Abstract
Background
There are no effective pharmacologic strategies for non-dependent methamphetamine (meth)-using and binge-drinking MSM at high-risk for HIV. We sought to determine the feasibility of enrolling and retaining this population in a pharmacologic trial; the acceptability of pharmacotherapy study procedures; and the tolerability of targeted naltrexone versus placebo.
Methods
Thirty meth-using and binge-drinking MSM were randomly assigned 1:1 to 50mg naltrexone or placebo for 8 weeks for targeted administration (i.e., during craving or in anticipation of meth or alcohol use). Substance use counseling and behavioral assessments were conducted every two weeks. Medication use was measured using WisePill dispensers.
Results
Trial completion was 93%; visit completion rate was 95%. Mean weekly number of medication pills taken was 2.2 and was similar between arms. Participant satisfaction rate was 96%. There were no serious adverse events nor differences in adverse event rates between arms. In exploratory intention-to-treat analyses, there were no differences in meth use and drinking. Naltrexone participants had greater reductions in serodiscordant receptive anal intercourse (IRR=0.15; 95%CI=0.05-0.42) and serodiscordant condomless receptive anal intercourse (IRR=0.11; 95%CI=0.03-0.37), compared to placebo. In subgroup analyses among frequent meth-users, naltrexone participants had greater reductions in meth-using days (IRR=0.78; 95%CI=0.62-0.99). In as-treated analyses, frequent study medication users in the naltrexone arm had greater reductions in binge drinking days (IRR=0.72; 95%CI=0.54-0.97).
Conclusions
Targeted naltrexone is a feasible, acceptable and tolerable intervention strategy for non-dependent meth-using and binge-drinking MSM. Naltrexone was associated with significant sexual risk reductions; and for some individuals, naltrexone was associated with meth and binge-drinking reductions.
Keywords: methamphetamine, alcohol, HIV, men who have sex with men, HIV prevention, pharmacotherapy
Introduction
Methamphetamine (meth) use and heavy episodic drinking (i.e., “binge drinking,” defined as having 5 or more drinks on a single occasion) are associated with HIV risk behaviors and are highly prevalent among men who have sex with men (MSM).1-22 Most MSM who report meth use and binge drinking are not dependent users.23,24 Moreover, non-dependent meth use and binge drinking have both been independently associated with new HIV infections.8,25-27 Thus, interventions that reduce meth use and/or binge drinking are likely to have an impact on HIV transmission among MSM.
There are currently no effective pharmacologic strategies for non-dependent meth-using and binge-drinking MSM.28,29 Although most meth users and binge drinkers are non-dependent episodic users, pharmacologic studies have focused on substance-dependent individuals.23,30 While some behavioral interventions for substance-using MSM report reduced substance use and HIV risk behaviors, behavioral interventions alone have limited efficacy and may benefit from adjuvant pharmacologic agents.29,31-33
Naltrexone, a μ-opioid antagonist, is postulated to block the rewarding effects resulting from both amphetamine and alcohol intoxication. Meth use enhances release of mRNA precursors for endogenous opioids that activate μ-opioid receptors and increase extracellular dopamine levels.34-36 Similarly, alcohol consumption results in the release of β-endorphins that activate μ-opioid receptors and increase dopamine levels.37 Naltrexone competitively blocks these endogenous opioids and β-endorphins from activating μ-opioid receptors 38, which mediate dopamine release. Thus, naltrexone may decrease the activity of dopamine reward pathways, potentially tempering the positive neurobiological effects of both meth and alcohol use.36,38-40 Administration of naltrexone before amphetamine exposure significantly reduces the subjective effects of amphetamine;41,42 while administration of naltrexone before alcohol exposure results in decreased craving and desire to drink, and slower alcohol consumption.43 Daily naltrexone use has also significantly reduced relapse to amphetamine and heavy alcohol use in randomized trials.44,45 Beyond alcohol and substance use, naltrexone's mechanism of action is also postulated to inhibit increase of dopamine levels thought to play a role in other urge-driven problematic disorders. Limited evidence from open label studies and case reports suggest that naltrexone may be potentially helpful in treating self-injurious behaviors46, internet sex addictions,47 and compulsive sexual behaviors (characterized by a failure to resist the impulse of sex).48-51
Additionally, naltrexone's pharmacokinetic properties support intermittent, targeted administration (i.e., taking the medication during craving or in anticipation of meth or heavy alcohol use) as it reaches peak plasma levels within an hour of oral administration and a single 50 mg dose of naltrexone can block μ-opioid receptors for up to 72 hours.52 Naltrexone's long-acting activity is believed to be due to the half-life of both the parent and the 6-β-naltrexol metabolite.53 The mean elimination half-life for naltrexone and 6-β-naltrexol are four and 13 hours, respectively, making it an appropriate medication for targeted administration.53 Studies have shown the efficacy of intermittent targeted naltrexone in reducing drinking among those with alcohol abuse disorders.54,55 However, despite these encouraging pre-clinical and clinical data, no studies to-date have explored the use of targeted, as-needed naltrexone for non-dependent meth-using and binge-drinking MSM, and it is unclear whether this population would participate and remain engaged in a placebo-controlled pharmacologic trial using naltrexone.
To address these gaps, we conducted a pilot study among non-dependent, dual meth-using and binge-drinking MSM with high-risk sexual behaviors and evaluated the feasibility, acceptability, and tolerability of targeted naltrexone compared to placebo. In exploratory analyses, we also evaluated the preliminary efficacy of targeted naltrexone on meth use and craving; alcohol use and craving; and sexual risk outcomes.
Methods
Study Design and Recruitment
This is a randomized, double-blind, placebo-controlled, two-arm pilot study with 1:1 random assignment to oral naltrexone 50mg versus placebo. Participants were recruited via street outreach, recruitment flyers, STD and HIV clinics, needle-exchanges, community organizations, MSM bars and events, online websites, and social media. Potential participants completed a brief telephone screen to assess initial eligibility and, if eligible, were scheduled for an in-person screening visit. All participants gave informed consent using IRB-approved consent forms. A 10-item true/false questionnaire was used to verify participants' understanding of the trial.
Study participants
Thirty meth-using and binge-drinking, sexually active MSM were randomly assigned to receive oral naltrexone (n=15) or placebo (n=15) for 8 weeks. Participants were eligible if they reported active meth use (at least twice per month) and binge-drinking (at least weekly); had anal intercourse with men in past 3 months while under the influence of meth or alcohol; expressed interest in reducing or stopping their meth use and binge drinking; were 18–70 years of age; did not have any acute medical or psychiatric illnesses; and had baseline safety labs without clinically significant abnormalities. We excluded individuals for any psychiatric (e.g., depression with suicidal ideation) or medical condition that would preclude safe study participation or compliance to procedures; known allergy or adverse reaction to naltrexone; current opioid use or dependence or having a known medical condition that may likely require opioid analgesics; opioid-positive urine at enrollment; HIV-positive with current CD4 count <200 cells/mm3; moderate-severe liver disease (AST, ALT > 3 times upper limit of normal); impaired renal function (estimated glomerular filtration rate (eGFR)<50 ml/min); current participation in another intervention research study with potential overlap; alcohol or meth dependence determined by Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders IV-TR (SCID) criteria; and no cell-phone access. Alcohol- and meth-dependent participants were excluded because the purpose of this study was to conduct a pharmacologic intervention for non-dependent users whom are otherwise not eligible for other addiction intervention trials, despite the risk associated with their alcohol and substance use. Alcohol-dependent participants were also excluded because naltrexone is FDA-approved to treat alcohol dependence and it would not be ethical to randomize these participants to placebo.
