Abstract
Recent work suggests that dissociable activity in theta and delta frequency bands underlies several common event-related potential (ERP) components, including the nogo N2/P3 complex, which can better index separable functional processes than traditional time-domain measures. Reports have also demonstrated that neural activity can be affected by stimulus sequence context information (i.e., the number and type of preceding stimuli). Stemming from prior work demonstrating that theta and delta index separable processes during response inhibition, the current study assessed sequence context in a Go/Nogo paradigm in which the number of go stimuli preceding each nogo was selectively manipulated. Principal component analysis (PCA) of time-frequency representations revealed differential modulation of evoked theta and delta related to sequence context, where delta increased robustly with additional preceding go stimuli, while theta did not. Findings are consistent with the view that theta indexes simpler initial salience-related processes, while delta indexes more varied and complex processes related to a variety of task parameters.
Keywords: Sequential effects, response inhibition, time-frequency, EEG, theta, delta
Amplitude of the P3(00) event-related potential (ERP) elicited during target detection tasks is modulated by the number and nature of preceding stimuli sequences, such that an alteration in a sequence of stimuli repetitions elicits a larger P3 than a continuation of the repetition (Gonsalvez, Gordon, Grayson, Barry, Lazzaro, & Bahramali, 1999; Johnson & Donchin, 1980; Squires, Petuchowski, Wickens, & Donchin, 1977; Squires, Wickens, Squires, & Donchin, 1976). It has been proposed that (at least) two co-occurring subcomponents may be active during cognitive/sensory processing, namely an early generic salience detection/attentional allocation process and a later process related to more elaborative stimulus processing/memory context updating (Polich, 2007). This suggests that ERPs may be mixtures of separable spatiotemporally overlapping processes that are difficult to disentangle using conventional time-domain component measures (e.g., midfrontal P3a and centroparietal P3b; Debener, Makeig, Delorme, & Engel, 2005; Spencer, Dien, & Donchin, 2001; Squires, Squires, & Hillyard, 1975). Furthermore, recent work using time-frequency analysis applied to ERP signals has offered increased evidence for this two-process model, with several studies suggesting that that many common ERP components (e.g., N2[00], P3) consist of multiple temporally superimposed processes operating in different frequency bands (e.g., delta, theta) that often uniquely relate to different stimulus/task dimensions, with midfrontal theta possibly reflecting a simple detection of stimulus salience/conflict and centroparietal delta indexing more complex and multifaceted stimulus evaluation/processing (Bernat, Malone, Williams, Patrick, & Iacono, 2007; Bernat, Nelson, Steele, Gehring, & Patrick, 2011; Demiralp, Ademoglu, Comerchero, & Polich, 2001; Harper, Malone, & Bernat, 2014; Karakaş, Erzengin, & Başar, 2000a, 2000b; Kolev, Demiralp, Yordanova, Ademoglu & Isoglu-Alkaç, 1997; Spencer & Polich, 1999; Yordanova, Devrim, Kolev, Ademoglu, & Demiralp, 2000). It remains unclear to what extent stimulus sequence effects differentially engage these two subcomponents (i.e., early attentional allocation, later elaborative processing), and how time-frequency specific activity (e.g., theta, delta) may be sensitive to sequence effects.
Stimulus Sequence Context Modulates Brain Responses
Several studies have demonstrated that stimulus sequence context can modulate brain responses to target stimuli. Evidence from studies using electroencephalography suggests that centroparietal P3b amplitude to target stimuli increases in a linear fashion as the number of preceding nontargets increases, or when a repetition of identical stimuli is terminated by an alternative stimulus (Giese-Davis, Miller, & Knight, 1993; Gonsalvez, Gordon, Anderson, Pettigrew, Barry, Rennie, & Meares, 1995; Gonsalvez et al., 1999; Gilmore, Malone, & Iacono, 2012; Johnson & Donchin, 1980; Polich & Bondurant, 1997; Squires et al., 1977; Squires et al., 1976). This effect is often framed in terms of contextual updating of working memory when a sequence of repetitions is broken by a stimulus alteration (Donchin, 1981), or increased attentional resource allocation to target stimuli (Polich, 2007). These findings suggest that the context of a stimulus is influenced in part by information related to the nature of prior stimuli. Sequence effects have also been shown to affect brain activity during inhibitory processing. A pair of studies using functional magnetic resonance imaging during a response inhibition task which modulated sequence context by increasing the number of go trials preceding nogo trials, indicated a variety of regions, including the ventral prefrontal cortex, cingulate gyrus, supplementary motor regions, and superior parietal regions, that exhibited increases in BOLD activation during demands of response inhibition following longer preceding repetitions of response commission (Durston, Thomas, Yang, Uluğ, Zimmerman, & Casey, 2002; Durston, Thomas, Worden, Yang, & Casey, 2002). These findings suggest that regions associated with the online maintenance of task demands are sensitive to stimulus context based on preceding stimulus sequences. Interestingly, these sequence effects were not observed in children, suggesting that this fronto-striatal circuitry is sensitive to developmental changes in stimulus sequence processing (Durston, Thomas, Yang, et al., 2002). While general sequence effects have been called into question by findings suggesting that the target-to-target interval (i.e., the time between two target stimuli) may be the driving factor behind centroparietal P3b sequence effects (Gonsalvez & Polich, 2002), it remains unclear whether sequence effects affect the P3a-like response during motor inhibition/target detection (e.g., no-go P3), and to what degree different sub-processes (e.g., early attention, later elaborative stimulus processing) may be modulated by classical sequence effects when stimuli are presented with short interstimulus intervals (as is common in most cognitive tasks).
Multiple Subcomponents of Cognitive Processing
The idea that ERPs contain multiple overlapping subcomponents related to cognitive processing has long been understood to be a confounding factor in work with time-domain ERP components. Beyond simple activity in perceptual cortices in response to incoming stimulation, convergent evidence now suggests that two separable processes overlapping in time and topography contribute to time-domain ERP measures, where the relative weight of their contribution is based on their unique sensitivity to stimulus dimensions in a specific task paradigm (e.g., novelty, target detection; loss/gain processing). The first is related to early salience detection or attentional allocation with a frontocentral distribution, and the second is related to later higher-order stimulus evaluation and memory updating which exhibits a centroparietal distribution (Polich, 2007). This two-process model is related to a central hypothesis regarding P3 function (Polich, 2007), wherein the P3 response may be a mechanism of neural inhibition of ongoing brain activity during events that require deployment of attentional resources, and is subserved by two subcomponents, the midfrontal P3a (reflecting initial attentional or orienting response, with a possible source in anterior regions [Bledowski et al., 2004; Dien, Spencer, and Donchin, 2003]) and the centroparietal P3b (involved in elaborative stimulus processing or context updating, with possible sources in parietal regions [Bledowski et al., 2004; Dien et al., 2003]). Using spatiotemporal PCA applied to the P3 elicited during a novelty oddball task, Spencer and colleagues (2001) identified two spatially distinct subcomponents (i.e., one earlier anterior component and one later centroparietal component), which occurred together in both target and novel conditions but with different weights depending on the stimuli category. This finding suggests the presence of two overlapping, yet separable, processes related to task-relevant and task-irrelevant processing, which co-occur in time and space but are summed together in traditional time-domain component measures. Independent component analysis (ICA) has also been useful in disentangling target-related centroparietal activity from earlier frontocentral novelty-related activity, and findings suggest that both components contribute in varying degree to both target and nontarget novelty conditions (Debener et al., 2005), again supporting the idea that EEG activity as measured in the time-domain contains mixtures of these two subcomponents.
Two ERP components related to inhibitory processing, the nogo N2 and nogo P3, also offer support for this two-process model. The nogo-N2 is a frontocentral negative voltage deflection occurring around 250 ms, while the nogo-P3 is a centroparietal positive voltage deflection occurring later during the response (300-500 ms). Both components are sensitive to demands of response inhibition (Fallgatter, Brandeis, & Strik, 1997; Kok, 1986; Kopp, Mattler, Goertz, & Rist, 1996; Pfefferbaum, Ford, Weller, & Kopell, 1985), but they exhibit differential relations to stimulus probability (Lavric, Pizzagalli, & Forstmeier, 2004), discriminability (Pfefferbaum et al., 1985), modality (i.e, visual vs. auditory; Falkenstein, Hoormann, & Hohnsbein, 1999), and response type (i.e., covert vs. overt inhibition; Smith, Johnstone, & Barry, 2008). These two components have distinct, yet slightly overlapping, scalp topographies and neural generators identified via source reconstruction (Bokura, Yamaguchi, & Kobayashi, 2001). Thus, the nogo-N2 and P3 support the existence of two spatiotemporally overlapping subcomponents related to inhibitory processing and target detection, one reflecting early midfrontal attentional orienting response related to conflict monitoring and signaling the need for inhibition (nogo-N2; Nieuwenhuis, Yeung, Van den Wildenberg, & Ridderinkhof, 2003; Smith et al., 2008), and a later centroparietal response (nogo-P3) reflecting more elaborative processing, such as cognitive/motor inhibition or stimulus probability (Smith et al., 2008; Lavric et al., 2004). Taken together, these findings offer support that scalp-recorded EEG activity related to cognitive processes contains a mixture of frontal orienting/salience detection and parietal evaluative stimulus processing subcomponents which are non-orthogonal, and are instead better thought of as co-occurring processes which combine in a weighted fashion depending on the current task demands.
