Abstract
Background
In Duchenne muscular dystrophy (DMD), abnormal cardiac function is typically preceded by a decade of skeletal muscle disease. Molecular reasons for differences in onset and progression of these muscle groups are unknown. Human biomarkers are lacking.
Methods
We analyzed cardiac and skeletal muscle microarrays from normal and golden retriever muscular dystrophy (GRMD) dogs (ages 6, 12, or 47+ months) to gain insight into muscle dysfunction and to identify putative DMD biomarkers. These biomarkers were then measured using human DMD blood samples.
Results
We identified GRMD candidate genes that might contribute to the disparity between cardiac and skeletal muscle disease, focusing on brain-derived neurotropic factor (BDNF) and osteopontin (OPN/SPP1). BDNF was elevated in cardiac muscle of younger GRMD but was unaltered in skeletal muscle, while SPP1 was increased only in GRMD skeletal muscle. In human DMD, circulating levels of BDNF were inversely correlated with ventricular function and fibrosis, while SPP1 levels correlated with skeletal muscle function.
Conclusion
These results highlight gene expression patterns that could account for differences in cardiac and skeletal disease in GRMD. Most notably, animal model-derived data were translated to DMD and support use of BDNF and SPP1 as biomarkers for cardiac and skeletal muscle involvement, respectively.
INTRODUCTION
Duchenne muscular dystrophy (DMD) is caused by mutations in the DMD gene, resulting in severely reduced or absent dystrophin protein, which primarily affects striated muscle function (1). DMD natural history involves progressive skeletal muscle weakness leading to loss of ambulation, respiratory failure, and death in the second to third decade of life (2, 3). Although progressive respiratory failure was long the primary cause of DMD mortality, the advent of corticosteroid therapy and non-invasive ventilatory support has increased overall survival (4) such that cardiomyopathy is now the leading cause of death (5). This has heightened the importance of early identification of cardiomyopathy. Currently, prediction models incorporating advanced imaging can define abnormalities, but identifying which patients will exhibit the earliest onset and rapid progression has been elusive (6-8).
Despite tremendous progress in defining the molecular basis and pathogenesis of DMD since the identification of dystrophin (9), major gaps remain in our understanding of factors that contribute to disease progression. Animal models have been useful in studying the pathophysiologic mechanisms of DMD. The mdx mouse, the most widely used animal model of muscular dystrophy, has proven invaluable in a range of pre-clinical studies. However, the subtle nature of cardiac abnormalities (10) limits extrapolation to human disease (11-13). The golden retriever muscular dystrophy (GRMD) model closely approximates the progressive skeletal muscle involvement of human disease (12-14). Moreover, onset and progression of cardiac involvement in GRMD is delayed compared with skeletal muscle (12, 13) and follows a course more in line with that of human DMD (11, 15) (reviewed in reference (16)). Importantly, the severity of the cardiac and skeletal phenotypes varies markedly among dogs, similar to humans (12, 13). We used gene expression studies of GRMD cardiac and skeletal muscle to gain insights into the molecular pathways that might contribute to differences in onset and progression of cardiac versus skeletal muscle dysfunction. Because the GRMD model closely approximates human disease, we sought to identify biomarkers of dystrophin-associated cardiomyopathy in this model, and then translate our findings by studying sera from adolescent patients with DMD.
RESULTS
GRMD gene expression profiles are age-dependent and tissue-specific
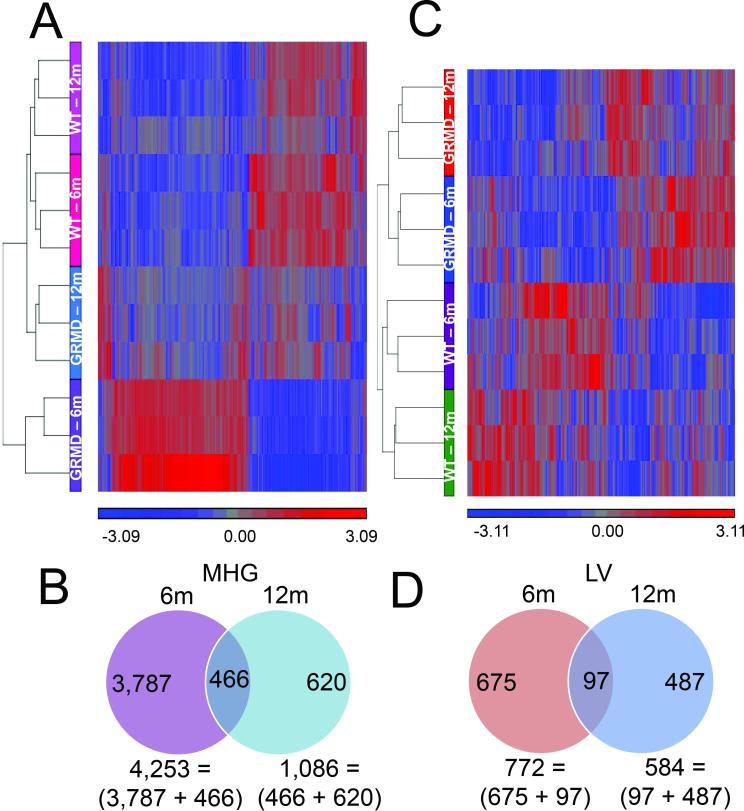
A total of 30 tissues (LV and MHG) from 15 dogs (6 normal and 9 GRMD) were grouped and analyzed according to age, disease, and tissue type (Table 1). For GRMD dogs versus age-matched controls, there were 4,873 probes detected at disparate levels between dystrophic and wild type MHG. The vast majority (~80%) were detected for the younger animals only, as shown by hierarchical clustering in Figure 1A, with only 466 probes altered in GRMD dogs of both ages (Figure 1B). These results suggest that age strongly influences the transcriptional processes that drive disease progression in dystrophic skeletal muscle, which is not surprising, given that the clinical course of disease is strongly age-dependent.