Study procedures
At screening visits, after informed consent, participants received a complete history and physical, complete blood count and a comprehensive metabolic panel. To rule out opioid use, rapid qualitative urine testing were used (Medtox Diagnostics, Burlington, NC). Participants reporting HIV-negative or unknown HIV status received HIV rapid testing; HIV-positive participants received CD4 and HIV viral load tests. Participants received HIV risk-reduction counseling based on CDC guidelines.56 Staff collected extensive participant contact information and two back-up contacts. Eligible participants were scheduled for an enrollment visit.
At enrollment, treatment was assigned using double-blinded block-randomization. The study statistician provided the randomization codes to the Drug Product Services Laboratory at University of California, San Francisco (UCSF), which prepared kits corresponding with the treatment assignment (naltrexone 50 mg or matching placebo) in the randomization code.
Participants were seen every two weeks for substance-use counseling and behavioral assessments. HIV risk-reduction counseling and testing was repeated for HIV-negative participants at final visit. Participants were paid $25 for screening, $35 for enrollment, $10 for the visits every 2 weeks and $35 for final visits. Procedures were approved by UCSF's Committee on Human Research (IRB Number=12-09809) and the trial was registered at clinicaltrials.gov (Identifier=NCT01723384).
Trained staff, supervised by a clinical psychologist, administered brief (15-20 minutes) substance use counseling at follow-up visits, which was modified from a standardized, manual-driven psychosocial treatment program using cognitive behavioral therapy 57and motivational interviewing techniques 58,59 and incorporated the Stages of Change Model.60 This platform has been used in brief substance use behavioral interventions and has high acceptability among substance-using MSM. 61-64
Consistent with other targeted pharmacologic trials,54,65 participants were instructed to take one pill on days they anticipate heavy drinking, using meth, or when craving alcohol and/or meth (i.e., on an as needed, intermittent basis). For this study, participants did not have to take the study medication on days when they did not anticipate the risk events mentioned above, nor on days when they did not crave alcohol and meth. Study clinicians provided training and instructions on targeted dosing of medication during enrollment. Wireless medication monitoring devices, were used to record each opening as a real-time medication event.66 Medication use was tabulated as the number of distinct days in which the WisePill dispensers were opened, divided by weeks of follow-up, to estimate the average weekly pills used. We also assessed the number of days when participants reported using the medication as-needed, per-protocol.
All participants were asked about potential adverse events at each follow-up visit; symptom-driven physical exams and safety laboratory monitoring were done at weeks 4, 8. Adverse events were classified using the Division of AIDS (DAIDS) Table for Grading Severity of Adult Adverse Experiences for HIV Prevention Trials Network .67
Audio-computer assisted self-interview (ACASI) was used to standardize data collection and minimize reporting bias.68 Standardized measures were used to assess drug and alcohol use, substance use treatment, and sexual risk behavior.10 Acceptability measures included questions on attitudes about trial participation, level of satisfaction with trial procedures and likelihood of participating in a similar trial in the future.
Statistical analyses
Data were analyzed by intention-to-treat, without regard to study protocol compliance for primary outcomes. To assess feasibility of enrolling and retaining non-dependent, dual meth-using and binge-drinking MSM, we computed the proportions of participants eligible and enrolled among those recruited and screened, the proportion of scheduled visits completed, and the proportion of participants retained to the end of the study. We used Wilcoxon and Fisher's exact tests, as appropriate, to assess the comparability of participants by treatment assignment at baseline. To assess acceptability of naltrexone and placebo, we examined the frequency of taking the study drug; weekly number of WisePill dispenser openings was compared by study arm using the Wilcoxon test. To explore safety and tolerability, we computed the proportions of those experiencing adverse events and compared adverse event rates by treatment assignment using Fisher's exact test.
In exploratory analyses, which were planned before study unblinding, we used generalized estimating equations (GEE) models to evaluate group-specific linear trends in self-reported meth use, alcohol use and HIV related sexual risk behaviors, with robust standard errors to account for within-subject correlation as well as potential over-dispersion of count outcomes. Binary and count outcomes were examined using Poisson 69 and negative binomial models, respectively. In all models, the effect of the intervention was estimated by the interaction between treatment assignment and a linear term in time; departures from linear trends were evaluated. In as-treated analyses, frequent study medication use based on WisePill dispenser data was defined as a visit-specific indicator of medication use. This indicator variable for frequent use was coded as “1” for participants in the uppermost quartile of medication use (i.e., at least three or more openings per week) and “0” for participants with less frequent medication use (i.e., less than three openings per week). We then included this indicator and its interaction with treatment assignment as time-dependent covariates in negative binomial models for number of meth use and binge drinking days, providing as estimate of the effect of frequent naltrexone use, controlling for the placebo effects of frequent study medication use. Subgroup analyses among the subset of participants who reported more frequent (at least weekly) meth use and binge drinking at baseline were also conducted. Analyses were conducted with STATA 12.0 (College Station, TX).
Results
Screening, recruitment, and randomization
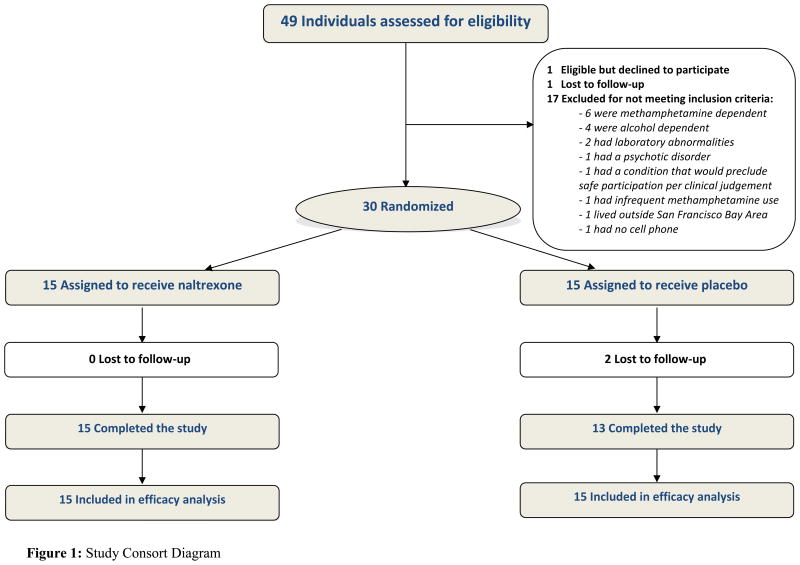
Figure 1 shows results for screening, recruitment, assignment and retention for the study period from June 2013 to September 2014. One hundred and thirty-five people were assessed for initial eligibility by a telephone prescreen (Figure 1). Among those ineligible, the most common reasons for ineligibility by phone were less frequent binge drinking (50%), not using meth or alcohol during sex (33%), less frequent meth use (25%) and no interest in reducing binge drinking (20%). Of those eligible by telephone prescreen, 49 (36%) signed informed consent and were assessed further for eligibility. Of the 49 individuals who consented to participate, 17 were deemed ineligible (6 due to meth-dependence, 4 due to alcohol-dependence), 1 was lost to follow-up during screening, 1 was eligible but declined participation and 30 were randomized. Thus, 61% of the 49 screened were randomized. During the screening and enrollment visits, 37% of participants tested positive for meth in urinalyses. There were no significant differences among those enrolled and those ineligible in screening with respect to age, race, ethnicity, HIV status, proficiency in English, cell phone access, participation in substance use treatment and self-help programs, alcohol use frequency and meth use frequency (all P>0.05).