Theta and Delta Underlie Common ERP Components
Conventionally, EEG data is analyzed in the time-domain, where component measures are often defined based on positive and negative voltage deflections of a signal averaged over many trials, which (mainly) represents time-locked (e.g., stimulus onset, motor or sensory events, etc.) evoked activity. Using time-frequency (TF) analysis, which parses activity that varies in spectral and temporal qualities, recent research has begun to suggest that time-domain ERPs consist of multiple superimposed processes that span across several ERP components (e.g., N2, P3), offering evidence that conventional time-domain measures only offer a limited view of the rich and complex nature of EEG signals. A large body of work investigating the underlying time and frequency characteristics of ERP activity has suggested that, across a broad array of experimental tasks, several common ERP components contain mixtures of spectrally unique, but temporally overlapping, processes operating in the theta and delta frequency bands that are often sensitive to different experimental manipulations. It has been demonstrated that midfrontal theta and centroparietal delta activity underlie the common N2 and P3a/P3b components elicited during a standard target detection/oddball task (Başar, Başar-Eroglu, Karakaş, & Schürmann, 1999, 2001; Başar-Eroglu and Demiralp, 2001; Demiralp, Ademoglu, Comerchero et al., 2001; Demiralp, Ademoglu, Istefanopulos, Başar-Eroglu, & Başar, 2001; Karakaş et al., 2000a, 2000b; Kolev et al., 1997; Spencer & Polich, 1999; Yordanova et al., 2000). This work has suggested that theta and delta are not simply yoked expressions of a single underlying process, but rather are indicative of separable processes, often exhibiting differential relations to experimental manipulations. Additional work has suggested that theta and delta contribute to similar ERP components from other tasks, such as the feedback- negativity (FN; Bernat et al., 2011; Bernat, Nelson, & Baskin-Sommers 2015; Foti, Weinberg, Bernat, & Proudfit, 2014; Miltner, Braun, & Coles, 1997; Nelson, Patrick, Collins, Lang, & Bernat, 2011), the error-related negativity (ERN; Bernat, Williams, & Gehring, 2005; Gehring, & Willoughby, 2004; Yordanova, Falkenstein, Hohnsbein, & Kolev, 2004), and, most applicable to the current report, the nogo-N2 and P3 (Barry, 2009; Harper et al., 2014; Kamarajan et al., 2004; Kamarajan et al., 2006; Kirmizi-Alsan, Bayraktaroglu, Gurvit, Keskin, Emre, & Demiralp, 2006). These findings offer further evidence that theta and delta are the main contributors to evoked ERP responses as observed in the time-domain, and may thus serve as useful indices to investigate subcomponents related to cognitive processes (e.g., theta – early attention/salience detection, delta – later evaluative processes/memory updating).
While a majority of TF research has used the common wavelet transform to model time- and frequency-varying activity from ERPs, this method characteristically smears low frequency (0-3 Hz) activity in time, producing poor temporal resolution at lower frequencies. To address this problem, recent work from our group and others has focused on the use of the reduced interference distribution (RID) transforms from Cohen's class of TF transforms, which can improve the modeling of low frequency temporal dynamics due to uniform time/frequency resolution and increase the TF specificity of delta and theta (Aviyente, Bernat, Evans, & Sponheim, 2011; Bernat et al., 2005; Jannek, Roemer, Weis, Haardt, & Husar, 2009; Weis, Roemer, Haaardt, Jannek, & Husar, 2009). Using this TF approach, theta and delta have been successfully represented across several tasks, including feedback processing (Bernat et al., 2011; Bernat et al., 2015; Liu, Nelson, Bernat, & Gehring, 2014; Nelson et al., 2011), error processing (Bernat et al., 2005; Hall, Bernat, & Patrick, 2007), target detection (Bernat et al., 2007; Gilmore, Malone, & Iacono, 2010; Gilmore, Malone, Bernat, & Iacono, 2010), facial affect processing (Eisenbarth, Angrilli, Calogero, Harper, Olson, & Bernat, 2013) and most recently, response inhibition (Harper et al., 2014). A recent report utilizing this TF method to identify the underlying TF characteristics of two electrophysiological indices of response inhibition, the midfrontal nogo N2 and centroparietal nogo-P3, suggested that while the time-domain results indicated a robust effect at P3 but a non-significant go/nogo effect at N2, TF analysis revealed separable, yet temporally overlapping, midfrontal theta and centroparietal delta components during the N2/P3 complex that were both sensitive to demands of response inhibition and demonstrated greater specificity than N2 or P3, which were confounded by the phase and amplitude of theta and delta (Harper et al., 2014). This bolsters the idea that TF analysis, particularly when employing the RID, is a useful analytic tool for studying the dynamic processes underlying EEG signals.
These findings suggest that the common method of quantifying components based on peaks and troughs of the ERP signal may artificially separate singular frequency-specific processes which operate throughout the ERP response, as was the case with theta and delta activity during the nogo N2/P3 (Harper et al., 2014) and FN and P3 (Bernat et al., 2011; Bernat et al., 2015; Nelson et al., 2011), which would have been artificially split in time by windowed ERP measures. In several of these reports, regression analysis predicting the time-domain components with theta and delta measures confirmed that the time-domain components can be understood as a mixture of theta and delta activity (Bernat et al., 2011; Bernat et al., 2015; Harper et al., 2014; Karakaş et al., 2000a, 2000b). Together, this work offers evidence that theta and delta may better index separable functional processes during action monitoring and cognitive control due to their separability and specificity when compared to standard time-domain component measures.
Functional Significance of Separable Theta and Delta Processes
Using TF methodology, recent research has further detailed the independent contributions of theta and delta during cognitive and sensory processing, suggesting that theta and delta may be better index of the two subcomponents (early attention/salience, later evaluative processing) detailed above than traditional ERP measures such as the N2 and P3. An emerging body of literature, for example, suggests that midfrontal theta activation is related to several facets of cognitive control, such as error monitoring (Bernat et al., 2005; Cavanagh, Cohen, & Allen, 2009; Cohen 2011; Gehring & Willoughby, 2004; Hall et al., 2007; Trujillo & Allen, 2007; Yordanova et al., 2004), reinforcement learning (Cavanagh, Figueroa, Cohen & Frank, 2011; Cavanagh, Frank, Klein, & Allen, 2010), negative feedback evaluation (Bernat et al., 2011, Bernat et al., 2015; Gehring & Willoughby, 2004; Nelson et al., 2011; Liu et al., 2014), novelty detection during the P3a and target discrimination difficulty during the P3b (Demiralp, Ademoglu, Comerchero, et al., 2001; Kolev et al., 1997), and response inhibition/performance monitoring (Barry, 2009; Harper et al., 2014; Kamarajan et al., 2004; Kamarajan et al., 2006; Nigbur, Ivanova, & Stürmer, 2011; Yamanaka & Yamamoto, 2009). These findings suggest that theta may be part of a general performance monitoring system underling various forms of cognitive control, which subserves efforts towards performance monitoring in a generic and reactive fashion, such as signaling the need for increased attention or conflict detection (Cavanagh & Frank, 2014; Cavanagh, Zambrano-Vazquez, & Allen, 2011). A recent report by Bernat and colleagues (Bernat et al., 2015) offers further evidence suggesting that theta primarily indexes a general response to the most critical task dimension, which demonstrated that only the primary (gain, loss), and not secondary (relative outcome, outcome magnitude), feedback-stimulus attribute reliably modulated theta activity, suggesting that midfrontal theta likely reflects an initial, low-level response related to the primary or critical aspect of a stimuli. Theta may also serve as a simple orienting-like response to stimuli that require enhanced cognitive control or attentional allocation, such as during demands of response inhibition or novel detection, similar in function to the nogo-N2 or P3a (Demiralp, Ademoglu, Comerchero, et al., 2001; Harper et al., 2014).
