Abstract
A rapidly growing body of research suggests that meditation can change brain and cognitive functioning. Yet little is known about the neurochemical mechanisms underlying meditation-related changes in cognition. Here we investigated the effects of meditation on spontaneous Eye Blink Rates (sEBR), a non-invasive peripheral correlate of striatal dopamine activity. Previous studies have shown a relationship between sEBR and cognitive functions such as mind-wandering, cognitive flexibility, and attention–functions that are also affected by meditation. We therefore expected that long-term meditation practice would alter eye-blink activity. To test this, we recorded baseline sEBR and Inter Eye-Blink Intervals (IEBI) in long-term meditators (LTM) and meditation naive participants (MNP). We found that LTM not only blinked less frequently, but also showed a different eye-blink pattern than MNP. This pattern had good to high degree of consistency over three time points. Moreover, we examined the effects of an 8 week-course of Mindfulness Based Stress Reduction (MBSR) on sEBR and IEBI, compared to an active control group and a waitlist-control group. No effect of short-term meditation practice was found. Finally, we investigated whether different types of meditation differentially alter eye blink activity by measuring sEBR and IEBI after a full day of two kinds of meditation practices in the LTM. No effect of meditation type was found. Taken together, these findings may suggest either that individual difference in dopaminergic neurotransmission is a self-selection factor for meditation practice, or that long-term, but not short-term meditation practice induces stable changes in baseline striatal dopaminergic functioning.
Introduction
Within the past few decades, there has been a surge of interest in the effects of mindfulness meditation on brain and cognitive functioning. A common aim of various styles of mindfulness meditations is to intentionally bring one’s attention to the internal and external experiences occurring in the present moment, without being caught up in the contents of experience. Mindfulness meditation is often taught through a variety of techniques such as mindful breathing or mindful walking exercises and can be used with various intentions, such as stress reduction, increasing well being, or caring for others (Lutz, Jha, Dunne, & Saron, 2015). Numerous studies have demonstrated positive effects of mindfulness meditation training on cognitive functions such as resistance to distraction, mind wandering, attentional control, self-regulation, working memory and impulsivity (Chambers, Lo, & Allen, 2007; Jha, Krompinger, & Baime, 2007; Lattimore, Fisher, & Malinowski, 2011; Lutz et al., 2009; MacLean et al., 2010; Slagter et al., 2007; Tang et al., 2007). Moreover, brain imaging studies have revealed changes in brain activity and function resulting from contemplative training such as mindfulness (e.g. Dillbeck & Bronson, 1981; Farb et al., 2007; Fox et al., 2014; Gaylord, Orme-Johnson, & Travis, 1989; Goldin & Gross, 2010; Lutz, Brefczynski-Lewis, Johnstone, & Davidson, 2008; Tang, Hölzel, & Posner, 2015; Travis & Wallace, 1999). There is also evidence that suggests that these cognitive and neural effects may persist during resting conditions (Lutz, Slagter, Dunne, & Davidson, 2008). For instance, long-term meditators exhibit a different electroencephalography (EEG) spectral profile than novices at rest (Lutz, Greischar, Rawlings, Ricard, & Davidson, 2004).
Although a rapidly growing body of work suggests that meditation can produce changes in brain and cognitive function, very little is known to date about the neurochemical mechanisms that may underlie these effects. Dopamine (DA) plays an important role in complex cognitive functions that also have been found to be affected by meditation practice, such as working memory, attention regulation and cognitive control (Braver & Cohen, 1999; Roshan Cools & D’Esposito, 2011; Nieoullon, 2002). Many studies have found a relation between dopaminergic function and cognitive performance, in humans, non-human primates, and rodents (Braver & Cohen, 1999; R. Cools, 2001; Kehagia, Murray, & Robbins, 2010; Müller et al., 2007). Specifically, this research has associated low striatal dopamine levels with increased cognitive stability (e.g., maintenance of information, resistance to distraction), whereas high striatal dopamine levels are associated with increased cognitive flexibility (e.g., updating of information, attention shifting) (Roshan Cools & D’Esposito, 2011; Frank, 2005). As meditation practice has been associated with changes in both cognitive stability and flexibility, this raises the intriguing possibility that changes in dopaminergic neurotransmission may underlie - at least in part - some of the observed meditation-related changes in cognitive functioning. Specifically, given that meditation affects both cognitive stability and cognitive flexibility (needed for sustained attention and attentional switching when distractions arise, respectively), it is possible that meditation allows for an optimization of the balance between cognitive stability (reflected by low striatal dopaminergic levels) and flexibility (reflected by high striatal dopaminergic levels), depending on the attentional need moment by moment.
