and subcortical areas of the brain also have different molecular targets. Based on neuroimaging research and due to pharmacokinetic similarities between illicit stimulant substances and central stimulant medication such as MPH (27, 28), we hypothesize that individuals with stimulant use disorder might be different from other SUD subtypes. In addition, individuals with combination of several drugs of abuse might represent a more severe SUD subtype, since previous research show that polysubstance abuse is associated with increased risk for adverse outcomes including SUD severity (29, 30).
Treatment related factors
According to The Swedish Medical Products Agency (MPA), license to prescribe MPH is restricted to specialists in psychiatry and specialists in child- and adolescent neurology (31). Addiction medicine is a (sub)specialty of general adult psychiatry, and should according to the National Board of Health and Welfare provide treatment interventions targeting both the SUD and the coexisting psychiatric comorbidity simultaneously (32), whereas general psychiatric clinics might focus primarily and more specifically on the aspects related to the psychiatric disorders. Subsequently, we assume that individuals with a first prescription from a specialist in addiction medicine might be different from those receiving their prescriptions from a specialist in general psychiatry. The information of the prescribing physicians (sub)specialty can be retrieved from the PDR.
The individual daily MPH dose at day 100 was estimated through the text variable in the PDR. According to Swedish treatment guidelines for initiation of ADHD medication, individualized titration regimens should be applied, and ADHD symptom relief and adverse effects are monitored, guiding the clinician to arrive at an effective and well-tolerated MPH dose (33). This process can continue for several weeks and we used day 100 as start of follow-up to avoid misclassification of doses due to inadequate register quality during the titration phase and misclassification of treatment adherence due to the fact that one single prescription filling can cover medication for a treatment period of up to 90 days. A prescription in the PDR contains an unstructured text variable containing both amount of medication prescribed and other more individualized instructions including how the prescribed drug is to be used and consumed. By making use of a semi-manual method for extracting prescribed daily doses, the text added by the prescribing physician was removed, leaving only the part of the text containing the prescribed dose. From this text the individual doses were manually retrieved. The semi-manual method has previously been validated by an independent clinician who proofread randomly selected records in the material. A total of 0.3% of the prescriptions were not coded correctly or were interpreted differently by the proofreader.
We stratified MPH doses into six groups (the percentages of the entire sample are given within brackets). MPH doses of ≤ 36mg (30%), ≥37mg – ≤54mg (25%), ≥55mg – ≤72mg (16%), ≥73mg – ≤90mg (11%), ≥91mg – ≤108mg (8%) and ≥109mg (11%). Since the initial titration phase consequently has shown the imposition of an increased risk of treatment discontinuation (19, 25, 34) and to ensure that individuals who discontinued treatment after only one prescription were not included in the analyses, we set start of follow up at day 100. Individuals were considered to be in active treatment if a prescription was refilled within the number of days that the prescription would last according to the text variable on the prescription (plus an additional 25% of this time sequence to account for irregularities in dispensing patterns). During subsequent periods or those without any new prescription, the patient was assumed to be off treatment.
If prescriptions of different dosage were filled on the same occasion, we assumed them to have been consumed simultaneously according to the text variable on each prescription. To ensure that participants had not been receiving MPH treatment prior to follow-up, we used information about prescription dates six months before follow-up. Since the Prescribed Drug Register covered July 1, 2005 – December 31, 2009, start of follow-up was set at January 1, 2006.
Statistical analyses
We used Cox regression models to estimate time to first treatment discontinuation in relation to six categories of MPH doses. The model was adjusted for all individual baseline characteristics listed in tables 1 and 2. Since the crude and adjusted models were almost identical we chose to only present the adjusted results.
Table 1.