Table 1.
Overview of Gene Expression Analysis Results
| Pairwise Comparisons and Resulting Number of Significantly Differential Probes (p value < 0.01, fold-difference > 1.5) | ||||||
|---|---|---|---|---|---|---|
| Experiment | Baseline | No. Probes | ||||
| Age-matched comparisons | ||||||
| GRMD | 6m | MHG | WT | 6m | MHG | 4,253 |
| LV | LV | 772 | ||||
| 12m | MHG | 12m | MHG | 1,086 | ||
| LV | LV | 584 | ||||
| Tissue-matched comparisons | ||||||
| GRMD | 12m | MHG | GRMD | 6m | MHG | 3,154 |
| LV | LV | 627 | ||||
| 47-93m | MHG | MHG | 2,959 | |||
| LV | LV | 1,796 | ||||
| MHG | 12m | MHG | 783 | |||
| LV | LV | 1,175 | ||||
Figure 1. Microarray analysis of GRMD skeletal muscle.
Hierarchical clusters of 5,339 (A) or 1,259 (C) probes differentially expressed between GRMD and WT in skeletal muscle or LV, respectively. Vertical columns represent each of the clustered probes, and horizontal rows correspond to individual samples. Gene expression patterns are shown by color, with bright blue representing the lowest standardized signals, bright red the highest, and gray for median values as indicated by the scale bar beneath the cluster. Venn diagrams show distribution of gene expression differences among MHG (B), or LV (D) samples in 6 and 12 month-old dogs.
Dogs with GRMD typically do not have impaired ventricular function detectable by imaging, or symptoms of heart failure, until 2 years of age or considerably later (16), well beyond the onset of skeletal muscle involvement and consistent with the relatively delayed onset of cardiomyopathy in human DMD. Accordingly, cardiac function was not assessed for the 6-12 month-old GRMD dogs. No animal had overt evidence of clinical cardiac disease. Of the three oldest dogs, one had clinical evidence of heart failure and another died suddenly concerning for arrhythmia. The heart of these two dogs was not assessed by histopathology. The 93-month-old dog had severe cardiomyopathy. Necropsy showed cardiomegaly and marked myocardial fibrosis and mineralization was seen microscopically.
Despite the later onset of clinical cardiac versus skeletal muscle disease in GRMD dogs, LV gene expression patterns differed strongly at 6 and 12 months. Pairwise comparisons of GRMD versus wild type LV samples yielded a total of 1,259 differentially expressed probes (Figure 1C), 772 and 584 of which were significantly different between dogs aged 6 and 12 months-old, respectively (Figure 1D). Consistent with an age effect, < 8% of these 1,259 significantly differential probes were altered at both ages (Figure 1D, examples shown in Supplemental Table S1 (online)).
Identification of GRMD cardiomyopathy gene candidates
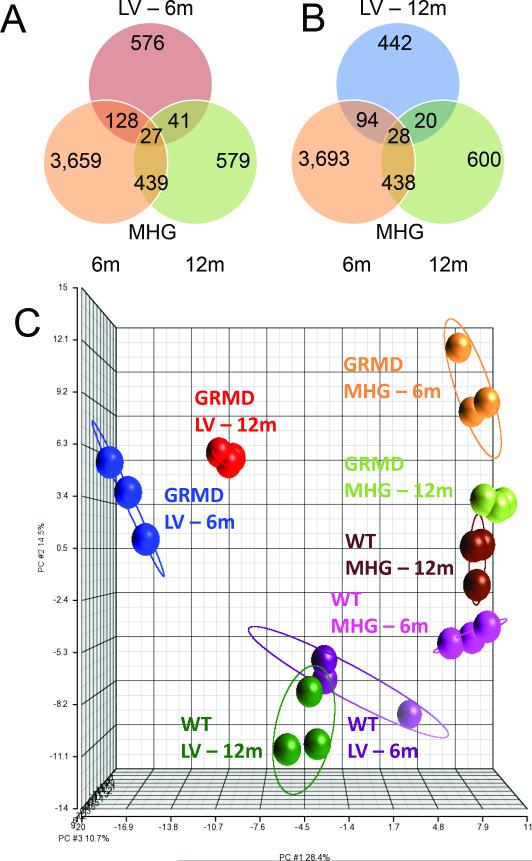
There was very little overlap (~3%) in the expression profiles of GRMD LV and MHG tissues for either age group (Figures 2A and 2B). Moreover, GRMD LV expression profiles were distinct from MHG, suggesting a cardiac-specific transcriptional program (Figure 2C). We also noted a small subset of genes altered in both LV and MHG tissues, relative to normal controls, but in the opposite direction (Supplemental Figure S1 (online)). For example, the gene encoding HMGB3, which was recently shown to be suppressed in regenerating skeletal muscle (17), was down-regulated in younger and older LV but up-regulated in MHG from 6 month-old dogs.
