Abstract
Background
Anesthetic preconditioning (APC) is a clinically important phenomenon in which volatile anesthetics (VA) protect tissues such as heart against ischemic injury. The mechanism of APC is thought to involve potassium (K+) channels encoded by the Slo gene family, and we showed previously that slo-2 is required for APC in C. elegans. Thus, we hypothesized that a slo-2 ortholog may mediate APC-induced cardioprotection in mammals.
Methods
A perfused heart model of ischemia-reperfusion (IR) injury, a fluorescent assay for K+ flux, and mice lacking Slo2.1 (Slick), Slo2.2 (Slack) or both (double knockouts, Slo2.x dKO) were employed, to test whether these channels are required for APC induced cardioprotection, and for cardiomyocyte or mitochondrial K+ transport.
Results
In wild-type (WT) hearts APC improved post-IR functional recovery (APC = 39.5 ± 3.7 % of pre-ischemic rate x pressure product, vs. 20.3 ± 2.3 % in controls, means ± SEM, p=0.00051, non-paired two-tailed t-test, n=8) and lowered infarct size (APC = 29.0 ± 4.8 % of LV area, vs. 51.4 ± 4.5 % in controls, p=0.0043, n=8). Protection by APC was absent in hearts from Slo2.1−/− mice (% recovery APC = 14.6 ± 2.6 vs. 16.5 ± 2.1 % in controls, p=0.569, n=8–9, infarct APC = 52.2 ± 5.4 % vs. 53.5 ± 4.7 % in controls, p=0.865, n=8–9). APC protection was also absent in Slo2.x dKO hearts (% recovery APC = 11.0 ± 1.7 vs. 11.9 ± 2.2 % in controls, p=0.725, n=8, infarct APC = 51.6 ± 4.4 % vs. 50.5 ± 3.9 % in controls, p=0.855, n=8). Meanwhile, Slo2.2−/− hearts responded similar to WT (% recovery APC = 41.9 ± 4.0 vs. 18.0 ± 2.5 % in controls, p=0.00016, n=8, infarct APC = 25.2 ± 1.3 % vs. 50.8 ± 3.3 % in controls, p<0.000005, n=8). Furthermore, VA stimulated K+ transport seen in cardiomyocytes or mitochondria from WT or Slo2.2−/− mice, was absent Slo2.1−/− or Slo2.x dKO.
Conclusion
Slick (Slo2.1) is required for both VA stimulated K+ flux and for the APC induced cardioprotection.
Introduction
Clinically-relevant doses of halogenated volatile anesthetics (VA) can protect tissues such as the heart from ischemia and reperfusion (IR) injury,1–3 a phenomenon known as “anesthetic preconditioning” (APC). APC is evolutionarily conserved from C. elegans4 to humans,3 and current AHA/ACC guidelines specifically recommend the use of VAs during cardiac surgery for their protective effects.5
The mechanism of APC is complex, and involves many of the same effector signaling pathways as ischemic preconditioning (IPC), including protein kinases C6 and A,7,8 inhibitory G-proteins,9 adenosine,10,11 nitric oxide,12 and mitochondrial ROS generation.13,14 These signals are thought to converge at the level of mitochondria through distinct K+ channels, but the molecular identity of these channels is a subject of debate.
Several K+ channel types have been proposed to exist in mitochondria, including: ATP sensitive (KATP),15 voltage sensitive (KV)16 and large conductance (BK) channels of the Slo gene family,17 as well as a K+/H+ exchanger (KHE)18 (see 19,20 for review). Current evidence favors an involvement of mitochondrial KATP channels in IPC,21 while a mitochondrial Slo channel is thought to underlie APC.7,22,23
The mammalian Slo channel family comprises Slo1 (KCNMA1), Slo2.1 (KCNT2, Slick), Slo2.2 (KCNT1, Slack) and Slo3 (KCNU1).24 Slo3 expression is germline restricted24,25 but the others are widely expressed. Since pharmacologic Slo1 activators can protect the heart against IR injury,17,26 it was widely assumed that a mitochondrial Slo1 channel (also termed BK, KCa or BKCa) was responsible for APC. However, we recently showed that both C. elegans and mice lacking Slo1 can still be protected by APC, and mitochondria from these organisms contain a K+ channel activated by VA.22
We have also previously demonstrated that the KCa channel Slo2 is required for VA induced protection of C. elegans against IR injury, with Slo2 mutant nematodes also lacking VA-stimulated mitochondrial K+ flux.22 Thus, we hypothesized that one of the mammalian Slo2 orthologs, Slick or Slack (which are KNa, not KCa channels24,24,27), may underlie APC in mammals. Herein we used novel gene deleted mice (Slo2.1−/−, Slo2.2−/−, and Slo2.x double knockout) to conclusively demonstrate that Slo2.1 is the channel required for APC and for VA stimulated K+ flux in cardiomyocytes and mitochondria.
Methods
(*Cut from 5.5 to 4 pages. Changes too numerous to list. Only requested additions are noted in red).
Animals
Mice were housed in an AAALAC-accredited pathogen-free facility with food and water available ad libitum. All procedures were approved by the University of Rochester’s Committee on Animal Resources (protocol #2010-030) in accordance with the NIH Guide for the Care and Use of Laboratory Animals (2011 revision). A Slo2.x dKO strain containing both mutant Slo2.x alleles28 was provided by Dr. Chris Lingle, Ph.D (Washington University, St. Louis MO). Mice were out-crossed >6x to a C57/BL6 background, and conventional breeding was employed such that experimental WT and knockout mice (males, 8–10 weeks old) were littermates. All animals were genotyped by tail clip PCR (Kapa Biosystems, Woburn MA)29 with the following primers: Slo2.1 (Slick), C523-24C 5′-AACTTTATGAGTTCCTCTTCCATG-3′, C320-24F 5′-GAGCATCATACTTTGCTTTTTGGG-3′, KO=269bp, WT=579bp; Slo2.2 (Slack) C311-30 5′-CCCATTCCACACTGCAGCCCTGTCTCTTTC-3′, C315-30 5′-TGTTTACTAGGGTCCAGGGAGAACCTATGA-3′, KO=200bp, WT=607bp. Due to personnel limitations (i.e., same person handling mice and doing experiments), it was not feasible to blind the experimenter to animal genotype. However, for all experiments mice of varying genotypes were randomly assigned to experimental groups, with treatments in randomized order across experimental days.
Ex-vivo perfused heart
Mouse hearts were perfused as previously described.22,26,29 Briefly, following tribromoethanol anesthesia (100mg/kg ip) the aorta was rapidly cannulated and perfused without pacing at a constant flow of 4 ml/min/100mg with Krebs-Henseleit buffer (KH, in mmol·L−1: 118 NaCl, 4.7 KCl, 25 NaHCO3, 10 D-glucose, 1.2 mM MgSO4, 1.2 KH2PO4, and 2.5 CaCl2, gassed with 95% O2, 5% CO2 at 37°C). Left ventricular pressure was measured via a water-filled transducer-linked LDPE balloon. LV and coronary root pressures were monitored and digitally recorded at 1 kHz (DATAQ, Akron OH). Parameters calculated from the LV balloon throughout perfusion included: heart rate, systolic and end diastolic pressures, LV developed pressure (systolic minus diastolic), rate x pressure product, dP/dTMAX (contraction) and dP/dTMIN (relaxation).