Hazard ratios for MPH treatment discontinuation day 101 to 830
Proportional hazard regression for MPH treatment discontinuation day 101 to 830 across different demographic variables and MPH dose groups.
| N Day 100 |
HR Treatment Discontinuationc |
|
|---|---|---|
| Prescribed dose day 100 | ||
| ≤36 mg | 789 | 1.67 (1.29–2.15) |
| 37–54 mg | 653 | 1.37 (1.06–1.48) |
| 55–72 mg | 447 | 1.36 (1.04–1.78) |
| 73–90 mg | 280 | 1.19 (0.88–1.59) |
| 90–108 mg | 202 | ref=1 |
| ≥108 mg | 288 | 1.09 (0.81–1.48) |
| Sex | ||
| Male | 1654 | 1.12 (0.99–1.27) |
| Female | 1005 | ref=1 |
| Age | ||
| 18–26 | 734 | 1.33 (1.15–1.55) |
| 27–39 | 1046 | 0.97 (0.84–1.11) |
| 40–59 | 879 | ref=1 |
| Prescribing Physician’s Specialty | ||
| Addiction | 433 | 1.25 (1.03–1.51) |
| Psychiatry | 2002 | ref=1 |
| Other | 224 | 1.07 (0.91–1.26) |
| Psychiatric Diagnosis from 1997 | ||
| Non of following | 777 | 1.06 (0.80–1.39) |
| Schizophrenia | 72 | 1.01 (0.72–1.43) |
| Mood or Anxiety disorders | 1716 | 0.89 (0.70–1.14) |
| Eating disorders | 108 | 1.24 (0.91–1.67) |
| Personality disorders/Conduct disorder | 742 | 1.02 (0.87–1.18) |
| SUD Diagnosis | ||
| ATC-code SUD medication | 256 | 1.01 (0.80–1.29) |
| Alcohol Use Disorder | 527 | ref=1 |
| Stimulant Use Disordera | 862 | 1.26 (1.06–1.49) |
| Combinedb | 1014 | 1.01 (0.85–1.19) |
Proportional hazard regression
F14 Amphetamine Use Disorder and F15 Cocaine Use Disorder
Substance Use Disorder including F11 OpiatesF12 Cannabis, F13 Hypnotics and Sedatives, F16 Hallucinogens, F18 Inhalants, F19 Several drugs in combination.
Adjusted for all variables listed in the table
Table 2.
Odds ratios for early treatment discontinuation (before day 100 after the first dispensed MPH prescription).
Differences in patient-related factors between individuals with and without on going MPH treatment day 100.
| Total (N=4280) % |
Discontinuation < 100 (N=1134) % |
ORc (95% CI) | |
|---|---|---|---|
| Male | 64.4 | 67.3 | 1.19 (1.03–1.38) |
| Female | 35.6 | 32.7 | ref=1 |
| Age | |||
| 18–26 | 27.0 | 29.8 | 1.20 (1.01–1.42) |
| 27–39 | 38.6 | 36.9 | 0.98 (0.84–1.16) |
| 40–59 | 34.3 | 33.2 | ref=1 |
| Comorbid Psychiatric Disorder | |||
| None of following | 29.9 | 31.2 | 1.09 (0.94–1.26) |
| Schizophrenia | 3.2 | 3.5 | 1.13 (0.77–1.64) |
| Mood or anxiety disorders | 63.3 | 62.0 | 0.92 (0.80–1.06) |
| Eating disorders | 3.5 | 2.5 | 0.63 (0.42–0.96) |
| Personality disorders/Conduct disorder | 28.5 | 28.9 | 1.03 (0.89–1.20) |
| Prescribing Physician’s Specialty | |||
| Addiction | 19.3 | 20.5 | 1.28 (1.01–1.62) |
| Psychiatry | 72.0 | 69.5 | ref=1 |
| Other | 8.7 | 10.1 | 1.14 (0.96–1.35) |
| SUD Diagnosis | |||
| ATC-code SUD medication | 9.0 | 8.4 | 0.85 (0.64–1.12) |
| Alcohol Use Disorder | 56.1 | 59.1 | ref=1 |
| Stimulant Use Disordera | 17.9 | 18.7 | 0.98 (0.81–1.18) |
| Combinedb | 15.0 | 16.1 | 0.88 (0.73–1.07) |
F14 Amphetamine Use Disorder and F15 Cocaine Use Disorder
Substance Use Disorder including F11 OpiatesF12 Cannabis, F13 Hypnotics and Sedatives, F16 Hallucinogens, F18 Inhalants, F19 Several drugs in combination.