Whereas theta may be a reflection of a more narrow generic response to salient events which signals the need for enhanced cognitive control or attentional deployment, centroparietal delta-band activity seems to be sensitive to a multitude of cognitive functions and task demands which often indexes more elaborative processes related to cognitive control and sensory processing. For example, TF delta has been shown to underlie the P3b response to infrequent target stimuli and be modulated by discrimination difficulty (Bernat et al., 2007; Demiralp, Ademoglu, Comerchero, et al., 2001; Gilmore, Malone, Bernat, et al. 2010; Gilmore, Malone, et al., 2010). Errors of commission in a speeded response task elicit strong centroparietal activity in the delta-band that is not present during correct responses, which is distinct from error-related midfrontal theta (Cavanagh, Zambrano-Vazquez, et al., 2011; Yordanova et al., 2004). Delta is increased for positive outcomes during a simulated gambling task (i.e., reward processing; Bernat et al., 2011; Nelson et al., 2011), and, unlike theta, is sensitive to both primary (gain, loss) and secondary feedback attributes (relative outcomes, monetary magnitudes; Bernat et al., 2015), suggesting that delta may represent a multifaceted index of various stimulus attributes in a more evaluative fashion than midfrontal theta. Delta is also increased during stimuli signaling the need for response inhibition during go/nogo tasks (Barry, 2009; Harper et al., 2014), and given the similarity in latency and topography to the nogo-P3 component, this delta activity may reflect both motor/cognitive inhibition and stimulus context updating rather than a simple indication of conflict (Smith et al., 2008).
As a whole, this evidence offers further support for the notion of two overlapping subcomponents related to cognitive processing, and that TF analysis of theta and delta activity is a valuable methodology to help parse the unique sensitivity and relation of these subcomponents to various forms of cognitive processing.
Current Study
The current study sought to further elucidate the functional separation of theta and delta during the nogo N2/P3 complex (Harper et al., 2014) by investigating sequential context effects in a go/nogo task. In light of findings detailing the effects of stimulus sequence effects on brain responses during target processing (Squires et al., 1976) and response inhibition (Durston, Thomas, Worden, et al., 2002; Durston, Thomas, Yang, et al., 2002), the present study was designed to evaluate the effect of sequence context effects on evoked TF nogo theta and delta energy via modulation of preceding go sequences during a complex go/nogo design involving trial-by-trial stimulus-response updating. Given that theta and delta have been reliably shown to carry separable information related to inhibitory processes and contribute uniquely to the N2/P3 complex (Harper et al., 2014), it was hypothesized that sequence effects would differentially modulate nogo theta and delta. Specifically, given evidence suggesting that midfrontal theta may index a more generic orienting/salience detection process, while centroparietal delta seems to be related to more elaborative stimulus processing, we hypothesized that nogo delta activity would exhibit an increase for longer preceding sequences (i.e., a higher-order stimulus attribute), while theta would simply reflect a generic signal of conflict and attentional allocation (i.e., lower-order signal related to response inhibition).
Method
Participants
A total of 146 undergraduate and community participants were recruited and compensated with course credit or money ($10/hr). Three subjects were excluded due to excessive artifacts and nine due to recording problems. The final sample contained 134 participants (70 females, age: M = 19.99, SD = 3.69). A subset of this sample (N = 66) has been reported elsewhere (Harper et al., 2014).
Experimental Procedure
Testing was conducted in a sound-attenuated, dimly lit room, with stimuli presented centrally on a 21’’ CRT monitor at a viewing distance of 100 cm. Stimuli were delivered via E-Prime 1.1, and behavioral responses were made using a PST Serial Response Box (Psychology Software Tools, Inc.).
The experimental task was a complex go/nogo task similar to the design used by Roche and colleagues (2005) in which two different white letters (font: Courier New; point size: 57) were sequentially displayed centrally on a black background. Subjects were instructed to respond with a button press when the letter presented differed from the previous letter (go) and to withhold responses if the letter presented was identical to the preceding letter (nogo; e.g., the third letter in the sequence X-Y-Y-X). An experimental manipulation was used in which one, three, or five go trials always preceded a nogo trial (Durston, Thomas, Worden, et al., 2002; Durston, Thomas, Yang, et al., 2002). Three blocks of 144 pseudorandomly delivered trials (108 go and 36 nogo trials [12 of each sequence type] per block) were administered, with a different letter pair (X-Y, O-P, D-U) used for each block. Stimulus duration was 300 ms, the response window was 1150 ms, and inter-trial interval was 900 ms. Participants completed a set of 20 practice trials prior to electroencephalographic recording. Following each block, participants were presented with their mean accuracy and allowed to rest before beginning the next block.
Electroencephalographic Recordings
Recordings were collected using a 128-channel Synamps RT amplifier (Neuroscan, Inc.) with Neuroscan 128-channel Quik-Caps (sintered Ag-Ag/Cl; non-standard layout). Ten channels around the ears were removed due to inadequate scalp connection, resulting in 113 EEG channels available for analysis. Bipolar horizontal and vertical electrooculogram activity were recorded from electrodes on the outer canthus of both eyes, and above and below the left eye, respectively. Impedances were kept below 10 kΩ. EEG signals were referenced to the vertex electrode (directly between Cz and CPz) during recording and rereferenced to averaged mastoid signals offline. Data were collected using an analog 0.05 to 200 Hz bandpass filter and digitized online at 1000 Hz.
Signal Preprocessing
Epochs 3 s in length were taken from the continuous data from 1000 ms pre- to 2000 ms post-stimulus with a 150 ms pre-stimulus baseline (during which participants were asked to focus on a fixation dot), and corrected for ocular artifacts using an algorithm developed by Semlitsch and colleagues (1986), as implemented in the Neuroscan Edit 4.5 software (Neuroscan, Inc.). Data were then downsampled to 128 Hz using the Matlab resample function (Mathworks, Inc.), which applies an anti-aliasing filter during resampling. Two methods of data cleaning were performed. First, a two-step process for trial-level artifact rejection was undertaken: (1) whole trials were rejected if activity at F3 or F4 exceeded ±100 μV in either the pre- (−1000 to −1 ms) or post-stimulus (1 to 2000 ms) time windows (relative to one another), and (2) within-trial individual electrodes were rejected if activity exceeded ±100 μV based on the same pre- and post-stimulus time regions. Using this method, 9.1% of all trials were removed. Following the automated procedure detailed above, visual inspection of the averaged signals (see below) indicated that 84 electrodes (out of 15008) became disconnected during recording and were removed from analysis. Trials in which an error was made (error of commission on a nogo trial, or error of omission on a go trial) were excluded from analysis, with 8.06% of trials removed. After preprocessing, the data were averaged according to stimulus type (go, nogo) and sequence context (one, three, five). In detail, go trials at the end of each sequence were averaged separately (G1, G3, G5), and nogo trials preceded by either one, three, or five go trials were also averaged separately (N1, N3, N5), resulting in an equal number of trials (36) per condition in the experimental design.