In the current study, we examined the relationship between meditation experience and spontaneous eye blink rate (sEBR), a marker of striatal dopaminergic activity (Karson, 1983). sEBR can be obtained by counting the number of eye blinks per minute under resting conditions, which can be measured using facial electrodes or a video camera. As such sEBR may provide a relatively cheap, non-invasive, and simple measure for assessing the effect of meditation on striatal dopamine. Converging evidence from different lines of research indicates that sEBR is directly related to striatal dopaminergic activity. First, studies in human patients show that disorders characterized by abnormal dopaminergic activity are also associated with altered blinking. For example, blink rates are significantly reduced in Parkinson’s disease, a neurological disorder characterized by low striatal dopamine levels (Karson, Burns, LeWitt, Foster, & Newman, 1984), and enhanced by administration of L-DOPA (Karson, LeWitt, Calne, & Wyatt, 1982). Blink rates are also altered in schizophrenia; schizophrenic patients display both an elevated sEBR (Mackert, Flechtner, Woyth, & Frick, 1991) and elevated striatal dopamine uptake (Lindström et al., 1999). Second, pharmacological studies in both animals and healthy humans show that sEBR is elevated by dopamine agonists and reduced by dopamine antagonists (Cavanagh, Masters, Bath, & Frank, 2014; Elsworth et al., 1991; Jutkiewicz & Bergman, 2004; Kaminer, Powers, Horn, Hui, & Evinger, 2011; C N Karson, 1988; Kleven & Koek, 1996; Lawrence & Redmond, 1991; Taylor et al., 1999). It is of note in this regard that Kaminer et al. (2011) found that the temporal organization of spontaneous blinks (measured in frequency and variability) of rats and humans are qualitatively similar, suggesting that sEBR results obtained in rats may be extended to humans. Third, a link between dopamine and sEBR in healthy humans is supported by studies reporting a relationship between cognitive functions known to be dopamine-dependent (such as mind wandering, attentional regulation, and cognitive flexibility) and sEBR (Chermahini & Hommel, 2010; Colzato, Slagter, Spapé, & Hommel, 2008; Dreisbach et al., 2005; Holland & Tarlow, 1975; Oh, Han, Peterson, & Jeong, 2012). Fourth and lastly, a recent PET study in monkeys found a strong correlation between sEBR and D2-like receptor availability in the ventral striatum and caudate nucleus (Groman et al., 2014). Furthermore, in this study, D2-like receptor availability correlated with D2-like receptor agonist-induced changes in eye blink rate and the density of D2-like receptors determined in vitro. Thus, convergent evidence from different lines of research indicates that striatal dopamine activity regulates sEBR and that sEBR provides a valid measure of dopaminergic functioning in humans. The location of the spontaneous blink generator circuit is, however, still unknown, although the spinal trigeminal complex may play a direct role in the circuit (Kaminer et al., 2011; Kaminer, Thakur, & Evinger, 2015). As the basal ganglia regulate spinal trigeminal activity, this would enable dopamine to modify eye blink rate.
The current study tested the hypothesis that meditation is associated with changes in dopaminergic activity, as indexed by sEBR or the frequency of eye blinks per minute under resting conditions. To this end, sEBR was recorded at rest in long-term meditators (LTM) and in meditation naïve practitioners (MNP) before and after an 8-week Mindfulness Based Stress Reduction (MBSR) training. This design allowed examination of the effects of both long- and short-term meditation training on measures of sEBR and IEBI following three main questions as detailed below.
First, is long-term meditation experience associated with changes in spontaneous eye blink activity? To this end, we investigated whether any trait differences in eye blink patterns can be found between LTM and MNP. Here we hypothesized that mindfulness-related meditation cultivates cognitive skills to maintain a stable monitoring state from moment to moment, while flexibly disengaging attention from distractions or habitual impulses that automatically arise in the mind. Thus, we expected the cognitive state of LTM to be relatively more stable at baseline compared to MNP, and therefore, sEBR in LTM to be low for long periods at rest, reflecting mental stability, except during short, alternating periods of active cognitive regulation, for instance in response to a distractor. Specifically, we predicted that LTMs would show overall low sEBR but high variance in IEBI, compared to MNP.
Second, does short-term meditation training change spontaneous eye blink activity? To this end, we examined whether an 8-week MBSR training leads to changes in sEBR. We specifically predicted that sEBR would decrease after the 8-week MBSR training compared to a non-active control group as well as an active control-group following a similar intervention but without mindfulness practice. Thus, we expected that, following an MBSR intervention, the eye-blink patterns of MNP would look more similar to the eye-blink patterns of LTM. However, since 8 weeks of contemplative practice is relatively short, we expected that the effect of training on eye blink activity would be relatively small.