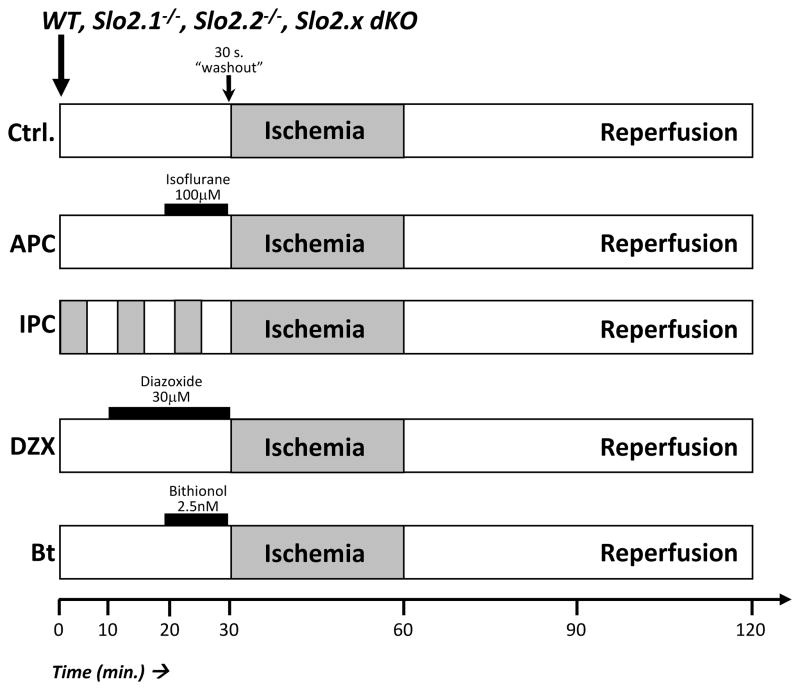
Hearts were equilibrated for 20 min. prior to initiating data acquisition. 163 total hearts were analyzed, and none were excluded from the study. A 30 min. normoxic perfusion was followed by 30 min. of no flow global ischemia, then 60 min. of reperfusion (see Figure 1). Treatment groups were: (i) Control IR injury. (ii) IPC comprising 3 cycles of 5 min. ischemia plus 5 min. reperfusion, to replace the 30 min. perfusion. (iii) 10 min. isoflurane (Henry Schein animal health, Dublin OH) infusion (100 μmol·L−1 or ~0.35 MAC for a C57/BL6 mouse.30,31), (iv) 20 min. 7-Chloro-3-methyl-4H-1,2,4-benzothiadiazine 1,1-dioxide (Diazoxide, a K+ channel activator32; Sigma, St. Louis MO) infusion (30 μmol·L−1). (v) 10 min. 2,2′-sulfanediylbis(4,6-dichlorophenol) (Bithionol a Slo channel activator33; TCI America, Portland OR) infusion (2.5 nmol·L−1). Pharmacologic agents were delivered via syringe pump just above the perfusion cannula for the indicated times, followed by 30 sec. washout prior to index ischemia. At the end of IR protocols, hearts were sliced, stained in 1% (wt/vol) 2,3,5-triphenyltetrazolium chloride (TTC) for 20 min., and fixed in 10% neutral buffered formalin for 24 hr. Heart slices were imaged and infarct size analyzed by planimetry as previously described.22,26,29
Figure 1. Experimental Protocol.
Schematic shows the 5 perfused heart experimental conditions tested. Gray shading represents ischemia. 30s. washout was performed after administration of pharmacologic agents, before ischemia.
Plasma membrane thallium (Tl+) flux assay on isolated mouse cardiomyocytes and HEK293 cells
Mouse adult ventricular cardiomyocytes were isolated by collagenase perfusion as previously described26. Briefly, following anesthesia (tribromoethanol as above), hearts were cannulated and perfused with isolation buffer (IB) at 37 °C (IB; in mmol·L−1: 120 NaCl, 15 KCl, 0.6 Na2HPO4, 0.6 KH2PO4, 1.2 MgSO4, 10 HEPES, 4.6 NaHCO3, 30 taurine, 5.5 D-glucose, and 10 2,3-butanedione monoxime (BDM), pH =7.4) for <2 minutes before switching to digestion buffer (35 ml of IB plus CaCl2 12.5 μmol·L−1 final, trypsin 400 μL at 2.5% (w/v), collagenase A 6.525 units, collagenase D 15.375 units), and 8 minutes later placed in 2–3 ml of stop buffer (IB plus CaCl2 12.5μmol·L−1, 10% heat inactivated FBS). Cardiomyocytes were dissociated and filtered through 75 μm mesh, then gravity sedimentation and resuspension were used to bring [Ca2+] step-wise to 1.8mmol·L−1, and the final pellet was placed into 1 ml of Minimal Essential Media (MEM; GIBCO, Grand Island NY). Cell viability and yield were determined using Trypan blue and a hemocytometer, and preparations with >85% viable rod-shaped cells were used experimentally. Cells were seeded (2500 cells/well) in a Falcon 96 well plate and incubated at 37 °C for 1 hr, then loaded with Thallos reagent (2 μmol·L−1; Teflabs, Austin TX) in 65 μL Hanks Buffered Salt Solution (HBSS; GIBCO, Grand Island NY), and monitored for thallium (Tl+) uptake by addition of 4 mmol·L−1 final Tl2SO4 in stimulus buffer (SB, in mmol·L−1: 276 Na+ gluconate, 2.6 CaSO4, 1.6 MgSO4, 11.2 D-glucose, 40 HEPES, pH 7.3) in a BioTek Synergy plate reader in kinetic mode measuring every 7 s. (λex 488 nm; λem 525 nm) in the presence or absence of channel modulators. N-benzyl-N-(3-isobutoxy-2-pyrrolidin-1-yl-propyl)aniline (Bepridil, Sigma, St. Louis MO) is a calcium channel blocker that also inhibits Slo2 channels33. Eight measurements were taken prior to additions. Paired control wells contained Tl+ free SB, enabling determination of Tl+-specific fluorescence changes.
HEK 293 cells were seeded on glass 12-mm coverslips and transfected using Lipofectamine 3000 (Invitrogen, Carlsbad, CA) with the expression vector pKT122 expressing an mCherry::rat Slick cDNA fusion in a pcDNA5/TO vector backbone (Invitrogen, Carlsbad CA). After 24 hours, cells were incubated in 1 ml of Hanks Buffered Saline Solution containing 2 μmol·L−1 Thallos reagent (TefLabs, Austin TX) for 30 min., then transferred to an open flow perfusion rig attached to a Nikon TE2000 microscope equipped with a monochromator (Till Photonics, Germany), CCD camera (PCO-TECH, Romulus MI) and appropriate filter sets. Transfected cells were identified via mCherry fluorescence and perfused with SB (as described above), with or without Slo2 channel modulators, then SB with 4 mmol·L−1Tl2SO4, and images acquired every 5 s. (λex 488 nm; λem 535 nm). Changes in fluorescent emission were quantified for all cells in the field of view, both transfected and untransfected, using TILLvisION software (TILL Photonics).
Mitochondrial Thallium (Tl+) flux assay on isolated mouse heart mitochondria
Following anesthesia (tribromoethanol as above), mitochondria were isolated from 3 pooled mouse hearts, loaded with BTC-AM (Life Technologies, Carlsbad CA) and monitored for Tl+ uptake (a surrogate for K+ channel flux) as previously described.22,29,34
Isoflurane dosing
In perfused hearts, isoflurane infusion via syringe pump afforded no possibility for volatilization before entering the heart. For C57BL/6 mice, 100 μmol·L−1 isoflurane equates to ~0.35 MAC.30,31 For cell and mitochondrial experiments, incubations generally lasted <1min. such that isoflurane volatilization was considered negligible. As such, isoflurane concentrations are denoted as “initial”.