Figure 1. Study Consort Diagram.
Participant Baseline Characteristics
We recruited a diverse sample of MSM (40% White, 17% Hispanic/Latino, 30% Black, 7% Asian or Pacific Islander, and 6% Mixed or Other race), of whom 40% were HIV-positive (Table 1). Baseline demographic characteristics were similarly distributed in both arms.
Table 1. Baseline Characteristics of Trial Participants.
| Demographics | No. (%) | p value‡ | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Placebo (n=15) | Naltrexone (n=15) | Overall (n=30) | ||||||
| Age, mean (SD), y | 42.3 | (10.0) | 43.7 | (8.8) | 43 | (9.3) | 0.54 | |
|
| ||||||||
| Race/Ethnicity | ||||||||
| White | 6 | (40.0) | 6 | (40.0) | 12 | (40.0) | ≥ 0.99 | |
|
|
||||||||
| African-American | 5 | (33.3) | 4 | (26.7) | 9 | (30.0) | ||
|
|
||||||||
| Latino | 2 | (13.3) | 3 | (20.0) | 5 | (16.7) | ||
|
|
||||||||
| Asian and Pacific Islander | 1 | (6.7) | 1 | (6.7) | 2 | (6.7) | ||
|
|
||||||||
| Other | 1 | (6.7) | 1 | (6.7) | 2 | (6.7) | ||
|
| ||||||||
| Education | ||||||||
| High school or less | 2 | (13.3) | 2 | (13.3) | 4 | (13.3) | ≥ 0.99 | |
|
|
||||||||
| Some college | 8 | (53.3) | 9 | (60.0) | 17 | (56.7) | ||
|
|
||||||||
| College or above | 5 | (33.3) | 4 | (26.7) | 9 | (30.0) | ||
|
| ||||||||
| Income | ||||||||
| under $20,000 | 9 | (60.0) | 9 | (60.0) | 18 | (60.0) | 0.68 | |
|
|
||||||||
| $20,000-39,999 | 3 | (20.0) | 5 | (33.3) | 8 | (26.7) | ||
|
|
||||||||
| $40,000 and above | 3 | (20.0) | 1 | (6.7) | 4 | (13.3) | ||
|
|
||||||||
| refused to answer | 1 | (6.7) | 1 | (6.7) | 2 | (6.7) | ||
|
| ||||||||
| Employment status | ||||||||
| Not employed | 8 | (53.3) | 13 | (86.7) | 21 | (70.0) | 0.13 | |
|
|
||||||||
| Part-time | 3 | (20.0) | 2 | (13.3) | 5 | (16.7) | ||
|
|
||||||||
| Full-time | 3 | (20.0) | 0 | 0.0 | 3 | (10.0) | ||
|
|
||||||||
| Employed student | 1 | (6.7) | 0 | 0.0 | 1 | (3.3) | ||
|
| ||||||||
| Alcohol Use | ||||||||
|
| ||||||||
| Frequency of alcohol use (past 4 weeks) | ||||||||
| 1 day per week or less | 1 | (6.7) | 5 | (33.3) | 6 | (20.0) | 0.17 | |
|
|
||||||||
| 2 days per week or more | 14 | (93.3) | 10 | (66.7) | 24 | (80.0) | ||
|
| ||||||||
| Alcohol use during sex (past 4 weeks) | ||||||||
| 50% or less of the time | 8 | (53.3) | 11 | (73.3) | 19 | (63.3) | 0.45 | |
|
|
||||||||
| Greater than 50% of time | 7 | (46.7) | 4 | (26.7) | 11 | (36.7) | ||
|
| ||||||||
| Number of Drinks in Typical Drinking Days (past 4 weeks), mean (SD) | 5.9 | (2.2) | 5.1 | (2.9) | 5.5 | (2.5) | 0.32 | |
|
| ||||||||
| Number of Binge Drinking Days (past 4 weeks), mean (SD) | 9.3 | (9.0) | 3.9 | (3.1) | 6.6 | (7.2) | 0.13 | |
|
| ||||||||
| Alcohol visual analog scale (VAS) craving score, mean (SD) | 59.3 | (26.0) | 38.9 | (29.3) | 49.1 | (29.1) | 0.06 | |
|
| ||||||||
| History of alcohol self-help or treatment program | 6 | (40.0) | 10 | (66.7) | 16 | (53.3) | 0.27 | |
|
| ||||||||
| Methamphetamine use | ||||||||
|
| ||||||||
| Frequency of methamphetamine use (past 4 weeks) | ||||||||
| less than 1 day per week | 6 | (40.0) | 10 | (66.7) | 16 | (53.3) | 0.47 | |
|
|
||||||||
| at least 1 day per week | 9 | (60.0) | 5 | (33.3) | 14 | (46.7) | ||
|
| ||||||||
| Methamphetamine use during sex (past 4 weeks) | ||||||||
| 50% or less of the time | 10 | (66.7) | 11 | (73.3) | 21 | (70.0) | ≥0.99 | |
|
|
||||||||
| More than 50% of time | 5 | (33.3) | 4 | (26.7) | 9 | (30.0) | ||
|
| ||||||||
| Methamphetamine visual analog scale (VAS) craving score, mean (SD) | 41.6 | (23.0) | 35.7 | (25.5) | 38.7 | (24.1) | 0.53 | |
|
| ||||||||
| History of methamphetamine self-help or treatment program | 6 | (40.0) | 10 | (66.7) | 16 | (53.3) | 0.27 | |
|
| ||||||||
| Clinical | ||||||||
|
| ||||||||
| HIV serostatus | HIV positive | 5 | (33.3) | 7 | (46.7) | 12 | (40.0) | 0.71 |
|
|
||||||||
| HIV negative | 10 | (66.7) | 8 | (53.3) | 18 | (60.0) | ||
|
| ||||||||
| Has regular health care provider | 10 | (66.7) | 10 | (66.7) | 20 | (66.7) | ≥0.99 | |
|
| ||||||||
| Has health insurance | 11 | (73.3) | 12 | (80.0) | 23 | (76.7) | ≥0.99 | |
|
| ||||||||
| Center for epidemiologic studies depression scale (CES-D) score, mean (SD) | 18.1 | (7.5) | 20.3 | (13.9) | 19.2 | (11.0) | 0.76 | |
binary and categorical characteristics compared using the Fisher's exact test, and group medians compared using the Wilcoxon rank-sum test
Retention
Twenty-eight participants (93%) completed the trial (i.e., retained until final visit) with no significant differences by treatment assignment. Overall, 95% (143/150) of ACASI risk assessments were completed (naltrexone=97%, placebo=93%; P=0.99) and 95% of study visits were attended (naltrexone 97%, placebo 93%; P=0.99).