Signal Reduction/Quantification
Time-frequency evoked energy: theta and delta
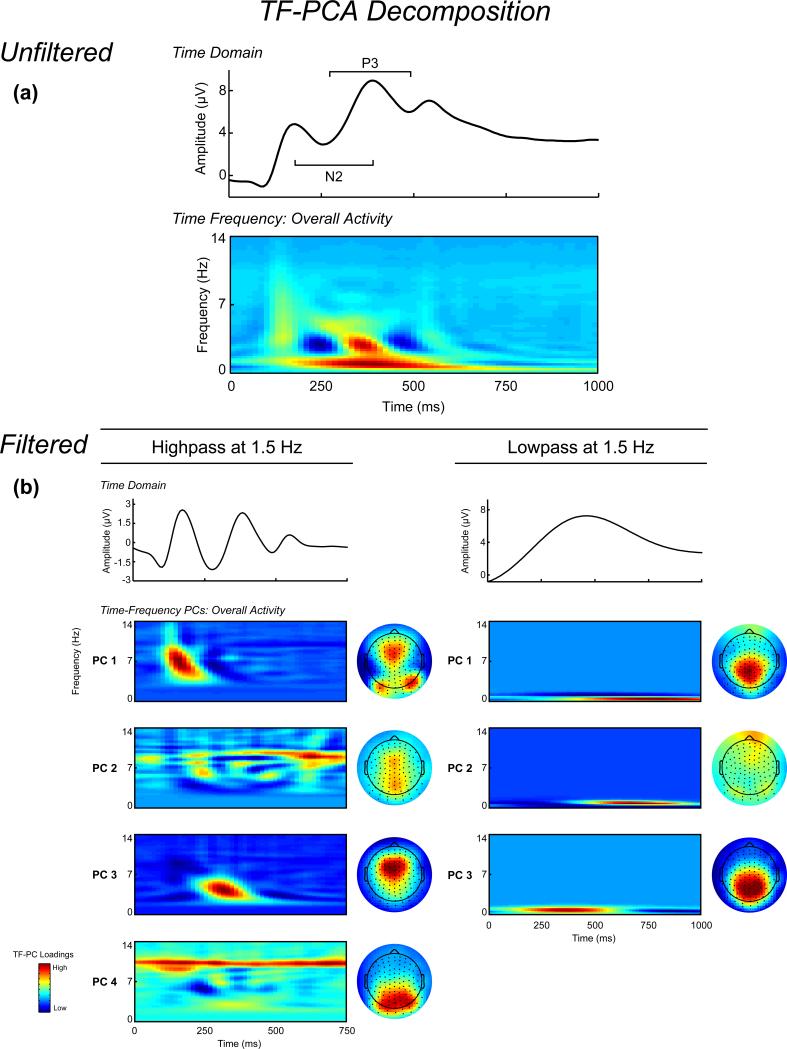
Time-frequency decomposition was performed according to our previous report (Harper et al., 2014). Trial-averaged ERPs were independently pre-filtered using separate 1.5 Hz highpass and lowpass 3rd order Butterworth filters to isolate theta and delta activity, respectively (Bernat et al., 2011, 2015; Harper et al., 2014; Nelson et al., 2011). Performing the TF analysis on averaged signals allows for the representation of the phase-consistent evoked ERP activity (which constitutes a majority of ERP variance) in the TF domain, allowing for the evaluation of evoked TF phase dynamics directly related to time-domain ERP signals. The filter cutoffs were chosen based on visual inspection of the unfiltered grand average time-frequency energy representation, shown in Figure 1, which indicated a natural separation at 1.5 Hz between lower frequency activity spanning the ERP and higher frequency activity in the N2-P3 complex time range. Filtered signals were then transformed to time-frequency representations using the binomial reduced interference distribution (RID) variant of Cohen's class of time-frequency transformations using the full epochs to provide sufficient data to resolve low frequencies, with 64 time bins per second (−1 to 2 s, 15.625 ms steps) and 4 frequency bins per Hz (DC to 32 Hz, .25 Hz steps). The RID transform benefits from uniform time-frequency resolution, which offers improved modeling of time-limited low-frequency activity compared to more common wavelet methods which characteristically smear low-frequency activity in time (Aviyente et al., 2011; Bernat et al., 2005). Principal component analysis (based on the covariance matrix with Varimax rotation; Bernat et al., 2005) was applied to the evoked energy time-frequency representations of the theta and delta filtered signals separately, with a 0-14 Hz frequency window and 0-750 or 0-1000 ms post-stimulus time window to the higher- and lower-frequency TF evoked energy, respectively. PCA decomposition as applied to the time-frequency domain is equivalent to its application in the frequency or time domain. The data matrix consists of time-frequency points as vectors and subject/electrodes/trial-averaged scores as rows (see Bernat et al., 2005 for a detailed explanation of this methodology).
Figure 1.
Section A, Time-domain and time-frequency (TF) decomposition of stimulus-locked ERPs. All ERPs and TF representations are plotted as the average across corresponding channel clusters (see Figure 2). Waveform plot: Averaged unfiltered stimulus-locked ERP across all trials (plotted as the average across the frontal and centroparietal clusters). TF representation: Averaged unfiltered TF representation of the ERP across all trials. Section B, waveform plots: Averaged filtered ERP signals across all trials, frequency-filtered using a 3rd order Butterworth filter to isolate higher-frequency (1.5 Hz highpass) and lower-frequency (1.5 Hz lowpass) signals. TF PCs: Principal component loadings of the high- and low-frequency filtered data across all trials, sorted by percentage variance accounted for pre-Varimax rotation. Topographic maps: Spatial distribution of the mean TF-PCA evoked energy component scores for each corresponding TF representation. For the high-frequency data, PC1 is a mixture of bilateral occipital and anterior activity during the P2, PCs 2 and 4 represent parietal alpha activity, and PC3 reflects midfrontal theta during the N2/P3. For the low-frequency data, PCs 1 and 2 reflect centroparietal and frontal post-P3 activity, respectively, while PC3 reflects centroparietal delta activity during the N2/P3 complex.
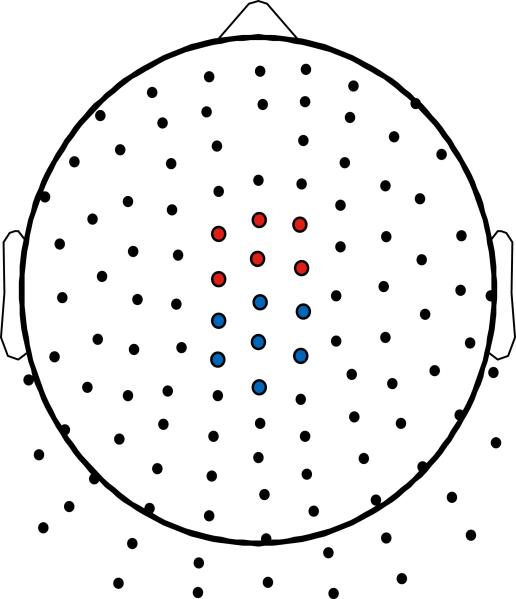
The grand average TF-PCA decomposition is presented in Figure 1. Four principal components (PCs) were extracted for the higher-frequency data explaining 24.70% of the total variance. PC1 represented occipital alpha activity during the P2 ERP component, PC2 reflected late low alpha activity, PC3 represented medial frontal theta during the N2-P3, and PC4 reflected parietal high alpha activity. For the lower-frequency data, three PCs were extracted which explained 90.81% of the variance. PC1 and PC2 represented parietal and frontal post-P3 activation, respectively, and PC3 represented centroparietal activity during the N2-P3 complex. PC3 from the higher-frequency data (Theta) and PC3 from the lower-frequency data (Delta) were chosen for further statistical analysis given a priori hypotheses regarding midfrontal theta and centroparietal delta activity underlying the N2/P3 complex. The topographic schematic in Figure 2 depicts the electrodes chosen for statistical analysis, where six frontocentral sites (including FCz) served as the midfrontal region, and seven centroparietal sites (including Cz and CPz) served as the centroparietal region. These channels were chosen based on a prior investigation (Harper et al., 2014), and the mean PC-weighted TF evoked energy component scores across the TF representation (equivalent in theory to the way PC scores are calculated with questionnaire data) at these two regions served as the unit of analysis in the statistical analyses.
Figure 2.
Schematic layout of the Neuroscan 128-Channel Non-Standard Layout Quik-Cap. Clusters of channels used for statistical analysis are highlighted. Red: Frontal cluster for TF evoked energy theta. Blue: Centroparietal cluster for TF evoked energy delta.
Statistical Analysis
Separate one-way MANOVAs were computed using Scheffé's contrasts for post-hoc comparisons to examine the effect of preceding sequence on task accuracy and reaction time (RT).
To evaluate the hypothesis that both theta and delta would be increased during response inhibition, but would differ in topographic distribution (midfrontal-theta, centroparietal-delta), 2 × 2 repeated measures MANOVAs were computed with region (midfrontal, centroparietal) and stimulus type (go, nogo; collapsed across sequence types) as within-subject factors separately for theta and delta component scores. To test the effect of preceding stimuli on TF theta and delta, 3 × 2 repeated measures MANOVAs with sequence (1, 3, 5) and stimulus type (go, nogo) as within-subject factors were computed individually for theta and delta component measures. Separate one-way MANOVAs with Scheffé's contrasts were used to elucidate the direction of effects. Spearman's rho was used to assess the association between TF component scores. All statistics were performed using SAS 9.3 (SAS Institute Inc., NC, USA).