Statistical analysis
Significance was determined using a two-way ANOVA; and when warranted post-hoc pair-wise t-testing (two-tailed) analysis using Bonferroni multiple comparisons correction. P values reported in the results are from t-tests, with p<0.05 (before multiple comparisons correction) considered statistically significant. Sample size was determined based upon experience and previous studies.
Results
Cardiac anesthetic preconditioning requires Slo2.1 (Slick)
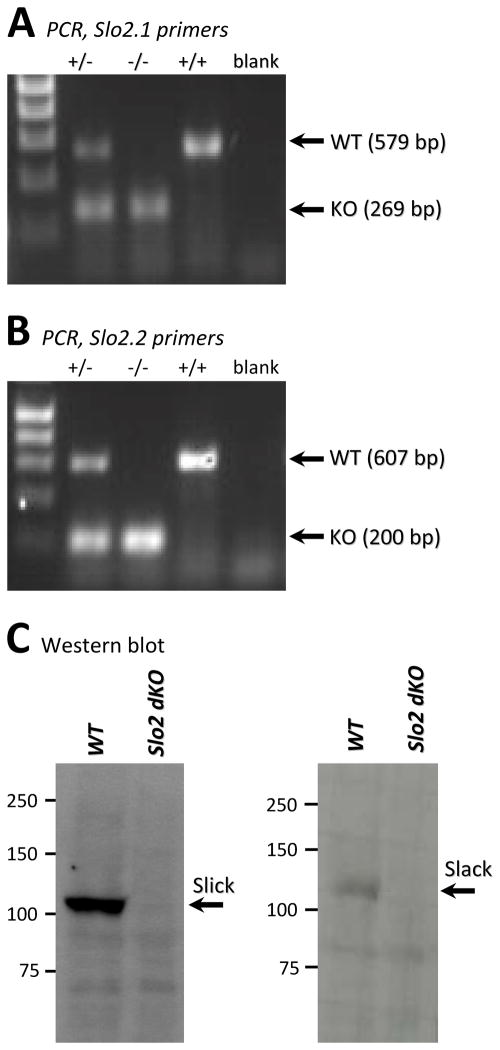
In C. elegans, Slo2 is encoded by a single gene, and contributes to VA-stimulated mitochondrial potassium flux and APC.22 However, in mammals Slo2 has diverged into two paralogs (Slo2.1 and Slo2.2) with differing ion sensitivity to the C. elegans channel. Using a recently developed mouse strain with the genes coding for Slick (Kcnt2 or Slo2.1) and Slack (Kcnt1 or Slo2.2) both deleted,28 our goal herein was to determine if either or both of these mammalian gene products are orthologous to the worm Slo2 channel, and facilitate K+ flux across the mitochondrial inner membrane in response to VA. First, we verified using genomic PCR that the Slo2.x double knockout (dKO) contained the expected lesions in each of the two genes, and that each allele was unambiguously detectable (Figures 2A and 2B). Next, we used Western blot analysis to query whether the channels themselves were similarly ablated. Slick and Slack are abundantly expressed in neural tissue, and our results demonstrate that we can readily detect both paralogs in brain lysates as well as their absence in the dKO background (Figure 2C). Finally, we report that Slo2.x dKO mice were viable and had normal cardiac electrical function (Table S1).
Figure 2. Slo2.1 and Slo2.2 genotyping and protein expression analysis of Slick and Slack in Slo2.x dKO mice.
(A): Tail clip PCR showing WT or knockout Slo2.1 products at the indicated mass, for WT (+/+), knockout (−/−), or heterozygous (+/−) animals. Primers were as described in the methods. Left lane = DNA ladder. (B): As in panel A, but with Slo2.2 primers. (C): Western blot for Slick and Slack (Neuromab antibody) on brain tissue lysates from wild-type (WT) and Slo2.x dKO mice.
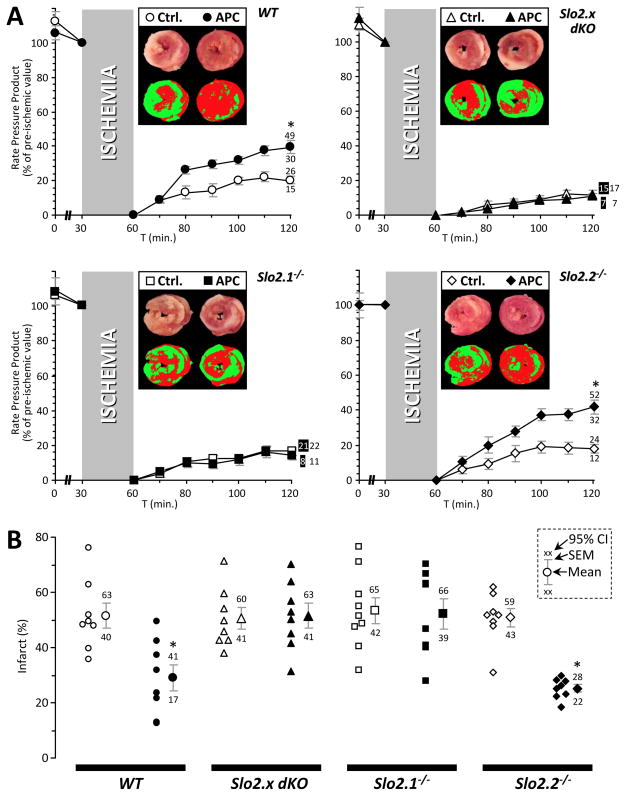
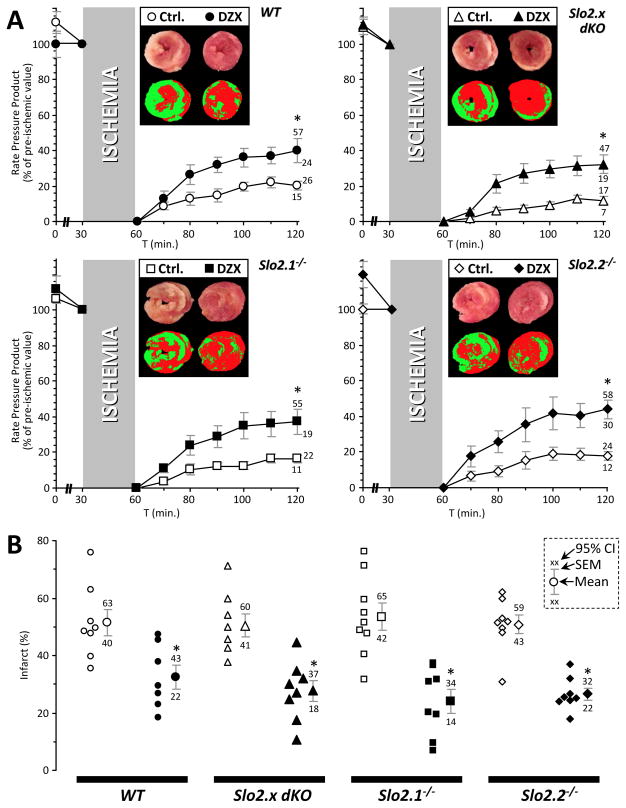
To interrogate the individual contribution of each Slo2.x paralog to APC, the mutant alleles were genetically separated through extensive backcrossing to a C57BL/6J background. Ex-vivo perfused hearts from wild type (WT) and Slo2 ablated mice (both the Slo2.x dKO and single mutant alleles) were then subjected to IR injury with optional APC (isoflurane in perfusion media). Injury was assessed by measuring post-IR functional recovery (Figure 3A) and infarct size (Figure 3B). APC was protective in WT hearts, with a 95% improvement in the post-IR recovery of Rate Pressure Product (RPP; heart rate x left ventricular developed pressure, p=0.0005) and a 44% reduction in infarct size (p=0.0043), vs. control hearts. Slo2.2−/− mice exhibited a similar degree of protection by APC (132 % improvement in recovery of RPP, p=0.00016, 50% reduction in infarct size, p=0.000005) vs. control hearts. However, APC in Slo2.1−/− mice resulted in no protection (12% worse recovery of RPP, p=0.057, 2% reduction in infarct size, p=0.817, compared to IR alone). As expected, APC in dKO mice was similarly ineffective (8% worse recovery of RPP, p=0.725, 2% increase in infarct size, p=0.854, compared to IR alone). None of the genotypes exhibited significant differences in baseline susceptibility to ischemia.