ORs adjusted for all covariates listed in the table
Sensitivity analyses
We performed a series of sensitivity analyses to test the robustness of our results. Firstly, logistic regression models were used to explore whether baseline characteristics such as sex, age, SUD subtype, comorbid psychiatric disorders, and the prescribing physician’s (sub)specialty (addiction medicine, general psychiatry or other) differed between the subsample of individuals who discontinued MPH treatment before the initial titration phase of 100 days was completed (early discontinuation) (n=1134), and individuals with ongoing MPH treatment day 100 (n=3146). Secondly, we stratified the sample on baseline characteristics to explore a possible modifying effect of these background factors on the association between MPH dose and treatment discontinuation. Thirdly, we controlled for the time dependent incline in prescribed MPH doses as a result of societal acceptance of, or clinicians becoming more experienced in pharmacological ADHD treatment in adults by analyzing separate subsamples of individuals prescribed MPH treatment year 2006–2007 and 2008–2009. Fourthly, to ensure that potential previous MPH treatment did not influence doses in individuals included in the study, we also calculated doses and treatment adherence for a sample followed from 2008 and onwards. Finally, since start of follow up was set at day 100 from the first prescription, and given that the risks of relapse into alcohol or drug use might be more pronounced during the first time of abstinence, we explored whether the dose at day 200 was an equally strong predictor of treatment adherence as dose at day 100.
RESULTS
The adjusted proportional hazard ratios for MPH treatment discontinuation day 101 to 830 are shown in Table 1. Hazard ratios for treatment discontinuation decrease along with increasing MPH dose up until doses exceeding 72 mg (HR≤36 mg 1.67 (1.29–2.15); HR37-54mg 1.37 (1.06–1.48)); HR55-72mg 1.36 (1.04–1.78); HR73-90mg 1.19 (0.88–1.59); HR≥108mg 1.09 (0.81–1.48). The results for doses exceeding 72 mg were non-significant compared to the reference selected but remained significant compared to other dose categories. A significant trend (linear trend, p<0.0001) towards decreased risk of treatment discontinuation along with increase MPH doses is shown across all dose categories. However, the point estimates for doses over 108 mg exceed the estimates for the reference category (90–108mg), possibly due to low sample size. Consistent with early discontinuation patterns explored in the sensitivity analyses below, an increased risk of treatment discontinuation day 101 to 830 was seen in individuals with a prescription of MPH from a physician specialized in addiction medicine (HRAddict 1.25 (1.03–1.51)).
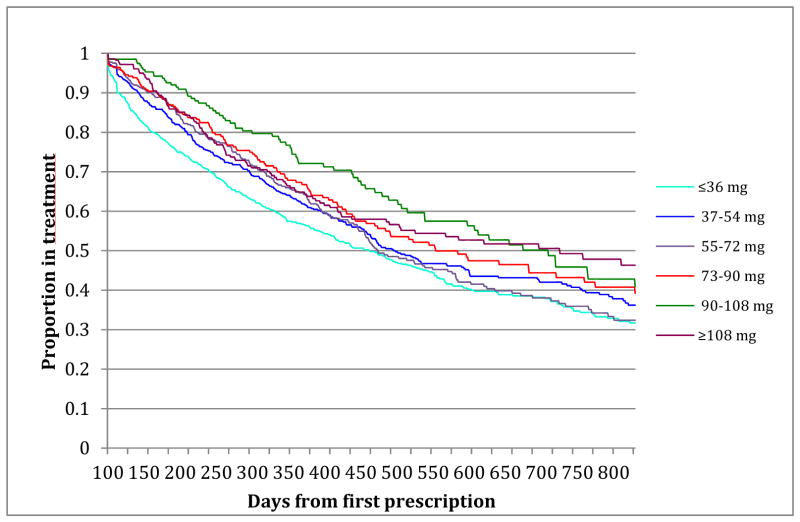
The only demographic patient related factor that influenced risk for treatment discontinuation was young age (i.e. age 18–25) (HR<26 1.33 (1.15–1.55). Neither a diagnosis of alcohol use disorder alone nor individuals with a SUD diagnosis of several drugs in combination were associated with an increased risk for treatment discontinuation, whereas individuals with a diagnosis of stimulant use disorder were at an 25% increased risk for treatment discontinuation (HRSU 1.26 (1.06–1.49)). Figure 2 shows a Kaplan Meier survival graph depicting crude treatment discontinuation rates among individuals with different MPH doses.