Medication Acceptability
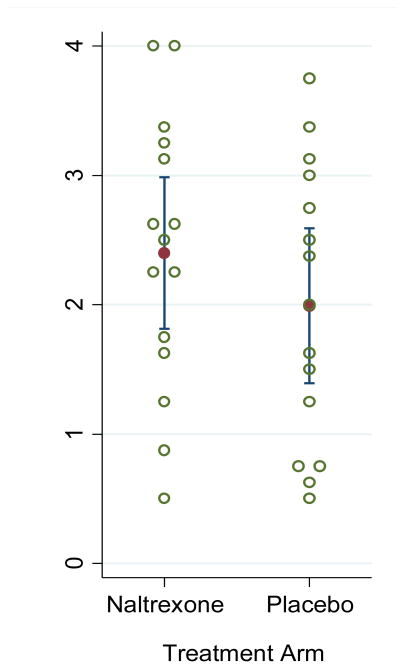
The mean cumulative number of study medication doses taken by participants, as measured WisePill data, was 16.7 (SD=8.5) and were similar by arm (naltrexone=18.0 [SD=8.8]; placebo=15.3 [SD=8.3]; P=0.36). The mean number of WisePill medication events each week of follow-up was 2.1 (SD=1.0) and were similar between arms (naltrexone=2.2 [SD=1.0]; placebo=1.9 [SD=1.0]; P=0.49; Figure 2). WisePill medication events were similar by day of the week, and similar between weekdays and weekends. Nearly half (46%) of opening events occurred on consecutive days. WisePill medication events were associated with time of day; events were more likely to occur between the hours of 12pm-5pm and less likely to occur between 12am-6am (P<0.001).
Figure 2. Average WisePill Dispenser weekly openings, by arm.
On average, participants reported taking study medication 64.0% (SD=37.3) of the days that they craved meth or anticipated meth use; results were similar by arm (naltrexone=66.6% [SD=40.4], placebo=61.2% [SD=34.0], P=0.24). Additionally, on average, participants reported taking study medication 53.8% of the days that they craved alcohol or anticipated a heavy alcohol drinking session; results were similar by arm (naltrexone=52.7% [SD=44.6], placebo=54.8% [SD=34.4], P=0.99).
Safety
There were no serious adverse events (AEs), no medication discontinuations due to side effects, and no differences in frequency of AEs between arms (P=0.39). One participant experienced moderate chest pain (grade 2) and another experienced severe hyperglycemia (grade 3); both of were unrelated to medication. Most frequently reported AEs were mild (grade 1) and unrelated to study drug: upper respiratory infection (n=6), nausea (n=6), fatigue/drowsiness (n=3), hyperglycemia (n=3) and headaches (n=3).
Procedures Acceptability
At study completion, 96% of participants were satisfied or highly satisfied with study participation. Eighty-four percent of participants found the study procedures were not at all or a little difficult. Ninety-six percent reported being somewhat likely or very likely to participate in future studies and one-hundred percent expressed being somewhat likely or very likely to recommend the study to friends.
Exploratory Analyses
In intention-to-treat analyses, there were no differences in meth and alcohol use between arms (Table 2). The naltrexone arm had significantly greater reductions in serodiscordant receptive anal intercourse and serodiscordant condomless receptive anal intercourse, versus placebo. In contrast, there were no differences in depression score, meth craving, and alcohol craving between arms.
Table 2. Exploratory Analyses on Evaluating Efficacy of Naltrexone compared to Placebo.
| Alcohol Use |
Baseline n (%) mean (SD) |
Month 1 n (%) mean (SD) |
Month 2 n (%) mean (SD) |
IRR | 95%-CI | p-value | |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Any alcohol use | Placebo | 15 (100%) | 14 (93.3%) | 12 (80.0%) | 0.92 | 0.78-1.08 | 0.33 |
|
|
|||||||
| Naltrexone | 15 (100%) | 10 (66.7%) | 12 (80.0%) | ||||
|
|
|||||||
| Number of binge drinking days | Placebo | 9.3 (9.0) | 7.8 (7.3) | 5.5 (6.4) | 0.94 | 0.68-1.32 | 0.74 |
|
|
|||||||
| Naltrexone | 3.9 (3.1) | 2.3 (2.8) | 1.7 (2.0) | ||||
|
|
|||||||
| Number of drinks in drinking days | Placebo | 5.9 (2.2) | 4.7 (2.8) | 4.6 (2.6) | 0.94 | 0.82-1.10 | 0.39 |
|
|
|||||||
| Naltrexone | 5.1 (2.9) | 9.1 (18.6) | 2.6 (2.2) | ||||
|
|
|||||||
| Methamphetamine Use | |||||||
| Any meth use | Placebo | 15 (100%) | 14 (93.3%) | 10 (66.7%) | 0.82 | 0.59-1.12 | 0.21 |
|
|
|||||||
| Naltrexone | 13 (86.7%) | 8 (53.3%) | 7 (46.7%) | ||||
|
|
|||||||
| Number of meth use days | Placebo | 3.7 (3.3) | 2.7 (3.1) | 2.0 (1.7) | 0.98 | 0.70-1.40 | 0.89 |
|
|
|||||||
| Naltrexone | 1.4 (1.8) | 1.0 (1.4) | 1.1 (2.2) | ||||
|
|
|||||||
| Sexual Risk Behaviors | |||||||
| Number of male partners | Placebo | 5.9 (8.8) | 3.7 (3.8) | 1.9 (1.9) | 0.69 | 0.35-1.36 | 0.28 |
|
|
|||||||
| Naltrexone | 5.6 (12.6) | 0.9 (1.3) | 1.0 (1.6) | ||||
|
|
|||||||
| Serodiscordant insertive anal intercourse events | Placebo | 2.5 (6.4) | 0.4 (0.9) | 0.2 (0.4) | 1.72 | 0.37-7.93 | 0.48 |
|
|
|||||||
| Naltrexone | 4 (12.8) | 0.5 (1.2) | 0.6 (1.5) | ||||
|
|
|||||||
| Serodiscordant condomless insertive anal intercourse events | Placebo | 1.7 (5.2) | 0.2 (0.6) | 0.2 (0.4) | 1.57 | 0.22-11.10 | 0.65 |
|
|
|||||||
| Naltrexone | 3.6 (12.8) | 0.3 (1.0) | 0.5 (1.4) | ||||
|
|
|||||||
| Serodiscordant receptive anal intercourse events | Placebo | 2.1 (4.4) | 1.5 (2.9) | 0.8 (1.5) | 0.15 | 0.05-0.42 | <0.01 |
|
|
|||||||
| Naltrexone | 1.3 (3.1) | 0.1 (0.5) | 0.0 (0.0) | ||||
|
|
|||||||
| Serodiscordant condomless receptive anal intercourse events | Placebo | 1.6 (4.7) | 1.3 (2.9) | 0.8 (1.5) | 0.11 | 0.03-0.37 | <0.01 |
|
|
|||||||
| Naltrexone | 0.8 (1.9) | 0.1 (0.3) | 0.0 (0.0) | ||||
|
|
|||||||
| Continuous Outcomes | Baseline mean (SD) | Month 1 mean (SD) | Month 2 mean (SD) | Coef | 95%-CI | p-value | |
|
|
|||||||
| Center for Epidemiologic Studies Depression Scale | Placebo | 18.1 (7.5) | 17.5 (12.8) | 20.8 (10.7) | 6.43 | -18.1 - 5.2 | 0.28 |
|
|
|||||||
| Naltrexone | 20.3 (13.9) | 19.7 (12.3) | 18.3 (12.0) | ||||
|
|
|||||||
| Methamphetamine Craving from Visual Analog Scale | Placebo | 41.6 (23.0) | 31.1 (26.0) | 22.1 (19.1) | 2.03 | -3.52 - 7.6 | 0.47 |
|
|
|||||||
| Naltrexone | 35.7 (25.5) | 28.6 (26.0) | 26.1 (30.6) | ||||
|
|
|||||||
| Alcohol Craving from Visual Analog Scale | Placebo | 59.3 (26.0) | 43.7 (28.8) | 48.0 (32.6) | 1.63 | -2.2 - 5.4 | 0.40 |
|
|
|||||||
| Naltrexone | 38.9 (29.3) | 28.0 (34.1) | 24.9 (34.6) | ||||
Notes: Serodiscordant partner is defined as HIV-positive person with HIV-negative or unknown HIV-status partner, or HIV-negative person with HIV-positive or unknown HIV-status partner.