Figure 3. Anesthetic preconditioning in wild-type (WT) and Slo2.x knockout mouse hearts.
Langendorff perfused hearts from WT (circles), Slo2.x dKO (triangles), Slo2.1−/− (squares) and Slo2.2−/− (diamonds) were subjected to ischemia-reperfusion (IR) injury alone (open symbols) or IR with anesthetic preconditioning (APC) comprising 100 μmol·L−1 isoflurane infusion for 10 min. prior to ischemia (filled symbols). (A): Cardiac function data (RPP, product of heart rate x left ventricular developed pressure) throughout perfusion. RPP is expressed as a percentage of the value immediately prior to ischemia. Data are means ± SEM, with 95% confidence intervals for the 60 min. reperfusion time point shown adjacent to error bars (numbers shaded where appropriate to indicate which data set they belong to – see inset to panel B). Insets: representative heart cross-sections stained with TTC (upper images), and threshold pseudo-colored images used to quantify infarct size (lower images). (B): Infarct size for control IR and APC+IR treated hearts from each genotype. Within each group, individual data points are on the left, thereby indicating the number of replicates (N). Means ± SEM are on the right, with 95% confidence intervals shown adjacent. Inset key shows position of mean, SEM, and 95% CIs on the graphs. *Statistically significant difference between control IR and APC+IR groups at 60 min. of reperfusion (ANOVA, with post-hoc unpaired t-test).
Cardioprotection by ischemic preconditioning (IPC) or the mKATP channel opener diazoxide (DZX) are independent of Slo2.x channel genotype
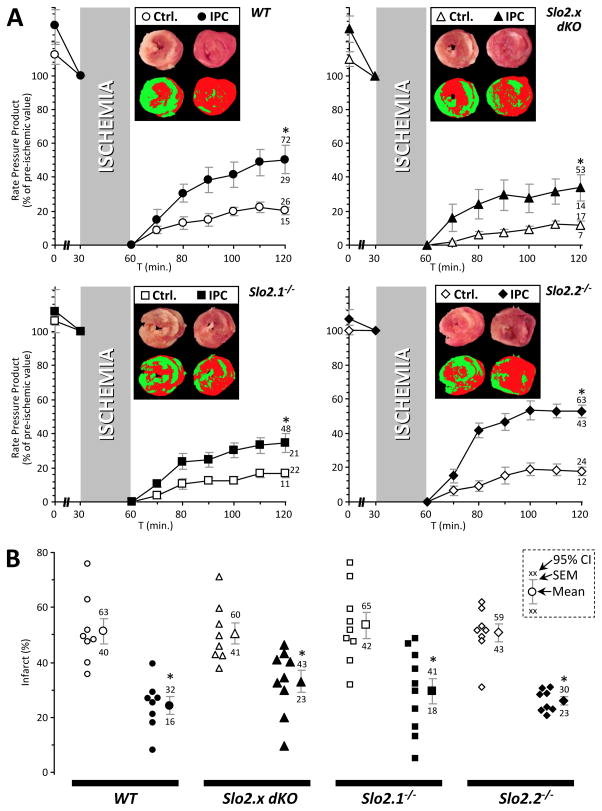
To discount the possibility that lack of protection by APC in the Slo2.1−/− or dKO hearts was due to an inability of these hearts to be protected at all, we examined two additional cardioprotective treatments that are unrelated to VA: namely IPC (Figure 4) and the mitochondrial KATP channel opener diazoxide (DZX) (Figure 5).
Figure 4. Cardioprotection by ischemic preconditioning (IPC) in wild-type (WT) and Slo2.x knockout mouse hearts.
Langendorff perfused hearts were subjected to control ischemia-reperfusion (IR) injury alone (open symbols) or IR+IPC (filled symbols). Symbols for genotypes are the same as Figure 3. Data for controls (IR alone, no treatment) are reproduced from Figure 3 and shown for comparative purposes. (A): Cardiac function data (RPP, product of heart rate x left ventricular developed pressure) throughout perfusion. RPP is expressed as a percentage of the value immediately prior to ischemia. Data are means ± SEM, with 95% confidence intervals for the 60 min. reperfusion time point shown adjacent to error bars (see inset to panel B). Insets: representative heart cross-sections stained with TTC (upper images), and threshold pseudo-colored images used to quantify infarct size (lower images). (B): Infarct size for control IR and IPC+IR treated hearts from each genotype. Within each group, individual data on the left, thereby indicating the number of replicates (N). Means ± SEM are on the right, with 95% confidence intervals shown adjacent. Inset key shows position of mean, SEM, and 95% CIs on the graphs. *Statistically significant difference between control IR and IPC+IR groups at 60 min. of reperfusion (ANOVA, with post-hoc unpaired t-test).
Figure 5. Cardioprotection by diazoxide in wild-type (WT) and Slo2.x knockout mouse hearts.
Langendorff perfused hearts were subjected to control ischemia-reperfusion (IR) injury alone (open symbols) or diazoxide+IR (DZX, filled symbols). Symbols for genotypes are the same as Figure 3. Data for controls (IR alone, no treatment) are reproduced from Figure 3 and shown for comparative purposes. (A): Cardiac function data (RPP, product of heart rate x left ventricular developed pressure) throughout perfusion. RPP is expressed as a percentage of the value immediately prior to ischemia. Data are means ± SEM, with 95% confidence intervals for the 60 min. reperfusion time point shown adjacent to error bars (see inset to panel B). Insets: representative heart cross-sections stained with TTC (upper images), and resulting pseudo-colored images calculated via threshold masks and used to quantify infarct size (lower images). (B): Infarct size for control IR and DZX+IR treated hearts from each genotype. Within each group, individual data are shown on the left, thereby indicating the number of replicates (N). Means ± SEM are on the right, with 95% confidence intervals shown adjacent. Inset key shows position of mean, SEM, and 95% CIs on the graphs. *Statistically significant difference between control IR and DZX+IR groups (ANOVA, with post-hoc unpaired t-test).