Third, is spontaneous eye blink activity affected by the kind of meditation most recently practiced in LTM? To this end, we measured the effect of an intensive day of practice in one of two meditation styles (mindfulness and compassion meditations) on sEBR and IEBI in LTM, compared to a waitlist control group having a day of leisure in a controlled laboratory environment. Here, we expected that if spontaneous eye blink activity indexes trait baseline dopaminergic activity, it should be relatively unaffected by day of practice, regardless of style of practice (i.e., no meditation state effect). In this case, one may expect a decrease in sEBR and an increase in IEBI in LTM, regardless of meditation styles practiced, compared to the waitlist control group.
Method
Participants
Participants provided written informed consent for study procedures that were approved by the UW-Madison Health Sciences Internal Review Board. Meditation-naïve participants were recruited for a study on “health and well-being” through advertisements in Madison, WI area newspapers, emails, and through postings and discussions with meditation teachers and groups. LTM were recruited in the US at meditation centers and through related mailing lists, in addition to flyers and advertisements in newspapers. In total, 155 healthy human subjects were recruited, which comprised 124 meditation-naïve participants (MNP) (average age 48.1±10.7 years, 81 female) and 31 long-term meditators (LTM) (average age 50.7± 10.1 years, 17 female). Meditation recruitment criteria for LTM included at least three years of daily meditation practice (at least 30 minutes per day), and at least 3 intensive retreats lasting 5 or more days. LTM had an average of 9,154 lifetime hours of meditation practice, ranging from 1,439 to 29,046 total hours. Lifetime hours of practice were calculated based on subjects reports of their average hours of formal (sitting and walking) meditation practice per week and the total years of practice, including time spent in meditation retreats.
All LTM participants were proficient in meditation practices, as taught within the framework of either Theravada or Tibetan Buddhism. These practices included two attention-based meditations, which we referred to as Open Monitoring (OM) and Focused Attention (FA) meditations, as well as one compassion/loving kindness meditation referred to as metta meditation. Briefly, FA meditation involves directing and sustaining attention on a selected object (e.g., breathing), detecting mind wandering and distractors (e.g., thoughts), as well as disengagement of attention from distractors and shifting of the focus of attention back to the selected object. By contrast, OM meditation has no explicit focus of attention, but rather requires nonreactive meta cognitive monitoring of anything that is experienced, thus replacing the “effortful” selection of an object as primary focus with an “effortless” sustained awareness of the rich features of each experience (Lutz, Slagter, et al., 2008). The practice of compassion/loving kindness meditation is a form of concentration practice where the practitioner focuses his/her mind on the suffering of oneself or others and then on the wish that the individual(s) in question may be happy and free from suffering.
All MNP participants were screened and criteria for exclusion included: significant previous experience with meditation or other mind–body techniques (e.g. tai-chi, Qigong), remarkable exercise habits (engagement in moderate sport or recreational activities >5hrs per week; engagement in vigorous sport or recreational activities >4hrs per week), and inability to walk. Criteria for exclusion for all participants included: use of psychotropic or steroid drugs, night-shift work, diabetes, peripheral vascular disease or other diseases affecting circulation, needle phobia, pregnancy, current smoking habit, alcohol or drug dependency, and inability to attend weekly class and full-day group sessions.
MNP participants were scheduled into 3 cohorts. In each cohort, they were randomized into 3 groups by a logistical staff member through a random-number generator: an 8-week Mindfulness Based Stress Reduction (MBSR) class, an 8-week Health Enhancement Program (HEP) as an active control group, or a no-intervention waiting list control group (WL).
Study Design and Interventions
In LTM, spontaneous eye blink activity was measured three times during resting conditions. The first measure was taken after a regular day, and the second and third time after a day of either mindfulness or compassion meditation (order randomized across participants; see below). In MNP, spontaneous eye blink activity was measured two or three times. Eye blink activity was measured twice in MBSR and HEP participants, once before and once after their respective intervention. Eye blink activity was also assessed at these time points in the WL, and in addition, in a subgroup of WL at a third time point after a day of leisure (see below). An overview of the study design as well as numbers of participants completing each time point are documented in Figure 1.
Figure 1.