In as-treated analyses, we evaluated the associations of frequent naltrexone use (those in the uppermost quartile of WisePill dispenser openings, defined as three or more openings per week) with numbers of meth-using and binge-drinking days, controlling for frequency of any study medication use. The as-treated incident rate ratios (IRRs) were 0.60 (95%CI=0.31-1.19; P=0.14) for meth use, and 0.72 (95%CI=0.54-0.97; P=0.03) for binge drinking. In the subgroup analysis among participants who reported at least weekly methamphetamine use at baseline, those randomized to naltrexone had significantly greater reductions in number of meth using days, compared to placebo (IRR=0.78, 95%CI=0.62-0.99; P=0.04).
Assessment of blinding
Treatment guessing accuracy between participants in the two groups did not differ significantly (P=0.64). In the placebo group, 54% guessed they were on placebo. In the naltrexone group, 54% guessed they were on naltrexone.
Discussion
To our knowledge, this is the first study to enroll an actively using sample of non-dependent, dual meth- and alcohol-using MSM in a placebo-controlled pharmacologic trial. Our data suggests that it is feasible, acceptable and tolerable to utilize targeted, as-needed naltrexone in this population. Given the high prevalence of non-dependent patterns of meth use and binge drinking among MSM; the associations among meth use, binge drinking, sexual risk behavior and HIV; and the fact that most substance-using MSM do not access current treatment options,23,24 it is important to demonstrate that non-dependent, actively using MSM are willing to participate in pharmacologic studies. Moreover, pharmacologic research for substance use has been primarily focused on dependent individuals, and rarely includes non-dependent users with a desire to cut down or address their use before the possibility of transitioning to becoming substance dependent. This study demonstrates that MSM who are current substance users without dependence can be enrolled in pharmacologic intervention trials. Our high completion and retention rates suggest that this population is willing to engage and can be retained in pharmacologic research studies. The completion and retention rates in this pharmacologic study are comparable to or better than other studies involving behavioral interventions for non-dependent samples of substance-using MSM 70-72 and suggest that this population may be as amenable to pharmacotherapy as they are to behavioral strategies.
Additionally, we observed high acceptability with regard to our study procedures, and intermittent, as-needed medication use. Although there have been pharmacotherapy studies with as-needed dosing for those with alcohol use disorders or dependence, this is the first study to demonstrate acceptability of this dosing procedure among non-dependent, substance-using MSM. Given the challenges with daily dosing in pharmacotherapy, as-needed dosing may present a viable substitute that could expand the population that may benefit from therapy.54 Indeed, among MSM in general, intermittent dosing of other chemoprophylaxis interventions has been explored, including the use of intermittent HIV antiretroviral medications for Pre-Exposure Prophylaxis (PrEP).73 The acceptability of taking study drug for meth and alcohol use among MSM in our study is broadly consistent with these other intermittent medications strategies and suggests that MSM are willing and able to take medications in anticipation of risky events (whether related to substance use or sexual risk).
Our pilot study was not powered to detect treatment effects between naltrexone and placebo on meth use, alcohol use, and sexual risk outcomes. Nevertheless, in our exploratory intention-to-treat analyses, we observed point estimates that suggest a protective effect of naltrexone compared to placebo for meth use. Although the reduction in meth use is not statistically significant in the entire sample, the numerically lower frequency of meth use in the naltrexone group is somewhat encouraging, in light of the findings from another larger oral naltrexone trial by Jayaram-Lindstrom et al. which observed that daily oral naltrexone was associated with a “reasonably strong” to “strong” effect size in reducing amphetamine relapse.44 Key differences in our study design (as-needed versus daily) and population may explain why we did not observe a similarly strong effect size in favor of oral naltrexone. Unlike Jayaram-Lindstrom et al., our study was restricted to non-dependent individuals who may use meth less frequently. However, when we conducted a sub-group analysis specifically looking at individuals with more frequent baseline meth use, we did observe statistically significant reductions in number of meth-using days in the naltrexone group compared to placebo. Hence, our findings among frequent meth users are generally in line with the Jayaram-Lindstrom study which had individuals who reported more frequent amphetamine use prior to their baseline visit.
Additionally, we did not observe significant reductions in alcohol use, number of binge drinking days and drinks on drinking days in the naltrexone group compared to placebo, although our point estimates suggest small protective effects. As-needed naltrexone was shown in Kranzler et al's study to significantly reduce mean drinks per day, and number of drinks during drinking days.54 In that study, authors reported higher medication use since their protocol instructed participants to take medication at least thrice per week, in addition to as-needed use. In our as-treated analyses, those who reported more frequent medication use did have significantly greater reductions in number of binge-drinking days, which is broadly consistent with Kranzler et al. There may be a minimum threshold for naltrexone use that needs to be met in order for it to be beneficial and substance-using MSM may require reminders for medication use to facilitate greater uptake of targeted dosing strategies.
Furthermore, we observed statistically significant reductions in both serodiscordant receptive anal intercourse and serodiscordant condomless receptive anal intercourse in our intention-to-treat analysis. It is unclear why we observed reductions in these two specific sexual behavior endpoints. As mentioned, among frequent meth users, we found that naltrexone was significantly associated with reductions in meth use days. Meth's physiological effects include impotence, which limits the practice of insertive anal intercourse among MSM who use meth.74-76 Additionally, MSM have reported that their use of meth makes receptive anal intercourse more pleasurable and less painful.76,77 Hence, meth use have been associated with greater receptive anal intercourse, but not insertive anal intercourse in prior MSM studies.7,76,78-80 We speculate that as meth use decreased in the naltrexone group, impotence may has also decreased. Additionally, pleasure from receptive anal intercourse may have also been reduced in the naltrexone group, as meth use decreased. These changes may had led to less receptive anal intercourse events, as participants were able to engage in more insertive anal intercourse events and experienced reductions in pleasure from receptive anal intercourse. This hypothesis is corroborated by another study which observed reductions in receptive anal intercourse among MSM after reductions in meth use and receipt of meth treatment.31 Broadly, it is plausible that these results may be partially explained by the purported effects of naltrexone on sexual urges and compulsive sexual behaviors, as noted in case reports of off-label naltrexone use. One report has noted significant reductions sexual behaviors among multiple sex partners due to naltrexone use.51 Additional research is needed to clarify whether these reductions were directly from naltrexone, or an indirect result of either meth or alcohol use reductions among individuals responding to naltrexone. In any case, condomless receptive anal intercourse is estimated to have the highest per contact risk for HIV infection via sexual transmission.81 Substance use is a major driver of sexual risk transmission among MSM and there are few evidence-based interventions shown to efficaciously reduce high-risk sexual behaviors among substance-using MSM. Behavioral intervention trials to date have yielded mixed results in reducing HIV-related sexual risk behaviors among substance-using MSM 70-72; there remains a great need to identify new strategies, especially for non-dependent substance-using MSM. To our knowledge, this is the first study of oral naltrexone to demonstrate significant reductions in HIV-related sexual risk behaviors among substance-using MSM. Our findings highlight new opportunities to utilize pharmacotherapy as a potential HIV prevention strategy among substance-using MSM.