The improvement in functional recovery (RPP) induced by IPC for each genotype was: WT 148%, dKO 184%, Slo2.1−/− 108%, Slo2.2−/− 193%, compared to IR alone, p<0.025 for all genotypes (Figure 4A). The reduction in infarct size induced by IPC was: WT 53%, dKO 34%, Slo2.1−/− 45%, Slo2.2−/− 49%, compared to IR alone, p<0.0025 for all genotypes (Figure 4B). Similar to IPC, DZX delivered consistent cardioprotection across all genotypes. Namely, the improvement in functional recovery (RPP) induced by DZX: WT 98%, dKO 171%, Slo2.1−/− 125%, Slo2.2−/− 143%, compared to IR alone, p<0.01 for all genotypes (Figure 5A). The reduction in infarct size induced by IPC was: WT 37%, dKO 45%, Slo2.1−/− 55%, Slo2.2−/− 48%, compared to IR alone, p<0.001 for all genotypes (Figure 5B). Thus, cardioprotection by either IPC or DZX was consistent regardless of genotype, suggesting Slo2.1−/− hearts are still capable of being protected by these interventions and that cardioprotection, even via mechanisms thought to involve mitochondria, does not invariably require Slick.
In addition to functional recovery measured as rate x pressure product, data for dP/dTMAX anddP/dTMIN, LV developed pressure, and LV end diastolic pressure, for all genotypes and treatment regimens, are provided in Supplemental Tables 2, 3, and 4 respectively.
VA stimulated K+ transport in cardiomyocytes and mitochondria requires Slo2.1 (Slick)
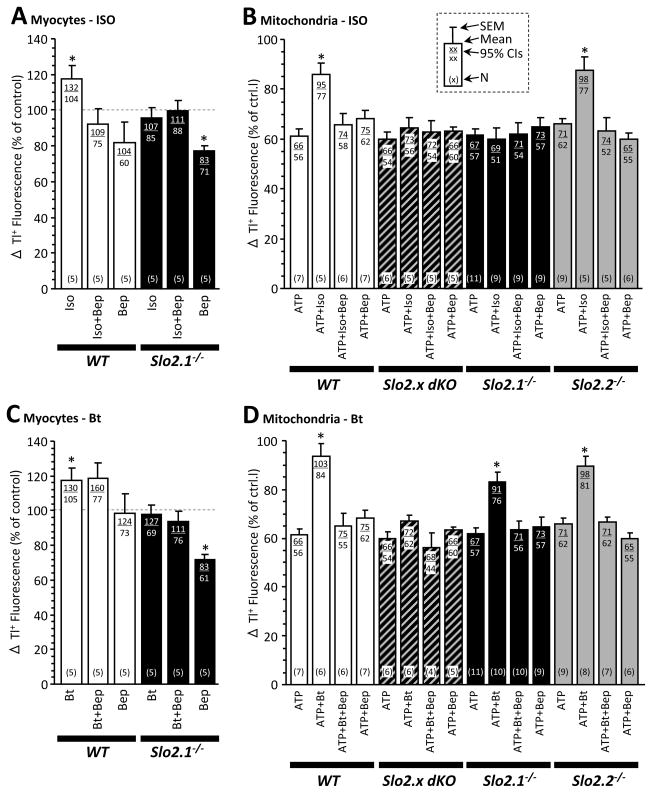
Given the proposed role of a mitochondrial large conductance K+ channel in APC,7,22,23 we examined VA-stimulation of K+ flux in both primary cardiomyocytes and isolated cardiac mitochondria, using a thallium (Tl+) based fluorescence assay.22,34 All four genotypes were studied at the isolated mitochondrial level, but due to our initial results (vide supra) indicating that Slick but not Slack is involved in APC, studies in cardiomyocytes were limited to WT and Slo2.1−/−.
The VA isoflurane was found to stimulate K+ flux in cardiomyocytes (Figure 6A) as well as mitochondria (Figure 6B) from WT mice, and this effect was blocked by the inhibitor bepridil. Bepridil is thought to target cardiac KNa35 and other channels, 36 and has recently been shown to function as an Ebola anti-viral.37 Its use here was motivated by its ability to inhibit Slo2 channels33, which we validated using recombinant Slick expressed in HEK 293 cells (Figure S1). Notably, VA stimulation of K+ flux was absent in both cardiomyocytes and mitochondria from Slo2.1−/− hearts (Figure 6A/B). At the mitochondrial level, the Tl+ flux pattern in Slo2.2−/− was similar to WT, and the pattern in dKO was similar to Slo2.1−/−. These data indicate that isoflurane induces a Slo2.1 dependent K+ flux in both cardiomyocytes and mitochondria.
Figure 6. Thallium (Tl+) flux in cardiomyocytes and mitochondria from mice of varying Slo2 genotype.
(A): Cardiomyocytes were isolated from wild-type (WT) (white bars) and Slo2.1−/− (black bars) mice, and surface K+ channel activity assayed via thallium (Tl+) uptake. Where indicated, isoflurane (Iso, 150 μmol·L−1 initial) and/or bepridil (Bep, 10 μmol·L−1) were added. Data are normalized to control (no addition, dashed line), and are means ± SEM. 95% confidence intervals are shown at top, number of replicates (N) at bottom, of each graph bar – see inset key in panel B. *Statistically significant difference versus control within a given genotype (ANOVA, with post-hoc unpaired t-test). (B): Mitochondria were isolated from WT (white bars) Slo2.x dKO (hashed), and Slo2.1−/− (black), and Slo2.2−/− (gray) hearts, and K+ channel activity assayed via Tl+ uptake. ATP was present throughout to block mKATP channels. Where indicated, isoflurane (Iso, 300 μmol·L−1 initial) and/or bepridil (Bep, 10 μmol·L−1) were added. Data are normalized to control (no addition), and are means ± SEM. 95% confidence intervals are shown at top, number of replicates (N) at bottom, of each graph bar – see inset key. *Statistically significant difference versus ATP condition within a given genotype (ANOVA, with post-hoc unpaired t-test). (C): Cardiomyocyte Tl+ flux, as in panel A, but stimulated with bithionol (Bt, 0.25 μmol·L−1) instead of isoflurane. Data are normalized to control (no addition, dashed line), and are means ± SEM. 95% CIs and N as per inset key in panel B. *Statistically significant difference versus control within a given genotype (ANOVA, with post-hoc unpaired t-test). (D): Mitochondrial Tl+ flux as in panel B, but stimulated with bithionol (Bt, 2.5 μmol·L−1) instead of isoflurane. Data are normalized to control (no addition), and are means ± SEM. 95% CIs and N as per inset key in panel B. *Statistically significant difference versus ATP condition within a given genotype (ANOVA, with post-hoc unpaired t-test).