Figure 2.
Crude MPH discontinuation rates day 101–830
A Kaplan Meier survival graph depicting crude treatment discontinuation rates among individuals with different MPH doses.
Sensitivity analyses
Differences in patient and treatment-related factors between individuals with and without ongoing MPH treatment day 100 are shown in Table 2. Males, young individuals and patients receiving their prescriptions from a physician specialized in addiction medicine were at increased risk of early treatment discontinuation (i.e. before day 100) (ORMale 1.19 95% CI 1.03–1.38; OR<26 1.20 95 % CI 1.01–1.42; ORAddict 1.28 95% CI 1.01–1.62). However, coexisting psychiatric comorbidity or SUD diagnosis did not significantly influence the risk of early treatment discontinuation. Furthermore, the sensitivity analyses show that the dose dependent pattern for treatment discontinuation remains within subsamples of only women and different SUD subtypes. No time dependent incline in treatment discontinuation among individuals prescribed MPH treatment year 2006–2007 and 2008–2009 was found (test for linear trend in adjusted model p<0.0001, for both groups) and treatment discontinuation rates were similar among individuals who filled their first prescription of MPH 2008 or later compared to individuals prescribed MPH from 2006 and onwards. Finally, Table 3 shows that individual MPH dose day 200 was equally associated with treatment discontinuation as dose at day 100. (Test for linear trend in crude model p=0.02 and in adjusted model p=0.04).
Table 3.
Hazard ratios for treatment discontinuation day 201 to 830.
Individual MPH dose as predictor of treatment discontinuation day 200 compared to day 100. (Test for linear trend in crude model p=0.02 and in adjusted model p=0.04).
| Prescribed dose day 200 | N Day 200 |
HR Discontinuation Day 201 to 830a |
|---|---|---|
| ≤36 mg | 297 | 1.29 (0.85–1.98) |
| 37–54 mg | 282 | 1.22 (0.79–1.88) |
| 55–72 mg | 228 | 1.43 (0.92–2.22) |
| 73–90 mg | 149 | 1.02 (0.63–1.64) |
| 90–108 mg | 100 | ref=1 |
| ≥108 mg | 171 | 0.83 (0.51–1.36) |
Proportional hazard regression.
Test for linear trend in crude model p=0.02 and in adjusted model p=0.04
Adjusted for sex, age, comorbid psychiatric disorder, prescribing physician’s (sub)specialty, SUD diagnosis
DISCUSSION
The main finding in this register-based nation-wide cohort study is that MPH doses were associated with increased retention to treatment in individuals with ADHD and SUD. These findings are consistent with both clinical observations and recent randomized controlled trials showing significant improvements in ADHD symptoms and SUD outcomes using higher stimulant doses than previous studies (10, 11). The Kaplan Meier estimates for retention in treatment at day 800 were 47.8% (95%CI 39.8–55.5) for individuals with doses over 108 mg/day compared to 32.9% (95% CI 27.3–36.2) for those with doses less than 36 mg/day indicating a difference in treatment retention proportion of approximately 15%. One interpretation of our findings is that individuals with comorbid SUD have developed tolerance to stimulant compounds calling for higher MPH doses to achieve optimal ADHD symptom control. In accordance with such a hypothesis (35), an important question is whether MPH primarily targets ADHD symptoms, modulates the pathophysiology of SUD directly, or both. While the efficacy of agonist maintenance therapy available for opioid addiction has been extensively studied (36–38), substitution treatment for central stimulant dependence has been less investigated (35, 39–41). Research targeting the direct effect of stimulant medication on SUD pathophysiology is as yet inconclusive (35, 39–41). Adequate treatment, and more specifically adequate dosage, may increase motivation to stay in treatment and prevent relapse to illegal drug use.
Individuals with a diagnosis of stimulant use disorders, when analyzed separately and in contrast to individuals with both alcohol use disorder and polysubstance abuse, showed an increased risk of treatment discontinuation. One explanation to as why individuals with a diagnosis of amphetamine and/or cocaine use disorder distinguish themselves from other SUD subtypes might be associated with the often unstable living conditions among active amphetamine users (42). Socializing with substance using peers, low rates of permanent employment and high prevalence of criminal activities are all factors known to negatively impact treatment outcome and adherence (43). Also, imaging studies show that individuals with stimulant use disorder present a particularly week dopamine responses to MPH exposure (27, 28). This might, due to similarities in pharmacokinetic properties of illicit stimulants of abuse and MPH, lead to a more severe form of tolerance to stimulant compounds.