Figure 2. Microarray analysis of GRMD cardiac muscle and comparison with skeletal muscle.
A, Venn diagram showing overlap of genes differentially expressed between GRMD versus WT in LV at 6 months (red circle), and in MHG at 6 months (orange circle) and 12 months (light green circle). B, Venn diagram showing overlap of genes differentially expressed between GRMD versus WT in LV at 12 months (blue circle), and in MHG at 6 months (orange circle) and 12 months (light green circle). C, Principal components analysis (PCA) of 1,259 transcripts detected as significantly differential in GRMD versus WT LV. The first three principle components for these 1,259 probes were plotted to show the spatial relationships among all 8 groups included in the study.
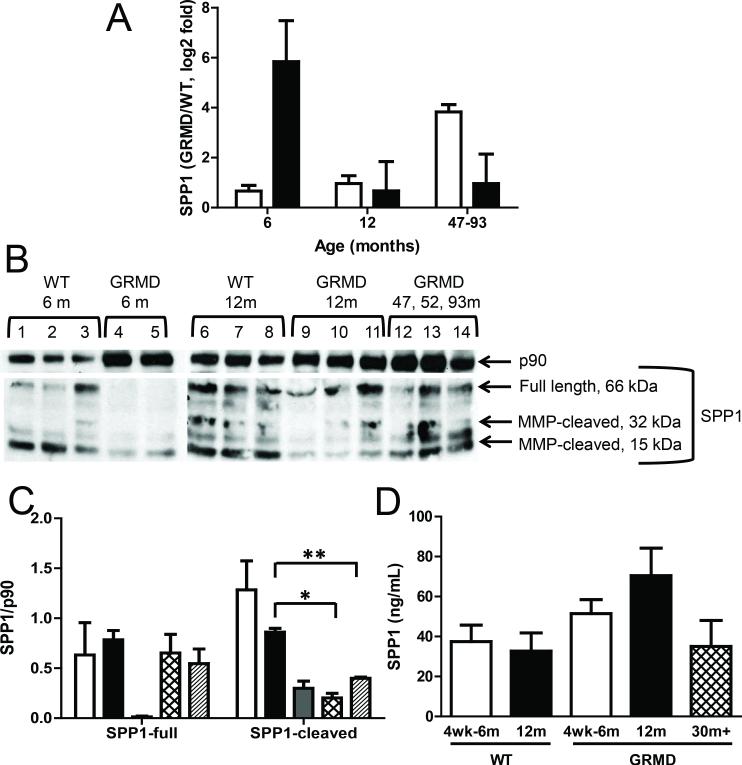
The most highly induced transcript in our MHG data set was SPP1, which is a strong indicator of muscle injury (18). In contrast, SPP1 was unaltered in matched LV from these dogs, consistent with a lack of cardiac muscle injury in the earlier stages of muscular dystrophy. Interestingly, SPP1 was increased in the LV of the three additional dogs aged 47 to 93 months (Figure 3A) and thus appeared to track with the progression of disease (e.g. earlier for skeletal versus cardiac muscle). SPP1 protein levels, on the other hand, were inversely related to transcript levels (Figure 3B and 3C), perhaps pointing to a feedback mechanism. Consistent with secretion of SPP1 protein into the blood, we detected higher levels of serum SPP1 (Figure 3D), although this difference was not quite significant (p=0.052) using the standard p value cut off. Given the proportionally greater total body mass of skeletal versus cardiac muscle, one would expect serum levels of SPP1 protein to track with skeletal myopathy, which appeared to be the case (Figure 3D). As an example, SPP1 protein levels in the three 47 to 93 month-old GRMD dogs were similar to levels detected in WT animals (Figure 3B and3C). We did not observe any differences in SPP1 protein levels in LV tissues (data not shown).
Figure 3. SPP1 transcript and protein levels in GRMD.
A, Bar graph shows qPCR results for SPP1 mRNA in LV (white bars) and MHG (black bars) tissues from GRMD versus WT dogs. Each group shown includes data from three animals, and error bars represent SEM. B, Western blots for SPP1 and p90 (loading control) in MHG tissues of WT and GRMD dogs are shown. Insufficient protein levels were obtained for assessing SPP1 protein levels in MHG tissues from one of the 6 month-old GRMD dogs, and this animal was therefore excluded. The anti-SPP1 antibody used recognizes both the full length and two MMP-cleaved fragments (middle blot). C, Bar graph shows semi-quantification of Western blot results for SPP1 (full-length and cleaved forms) and total p90, as calculated by densitometry for WT MHG tissues from dogs aged 6 months (white bars) or 12 months (back bars) and for GRMD MHG tissues from dogs aged 6 months (gray bars), 12 months (hatched bars), or aged 47-93 months (diagonally striped bars). Asterisks indicate significance (p < *0.05 or **0.005). D, Bar graph shows serum levels of SPP1 protein as measured by ELISA for young (white bars), 12 month-old (black bars), and considerably aged (hatched bars) animals.