This pilot study has several limitations. As mentioned, this study was not powered to assess the efficacy of oral naltrexone versus placebo, which should be kept in mind while interpreting the exploratory analyses between treatment groups for meth and alcohol use and sexual risk behaviors. Furthermore, given the exploratory nature of the between-group analyses, we did not formally control for multiple hypotheses testing. Moreover, these exploratory analyses utilized GEE models which have been shown to result in standard errors that are too small for small studies, and may bias findings away from the null.82 Taken together, the significant results from these exploratory analyses (reductions in binge drinking days in as-treated analyses; meth using days in sub-group analyses; and serodiscordant receptive anal intercourse events) should be interpreted with caution, as they may be subject to both type-I error and error from bias. While we did not find significant differences between those enrolled versus those excluded during screening, our small sample size and use of non-probability sampling may nevertheless limit the generalizability of our findings. In addition, our follow-up was limited to 8 weeks and our study design was limited to assessments and monitoring every two weeks. It is possible that longer and more frequent follow-ups may be needed to observe significant treatment effects for naltrexone and definitively establish its safety. Despite these limitations, the results of this randomized, double-blind, placebo-controlled pilot study support conducting a larger efficacy trial. Ideally, this trial should utilize frequent assessments with objective measures (urine drug screens) on outcomes preferred by regulatory agencies and more regular adverse event monitoring.
In summary, we found that it is feasible, acceptable, and tolerable to conduct a placebo-controlled pharmacologic trial for non-dependent substance-using MSM. In this pilot study, naltrexone was associated with significant reductions in meth-using days and binge-drinking days among some individuals, and was associated with significant reductions in high-risk sexual behaviors in intention-to-treat analyses. Results of this study support further evaluation of naltrexone in larger and more diverse populations of meth-using and binge-drinking MSM, including those with substance use disorders.
Acknowledgments
All authors participated in the revision of the manuscript for important intellectual content and provided approval of the final version of the manuscript.
Support: This study was funded by the National Institutes of Drug Abuse (NIDA), grant number R36DA035109. JAH was funded by K24AA022586.
Footnotes
ClinicalTrials.gov Identifier: NCT01723384
Conflicts of Interests and source of funding statements: All authors declare no conflicts of interest. The funder did not play any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; nor in the preparation, review, or approval of the manuscript.
References
- 1.Sanchez T, Finlayson T, Drake A, et al. Human immunodeficiency virus (HIV) risk, prevention, and testing behaviors--United States, National HIV Behavioral Surveillance System: men who have sex with men, November 2003-April 2005. MMWR Surveill Summ. 2006;55(6):1–16. [PubMed] [Google Scholar]
- 2.Finlayson TJ, Le B, Smith A, et al. HIV risk, prevention, and testing behaviors among men who have sex with men--National HIV Behavioral Surveillance System, 21 U.S. cities, United States, 2008. MMWR Surveill Summ. 2011;60(14):1–34. [PubMed] [Google Scholar]
- 3.Semple SJ, Zians J, Strathdee SA, Patterson TL. Sexual marathons and methamphetamine use among HIV-positive men who have sex with men. Arch Sex Behav. 2009;38(4):583–590. doi: 10.1007/s10508-007-9292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor MM, Aynalem G, Smith LV, Montoya J, Kerndt P. Methamphetamine use and sexual risk behaviours among men who have sex with men diagnosed with early syphilis in Los Angeles County. Int J STD AIDS. 2007;18(2):93–97. doi: 10.1258/095646207779949709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ober A, Shoptaw S, Wang PC, Gorbach P, Weiss RE. Factors associated with event-level stimulant use during sex in a sample of older, low-income men who have sex with men in Los Angeles. Drug Alcohol Depend. 2009;102(1-3):123–129. doi: 10.1016/j.drugalcdep.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawstorne P, Digiusto E, Worth H, Zablotska I. Associations between crystal methamphetamine use and potentially unsafe sexual activity among gay men in Australia. Arch Sex Behav. 2007;36(5):646–654. doi: 10.1007/s10508-007-9206-z. [DOI] [PubMed] [Google Scholar]
- 7.Koblin BA, Murrill C, Camacho M, et al. Amphetamine use and sexual risk among men who have sex with men: results from the National HIV Behavioral Surveillance study--New York City. Subst Use Misuse. 2007;42(10):1613–1628. doi: 10.1080/10826080701212519. [DOI] [PubMed] [Google Scholar]
- 8.Plankey MW, Ostrow DG, Stall R, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. Journal of acquired immune deficiency syndromes. 2007;45(1):85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irwin TW, Morgenstern J, Parsons JT, Wainberg M, Labouvie E. Alcohol and sexual HIV risk behavior among problem drinking men who have sex with men: An event level analysis of timeline followback data. AIDS Behav. 2006;10(3):299–307. doi: 10.1007/s10461-005-9045-7. [DOI] [PubMed] [Google Scholar]
- 10.Colfax G, Vittinghoff E, Husnik MJ, et al. Substance use and sexual risk: a participant- and episode-level analysis among a cohort of men who have sex with men. Am J Epidemiol. 2004;159(10):1002–1012. doi: 10.1093/aje/kwh135. [DOI] [PubMed] [Google Scholar]
- 11.Prestage G, Grierson J, Bradley J, Hurley M, Hudson J. The role of drugs during group sex among gay men in Australia. Sex Health. 2009;6(4):310–317. doi: 10.1071/SH09014. [DOI] [PubMed] [Google Scholar]
- 12.Benotsch EG, Nettles CD, Wong F, et al. Sexual risk behavior in men attending Mardi Gras celebrations in New Orleans, Louisiana. J Community Health. 2007;32(5):343–356. doi: 10.1007/s10900-007-9054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benotsch EG, Mikytuck JJ, Ragsdale K, Pinkerton SD. Sexual risk and HIV acquisition among men who have sex with men travelers to Key West, Florida: a mathematical modeling analysis. AIDS Patient Care STDS. 2006;20(8):549–556. doi: 10.1089/apc.2006.20.549. [DOI] [PubMed] [Google Scholar]
- 14.Lambert G, Cox J, Hottes TS, et al. Correlates of unprotected anal sex at last sexual episode: analysis from a surveillance study of men who have sex with men in Montreal. AIDS Behav. 2011;15(3):584–595. doi: 10.1007/s10461-009-9605-3. [DOI] [PubMed] [Google Scholar]
- 15.Vanable PA, McKirnan DJ, Buchbinder SP, et al. Alcohol use and high-risk sexual behavior among men who have sex with men: the effects of consumption level and partner type. Health Psychol. 2004;23(5):525–532. doi: 10.1037/0278-6133.23.5.525. [DOI] [PubMed] [Google Scholar]
- 16.Folch C, Esteve A, Zaragoza K, Munoz R, Casabona J. Correlates of intensive alcohol and drug use in men who have sex with men in Catalonia, Spain. Eur J Public Health. 2010;20(2):139–145. doi: 10.1093/eurpub/ckp091. [DOI] [PubMed] [Google Scholar]
- 17.Reisner SL, Mimiaga MJ, Case P, Johnson CV, Safren SA, Mayer KH. Predictors of identifying as a barebacker among high-risk New England HIV seronegative men who have sex with men. J Urban Health. 2009;86(2):250–262. doi: 10.1007/s11524-008-9333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NYCDOHMH. Alcohol use and risky sex in New York City. New York City: New York City Department of Health and Mental Hygiene; 2008. [Google Scholar]