The sensitivity analyses showed that both patient-related factors such as sex and age, and treatment related factors such as the prescribing physicians (sub)specialty, had modifying effect on treatment adherence, mainly by increasing the risk of early treatment discontinuation. However, despite the fact that prescription of ADHD medication constantly increased during 2006–2009 (25) and the possibility that clinicians as a result could have become more experienced in prescribing central stimulant treatment, time trends did not seem to modify the dose effect on retention to pharmacological treatment. Due to the nature of the disorder, many individuals with SUD tend to drop out of treatment or relapse into active drug use early in treatment (13). The present study design allowed for an initial titration period and the dose used in the statistical analyses was measured at an arbitrarily chosen point estimate day 100. In addition a sensitivity analyses performed to assess the validity of the selected measurement time point showed that MPH dose at day100 and 200 were equally associated with treatment adherence.
The present results should be viewed in the light of some limitations. Firstly, the obvious disadvantages and limitations of observational research in general and more specifically of using rates of prescription fillings as a proxy for treatment adherence is that a prescription refill might not be equivalent to ingestion of medication since stimulant medications can be abused and diverted (44). In addition, results from register based studies must always be interpreted taking restrictions of internal validity due to the possibility of heterogeneity within groups of register based diagnoses into consideration. Secondly, most current treatment guidelines (20, 22, 23) recommend abstinence from abused substances prior to and during stimulant treatment. In the effort to prevent abuse and diversion of stimulant medication, MPH, a schedule II classed medication, is controlled (45) and individuals with higher MPH doses, might as a result be more closely monitored (46) and receive different or more intense psychosocial interventions that in turn will influence retention to treatment. Thirdly, rates of prescription fillings are only one of many possible measures of adherence to treatment (18). However, large register-based studies seldom provide detailed information on clinical remission or specified treatment outcome measures. All-cause treatment discontinuation rate can be a helpful study endpoint and one advantage with a register-based approach is that a closed pharmacy system and the unique Swedish Prescribed Drug register makes it possible to follow every single dispensed prescription without the risk of introducing recall bias or bias through different informants. Fourthly, to specifically address stimulant treatment initiated in adulthood, avoid bias from MPH dose adjustments for age and weight in growing individuals and since SUD is rare in childhood we restricted our sample to individuals over 18. However, excluding individuals in late adolescence that might be at particularly high risk for both premature treatment discontinuation and SUD will limit the generalizability of our results. Fifthly, the Prescribed Drug Register records prescriptions from July 2005 and onwards. Thus, we were not able to exclude the possibility that some individuals might have received MPH treatment in childhood. We however, made attempts to avoid misclassification of individuals with ongoing stimulant medication by defining start of follow up 6 months after the individuals entered the register. Finally, the data behind this study does not allow us to control for individual differences in ADHD severity. MPH dosage and prescription rates might not be randomly distributed and could be associated with the level of ADHD symptoms displayed. Future studies should consider other factors, including ADHD symptom severity that potentially can influence dosage, to better understand treatment discontinuation. Individuals with ADHD and SUD are, due to limited knowledge regarding safety and efficacy of stimulant treatment for this comorbid condition, exposed to a variety of misunderstandings and inconsistencies regarding pharmacological treatment of their disorders. Lingering societal and clinical concerns about the safety of stimulant treatment in individuals with coexisting SUD (34–36) and conflicting treatment guidelines (20, 22, 23, 37) give physicians little guidance in how to safely use stimulant medication in the clinically challenging and vulnerable group of patients affected with both ADHD and SUD. More specifically, given inconsistencies surrounding the dose dependent efficacy of stimulant treatment (6, 7) and individual characteristics on adherence to both ADHD (19, 34, 47) and SUD treatment (13), further exploration of variables associated with treatment adherence should be an important focus for future research. A clearer understanding of the impact of individual MPH doses in this population may provide an important contribution in identifying individual risk factors for poor adherence to treatment as well as in planning treatment for individuals with these dual diagnoses and increase acceptance for adequate dosing in individuals affected with SUD.