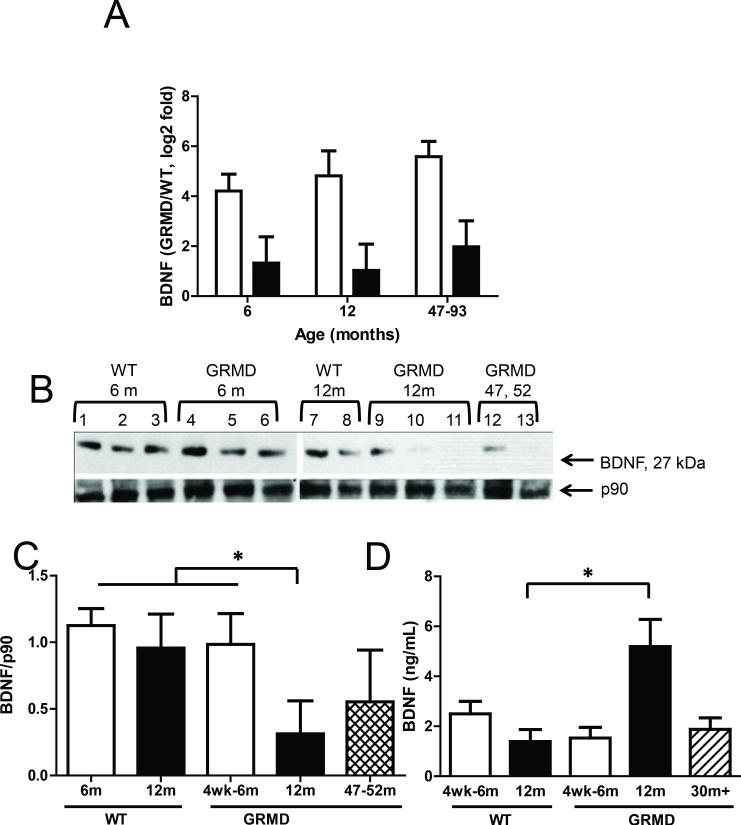
The most significantly altered ‘cardiac-specific’ transcript was BDNF, which was up-regulated more profoundly in 6 versus 12 month-old GRMD dogs and maintained at high levels in the 47 to 93 month-old animals (Figure 4A). BDNF is a particularly attractive candidate as a cardiac marker and modulator because it is a secreted factor that has been shown to contribute to cardiac angiogenesis (19) and muscle repair (20). Surprisingly, protein levels of BDNF were not increased in GRMD LV tissues relative to wild type controls. In fact, levels in the 47 to 93 month-old dogs were depressed, which could be consistent with cardiac disease progression at this later age (Figure 4B and 4C). BDNF protein was undetectable in skeletal muscle tissues (data not shown), consistent with low BDNF transcript levels as measured by microarrays and qPCR. Serum levels of BDNF on the other hand were significantly and steeply increased for 12 month-old GRMD dogs compared to age-matched control animals (Figure 4D). However, levels in the 47 to 93 month-old dogs were similar to those in 6 month-old GRMD and control animals. One interpretation for the inverse correlation between tissue and serum levels of BDNF is that LV mRNA production increases in dystrophic dogs but BDNF protein is secreted into the circulation rather than being retained in the tissue.
Figure 4. BDNF transcript and protein levels in GRMD.
A, Bar graph shows qPCR results for BDNF in tissues in LV (white bars) and MHG (black bars) tissues from GRMD versus WT dogs. Each group shown includes data from three animals, and error bars represent SEM. B, Western blots for BDNF and p90 (loading control) in LV tissues of WT and GRMD dogs are shown, excluding the 93 month-old GRMD dog for which LV protein was insufficient. C, Bar graph showing semi-quantification of Western blot results for BDNF, as calculated by densitometry, for LV tissues from WT and GRMD dogs aged 6 months (white bars), 12 months (back bars), or 47-93 months (diagonally striped bars). Asterisks indicate significance (p < *0.005). D, Bar graph shows serum levels of BDNF protein as measured by ELISA for 4 week or 6 month-old (white bars), 12 month-old (black bars), and considerably aged (hatched bars) animals. the groups indicated in the legend shown beneath the graph. Asterisks indicate significance (**p = 0.001).
Validation of GRMD biomarkers in a cohort of DMD patients and normal controls
In order to further evaluate the clinical implications of BDNF and SPP1 in DMD, we measured circulating protein levels in a cohort of 18 DMD subjects and 8 healthy controls. The mean CMR LVEF in the DMD cohort was 49.5% ± 9.2; 10 DMD subjects had abnormal LVEF (defined as LVEF < 55%). A total of 13 DMD subjects had at least one segment with fibrosis or LGE.