To substantiate the hypothesis that K+ flux and cardioprotection occur through Slick activation, we turned to a second distinct reagent. Bithionol has been shown to block Slo type channels,33 and inhibits recombinant Slick expressed HEK293 cells (Figure S1). At the cardiomyocyte level, bithionol induced a bepridil-sensitive K+ flux in WT cells that was absent in Slo2.1−/− cells (Figure 6C). In mitochondria, bithionol induced a bepridil-sensitive K+ flux in WT that was absent in the dKO mitochondria and reduced in both the Slo2.1−/− and Slo2.2−/− mitochondria (Figure 6D). The cell data fully support our hypothesis. However, the mitochondria data suggest that both Slick and Slack partially contribute to bithionol-stimulated Tl+ uptake, which is potentially interesting (see Discussion). However, it is likely that bithionol stimulates other channel activities or even mitochondrial function through unknown mechanisms of action, and very low levels of Slo2.2 are expressed in the heart.28
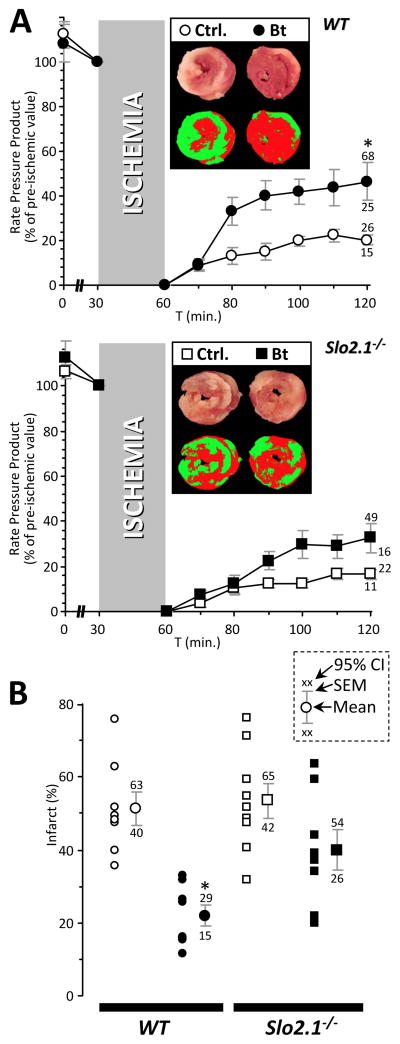
Finally, additional support for our hypothesis came from the observation that bithionol was cardioprotective in WT hearts (Figure 7A/B: 129% improvement in RPP recovery, p=0.0098, 57% reduction in infarct size, p=0.00008, compared to IR alone). This protection was partially lost in Slo2.1−/− hearts (bithionol induced 97% improvement in RPP recovery, p=0.028, 14% reduction in infarct size, p=0.15). Overall these data support a K+ transport pathway with the genetic and pharmacologic characteristics of Slick, being required for the cardioprotective effects of APC.
Figure 7. Effect of bithionol on IR injury outcomes in wild-type (WT) and Slo2.1 knockout mouse hearts.
(A): Langendorff perfused hearts from WT (circles) and Slo2.1−/− (squares) were subjected to ischemia-reperfusion (IR) injury alone (open symbols) or IR with bithionol (Bt, 2.5 nM for 20 min. prior to the onset of ischemia, filled symbols), as detailed in the methods and in Figures 3–5. The control groups are reproduced from Figure 3 and shown here for comparative purposes. RPP is expressed as a percentage of the value immediately prior to ischemia. Data are means ± SEM, with 95% confidence intervals for the 60 min. reperfusion time point shown adjacent to error bars (see inset, panel B). Insets: representative heart cross-sections stained with TTC (upper images), and resulting pseudo-colored images calculated via threshold masks and used to quantify infarct size (lower images). (B): Infarct size for control IR and Bt+IR treated hearts from each genotype. Within each group, individual data points are shown on the left, thereby indicating the number of replicates (N). Inset key shows position of mean, SEM, and 95% CIs on the graphs. *Statistically significant difference between control IR and Bt+IR groups at 60 min. of reperfusion (ANOVA, with post-hoc unpaired t-test).
Discussion
Herein we demonstrated that the KNa channel Slick, encoded by the Slo2.1 gene, is required for cardioprotection by APC in mice. We also showed that Slick is responsible for VA-stimulated K+ flux at both the cardiomyocyte plasma and mitochondrial membranes, and that pharmacologic Slick openers are cardioprotective.
While Slo2 is a KCa channel in C. elegans, in mammals it has diverged into two paralogs, Slick and Slack, that are KNa channels.24,24,27 These channels may contribute to various physiologic functions attributed to KNa channels, including metabolic sensing and neuronal plasticity,24,27,38 and although the major focus of KNa channel studies to date has been neuronal, KNa channels are found in cardiac myocytes.39 Notably, they have not been reported in mitochondria. While these results cement a cardioprotective role for Slick, the relative contribution of plasma membrane vs. mitochondrial Slick channels to this effect remains unclear, and is a topic of ongoing research.
Many signaling pathways such as anesthetic preconditioning (APC) and ischemic preconditioning (IPC) are evolutionarily conserved,22 and there are many redundancies in their signaling cascades. For example, the mechanism of APC and IPC share commonalities such as protein kinase C activation,12,40 reactive oxygen species (ROS) generation,14,41–43 and mitochondrial K+ channels.44–46 While the precise mechanism of APC is not well understood, evidence suggests that APC involves both altered mitochondrial function and K+ channel activity (reviewed in 47).
Studies have implicated the mitochondrial ATP-sensitive K+ channel (mKATP) in APC largely through the use of non-specific measures of channel activity (e.g., flavoprotein fluorescence) and drugs that are known to have off-target mitochondrial effects (e.g., diazoxide or 5-hydroxydecanoate).44–46,48,49 While these pharmacologic approaches suggest the involvement of the mKATP, the effects could have also been due to another K+ channel such as Slick. The current lack of a molecular identity for the mKATP channel precludes a definitive answer to this dilemma.
Moreover, the data presented herein do not rule out a potential cross-talk between the mKATP and Slick. For example, since both diazoxide- and IPC-mediated cardioprotection were preserved in Slo2.1−/− mice, Slick activation may be upstream of mKATP channel activation. Overall, while there are many similarities between APC and IPC, there is also evidence for distinct signaling pathways50, and herein we show that Slo2.1 appears to be involved only in APC, not IPC.
In the field of APC, the canonical BK channel Slo1 which may be present in cardiac mitochondria51 emerged as an early candidate effector of APC protection.52–54 However, the importance of this channel was recently questioned by evidence that both IPC and APC protection are robust in Slo1 ablated organisms.22 While the role of Slo1 in the heart remains to be fully elucidated, it is important to emphasize that our data do not preclude co-existence of Slo1 and Slick in cardiomyocytes or cardiac mitochondria. Rather, they conclude that only Slick is required for APC in the heart.
Our results are consistent with findings in C. elegans which showed that SLO-2 was required for APC.22 Despite the large sequence similarity (~74% identical) 38 between Slick and Slack, we found no role for Slack in cardiac APC or IPC. This is in agreement with the low expression levels of Slo2.2 in the heart.27 However, this does not rule out a role for Slo2.2 in protecting other tissues from ischemia. Both Slo2.1 and Slo2.2 are highly expressed in the nervous system24 and there is evidence that they may co-assemble into channels.55 In this respect, it is intriguing that both Slick and Slack appeared to contribute to bithionol-induced K+ flux in purified mitochondria (Figure 6D). However, this was the only condition where both Slick and Slack were additive in our hands. Moreover, the mechanism of action of bithionol is unknown, but its activity as an antihelmintic may rely on suppressing succinate oxidation.56 Hence, it is premature to conclude that Slack contributes to mitochondrial function. Nevertheless, it is possible that in other tissues such as the brain, Slo2.2 may play a role protecting against stress.
It is also interesting to speculate that Slick did not evolve to be opened by volatile anesthetics, and given our results we speculate that it may have a physiologic role in the mitochondrion. While some mitochondrial K+ channels are demonstrated to play roles in patho-physiology (e.g., protection from IR injury),19 most are only reported at the phenomenological level as mitochondrial swelling or K+ flux sensitive to activators/inhibitors, with very little known about the normal function of these channels. The activation of mitochondrial K+ channels is hypothesized to modulate mitochondrial matrix volume, Ca2+ uptake capacity, and ROS production,57 and while these events are involved in the pathophysiology of IR injury, they also have effects on regular mitochondrial function and metabolism. For example, Ca2+ stimulates oxidative phosphorylation, and matrix volume can determine the efficiency of high energy phosphate transport between the mitochondrion and cytosol. Ongoing research in our lab is aimed at determining the role of Slick in regulating physiologic mitochondrial function.