Acknowledgments
Role of the funding source: The funders of the study had no role in the design and conduct of the study, data gathering, management, analysis, and interpretation; or in the preparation, review, or approval of the report. CS had full access to all the data in the study and, together with LB, takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support was provided by the Regional Agreement on Medical Training and Clinical Research (K1426-2011; 20120334) between the Stockholm County Council and Karolinska Institutet. We acknowledge financial support from the Swedish Research Council (2013-2280; 2012-2607), the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework (Grant no. 340-2013-5867), and the National Institute of Mental Health (NIMH) (Grant no. 1R01MH102221). HL has served as a speaker for Eli-Lilly and has received a research grant from Shire; both outside the submitted work. The lead author takes responsibility for the integrity of the data and the accuracy of the data analysis
Footnotes
Contributors: CS, LB and HL conceived and designed the study. LB and CS did the analyses. CA, MK and JF assisted with the study design. CS did the search and drafted the research in context panel. CS drafted the report, which was revised by all co-authors.
Declaration of interests: We declare that we have no competing interests.
There were no financial or other conflicts of interest for the other authors.
Declaration of interests: Financial support was provided by the Regional Agreement on Medical Training and Clinical Research (K1426-2011; 20120334) between the Stockholm County Council and the Karolinska Institutet. We acknowledge financial support from the Swedish Research Council (2013-2280; 2012-2607), the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework (Grant no. 340-2013-5867), and the National Institute of Mental Health (NIMH) (Grant no. 1R01MH102221). H Larsson has served as a speaker for Eli-Lilly and has received a research grant from Shire; both outside the submitted work. There were no financial or other conflicts of interest for the other authors. The lead author takes responsibility for the integrity of the data and the accuracy of the data analysis and declares that we have no competing interests.
References
- 1.Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–23. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias AJ, Gelernter J, Chan G, et al. Correlates of co-occurring ADHD in drug-dependent subjects: prevalence and features of substance dependence and psychiatric disorders. Addict Behav. 2008;33(9):1199–207. doi: 10.1016/j.addbeh.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skoglund C, Chen Q, Franck J, et al. Attention-deficit/hyperactivity disorder and risk for substance use disorders in relatives. Biol Psychiatry. 2015;77:880–886. doi: 10.1016/j.biopsych.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Frodl T. Comorbidity of ADHD and Substance Use Disorder (SUD): a neuroimaging perspective. J Atten Disord. 2010;14(2):109–20. doi: 10.1177/1087054710365054. [DOI] [PubMed] [Google Scholar]
- 5.Cunill R, Castells X, Tobias A, et al. Pharmacological treatment of attention deficit hyperactivity disorder with co-morbid drug dependence. J Psychopharmacol. 2015;29(1):15–23. doi: 10.1177/0269881114544777. [DOI] [PubMed] [Google Scholar]
- 6.Koesters M, Becker T, Kilian R, et al. Limits of meta-analysis: methylphenidate in the treatment of adult attention-deficit hyperactivity disorder. J Psychopharmacol. 2009;23(7):733–44. doi: 10.1177/0269881108092338. [DOI] [PubMed] [Google Scholar]
- 7.Faraone SV, Spencer T, Aleardi M, et al. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2004;24(1):24–9. doi: 10.1097/01.jcp.0000108984.11879.95. [DOI] [PubMed] [Google Scholar]
- 8.Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21(2):RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- 10.Konstenius M, Jayaram-Lindstrom N, Guterstam J, et al. Methylphenidate for attention deficit hyperactivity disorder and drug relapse in criminal offenders with substance dependence: a 24-week randomized placebo-controlled trial. Addiction. 2014;109(3):440–9. doi: 10.1111/add.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin FR, Mariani JJ, Specker S, et al. Extended-Release Mixed Amphetamine Salts vs Placebo for Comorbid Adult Attention-Deficit/Hyperactivity Disorder and Cocaine Use Disorder: A Randomized Clinical Trial. JAMA psychiatry. 2015;72(6):593–602. doi: 10.1001/jamapsychiatry.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volkow ND, Wang GJ, Fowler JS, et al. Methylphenidate and cocaine have a similar in vivo potency to block dopamine transporters in the human brain. Life Sci. 1999;65(1):PL7–12. doi: 10.1016/s0024-3205(99)00225-8. [DOI] [PubMed] [Google Scholar]