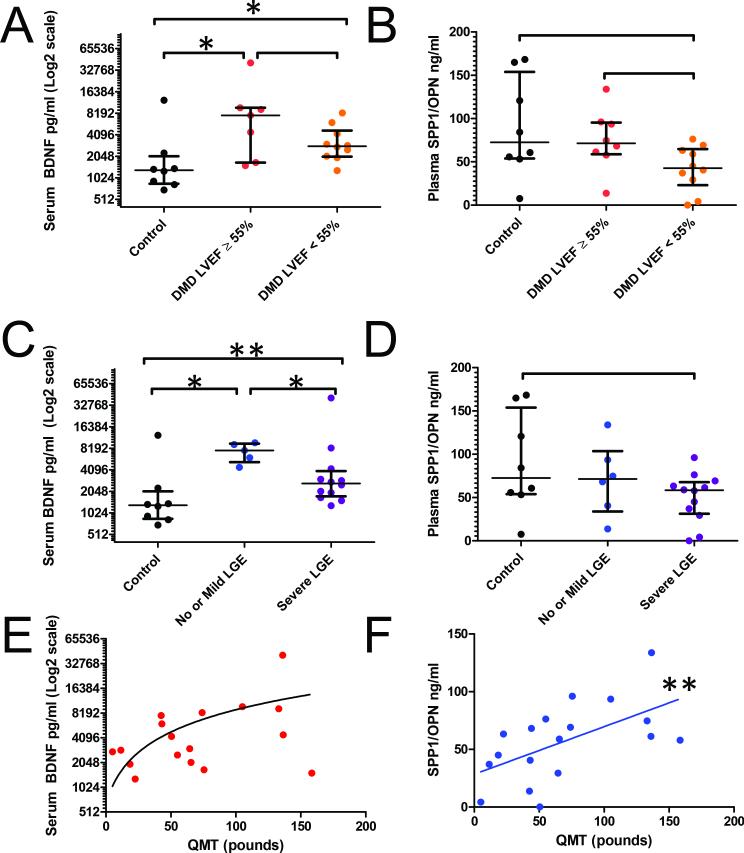
BDNF levels were significantly higher in DMD subjects when compared to controls (Figure 5A), while only a trend for statistical significance was noted for SPP1 (Figure 5B). To assess the relationship between BDNF levels and CMR measures, the DMD cohort was separated as follows: 1) LV function: DMD subjects with normal (>55%) or abnormal LVEF (<55%); and 2) fibrosis: DMD subjects with severe myocardial fibrosis, defined as >2 segments with LGE, and those with mild or no fibrosis. BDNF was higher in DMD subjects with normal versus abnormal LVEF but was not statistically significant (p = 0.193), presumably due to wide variation of the levels. There was also a significant difference among all groups for BDNF when stratified by level of fibrosis (Figure 5C), but again only a trend for significance for SPP1 (Figure 5D). DMD subjects with mild or no fibrosis had higher median serum BDNF levels compared with DMD subjects with severe fibrosis.
Figure 5. Serum levels of BDNF and SPP1 in DMD and control subjects.
BDNF and SPP1 proteins were measured by ELISA, and results are shown for individuals stratified by heart function, as measured by cardiac magnetic resonance (CMR) left ventricular ejection fraction (LVEF) (A and B) or fibrosis, measured by CMR late gadolinium enhancement (LGE) (C and D). Correlations of BDNF (E, r=0.3, p=0.248) and SPP1 (F, r=0.6, p=0.009) with Quantitative Muscle Testing (QMT) are also demonstrated. Asterisks indicate statistical significance (*p<0.05, **p<.01).
As would be expected, SPP1 blood levels were significantly and linearly correlated with muscle function, as assessed using Quantitative Muscle Testing (QMT) (Figure 5F). Serum levels of BDNF did not correlate significantly with QMT (Figure 5E).
DISCUSSION
This study was designed to identify gene expression differences between cardiac and skeletal muscle that could account for the later onset of cardiomyopathy. We reasoned that this could occur due to selective expression of injurious or protective proteins in cardiac and skeletal muscle. Gene expression was compared in samples of LV and MHG from 6 and 12 month-old GRMD and age-matched wild type dogs, as well as an older group of GRMD dogs. Whereas relatively few expression differences were seen between LV samples from 6 and 12 month-old dogs compared to wild type controls, there was an approximately 400% greater level of transcriptional perturbation in MHG from these same dogs (e.g., 4,253 and 1,086 differential transcripts in 6 versus 12 month-old GRMD animals when compared to age-matched controls) (Figure 1B). This is consistent with the more dramatic progression of skeletal muscle disease over this age period, while cardiac muscle function is relatively stable. More interestingly, fewer than 25% of the differentially expressed LV genes were also altered in MHG. Transcripts that were common to GRMD LV and MHG tissues were mainly those important for lipid metabolism, energy production, and inflammatory responses, consistent with the known pathophysiology of dystrophic muscle.