Results
Behavioral Analysis
The mean ratio of correct responses to go stimuli was 97.46% (SD = 3.83), and did not differ as a function of sequence effects (F(2,132) = 1.52, Wilk's λ = 0.98, p = 0.2223, ηp2 = .02). Unlike overall accuracy, reaction times for correct commissions on go trials did significantly vary by sequence (F(2,132) = 77.70, Wilk's λ = 0.46, p < 0.0001, ηp2 = .54). Mean RTs by condition were 397, 458, and 440 ms (SD = 78, 89, and 78), for G1, G3, and G5, respectively. Post-hoc tests indicated that participants responded quicker on G1 trials than both G3 (t(133) = −12.51, p < 0.0001) and G5 trials (t(133) = −9.30, p < 0.0001), but responded slower on G3 trials than G5 trials (t(133) = 5.17, p < 0.0001). Mean accuracy for nogo trials was 76.09% (SD = 15.17), and did not significantly differ by sequence (F(2,132) = 1.84, Wilk's λ = 0.97, p = 0.1632, ηp2 = .03).
Effects of Response Type and Topographic Region on TF Theta and Delta
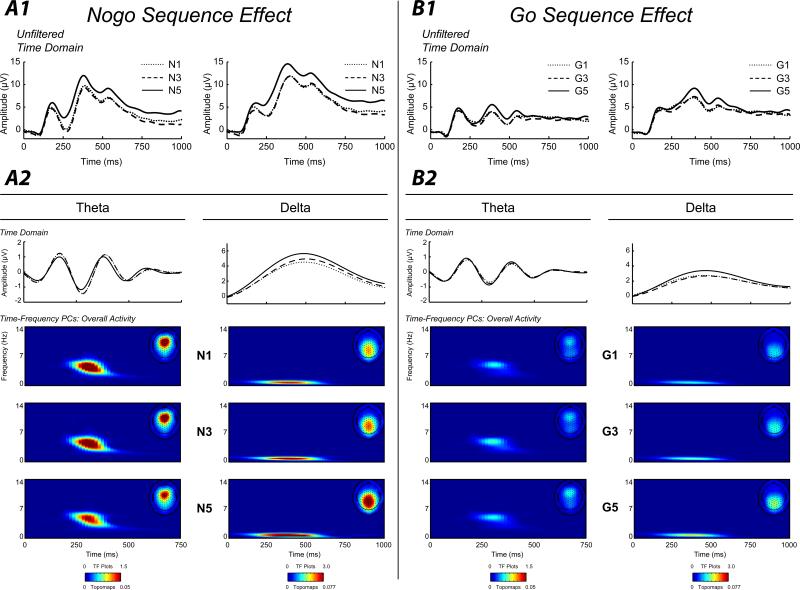
Figure 3 depicts the overall go and nogo effects for theta and delta (TF plots are scaled across condition within band [theta, delta] to facilitate visual comparisons between go and nogo activity). As hypothesized, theta activity was greater at midfrontal sites than centroparietal sites (F(1,133) = 56.72, Wilk's λ = 0.70, p < 0.0001, ηp2 = .30), and was greater on nogo trials than go trials (F(1,133) = 65.69, Wilk's λ = 0.67, p < 0.0001, ηp2 = .33). These effects were qualified by a significant region by go/nogo interaction, which indicated that go/nogo differences were greater at the midfrontal region (F(1,133) = 35.56, Wilk's λ = 0.79, p < 0.0001, ηp2 = .21).
Figure 3.
Section A1, waveform plot: Averaged unfiltered stimulus-locked ERPs for each nogo sequence condition (N1, N3, N5) separately, plotted at the midfrontal (left column) and centroparietal (right column) clusters. Section A2, waveform plots. Averaged ERPs for each nogo sequence condition plotted separately, frequency filtered using a 3rd order Butterworth filter to capture activity in the theta (left column) and delta (right column) TF-representations (3.50-4.75 and 0.5-1.0 Hz, respectively). TF representations: PC-weighted TF component scores of theta (left column) and delta (right column) evoked energy plotted separately for each nogo sequence. Topographic maps: Spatial distribution of the mean TF-PCA evoked energy component scores for each corresponding nogo sequence. Sections B1 and B2. Same as in Section A1 and A2, but for go sequences. Note that TF representations and topomaps within each frequency band are on the same scale across go/nogo and sequence to allow visual depiction of evoked energy changes across experimental conditions. Evident is the increase in activity for nogo stimuli for both theta and delta, and the strong increase in centroparietal delta activity for longer sequences. All ERPs and TF representations are plotted as the average across corresponding channel clusters (see Figure 2).
Delta activity was greater during nogo trials (F(1,133) = 85.36, Wilk's λ = 0.61, p < 0.0001, ηp2 = .39), and was greater at the centroparietal region (F(1,133) = 163.01, Wilk's λ = 0.45, p < 0.0001, ηp2 = .55). Once again, a significant interaction between region and go/nogo was found, although unlike theta, delta go/nogo differences were larger at the centroparietal region (F(1,132) = 45.79, Wilk's λ = 0.74, p < 0.0001, ηp2 = .26).
Given that theta and delta exhibited clear topographic differences at both overall and nogo-go difference levels, further statistics were computed using component scores at the respective maximal region for theta (midfrontal) and delta (centroparietal).
Association between TF Theta and Delta
Theta and delta evidenced a moderate association at the grand average (Spearman's rho(131) = 0.4322, p < 0.0001), and nogo minus go difference level (Spearman's rho(131) = 0.3068, p = 0.0003), suggesting that while theta and delta shared a moderate amount of variance, they were not simply expressions of the same underlying process (Harper et al., 2014).
Effects of Preceding Sequence on TF Theta and Delta
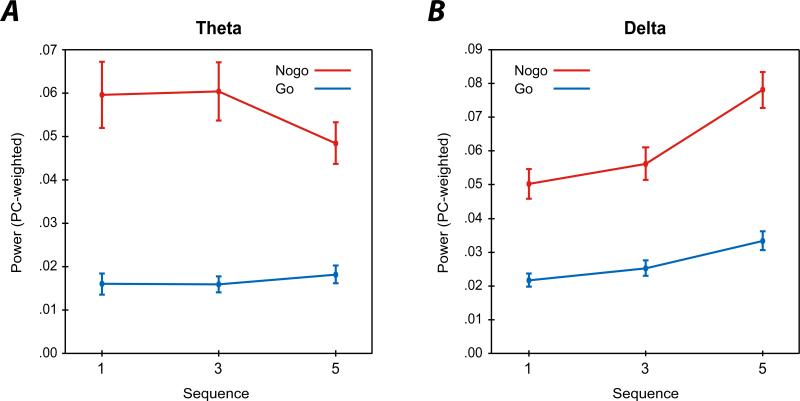
Figure 3 depicts the effect of preceding stimulus sequences for TF theta and delta for go and nogo conditions. Theta was greater following nogo stimuli compared to go stimuli (F(1,133) = 61.03, Wilk's λ = 0.69, p < 0.0001, ηp2 = .31), and did not differ as a function of sequence (F(2,132) = 1.85, Wilk's λ = 0.97, p = 0.1605, ηp2 = .03), although these effects were qualified by a significant go/nogo by sequence interaction (F(2,132) = 4.25, Wilk's λ = 0.94, p = 0.0163, ηp2 = .06). Examining this interaction by evaluating sequence effects within go and nogo stimuli separately, results indicated theta during go trials was not significantly modulated by sequence effects (F(2, 132) = 1.75, Wilk's λ = 0.97, p = 0.1786, ηp2 = .03), but that theta activity during nogo trials was significantly different across sequence effects (F(2, 132) = 3.12, Wilk's λ = 0.95, p < 0.0475, ηp2 = .05). Post-hoc comparisons revealed a trend level effect between N3 and N5 (t(133) = 2.41, p = 0.0589), which suggested that nogo theta was greater following three go trials than five go trials. No other post-hoc comparison was significant (ts(133) = −0.19 and 2.14, ps = 0.9827 and 0.1060, for N1-N3 and N1-N5, respectively). Figure 4 displays the profile plots for the sequence by go/nogo interactions for both theta and delta.
Figure 4.
Section A. Profile plot depicting the mean PC-weighted TF evoked energy theta component scores for go (blue) and nogo (red) conditions by sequence (x-axis). Bars represent standard error of the mean. Section B. Same as Section A, but for delta activity. Evident from the profile plots is the strong increase in nogo delta activity with increasing sequences, and the slight decrease in nogo theta activity following five go trials.