- 19.Mackesy-Amiti ME, Fendrich M, Johnson TP. Symptoms of substance dependence and risky sexual behavior in a probability sample of HIV-negative men who have sex with men in Chicago. Drug Alcohol Depend. 2010;110(1-2):38–43. doi: 10.1016/j.drugalcdep.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reisner SL, Mimiaga MJ, Bland S, et al. Problematic alcohol use and HIV risk among Black men who have sex with men in Massachusetts. AIDS Care. 2010;22(5):577–587. doi: 10.1080/09540120903311482. [DOI] [PubMed] [Google Scholar]
- 21.Mimiaga MJ, Thomas B, Mayer KH, et al. Alcohol use and HIV sexual risk among MSM in Chennai, India. Int J STD AIDS. 2011;22(3):121–125. doi: 10.1258/ijsa.2009.009059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson KM, Simoni JM, Pearson CR, Walters KL. ‘I've had unsafe sex so many times why bother being safe now?’: the role of cognitions in sexual risk among American Indian/Alaska Native men who have sex with men. Ann Behav Med. 2011;42(3):370–380. doi: 10.1007/s12160-011-9302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos GM, Das M, Colfax GN. Interventions for non-injection substance use among US men who have sex with men: what is needed. AIDS Behav. 2011;15(Suppl 1):S51–56. doi: 10.1007/s10461-011-9923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos GM, Coffin PO, Das M, et al. Dose-response associations between number and frequency of substance use and high-risk sexual behaviors among HIV-negative substance-using men who have sex with men (SUMSM) in San Francisco. J Acquir Immune Defic Syndr. 2013;63(4):540–544. doi: 10.1097/QAI.0b013e318293f10b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read TR, Hocking J, Sinnott V, Hellard M. Risk factors for incident HIV infection in men having sex with men: a case-control study. Sex Health. 2007;4(1):35–39. doi: 10.1071/sh06043. [DOI] [PubMed] [Google Scholar]
- 26.Koblin B, Chesney MA, Husnik MJ, et al. High-risk behaviors among men who have sex with men in 6 US cities: baseline data from the EXPLORE Study. American Journal of Public Health. 2006;93(6):926–932. doi: 10.2105/ajph.93.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koblin BA, Husnik MJ, Colfax G, et al. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20(5):731–739. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 28.Kampman KM. The search for medications to treat stimulant dependence. Addict Sci Clin Pract. 2008;4(2):28–35. doi: 10.1151/ascp084228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoptaw S, Reback CJ, Peck JA, et al. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug and Alcohol Dependence. 2005;78(2):125–134. doi: 10.1016/j.drugalcdep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Colfax G, Santos GM, Chu P, et al. Amphetamine-group substances and HIV. Lancet. 2010;376(9739):458–474. doi: 10.1016/S0140-6736(10)60753-2. [DOI] [PubMed] [Google Scholar]
- 31.Reback CJ, Larkins S, Shoptaw S. Changes in the meaning of sexual risk behaviors among gay and bisexual male methamphetamine abusers before and after drug treatment. AIDS Behav. 2004;8(1):87–98. doi: 10.1023/b:aibe.0000017528.39338.75. [DOI] [PubMed] [Google Scholar]
- 32.Stall RD, Paul JP, Barrett DC, Crosby GM, Bein E. An outcome evaluation to measure changes in sexual risk-taking among gay men undergoing substance use disorder treatment. J Stud Alcohol. 1999;60(6):837–845. doi: 10.15288/jsa.1999.60.837. [DOI] [PubMed] [Google Scholar]
- 33.Rawson RA, Gonzales R, Brethen P. Treatment of methamphetamine use disorders: an update. J Subst Abuse Treat. 2002;23(2):145–150. doi: 10.1016/s0740-5472(02)00256-8. [DOI] [PubMed] [Google Scholar]
- 34.Tien LT, Ho IK, Loh HH, Ma T. Role of mu-opioid receptor in modulation of preproenkephalin mRNA expression and opioid and dopamine receptor binding in methamphetamine-sensitized mice. J Neurosci Res. 2007;85(3):673–680. doi: 10.1002/jnr.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horner KA, Adams DH, Hanson GR, Keefe KA. Blockade of stimulant-induced preprodynorphin mRNA expression in the striatal matrix by serotonin depletion. Neuroscience. 2005;131(1):67–77. doi: 10.1016/j.neuroscience.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 36.Schad CA, Justice JB, Jr, Holtzman SG. Endogenous opioids in dopaminergic cell body regions modulate amphetamine-induced increases in extracellular dopamine levels in the terminal regions. J Pharmacol Exp Ther. 2002;300(3):932–938. doi: 10.1124/jpet.300.3.932. [DOI] [PubMed] [Google Scholar]
- 37.Ulm RR, Volpicelli JR, Volpicelli LA. Opiates and alcohol self-administration in animals. J Clin Psychiatry. 1995;56(Suppl 7):5–14. [PubMed] [Google Scholar]
- 38.Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets. 2010;9(1):13–22. doi: 10.2174/187152710790966704. [DOI] [PubMed] [Google Scholar]
- 39.Magendzo K, Bustos G. Expression of amphetamine-induced behavioral sensitization after short- and long-term withdrawal periods: participation of mu- and delta-opioid receptors. Neuropsychopharmacology. 2003;28(3):468–477. doi: 10.1038/sj.npp.1300063. [DOI] [PubMed] [Google Scholar]
- 40.Chiu CT, Ma T, Ho IK. Methamphetamine-induced behavioral sensitization in mice: alterations in mu-opioid receptor. J Biomed Sci. 2006;13(6):797–811. doi: 10.1007/s11373-006-9102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jayaram-Lindstrom N, Wennberg P, Hurd YL, Franck J. Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. J Clin Psychopharmacol. 2004;24(6):665–669. doi: 10.1097/01.jcp.0000144893.29987.e5. [DOI] [PubMed] [Google Scholar]
- 42.Jayaram-Lindstrom N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2008;33(8):1856–1863. doi: 10.1038/sj.npp.1301572. [DOI] [PubMed] [Google Scholar]
- 43.O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160(1):19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- 44.Jayaram-Lindstrom N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- 45.Anton RF, O'Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jama. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 46.Roth AS, Ostroff RB, Hoffman RE. Naltrexone as a treatment for repetitive self-injurious behaviour:an open-label trial. J Clin Psychiatry. 1996;57(6):233–237. [PubMed] [Google Scholar]
- 47.Bostwick JM, Bucci JA. Internet sex addiction treated with naltrexone. Mayo Clinic proceedings. 2008;83(2):226–230. doi: 10.4065/83.2.226. [DOI] [PubMed] [Google Scholar]
- 48.Raymond NC, Grant JE, Coleman E. Augmentation with naltrexone to treat compulsive sexual behavior: a case series. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2010;22(1):56–62. [PubMed] [Google Scholar]
- 49.Grant JE, Kim SW. A case of kleptomania and compulsive sexual behavior treated with naltrexone. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2001;13(4):229–231. doi: 10.1023/a:1014626102110. [DOI] [PubMed] [Google Scholar]
- 50.Ryback RS. Naltrexone in the treatment of adolescent sexual offenders. J Clin Psychiatry. 2004;65(7):982–986. doi: 10.4088/jcp.v65n0715. [DOI] [PubMed] [Google Scholar]
- 51.Raymond NC, Grant JE, Kim SW, Coleman E. Treatment of compulsive sexual behaviour with naltrexone and serotonin reuptake inhibitors: two case studies. International clinical psychopharmacology. 2002;17(4):201–205. doi: 10.1097/00004850-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Lee MC, Wagner HN, Jr, Tanada S, Frost JJ, Bice AN, Dannals RF. Duration of occupancy of opiate receptors by naltrexone. J Nucl Med. 1988;29(7):1207–1211. [PubMed] [Google Scholar]
- 53. [April 22, 2010];Prescribing Information Naltrexone Hydrochloride Tablets USP. 2009 2010 http://pharmaceuticals.mallinckrodt.com/_attachments/PackageInserts/35_Naltrexone%20HCl%20Tablets_REV020509.pdf.