Based on GRMD expression analysis, we identified a putative cardiac-specific transcript, BDNF, which we further investigated for relevancy and translational potential. BDNF is increased after myocardial infarction and protects against ischemic injury (21). Low plasma levels of BDNF were also previously reported to be an indicator of poor prognosis in patients with angina pectoris (22) and more recently associated with adverse outcomes in human heart failure (23). In both GRMD dogs and DMD subjects, circulating levels of BDNF were higher in dystrophic versus control subjects. Although stratification by LV function did not result in statistically significant differences in circulating BDNF (p = 0.193), there was a large mean difference (7,630 pg/ml vs 2,830 pg/ml) for normal versus abnormal LVEF, respectively, in DMD subjects. Power analysis indicated that a larger cohort would yield significant results, assuming a similar pattern emerged (Supplemental Figure S2 (online)). Such results would strongly support a role for BDNF in DMD cardiac tissue and suggest a protective function for BDNF early in the disease when cardiac function is relatively preserved compared to skeletal muscle. The observed inverse correlation of DMD subject serum BDNF levels with fibrosis further supports this notion. In previous studies serum BDNF levels were also inversely correlated with frequency of exercise and exercise intolerance in human heart failure (23, 24), which makes sense given its role in skeletal muscle metabolism (25, 26).
SPP1 was previously shown to be increased and to seemingly track with severity of DMD and GRMD skeletal muscle disease (27, 28). The fact that SPP1 transcript and protein levels were selectively increased in GRMD skeletal versus cardiac muscle in this study strongly suggests that SPP1 contributes to the earlier onset of skeletal myopathy. In DMD subjects, serum SPP1 levels correlated with skeletal muscle function, pointing to its potential value as a skeletal muscle disease biomarker (Figure 5). On the other hand, SPP1 serum levels did not track with LV function and fibrosis in DMD subjects, indicating that SPP1 is probably not a reliable biomarker for cardiac function.
In conclusion, we have identified age-dependent and tissue-specific transcriptional differences in GRMD cardiac and skeletal muscle that could serve as predictive or prognostic biomarkers; this will facilitate future studies in which gene expression is more closely associated with specific phenotypes. We validated two of these potential biomarkers in human DMD subjects, which add credibility to the dog as a translational model. BDNF in particular is an attractive candidate as a cardiac-specific biomarker.
MATERIALS AND METHODS
Golden retriever muscular dystrophy dogs
The study was approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. All dogs were used and cared for according to principles outlined in the National Research Council Guide for the Care and Use of Laboratory Animals and housed at the University of North Carolina at Chapel Hill. Dogs were confirmed as affected by elevated serum creatine kinase (CK) and genotyping. A total of 15 animals were included: 3 young GRMD dogs (6-7.5 months), 3 older GRMD dogs (12-13 months), and 6 age-matched wild type control animals; plus 3 GRMD dogs of advanced age (47, 52, and 93 months-old).
From a physiologic standpoint, the first year of a golden retriever's life is analogous to the first 20 years of a human's (29). Thus, by dividing these ages into quartiles, 6 and 12 golden retriever months would equate to 10 and 20 human years. The natural disease course of GRMD tends to parallel DMD up to 6 months, with an onset of debilitating weakness and postural changes in both conditions(16).
Serum samples were collected by venipuncture from each dog at 4-6 weeks of age and at the time of necropsy, stored at −80° C, and shipped in cryovials to Vanderbilt. . Samples of the medial head of the gastrocnemius (MHG), a tibiotarsal joint extensor, and left ventricular (LV) free wall were removed at necropsy, snap frozen in a Freon substitute, cooled in liquid nitrogen, stored at −80° C, and shipped in cryovials to Vanderbilt for analysis. Tibiotarsal joint flexors and extensors are differentially affected in GRMD, with flexors showing weakness at 3 months and recovering somewhat by 6 months, and extensors such as the medial head of the gastrocnemius having a more delayed but progressive response over the first year (11).
Humans
After Institutional Review Board approval, a cohort of 18 human DMD subjects (16.1 +/− 5.6 years) and 8 healthy controls (14 ± 2.7 years, p = 0.23) were enrolled in the study. All subjects and/or parents provided informed consent and/or assent where appropriate. All DMD subjects had diagnosis confirmed by skeletal muscle biopsy or the presence of a DMD mutation in the setting of skeletal muscle weakness. A total of 16 DMD subjects were corticosteroid treated at some point with 15 receiving therapy for greater than 6 months and 9 presently taking corticosteroids at the time of enrollment. Only one DMD subject was ambulatory at the time of enrollment. Exclusion criteria were the following: inability to undergo cardiac magnetic resonance imaging (CMR) without sedation or other contraindication to CMR with contrast.
DMD subjects underwent CMR to evaluate left ventricular ejection fraction (LVEF) and late gadolinium enhancement (LGE) as previously described (30). Plasma was withdrawn from the peripheral intravenous line at the time of the CMR and stored at −80° C. Skeletal muscle function was assessed using Quantitative Muscle Testing (QMT).