For delta, main effects were found for stimulus type (nogo > go; F(1,133) = 96.68, Wilk's λ = 0.58, p < 0.0001, ηp2 = .42), and sequence (5 > 3 > 1; F(2,132) = 43.70, Wilk's λ = 0.60, p < 0.0001, ηp2 = .60), which were qualified by a significant go/nogo by sequence interaction (F(2,132) = 10.51, Wilk's λ = 0.86, p < 0.0001, ηp2 = .14), which suggested that nogo delta evidenced larger sequence effects than go delta. Again, this interaction was decomposed by evaluating sequence effects separately for go and nogo delta. Within nogo trials, delta was significantly different across sequence effects (F(2, 132) = 35.83, Wilk's λ = 0.65, p < 0.0001, ηp2 = .35), and post-test indicated that nogo delta following five gos exhibited greater energy than the one or three go sequences (ts(133) = −8.31 and −6.68, ps < 0.0001, for N1-N5 and N3-N5, respectively), but that nogo delta did not differ between one and three go sequences (t(133) = −2.13, p = 0.1073). Examining go trials, a main effect of sequence was again found (F(2, 132) = 14.63, Wilk's λ = 0.82, p < 0.0001, ηp2 = .18). Post-hoc comparisons indicated that delta on G5 trials was greater than G1 or G3 trials (ts(133) = −5.07 and −4.78, ps < 0.0001, for G1-G5 and G3-G5, respectively), and that G3 and G5 were not significantly different (t(133) = −2.00, p = 0.1407).
Given that delta evidenced similar sequence effects during both go and nogo trials, the cross-subject correlation between delta N5-N1 differences and delta G5-G1 differences was computed to assess whether a shared process accounted for the sequence effects during go and nogo trials. Results indicated no significant association (Spearman's rho(131) = 0.1612, p = 0.0628), suggesting that while sequence differences between go and nogo delta are mildly correlated, they are not simply yoked expressions of the same process underlying sequence effects.
Discussion
The present report demonstrated that stimulus sequence context differentially modulated nogo theta and delta activity, even though both evidenced similar robust go/nogo differences. Previous research has suggested that common time-domain measures, including the nogo-N2 and P3, are better thought of as mixtures of separable theta and delta activity (Bernat et al., 2011; Bernat et al., 2015; Harper et al., 2014; Nelson et al., 2011), which offer better specificity to investigate cognitive and sensory processes than ERP component measures. The goal of the current study was to evaluate the effects of preceding stimulus sequence context on theta and delta activity during a complex go/nogo task to further investigate the separable functional nature of theta and delta and their relation to the two-process model related to cognitive functioning.
In detail, findings indicated that nogo delta activity scaled in a linear manner following longer preceding sequences, while nogo theta activity showed a small decrease following long sequences (i.e., five preceding go trials). While delta activity during go trials also exhibited a significant increase for sequence context, the change from shorter to longer sequences between nogo and go delta were uncorrelated, suggesting that different processes may account for sequence effects during response commission and inhibition (e.g., motoric priming). These findings both replicate an earlier study demonstrating that separable theta and delta activity underlie the common N2/P3 complex associated with response inhibition (Harper et al., 2014), and expand on the dissociative nature of theta and delta activity related to target detection and response inhibition and as indices of the two-process model.
Separable Theta and Delta TF Dynamics During Response Inhibition/Target Detection
Findings from the current report suggest that theta may serve a more simple salience process, in this case the need for inhibition regardless of the sequence context, whereas delta was robustly sensitive to both the simple stimulus salience (i.e., nogo signal) and more complex sequence context information. This bolsters existing literature suggesting that delta may index a myriad of elaborative cognitive functions (e.g., action monitoring [Yordanova et al., 2004], reward processing [Bernat et al., 2011; Bernat et al., 2015; Nelson et al., 2011], target detection [Demiralp, Ademoglu, Istefanplulos, et al., 2001; Demiralp, Ademoglu, Comerchero, et al., 2001; Karakaş et al., 2000a, 2000b; Kolev et al., 1997]), while theta may reflect a more generic midfrontal salience response to conflict or increased attentional allocation (Bernat et al., 2015; Cavanagh, Zambrano-Vazquez, et al., 2011).
Relevant to the sensitivity of delta to sequence context, studies have demonstrated that P3 amplitude to target stimuli tends to increase in a linear fashion as the number of preceding nontargets increases (Gonsalvez et al., 1999; Squires et al., 1976). Because delta activation has been shown to explains a majority of variance in centroparietal P3b amplitude (Bernat et al., 2011; Harper et al., 2014; Karakaş et al., 2000a, 2000b), exhibit similar sensitivity to experimental manipulations as the P3b (Spencer & Polich, 1999), and nogo delta in the current report was similarly affected by sequential context (i.e., linear increase), it may be that the target-P3b sequence findings reported elsewhere were primarily due to selective increases in delta-band activation. Sequence effects have often been framed in terms of contextual working memory updating (Polich, 2007), which may indicate a role of delta activity in working memory updating of stimulus context during response inhibition, or a sensitivity to higher-order stimulus attributes (e.g., Bernat et al., 2015).
The more general theta response to nogo stimuli in this report is consistent with recent research suggesting that theta activity underlying midfrontal N2-like components may represent a more generic response to salient events (Cavanagh, Zambrano-Vazquez, et al., 2011; Cavanagh & Frank, 2014), bolstering the idea that theta is a more singular process compared to delta, likely signaling the need for enhanced cognitive control (possibly via recruitment of other brain regions, such as the lateral prefrontal cortex [Aviyente et al., 2011; Cavanagh et al., 2009]), or increased attentional allocation or orienting towards salient events. These two processes (theta and delta) may operate in conjunction (similar to the proposed relationship between P3a and P3b; Polich, 2007), where midfrontal theta reflects the initial orienting response towards the salient stimuli, and centroparietal delta evaluates the stimulus in a more detailed manner. The minor decrease in theta activity following longer sequences may reflect a decrease in salience or attentional allocation due to possible expectancy effects given the maximum of five go trials preceding a nogo trial in the task design, but without a more fine-grained analysis of sequential context effects during response inhibition this explanation is tentative.
Theta and Delta as Indices of the Two-Process Model
As detailed above, there is a wealth of evidence suggesting at least two hypothetical subcomponents, one reflecting an initial attentional/orienting response, and another involved in more elaborative stimulus evaluation or working memory/context updating, that are concurrently active during cognitive processing (Polich, 2007). These two subcomponents are not orthogonal, and tend to co-occur during several different experimental conditions (e.g., both novel processing and target detection conditions) with differing latencies and topographies and are weighted depending on a given experimental manipulation, which combine to produce the ERP response (Debener et al., 2005; Spencer et al., 2001). An example of this is offered by a recent report which suggests that secondary stimulus properties do not affect theta-band loss processing activity, but do selectively affect delta-band reward processing activity (Bernat et al., 2015), and the current report, where nogo theta activity did not show the same sensitivity to stimulus sequence context as nogo delta, which both provide increasing support that theta and delta may offer a better target to investigate the two-process subcomponents than traditional ERP measures.
These subcomponents of the two-process model map on well to TF theta and delta component activity, where midfrontal theta may reflect early activity signaling increased attentional allocation or degree of conflict, while centroparietal delta may engage later evaluative or memory-related processes associated with higher-level stimulus processing. The current report offers support for this distinction, where theta likely serves as a generic signal indicating response switching (i.e., was not sensitive to sequence context), with delta possibly indexing both cognitive/motor inhibition and contextual information related to previous trials (i.e., sensitivity to sequence effects). Given the evidence for the generic nature of midfrontal theta activity (Cavanagh, Zambrano-Vazquez, et al., 2011), and its role in novelty processing during the P3a (Demiralp, Ademoglu, Comerchero, et al., 2001), it is reasonable that theta could be the driving force behind P3a orienting signals, which may then engage a hypothetical network related to cognitive control (via prefrontal regions) and parietal regions involved in memory updating, which may include delta signals underling the P3b (Polich, 2007).
Limitations and Future Directions
Given the a priori hypotheses regarding theta and delta activity during response inhibition and their relation to the N2/P3 complex, the current report only investigated how those two frequency bands were modulated by sequence context. As it has been suggested that other frequency bands, such as gamma activity, also carry information related to response inhibition (Shibata et al., 1999) and memory (Klimesch, Fruenberger, & Sauseng, 2010), it would be valuable to investigate how higher frequency activity is affected by both inhibitory processes and stimulus sequence effects. A more practical limitation of the current study was that the limit of preceding go trials before a nogo was a maximum of five trials, which may have led to an expectancy effect following five go trials that could have affected activity on the subsequent nogo trial (e.g., decreased salience of the nogo stimulus). Although a previous study using the same design as the current report indicated that participants did not explicitly learn to expect a nogo stimulus following 1, 3, or 5 go trials (Durston, Thomas, Worden, et al., 2002; Durston, Thomas, Yang, et al., 2002), it is unclear whether participants in the reported sample consciously identified the sequences. Future studies could be designed to modulate sequence effects in a more fine-grained manner (e.g., varying the preceding sequences from 1-10), which could offer a way to evaluate expectancy and target-to-target interval on nogo theta and delta. Finally, further work would do well to investigate functional networks, phase consistency, and possible neural sources associated with theta and delta components to determine regions engaged during theta and delta activity and their respective roles during initial orienting and subsequent stimulus evaluation (Aviyente et al., 2011; Lachaux, Rodriguex, Martinerie, & Varela, 1999; Tallon-Baudry, Betrand, Delpuech, & Pernier, 1996), which could offer further support for the roles of theta and delta in the two-process model.