A potential limitation of this study is the low dose of isoflurane (~0.35 MAC) used to elicit cardioprotection. It is unclear if higher doses, or other volatile anesthetics (e.g., desflurane, sevoflurane) will exhibit similar Slo2.1 dependent cardioprotective effects. Nevertheless, we note that the current widespread clinical use of VAs, and the ACC/AHA recommendations for their use during cardiac surgery,5 suggests that the identification of Slick as a mediator of APC may drive development of novel cardioprotective drugs targeting this channel.
Supplementary Material
Acknowledgments
Funding: National Institutes of Health (Bethesda, Maryland; R01-GM087483) to PSB & KWN.
We thank Chris Lingle, Ph.D. (Department of Anesthesiology, Washington University School of Medicine, St. Louis, MO) for providing Slo2.x knockout mice.
Footnotes
Conflicts of Interest: None
Data Transparency: All original data used to prepare figures for this manuscript and for statistical analyses, are available at: https https://dx.doi.org/10.6084/m9.figshare.2062833
Literature Cited
- 1.Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology. 1997;86:699–709. doi: 10.1097/00000542-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Stadnicka A, Marinovic J, Ljubkovic M, Bienengraeber MW, Bosnjak ZJ. Volatile anesthetic-induced cardiac preconditioning. J Anesth. 2007;21:212–9. doi: 10.1007/s00540-006-0486-6. [DOI] [PubMed] [Google Scholar]
- 3.Landoni G, Biondi-Zoccai GG, Zangrillo A, Bignami E, D’Avolio S, Marchetti C, Calabro MG, Fochi O, Guarracino F, Tritapepe L, De HS, Torri G. Desflurane and sevoflurane in cardiac surgery: a meta-analysis of randomized clinical trials. J Cardiothorac Vasc Anesth. 2007;21:502–11. doi: 10.1053/j.jvca.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Jia B, Crowder CM. Volatile anesthetic preconditioning present in the invertebrate Caenorhabditis elegans. Anesthesiology. 2008;108:426–33. doi: 10.1097/ALN.0b013e318164d013. [DOI] [PubMed] [Google Scholar]
- 5.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM, Jr, Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Selnes O, Shahian DM, Trost JC, Winniford MD. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123–e210. doi: 10.1016/j.jacc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig LM, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Protein kinase C translocation and Src protein tyrosine kinase activation mediate isoflurane-induced preconditioning in vivo: potential downstream targets of mitochondrial adenosine triphosphate-sensitive potassium channels and reactive oxygen species. Anesthesiology. 2004;100:532–9. doi: 10.1097/00000542-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Redel A, Lange M, Jazbutyte V, Lotz C, Smul TM, Roewer N, Kehl F. Activation of mitochondrial large-conductance calcium-activated K+ channels via protein kinase A mediates desflurane-induced preconditioning. Anesth Analg. 2008;106:384–91. doi: 10.1213/ane.0b013e318160650f. table. [DOI] [PubMed] [Google Scholar]
- 8.Yang C, Talukder MA, Varadharaj S, Velayutham M, Zweier JL. Early ischaemic preconditioning requires Akt- and PKA-mediated activation of eNOS via serine1176 phosphorylation. Cardiovasc Res. 2013;97:33–43. doi: 10.1093/cvr/cvs287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toller WG, Kersten JR, Gross ER, Pagel PS, Warltier DC. Isoflurane preconditions myocardium against infarction via activation of inhibitory guanine nucleotide binding proteins. Anesthesiology. 2000;92:1400–7. doi: 10.1097/00000542-200005000-00031. [DOI] [PubMed] [Google Scholar]
- 10.Kersten JR, Orth KG, Pagel PS, Mei DA, Gross GJ, Warltier DC. Role of adenosine in isoflurane-induced cardioprotection. Anesthesiology. 1997;86:1128–39. doi: 10.1097/00000542-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Ishida T, Yarimizu K, Gute DC, Korthuis RJ. Mechanisms of ischemic preconditioning. Shock. 1997;8:86–94. doi: 10.1097/00024382-199708000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Novalija E, Fujita S, Kampine JP, Stowe DF. Sevoflurane mimics ischemic preconditioning effects on coronary flow and nitric oxide release in isolated hearts. Anesthesiology. 1999;91:701–12. doi: 10.1097/00000542-199909000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–8. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 14.Mullenheim J, Ebel D, Frassdorf J, Preckel B, Thamer V, Schlack W. Isoflurane preconditions myocardium against infarction via release of free radicals. Anesthesiology. 2002;96:934–40. doi: 10.1097/00000542-200204000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Garlid KD, Halestrap AP. The mitochondrial K(ATP) channel--fact or fiction? J Mol Cell Cardiol. 2012;52:578–83. doi: 10.1016/j.yjmcc.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabo I, Bock J, Jekle A, Soddemann M, Adams C, Lang F, Zoratti M, Gulbins E. A novel potassium channel in lymphocyte mitochondria. J Biol Chem. 2005;280:12790–8. doi: 10.1074/jbc.M413548200. [DOI] [PubMed] [Google Scholar]
- 17.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–33. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 18.Shi GY, Jung DW, Garlid KD, Brierley GP. Induction of respiration-dependent net efflux of K+ from heart mitochondria by depletion of endogenous divalent cations. J Biol Chem. 1980;255:10306–11. [PubMed] [Google Scholar]
- 19.Szabo I, Zoratti M. Mitochondrial channels: ion fluxes and more. Physiol Rev. 2014;94:519–608. doi: 10.1152/physrev.00021.2013. [DOI] [PubMed] [Google Scholar]
- 20.Szewczyk A, Jarmuszkiewicz W, Kunz WS. Mitochondrial potassium channels. IUBMB Life. 2009;61:134–43. doi: 10.1002/iub.155. [DOI] [PubMed] [Google Scholar]
- 21.Kohro S, Hogan QH, Nakae Y, Yamakage M, Bosnjak ZJ. Anesthetic effects on mitochondrial ATP-sensitive K channel. Anesthesiology. 2001;95:1435–340. doi: 10.1097/00000542-200112000-00024. [DOI] [PubMed] [Google Scholar]
- 22.Wojtovich AP, Sherman TA, Nadtochiy SM, Urciuoli WR, Brookes PS, Nehrke K. SLO-2 Is Cytoprotective and Contributes to Mitochondrial Potassium Transport. PLoS One. 2011;6:e28287. doi: 10.1371/journal.pone.0028287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stumpner J, Lange M, Beck A, Smul TM, Lotz CA, Kehl F, Roewer N, Redel A. Desflurane-induced post-conditioning against myocardial infarction is mediated by calcium-activated potassium channels: role of the mitochondrial permeability transition pore. Br J Anaesth. 2012;108:594–601. doi: 10.1093/bja/aer496. [DOI] [PubMed] [Google Scholar]
- 24.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–31. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber M, Wei A, Yuan A, Gaut J, Saito M, Salkoff L. Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J Biol Chem. 1998;273:3509–16. doi: 10.1074/jbc.273.6.3509. [DOI] [PubMed] [Google Scholar]