All control subjects received cardiovascular evaluation, the results of which were normal. Plasma was drawn using venipuncture before testing and stored at −80° C. Exclusion criteria for control subjects were: congenital heart disease, cardiomyopathy, or other secondary heart disease. Blood levels of osteopontin (OPN/SPP1, hereafter indicated as SPP1) were measured in all 26 individuals. BDNF was measured in only 25 individuals, as serum was unavailable for one DMD subject.
Microarrays
Total RNA was isolated from tissues according to the manufacturer's protocol (Qiagen, Germantown, MD). Quality assessment of RNA, further processing, and data acquisition were performed by the GSR Microarray Core at Vanderbilt. Canine Gene 1.0 Expression arrays (Affymetrix, Inc., Santa Clara, CA) were used, one array per sample. Raw data were normalized, followed by two-way ANOVA using Partek Genomics Suite 6.6 (Partek Incorporated, St. Louis, MO) and deposited in GEO (GSE68626). Only probes that resulted in a fold-change of at least 1.5 and p value of less than 0.05 were considered altered.
Quantitative PCR (qPCR)
Validated canine-specific primers (Quantitect Primer Assays) for BDNF and SPP1 were purchased from Qiagen. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Qiagen) served as an internal control. cDNA was synthesized from RNA using iScript (Bio-Rad Laboratories, Hercules, CA), and relative gene expression was assessed using Taq SYBR Green PCR (Bio-Rad Laboratories) in a Bio-Rad CFX instrument, as previously described (31).
Protein analyses
Western blot analysis was performed as previously described (31) using rabbit anti-BDNF (Aviva Systems Biology, San Diego, CA), anti-SPP1, Rockland Immunochemicals, Inc., Limerick, PA), or anti-p90 (Cell Signaling Technologies, Inc., Danvers, MA). Serum or plasma samples were assessed by enzyme-linked immunosorbent assay (ELISA) for BDNF and SPP1 with canine-specific (USCN Life Science Inc., Wuhan, Hubei, China) and human-specific (R&D Systems, Inc., Minneapolis, MN) ELISA kits. Each sample was run in duplicate, and average concentrations were determined based on a standard curve.
Statistics
Differences between groups were evaluated by t test, with data expressed as mean ± SEM, unless otherwise stated. Human ELISA data were analyzed using a Kruskal-Wallis to evaluate for significant differences among the three groups (DMD with normal LVEF, DMD with abnormal LVEF, and control) or Mann Whitney U to assess differences between groups. Analyses were performed with IBM SPSS statistics, version 21.0 (Armonk, NY: IBM Corp). G*Power version 3.1.5 (32) was used for power analysis. Patient data were collected and managed using Research Electronic Data Capture (REDcap) tools hosted at Vanderbilt (33).
Supplementary Material
Acknowledgments
Financial Support: This project was supported in part by the Fighting Duchenne Foundation and the Fight DMD/Jonah & Emory Discovery Grant (Nashville, TN) (Markham).
This work was supported by American Heart Association Grant 13CRP14530007 (Soslow) (Dallas, TX).
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL123938 (Bethesda, MD) (Soslow), Award Number K01HL121045 (Galindo), and U01 HL100398 (Sawyer).
The project was supported by the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06 (Bethesda, MD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures: The authors have no potential/perceived conflicts of interest or other disclosures to report.
REFERENCES
- 1.Moser H. Duchenne muscular dystrophy: pathogenetic aspects and genetic prevention. Human genetics. 1984;66:17–40. doi: 10.1007/BF00275183. [DOI] [PubMed] [Google Scholar]
- 2.Boland BJ, Silbert PL, Groover RV, Wollan PC, Silverstein MD. Skeletal, cardiac, and smooth muscle failure in Duchenne muscular dystrophy. Pediatr Neurol. 1996;14:7–12. doi: 10.1016/0887-8994(95)00251-0. [DOI] [PubMed] [Google Scholar]
- 3.Perloff JK, de Leon AC, Jr., O'Doherty D. The cardiomyopathy of progressive muscular dystrophy. Circulation. 1966;33:625–48. doi: 10.1161/01.cir.33.4.625. [DOI] [PubMed] [Google Scholar]
- 4.Markham LW, Spicer RL, Khoury PR, Wong BL, Mathews KD, Cripe LH. Steroid therapy and cardiac function in Duchenne muscular dystrophy. Pediatric cardiology. 2005;26:768–71. doi: 10.1007/s00246-005-0909-4. [DOI] [PubMed] [Google Scholar]
- 5.Bach JR, Martinez D. Duchenne muscular dystrophy: continuous noninvasive ventilatory support prolongs survival. Respiratory care. 2011;56:744–50. doi: 10.4187/respcare.00831. [DOI] [PubMed] [Google Scholar]
- 6.Ryan TD, Taylor MD, Mazur W, et al. Abnormal circumferential strain is present in young Duchenne muscular dystrophy patients. Pediatric cardiology. 2013;34:1159–65. doi: 10.1007/s00246-012-0622-z. [DOI] [PubMed] [Google Scholar]