Overall, these results provide additional support for the idea that theta and delta index dissociable processes related to cognitive/sensory processing and may serve as indices of the two-process model, and bolster the idea that TF analysis may broadly be an improved target of research for cognitive electrophysiology over traditional time-domain measures.
Acknowledgments
This work was supported by the National Institute of Mental Health Grant MH080239 (PI: Bernat).
References
- Aviyente S, Bernat EM, Evans WS, Sponheim SR. A phase synchrony measure for quantifying dynamic functional integration in the brain. Human Brain Mapping. 2011;32:80–93. doi: 10.1002/hbm.21000. doi: 10.1002/hbm.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Karakaş S, Schürmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neuroscience Letters. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. doi: 10.1016/S0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Karakaş S, Schürmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. International Journal of Psychophysiology. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. doi: 10.1016/S0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Başar-Eroglu C, Demiralp T. Event-related theta oscillations: an integrative and comparative approach in the human and animal brain. International Journal of Psychophysiology. 2001;39:167–195. doi: 10.1016/s0167-8760(00)00140-9. doi: 10.1016/S0167-8760(00)00140-9. [DOI] [PubMed] [Google Scholar]
- Barry RJ. Evoked activity and EEG phase resetting in the genesis of auditory go/nogo ERPs. Biological Psychology. 2009;80:292–299. doi: 10.1016/j.biopsycho.2008.10.009. doi: 10.1016/j.biopsycho.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Malone SM, Williams WJ, Patrick CJ, Iacono WG. Decomposing delta, theta, and alpha time-frequency ERP activity from a visual oddball task using PCA. International Journal of Psychophysiology. 2007;64:62–74. doi: 10.1016/j.ijpsycho.2006.07.015. doi: 10.1016/j.ijpsycho.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Baskin-Sommers AR. Time-frequency theta and delta measures index separable components of feedback processing in a gambling task. Psychophysiology. 2015;52:626–637. doi: 10.1111/psyp.12390. doi: 10.1111/psyp.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Steele VR, Gehring WJ, Patrick CJ. Externalizing psychopathology and gain-loss feedback in a simulated gambling task: dissociable components of brain response revealed by time-frequency analysis. Journal of Abnormal Psychology. 2011;120:352–364. doi: 10.1037/a0022124. doi: 10.1037/a0022124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Williams WJ, Gehring WJ. Decomposing ERP time-frequency energy using PCA. Clinical Neurophysiology. 2005;116:1314–1334. doi: 10.1016/j.clinph.2005.01.019. doi:10.1016/j.clinph.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Hoechstetter K, Scherg M, Wibral M, Goebel R, Linden DEJ. Localizing P300 generators in visual target and distracter processing: a combined event-related potential and functional mag- netic resonance imaging study. Journal of Neuroscience. 2004;24:9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clinical Neurophysiology. 2001;112:2224–2232. doi: 10.1016/s1388-2457(01)00691-5. doi: 10.1016/S1388-2457(01)00691-5. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJB. Prelude and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. The Journal of Neuroscience. 2009;29(98-105) doi: 10.1523/JNEUROSCI.4137-08.2009. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Figueroa CM, Cohen MX, Frank MJ. Frontal theta reflects uncertainty and unexpectedness during exploration and exploitation. Cerebral Cortex. 2011;22:2572–2586. doi: 10.1093/cercor/bhr332. doi: 10.1093/cercor/bhr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJB. Frontal theta links prediction errors to behavioral adaption in reinforcement learning. NeuroImage. 2010;49:3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences. 2014 doi: 10.1016/j.tics.2014.04.012. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJB. Theta lingua france: a common mid-frontal substrate for action monitoring processes. Psychophysiology. 2011;49:220–238. doi: 10.1111/j.1469-8986.2011.01293.x. doi:10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. Error-related medial frontal theta activity predicts cingulate-related structural connectivity. NeuroImage. 2011;55:1373–1383. doi: 10.1016/j.neuroimage.2010.12.072. doi: 0.1016/j.neuroimage.2010.12.072. [DOI] [PubMed] [Google Scholar]
- Debener S, Makeig S, Delorme A, Engel AK. What is novel in the novelty oddball paradigm? Functional significance of the novelty P3 event-related potential as revealed by independent component analysis. Cognitive Brain Research. 2005;22:309–321. doi: 10.1016/j.cogbrainres.2004.09.006. doi: 10.1016/j.cogbrainres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Comerchero M, Polich J. Wavelet analysis of P3a and P3b. Brain Topography. 2001;13:251–256. doi: 10.1023/a:1011102628306. doi:10.1023/A:1011102628306. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Istefanopulos Y, Başar-Eroglu C, Başar E. Wavelet analysis of oddball P300. International Journal of Psychophysiology. 2001;36:221–227. doi: 10.1016/s0167-8760(00)00143-4. doi: 10.1016/S0167-8760(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Localization of the event-related potential novelty response as defined by principal components analysis. Cognitive Brain Research. 2003;17:637–650. doi: 10.1016/s0926-6410(03)00188-5. doi: 10.1016/S0926-6410(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Donchin E. Surprise!... surprise?. Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: an event-related fMRI study. NeuroImage. 2002;16:559–453. doi: 10.1006/nimg.2002.1074. doi:10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5:F9–F16. doi: 10.1111/1467-7687.00235. [Google Scholar]
- Eisenbarth H, Angrilli A, Calogero A, Harper J, Olson LA, Bernat E. Reduced negative affect response in female psychopaths. Biological Psychology. 2013;94:310–318. doi: 10.1016/j.biopsycho.2013.07.007. doi: 10.1016/j.biopsycho.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychologica. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. doi: 10.1016/S0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Brandeis D, Strik PW. A robust assessment of the NoGo-anteriorisation of P300 microstates in a cued Continuous Performance Test. Brain topography. 1997;9:295–302. doi: 10.1007/BF01464484. doi: 10.1007/BF01464484. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Bernat EM, Proudfit GH. Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clinical Neurophysiology. 2014 doi: 10.1016/j.clinph.2014.08.025. doi: 10.1016/j.clinph.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring. In: Ullsperger M, Falkenstein M, editors. Errors, conflicts, and the brain: Current opinions on performance monitoring. MPI of Cognitive Neuroscience; Leipzig: 2004. pp. 14–20. [Google Scholar]
- Giese-Davis JE, Miller GA, Knight RA. Memory template comparison processes in anhedonia and dysthymia. Psychophysiology. 1993;30:646–656. doi: 10.1111/j.1469-8986.1993.tb02090.x. doi: 10.1111/j.1469-8986.1993.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Bernat EM, Iacono WG. Relationship between the P3 event-related potential, its associated time-frequency components, and externalizing psychopathology. Psychophysiology. 2010;47:123–132. doi: 10.1111/j.1469-8986.2009.00876.x. doi: 10.1111/j.1469-8986.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Iacono WG. Brain electrophysiological endophenotypes for externalizing psychopathology: a multivariate approach. Behavioral Genetics. 2010;40:186–200. doi: 10.1007/s10519-010-9343-3. doi: 10.1007/s10519-010-9343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Iacono WG. Is the P3 amplitude reduction seen in externalizing psychopathology attributable to stimulus sequence effects? Psychophysiology. 2012;49:248–251. doi: 10.1111/j.1469-8986.2011.01299.x. doi: 10.1111/j.1469-8986.2011.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalvez CJ, Gordon E, Anderson J, Pettigrew G, Barry RJ, Rennie C, Meares R. Numbers of preceding nontargets differentially affect responses to targets in normal volunteers and patients with schizophrenia: A study of event-related potentials. Psychiatry Research. 1995;58:69–75. doi: 10.1016/0165-1781(95)02315-n. doi: 10.1016/0165-1781(95)02315-N. [DOI] [PubMed] [Google Scholar]
- Gonsalvez CJ, Gordon E, Grayson S, Barry RJ, Lazzaro I, Bahramali H. Is the target-to-target interval a critical determinant of P3 amplitude? Psychophysiology. 1999;36:643–654. doi:10.1017/S0048577299971639. [PubMed] [Google Scholar]