- 26.Wojtovich AP, Nadtochiy SM, Urciuoli WR, Smith CO, Grunnet M, Nehrke K, Brookes PS. A non-cardiomyocyte autonomous mechanism of cardioprotection involving the SLO1 BK channel. PeerJ. 2013;1:e48. doi: 10.7717/peerj.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci. 2003;23:11681–91. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Espinosa PL, Wu J, Yang C, Gonzalez-Perez V, Zhou H, Liang H, Xia X-M, Lingle CJ. Knockout of Slo2.2 enhances itch, abolishes KNa current, and increases action potential firing frequency in DRG neurons. eLife. 2015;4:e10013. doi: 10.7554/eLife.10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wojtovich AP, Urciuoli WR, Chatterjee S, Fisher AB, Nehrke K, Brookes PS. Kir6.2 is not the mitochondrial KATP channel but is required for cardioprotection by ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2013;304:H1439–H1445. doi: 10.1152/ajpheart.00972.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonner JM, Gong D, Li J, Eger EI, Laster MJ. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth Analg. 1999;89:1030–4. doi: 10.1097/00000539-199910000-00039. [DOI] [PubMed] [Google Scholar]
- 31.Franks NP, Lieb WR. Temperature dependence of the potency of volatile general anesthetics. implications for in vitro experiments. Anesthesiology. 1996;84:716–20. doi: 10.1097/00000542-199603000-00027. [DOI] [PubMed] [Google Scholar]
- 32.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D’Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–82. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 33.Yang B, Gribkoff VK, Pan J, Damagnez V, Dworetzky SI, Boissard CG, Bhattacharjee A, Yan Y, Sigworth FJ, Kaczmarek LK. Pharmacological activation and inhibition of Slack (Slo2.2) channels. Neuropharmacology. 2006;51:896–906. doi: 10.1016/j.neuropharm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Wojtovich AP, Williams DM, Karcz MK, Lopes CM, Gray DA, Nehrke KW, Brookes PS. A Novel Mitochondrial KATP Channel Assay. Circ Res. 2010;106:1190–6. doi: 10.1161/CIRCRESAHA.109.215400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori K, Saito T, Masuda Y, Nakaya H. Effects of class III antiarrhythmic drugs on the Na(+)-activated K+ channels in guinea-pig ventricular cells. Br J Pharmacol. 1996;119:133–41. doi: 10.1111/j.1476-5381.1996.tb15686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yatani A, Brown AM, Schwartz A. Bepridil block of cardiac calcium and sodium channels. J Pharmacol Exp Ther. 1986;237:9–17. [PubMed] [Google Scholar]
- 37.Johansen LM, DeWald LE, Shoemaker CJ, Hoffstrom BG, Lear-Rooney CM, Stossel A, Nelson E, Delos SE, Simmons JA, Grenier JM, Pierce LT, Pajouhesh H, Lehar J, Hensley LE, Glass PJ, White JM, Olinger GG. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci Transl Med. 2015;7:290ra89. doi: 10.1126/scitranslmed.aaa5597. [DOI] [PubMed] [Google Scholar]
- 38.Kaczmarek LK. Slack, Slick and Sodium-Activated Potassium Channels. ISRN Neurosci. 2013 doi: 10.1155/2013/354262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kameyama M, Kakei M, Sato R, Shibasaki T, Matsuda H, Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984;309:354–6. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- 40.Novalija E, Kevin LG, Camara AK, Bosnjak ZJ, Kampine JP, Stowe DF. Reactive oxygen species precede the epsilon isoform of protein kinase C in the anesthetic preconditioning signaling cascade. Anesthesiology. 2003;99:421–8. doi: 10.1097/00000542-200308000-00024. [DOI] [PubMed] [Google Scholar]
- 41.Kevin LG, Novalija E, Riess ML, Camara AK, Rhodes SS, Stowe DF. Sevoflurane exposure generates superoxide but leads to decreased superoxide during ischemia and reperfusion in isolated hearts. Anesth Analg. 2003;96:949–55. doi: 10.1213/01.ANE.0000052515.25465.35. table. [DOI] [PubMed] [Google Scholar]
- 42.Novalija E, Kevin LG, Eells JT, Henry MM, Stowe DF. Anesthetic preconditioning improves adenosine triphosphate synthesis and reduces reactive oxygen species formation in mitochondria after ischemia by a redox dependent mechanism. Anesthesiology. 2003;98:1155–63. doi: 10.1097/00000542-200305000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka K, Weihrauch D, Kehl F, Ludwig LM, LaDisa JF, Jr, Kersten JR, Pagel PS, Warltier DC. Mechanism of preconditioning by isoflurane in rabbits: a direct role for reactive oxygen species. Anesthesiology. 2002;97:1485–90. doi: 10.1097/00000542-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 44.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–70. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka K, Weihrauch D, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mitochondrial adenosine triphosphate-regulated potassium channel opening acts as a trigger for isoflurane-induced preconditioning by generating reactive oxygen species. Anesthesiology. 2003;98:935–43. doi: 10.1097/00000542-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 46.Kapinya KJ, Lowl D, Futterer C, Maurer M, Waschke KF, Isaev NK, Dirnagl U. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke. 2002;33:1889–98. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal B, Stowe DF, Dash RK, Bosnjak ZJ, Camara AK. Mitochondrial targets for volatile anesthetics against cardiac ischemia-reperfusion injury. Front Physiol. 2014;5:341. doi: 10.3389/fphys.2014.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–41. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bantel C, Maze M, Trapp S. Neuronal preconditioning by inhalational anesthetics: evidence for the role of plasmalemmal adenosine triphosphate-sensitive potassium channels. Anesthesiology. 2009;110:986–95. doi: 10.1097/ALN.0b013e31819dadc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sergeev P, da SR, Lucchinetti E, Zaugg K, Pasch T, Schaub MC, Zaugg M. Trigger-dependent gene expression profiles in cardiac preconditioning: evidence for distinct genetic programs in ischemic and anesthetic preconditioning. Anesthesiology. 2004;100:474–88. doi: 10.1097/00000542-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Singh H, Lu R, Bopassa JC, Meredith AL, Stefani E, Toro L. MitoBK(Ca) is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc Natl Acad Sci U S A. 2013;110:10836–41. doi: 10.1073/pnas.1302028110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai MH, Wu Y, Gao Z, Anderson ME, Dalziel JE, Meredith AL. BK channels regulate sinoatrial node firing rate and cardiac pacing in vivo. Am J Physiol Heart Circ Physiol. 2014;307:H1327–H1338. doi: 10.1152/ajpheart.00354.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh H, Stefani E, Toro L. Intracellular BK(Ca) (iBK(Ca)) channels. J Physiol. 2012;590:5937–47. doi: 10.1113/jphysiol.2011.215533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siemen D, Loupatatzis C, Borecky J, Gulbins E, Lang F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem Biophys Res Commun. 1999;257:549–54. doi: 10.1006/bbrc.1999.0496. [DOI] [PubMed] [Google Scholar]
- 55.Chen L, Tian L, MacDonald SH, McClafferty H, Hammond MS, Huibant JM, Ruth P, Knaus HG, Shipston MJ. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) alpha-subunits generated from a single site of splicing. J Biol Chem. 2005;280:33599–609. doi: 10.1074/jbc.M505383200. [DOI] [PubMed] [Google Scholar]
- 56.Hamajima F. Studies on metabolism of lung fluke genus Paragonimus. VII. Action of bithionol on glycolytic and oxidative metabolism of adult worms. Exp Parasitol. 1973;34:1–11. doi: 10.1016/0014-4894(73)90056-8. [DOI] [PubMed] [Google Scholar]
- 57.Facundo HT, Fornazari M, Kowaltowski AJ. Tissue protection mediated by mitochondrial K+ channels. Biochim Biophys Acta. 2006;1762:202–12. doi: 10.1016/j.bbadis.2005.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.