- 13.Brorson HH, Ajo Arnevik E, Rand-Hendriksen K, et al. Drop-out from addiction treatment: a systematic review of risk factors. Clin Psychol Rev. 2013;33(8):1010–24. doi: 10.1016/j.cpr.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Simpson DD. The relation of time spent in drug abuse treatment to posttreatment outcome. Am J Psychiatry. 1979;136(11):1449–53. doi: 10.1176/ajp.136.11.1449. [DOI] [PubMed] [Google Scholar]
- 15.Simpson DD, Joe GW, Rowan-Szal GA. Drug abuse treatment retention and process effects on follow-up outcomes. Drug Alcohol Depend. 1997;47(3):227–35. doi: 10.1016/s0376-8716(97)00099-9. [DOI] [PubMed] [Google Scholar]
- 16.Elliott RA, Barber N, Horne R. Cost-effectiveness of adherence-enhancing interventions: a quality assessment of the evidence. Ann Pharmacother. 2005;39(3):508–15. doi: 10.1345/aph.1E398. [DOI] [PubMed] [Google Scholar]
- 17.Blaschke TF, Osterberg L, Vrijens B, et al. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 18.Osterberg L, Blaschke T. Adherence to medication. The New England journal of medicine. 2005;353(5):487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 19.Kooij JJ, Rosler M, Philipsen A, et al. Predictors and impact of non-adherence in adults with attention-deficit/hyperactivity disorder receiving OROS methylphenidate: results from a randomized, placebo-controlled trial. BMC psychiatry. 2013;13:36. doi: 10.1186/1471-244X-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolea-Alamanac B, Nutt DJ, Adamou M, Asherson P, Bazire S, Coghill D, et al. Evidence-based guidelines for the pharmacological management of attention deficit hyperactivity disorder: update on recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(3):179–203. doi: 10.1177/0269881113519509. [DOI] [PubMed] [Google Scholar]
- 21.Seixas M, Weiss M, Muller U. Systematic review of national and international guidelines on attention-deficit hyperactivity disorder. J Psychopharmacol. 2012;26(6):753–65. doi: 10.1177/0269881111412095. [DOI] [PubMed] [Google Scholar]
- 22.The Canadian ADHD Resource Alliance (CADDRA) [Accessed 2016-02-18];Practice Guidelines for ADHD. http://www.caddra.ca/practice-guidelines/download.
- 23.National Institute of Health and Care Excellence (NICE) [Accessed 2016-02-18];Attention deficit hyperactivity disorder: Diagnosis and management of ADHD in children, young people and adults. http://www.nice.org.uk/guidance/cg72.
- 24.McCarthy S, Asherson P, Coghill D, et al. Attention-deficit hyperactivity disorder: treatment discontinuation in adolescents and young adults. Br J Psychiatry. 2009;194(3):273–7. doi: 10.1192/bjp.bp.107.045245. [DOI] [PubMed] [Google Scholar]
- 25.Zetterqvist J, Asherson P, Halldner L, et al. Stimulant and non-stimulant attention deficit/hyperactivity disorder drug use: total population study of trends and discontinuation patterns 2006–2009. Acta Psychiatr Scand. 2013;128(1):70–7. doi: 10.1111/acps.12004. [DOI] [PubMed] [Google Scholar]
- 26.Wong IC, Asherson P, Bilbow A, et al. Cessation of attention deficit hyperactivity disorder drugs in the young (CADDY)--a pharmacoepidemiological and qualitative study. Health Technol Assess. 2009;13(50):iii–iv. ix–xi, 1–120. doi: 10.3310/hta13490. [DOI] [PubMed] [Google Scholar]
- 27.Wang GJ, Smith L, Volkow ND, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2012;17(9):918–25. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volkow ND, Wang GJ, Fowler JS, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–3. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 29.Lynskey MT, Agrawal A, Bucholz KK, et al. Subtypes of illicit drug users: a latent class analysis of data from an Australian twin sample. Twin Res Hum Genet. 2006;9(4):523–30. doi: 10.1375/183242706778024964. [DOI] [PubMed] [Google Scholar]
- 30.Kinner SA, Degenhardt L, Coffey C, et al. Substance use and risk of death in young offenders: a prospective data linkage study. Drug Alcohol Rev. 2015;34(1):46–50. doi: 10.1111/dar.12179. [DOI] [PubMed] [Google Scholar]
- 31.Swedish Medical Producs Agency (MPA) [Accessed 2016-02-18]; https://lakemedelsverket.se/english/overview/Legislation/The Swedish national authrity for regulation and surveillance of development, manufacturing and marketing of drugs and medicinal products.