- 54.Kranzler HR, Tennen H, Armeli S, et al. Targeted naltrexone for problem drinkers. J Clin Psychopharmacol. 2009;29(4):350–357. doi: 10.1097/JCP.0b013e3181ac5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernandez-Avila CA, Song C, Kuo L, Tennen H, Armeli S, Kranzler HR. Targeted versus daily naltrexone: secondary analysis of effects on average daily drinking. Alcohol Clin Exp Res. 2006;30(5):860–865. doi: 10.1111/j.1530-0277.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- 56.Revised guidelines for HIV counseling, testing, and referral. MMWR Recomm Rep. 2001;50(RR-19):1–57. quiz CE51-19a51-CE56-19a51. [PubMed] [Google Scholar]
- 57.Brief D, Bollinger A, Horton G, LoCastro JS. Relapse Prevention Treatment for Cocaine Addiction: the RPT-C Manual. Bethesda, MD: Medication Development Division, National Institute on Drug Abuse; 1998. [Google Scholar]
- 58.Miller W, Rollnick S. Motivational Interviewing. 2nd. New York: Guilford Press; 2002. [Google Scholar]
- 59.Miller WR. Motivational interviewing with problem drinkers. Behavioural Psychotherapy. 1983;11:147–172. [Google Scholar]
- 60.DiClemente CC, Prochaska JO, Gilbertini M. Self-efficacy and the stages of self change in smoking. Cognitive Therapy and Research. 1985;9:181–200. [Google Scholar]
- 61.Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction. 2001;96(12):1725–1742. doi: 10.1046/j.1360-0443.2001.961217253.x. [DOI] [PubMed] [Google Scholar]
- 62.Samet JH, Rollnick S, Barnes H, Beyond CAGE A brief clinical approach after detection of substance abuse. Arch Intern Med. 1996;156(20):2287–2293. doi: 10.1001/archinte.156.20.2287. [DOI] [PubMed] [Google Scholar]
- 63.Rollnick S, Miller W. What is motivational interviewing? Cognit Behav Psychother. 1995;25:325–334. [Google Scholar]
- 64.Das M, Santos D, Matheson T, et al. Feasibility and acceptability of a phase II randomized pharmacologic intervention for methamphetamine dependence in high-risk men who have sex with men. Aids. doi: 10.1097/qad.0b013e328336e98b. Publish Ahead of Print:10.1097/QAD.1090b1013e328336e328398b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karhuvaara S, Simojoki K, Virta A, et al. Targeted nalmefene with simple medical management in the treatment of heavy drinkers: a randomized double-blind placebo-controlled multicenter study. Alcohol Clin Exp Res. 2007;31(7):1179–1187. doi: 10.1111/j.1530-0277.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- 66.Haberer JE, Kahane J, Kigozi I, et al. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010;14(6):1340–1346. doi: 10.1007/s10461-010-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DAIDS. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. 2009 [Google Scholar]
- 68.Metzger DS, Koblin B, Turner C, et al. Randomized controlled trial of audio computer-assisted self-interviewing: utility and acceptability in longitudinal studies. HIVNET Vaccine Preparedness Study Protocol Team. Am J Epidemiol. 2000;152(2):99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- 69.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 70.Coffin PO, Santos GM, Colfax G, et al. Adapted personalized cognitive counseling for episodic substance-using men who have sex with men: a randomized controlled trial. AIDS Behav. 2014;18(7):1390–1400. doi: 10.1007/s10461-014-0712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mausbach BT, Semple SJ, Strathdee SA, Zians J, Patterson TL. Efficacy of a behavioral intervention for increasing safer sex behaviors in HIV-positive MSM methamphetamine users: results from the EDGE study. Drug Alcohol Depend. 2007;87(2-3):249–257. doi: 10.1016/j.drugalcdep.2006.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mansergh G, Koblin BA, McKirnan DJ, et al. An intervention to reduce HIV risk behavior of substance-using men who have sex with men: a two-group randomized trial with a nonrandomized third group. PLoS medicine. 2010;7(8):e1000329. doi: 10.1371/journal.pmed.1000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Molina JM, Capitant C, Spire B, et al. On Demand PrEP With Oral TDF-FTC in MSM: Results of the ANRS Ipergay Trial. 2015 Conference on Retroviruses and Opportunistic Infections (CROI); 02/24/2015; Seattle, WA. 2015. [Google Scholar]
- 74.Peugh J, Belenko S. Alcohol, drugs and sexual function: a review. J Psychoactive Drugs. 2001;33(3):223–232. doi: 10.1080/02791072.2001.10400569. [DOI] [PubMed] [Google Scholar]
- 75.Chou NH, Huang YJ, Jiann BP. The Impact of Illicit Use of Amphetamine on Male Sexual Functions. The journal of sexual medicine. 2015;12(8):1694–1702. doi: 10.1111/jsm.12926. [DOI] [PubMed] [Google Scholar]
- 76.Frosch D, Shoptaw S, Huber A, Rawson RA, Ling W. Sexual HIV risk among gay and bisexual male methamphetamine abusers. J Subst Abuse Treat. 1996;13(6):483–486. doi: 10.1016/s0740-5472(96)00098-0. [DOI] [PubMed] [Google Scholar]
- 77.Semple SJ, Patterson TL, Grant I. Motivations associated with methamphetamine use among HIV+ men who have sex with men. J Subst Abuse Treat. 2002;22(3):149–156. doi: 10.1016/s0740-5472(02)00223-4. [DOI] [PubMed] [Google Scholar]
- 78.Rusch M, Lampinen TM, Schilder A, Hogg RS. Unprotected anal intercourse associated with recreational drug use among young men who have sex with men depends on partner type and intercourse role. Sex Transm Dis. 2004;31(8):492–498. doi: 10.1097/01.olq.0000135991.21755.18. [DOI] [PubMed] [Google Scholar]
- 79.Halkitis PN, Parsons JT, Stirratt MJ. A double epidemic: crystal methamphetamine drug use in relation to HIV transmission among gay men. Journal of homosexuality. 2001;41(2):17–35. doi: 10.1300/J082v41n02_02. [DOI] [PubMed] [Google Scholar]
- 80.Mansergh G, Shouse RL, Marks G, et al. Methamphetamine and sildenafil (Viagra) use are linked to unprotected receptive and insertive anal sex, respectively, in a sample of men who have sex with men. Sexually transmitted infections. 2006;82(2):131–134. doi: 10.1136/sti.2005.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scott HM, Vittinghoff E, Irvin R, et al. Age, race/ethnicity, and behavioral risk factors associated with per contact risk of HIV infection among men who have sex with men in the United States. Journal of acquired immune deficiency syndromes. 2014;65(1):115–121. doi: 10.1097/QAI.0b013e3182a98bae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paul S, Zhang X. Small sample GEE estimation of regression parameters for longitudinal data. Statistics in medicine. 2014;33(22):3869–3881. doi: 10.1002/sim.6198. [DOI] [PubMed] [Google Scholar]