- Gonsalvez CJ, Polich J. P300 amplitude is determined by target-to-target interval. Psychophysiology. 2002;39:388–396. doi: 10.1017/s0048577201393137. doi: 10.1017/S0048577201393137. [DOI] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychological Science. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J, Malone SM, Bernat EM. Theta and delta band activity explain N2 and P3 ERP components in a go/no-go task. Clinical Neurophysiology. 2014;125:124–132. doi: 10.1016/j.clinph.2013.06.025. doi: 10.1016/j.clinph.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannek D, Roemer F, Weis M, Haardt M, Husar P. Identification of signal components in multi-channel EEG signals via closed-form PARAFAC analysis and appropriate preprocessing.. 4th European Conference of the International Federation for Medical and Biological Engineering; Springer Berlin Heidelberg. 2009. pp. 1226–1230. doi: 10.1007/978-3-540-89208-3_293. [Google Scholar]
- Johnson R, Donchin E. P300 and stimulus categorization: two plus one is not so different from one plus one. Psychophysiology. 1980;17:167–178. doi: 10.1111/j.1469-8986.1980.tb00131.x. doi: 10.1111/j.1469-8986.1980.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjez B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Begleiter H. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. International Journal of Psychophysiology. 2004;51:155–180. doi: 10.1016/j.ijpsycho.2003.09.004. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjez B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. International Journal of Psychophysiology. 2006;51:155–180. doi: 10.1016/j.biopsych.2005.08.017. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakaş S, Erzengin ÖU, Başar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clinical Neurophysiology. 2000a;111:1719–1732. doi: 10.1016/s1388-2457(00)00418-1. doi:10.1016/S1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Karakaş S, Erzengin ÖU, Başar E. The genesis of human event-related responses explained through the theory of oscillatory neural assemblies. Neuroscience Letters. 2000b;285:45–48. doi: 10.1016/s0304-3940(00)01022-3. doi:10.1016/S0304-3940(00)01022-3. [DOI] [PubMed] [Google Scholar]
- Kirmizi-Alsan E, Bayraktaroglu Z, Gurvit H, Keskin YH, Emre M, Demiralp T. Comparative analysis of event-related potentials during Go/NoGo and CPT: decomposition of electrophysiological markers of response inhibition and sustained attention. Brain Research. 2006;1104:114–128. doi: 10.1016/j.brainres.2006.03.010. doi: 10.1016/j.brainres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Freunberger R, Sauseng P. Oscillatory mechanisms of process binding in memory. Neuroscience and Biobehavioral Reviews. 2010;34:1002–1014. doi: 10.1016/j.neubiorev.2009.10.004. doi: 10.1016/j.neubiorev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Kok A. Effects of degradation of visual stimuli on components of the event-related potential (ERP) in go/nogo reaction tasks. Biological Psychology. 1986;23:21–38. doi: 10.1016/0301-0511(86)90087-6. doi: 10.1016/0301-0511(86)90087-6. [DOI] [PubMed] [Google Scholar]
- Kolev V, Demiralp T, Yordanova J, Ademoglu A, Isoglu-Alkaç Ü. Time-frequency analysis reveals multiple functional components during oddball P300. NeuroReport. 1997;8:2061–2065. doi: 10.1097/00001756-199705260-00050. doi: 10.1097/00001756-199705260-00050. [DOI] [PubMed] [Google Scholar]
- Kopp B, Mattler U, Goertz R, Rist F. N2, P3 and the lateralized readiness potential in a nogo task involving selective response priming. Electroencephalography and Clinical Neurophysiology. 1996;99:19–27. doi: 10.1016/0921-884x(96)95617-9. doi: 10.1016/0921-884X(96)95617-9. [DOI] [PubMed] [Google Scholar]
- Lachaux J-P, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Human Brain Mapping. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID- HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavric A, Pizzagalli DA, Forstmeier S. When ‘go’and ‘nogo’are equally frequent: ERP components and cortical tomography. European Journal of Neuroscience. 2004;20:2483–2488. doi: 10.1111/j.1460-9568.2004.03683.x. doi: 10.1111/j.1460-9568.2004.03683.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nelson LD, Bernat EM, Gehring WJ. Perceptual properties of the feedback stimuli influence the feedback-related negativity in the flanker gambling task. Psychophysiology. 2014 doi: 10.1111/psyp.12216. doi: 10.1111/psyp.12216. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, Collins P, Lang AR, Bernat EM. Alcohol impairs brain reactivity to explicit loss feedback. Psychopharmacology. 2011;218:419–428. doi: 10.1007/s00213-011-2323-3. doi: 10.1007/s00213-011-2323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. doi: 10.3758/CABN.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nigbur R, Ivanova G, Stürmer B. Theta power as a marker for cognitive interference. Clinical Neurophysiology. 2011;122:2185–2194. doi: 10.1016/j.clinph.2011.03.030. doi: 10.1016/j.clinph.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalography and clinical Neurophysiology. 1985;60:423–434. doi: 10.1016/0013-4694(85)91017-x. doi:10.1016/0013-4694(85)91017-X. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. doi:10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Bondurant T. P300 sequence effects, probability, and interstimulus interval. Physiology & Behavior. 1997;61:843–849. doi: 10.1016/s0031-9384(96)00564-1. doi: 10.1016/S0031-9384(96)00564-1. [DOI] [PubMed] [Google Scholar]
- Roche RAP, Garavan H, Foxe JJ, O'Mara SM. Individual differences discriminate event-related potentials but not performance during response inhibition. Experimental Brain Research. 2005;160:60–70. doi: 10.1007/s00221-004-1985-z. doi: 10.1007/s00221-004-1985-z. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shibata T, Shimoyama I, Ito T, Abla D, Iwasa H, Koseki K, Nakajima Y. Event-related dynamics of the gamma-band oscillation in the human brain: information processing during a GO/NOGO hand movement task. Neuroscience Research. 1999;33:215–222. doi: 10.1016/s0168-0102(99)00003-6. doi: 10.1016/S0168-0102(99)00003-6. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clinical Neurophysiology. 2008;119:704–714. doi: 10.1016/j.clinph.2007.11.042. doi: 10.1016/j.clinph.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. doi: 10.1111/1469-8986.3820343. [PubMed] [Google Scholar]
- Spencer KM, Polich J. Poststimulus EEG spectral analysis and P300: Attention, task, and probability. Psychophysiology. 1999;36:220–232. doi: 10.1111/1469-8986.3620220. [PubMed] [Google Scholar]
- Squires K, Petuchowski S, Wickens C, Donchin E. The effects of stimulus sequence on event related potentials: A comparison of visual and auditory sequences. Perception & Psychophysics. 1977;22:31–40. doi: 10.3758/BF03206077. [Google Scholar]
- Squires KC, Wickens C, Squires NK, Donchin E. The effect of stimulus sequence on the waveform of the cortical event-related potential. Science. 1976;193:1142–1146. doi: 10.1126/science.959831. doi:10.1126/science.959831. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and Clinical Neurophysiology. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. The Journal of Neuroscience. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJB. Theta EEG dynamics of the error-related negativity. Clinical Neurophysiology. 2007;118:645–669. doi: 10.1016/j.clinph.2006.11.009. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Weis M, Römer F, Haardt M, Jannek D, Husar P. Multi-dimensional space-time-frequency component analysis of event related EEG data using closed-form PARAFAC.. Acoustics, Speech and Signal Processing, 2009. ICASSP 2009. IEEE International Conference; IEEE. Apr, 2009. pp. 349–352. doi: 10.1109/ICASSP.2009.4959592. [Google Scholar]
- Yamanaka K, Yamamoto Y. Single-trial EEG power and phase dynamics associated with voluntary response inhibition. Journal of Cognitive Neuroscience. 2009;22:714–727. doi: 10.1162/jocn.2009.21258. doi: 10.1162/jocn.2009.21258. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Devrim M, Kolev V, Ademoglu A, Demiralp T. Multiple time-frequency components account for the complex functional reactivity of P300. NeuroReport. 2000;11:1097–1103. doi: 10.1097/00001756-200004070-00038. doi: 10.1097/00001756-200004070-00038. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Falkenstein M, Hohnsbein J, Kolev V. Parallel systems of error processing in the brain. Neuroimage. 2004;24:590–602. doi: 10.1016/j.neuroimage.2004.01.040. doi:10.1016/j.neuroimage.2004.01.040. [DOI] [PubMed] [Google Scholar]