- 32.Swedish National Board of Health and Welfare. [Accessed 2016-02-18]; http://www.socialstyrelsen.se/nationalguidelines http://www.socialstyrelsen.se/nationellariktlinjermissbrukochberoende(Swedish national guidelines for psychological and pharmacological treatment of substance use disorders. Only available in Swedish)
- 33.Swedish National Board of Health and Welfare. [Accessed 2016-02-18]; http://www.socialstyrelsen.se/nationalguidelines http://www.socialstyrelsen.se/riktlinjer/beslutsstodforbehandling/lakemedelsbehandlingavadhdSwedish national guidelines for psychological and pharmacological treatment of ADHD. Only available in Swedish.
- 34.Sobanski E, Retz W, Fischer R, et al. Treatment adherence and persistence in adult ADHD: results from a twenty-four week controlled clinical trial with extended release methylphenidate. Eur Psychiatry. 2014;29(5):324–30. doi: 10.1016/j.eurpsy.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Mariani JJ, Khantzian EJ, Levin FR. The self-medication hypothesis and psychostimulant treatment of cocaine dependence: an update. Am J Addict. 2014;23(2):189–93. doi: 10.1111/j.1521-0391.2013.12086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;6(2):CD002207. doi: 10.1002/14651858.CD002207.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Dennis BB, Naji L, Bawor M, et al. The effectiveness of opioid substitution treatments for patients with opioid dependence: a systematic review and multiple treatment comparison protocol. Syst Rev. 2014;19(3):105. doi: 10.1186/2046-4053-3-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakko J, Svanborg KD, Kreek MJ, et al. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361(9358):662–8. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- 39.Tiihonen J, Kuoppasalmi K, Fohr J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164(1):160–2. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- 40.Galloway GP, Buscemi R, Coyle JR, et al. A randomized, placebo-controlled trial of sustained-release dextroamphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89(2):276–82. doi: 10.1038/clpt.2010.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miles SW, Sheridan J, Russell B, et al. Extended-release methylphenidate for treatment of amphetamine/methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108(7):1279–86. doi: 10.1111/add.12109. [DOI] [PubMed] [Google Scholar]
- 42.Hakansson A, Schlyter F, Berglund M. Characteristics of primary amphetamine users in Sweden: a criminal justice population examined with the Addiction Severity Index. Eur Addict Res. 2009;15(1):10–8. doi: 10.1159/000173004. [DOI] [PubMed] [Google Scholar]
- 43.Degenhardt L, Baxter AJ, Lee YY, et al. The global epidemiology and burden of psychostimulant dependence: findings from the Global Burden of Disease Study 2010. Drug Alcohol Depend. 2014;137:36–47. doi: 10.1016/j.drugalcdep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 44.Kaye S, Darke S. The diversion and misuse of pharmaceutical stimulants: what do we know and why should we care? Addiction. 2012;107(3):467–77. doi: 10.1111/j.1360-0443.2011.03720.x. [DOI] [PubMed] [Google Scholar]
- 45.US Food and Drug Administration. [Accessed 2016-02-18];Controlled Substances Act. http://www.fda.gov/RegulatoryInformation/Legislation/ucm148726.htm.
- 46.Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57(7–8):608–18. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 47.O’Callaghan P. Adherence to stimulants in adult ADHD. Atten Defic Hyperact Disord. 2014;6(2):111–20. doi: 10.1007/s12402-014-0129-y. [DOI] [PubMed] [Google Scholar]