- 7.Hor KN, Wansapura JP, Al-Khalidi HR, et al. Presence of mechanical dyssynchrony in Duchenne muscular dystrophy. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2011;13:12. doi: 10.1186/1532-429X-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hor KN, Wansapura J, Markham LW, et al. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance tagging study. Journal of the American College of Cardiology. 2009;53:1204–10. doi: 10.1016/j.jacc.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonilla E, Samitt CE, Miranda AF, et al. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988;54:447–52. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- 10.McGreevy JW, Hakim CH, McIntosh MA, Duan D. Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Disease models & mechanisms. 2015;8:195–213. doi: 10.1242/dmm.018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valentine BA, Cummings JF, Cooper BJ. Development of Duchenne-type cardiomyopathy. Morphologic studies in a canine model. The American journal of pathology. 1989;135:671–8. [PMC free article] [PubMed] [Google Scholar]
- 12.McNally EM, Kaltman JR, Benson DW, et al. Contemporary cardiac issues in duchenne muscular dystrophy. Circulation. 2015;131:1590–8. doi: 10.1161/CIRCULATIONAHA.114.015151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swaggart KA, McNally EM. Modifiers of heart and muscle function: where genetics meets physiology. Experimental physiology. 2014;99:621–6. doi: 10.1113/expphysiol.2013.075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howell JM, Fletcher S, Kakulas BA, O'Hara M, Lochmuller H, Karpati G. Use of the dog model for Duchenne muscular dystrophy in gene therapy trials. Neuromuscular disorders : NMD. 1997;7:325–8. doi: 10.1016/s0960-8966(97)00057-6. [DOI] [PubMed] [Google Scholar]
- 15.Sharp NJ, Kornegay JN, Van Camp SD, et al. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992;13:115–21. doi: 10.1016/0888-7543(92)90210-j. [DOI] [PubMed] [Google Scholar]
- 16.Kornegay JN, Bogan JR, Bogan DJ, et al. Canine models of Duchenne muscular dystrophy and their use in therapeutic strategies. Mamm Genome. 2012;23:85–108. doi: 10.1007/s00335-011-9382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maciotta S, Meregalli M, Cassinelli L, et al. Hmgb3 is regulated by microRNA-206 during muscle regeneration. PloS one. 2012;7:e43464. doi: 10.1371/journal.pone.0043464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirata A, Masuda S, Tamura T, et al. Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: a role for osteopontin. The American journal of pathology. 2003;163:203–15. doi: 10.1016/S0002-9440(10)63644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends in cardiovascular medicine. 2007;17:140–3. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clow C, Jasmin BJ. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Molecular biology of the cell. 2010;21:2182–90. doi: 10.1091/mbc.E10-02-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada S, Yokoyama M, Toko H, et al. Brain-derived neurotrophic factor protects against cardiac dysfunction after myocardial infarction via a central nervous system-mediated pathway. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1902–9. doi: 10.1161/ATVBAHA.112.248930. [DOI] [PubMed] [Google Scholar]
- 22.Jiang H, Liu Y, Zhang Y, Chen ZY. Association of plasma brain-derived neurotrophic factor and cardiovascular risk factors and prognosis in angina pectoris. Biochemical and biophysical research communications. 2011;415:99–103. doi: 10.1016/j.bbrc.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Fukushima A, Kinugawa S, Homma T, et al. Serum Brain-Derived Neurotrophic Factor Level Predicts Adverse Clinical Outcomes in Patients with Heart Failure. Journal of cardiac failure. 2015 doi: 10.1016/j.cardfail.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima A, Kinugawa S, Homma T, et al. Decreased serum brain-derived neurotrophic factor levels are correlated with exercise intolerance in patients with heart failure. International journal of cardiology. 2013;168:e142–4. doi: 10.1016/j.ijcard.2013.08.073. [DOI] [PubMed] [Google Scholar]
- 25.Matthews VB, Astrom MB, Chan MH, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–18. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 26.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Medicine and science in sports and exercise. 2007;39:728–34. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 27.Zanotti S, Gibertini S, Di Blasi C, et al. Osteopontin is highly expressed in severely dystrophic muscle and seems to play a role in muscle regeneration and fibrosis. Histopathology. 2011;59:1215–28. doi: 10.1111/j.1365-2559.2011.04051.x. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura A, Kobayashi M, Kuraoka M, et al. Initial pulmonary respiration causes massive diaphragm damage and hyper-CKemia in Duchenne muscular dystrophy dog. Scientific reports. 2013;3:2183. doi: 10.1038/srep02183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. The journals of gerontology Series A, Biological sciences and medical sciences. 1997;52:B171–8. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- 30.Soslow JH, Damon BM, Saville BR, et al. Evaluation of post-contrast myocardial t1 in duchenne muscular dystrophy using cardiac magnetic resonance imaging. Pediatric cardiology. 2015;36:49–56. doi: 10.1007/s00246-014-0963-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galindo CL, Kasasbeh E, Murphy A, et al. Anti-remodeling and anti-fibrotic effects of the neuregulin-1beta glial growth factor 2 in a large animal model of heart failure. Journal of the American Heart Association. 2014;3:e000773. doi: 10.1161/JAHA.113.000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior research methods. 2009;41:1149–60. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.