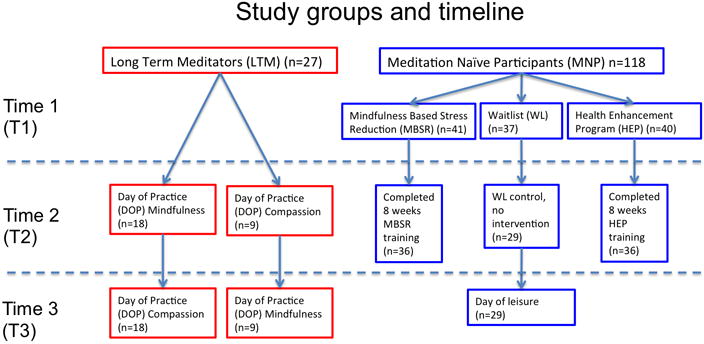
MBSR training consists of continuous focused attention on the breath, bodily sensations and mental content while in seated postures, walking, and yoga (Kabat-Zinn, 1990). This program can be conceptualized as incorporating OM-related meditations with FA-related meditations.
In order to isolate mindfulness as the agent of change, we designed an active comparison intervention to control for the aspects of MBSR that are known to promote positive outcomes, but are not specific to mindfulness such as a supportive group atmosphere, expert instruction, and engaging in activities that are believed to provide benefit. Our active comparison condition – the Health Enhancement Program (HEP) – matched MBSR in structure, instructor expertise, and content (see MacCoon et al., 2012, for more detailed information). Like MBSR, HEP consisted of four components: (1) physical activity (e.g. walking), (2) balance, agility, and core strength, (3) nutritional education, and (4) music therapy. Each of these components was chosen to match the collateral benefits that MBSR may produce that are not unique to mindfulness. For example, physical activity with a focus on walking was selected to control for the physical benefits of walking meditation. Each component was delivered by an expert in the respective practice, over 8 weekly 2.5 h sessions and one full-day session. Like those participating in MBSR training, HEP participants were assigned 45 to 60 minutes of daily at-home practice.
LTMs were not assigned to an intervention. Instead, they and a sub-group of WL controls matched to LTMs for age and gender, engaged in similar laboratory procedures and participated in a third assessment (Time 3) that occurred approximately 10 weeks after Time 2. In addition to the laboratory procedures that MNPs completed, the LTMs participated in either an 8-hour day of mindfulness practice, as taught in the Vipassana or Insight tradition (Goldstein, 2003), or a day of compassion meditation (short for “loving-kindness and compassion meditation”, or called also traditionally Metta meditation) at Time 2 (and the other day of practice type at Time 3). Over the course of Time 2 and Time 3, each LTM underwent both mindfulness and compassion training. For logistical reasons, at Time 2 some LTMs underwent the mindfulness DOP (n=18) while the rest (n=9) underwent the compassion DOP; at Time 3, the condition for each participant was reversed. Meanwhile, the matched WL controls participated in a day of relaxation that matched the LTM day of practice for activity and length. The two days of practice closely reproduced the structure of a meditation retreat. Six 45-min sessions of sitting meditation, three in the morning, three in the afternoon, were separated by four 30-min sessions of walking meditation. The first 45-min sitting meditation session began at 8:15 am with a short, guided meditation. The morning and afternoon sessions were separated by a 1-h lunch break, followed by a 30-min meditation and inspiration audio talk. The order of mindfulness and compassion meditation sessions was randomized across participants. Two subjects didn’t complete the mindfulness meditation session, and their data were excluded from the analyses.
Baseline Eye Blink Recording
Spontaneous eye-blink rates are affected by the time of day (Barbato et al., 2000). Data were collected around 7pm for all participants at every time-point, ensuring that differences in the time of data collection could not contribute to any observed difference in eye blink activity. Baseline sEBR was extracted from high-density, 256-channel EEG data that was collected during a 10-minute baseline EEG recording preceding a fear-conditioning task. Participants were seated in front of a computer screen. During the first 2 minutes and last 2 minutes of the baseline recording, participants were instructed to keep their eyes closed. During the 6 minutes in between these periods, participants were instructed to keep their eyes open while looking at a cross in the middle of a fixation screen. No explicit instruction was given about blinking-behavior to insure its spontaneity. Eye-blink data were extracted from the 6 minutes baseline recording with eyes open. Artifacts and bad channels (i.e. channels with high impedance/poor contact with the scalp) were removed from the raw EEG data using EEGLAB, and a low-pass filter of 100Hz was applied before data-analysis.
After performing an Independent Component Analysis (ICA) in MATLAB, maximally independent ICA components were selected based on the presence of eye-blink activity, its temporal activity and its frontal distribution. Based on the time-points of the individual eye-blinks, sEBR per minute was computed as well as different IEBI variables, including average, variance, standard deviation, maximum, and median of the IEBI distribution, replicating the eye-blink measures used by Doughty and colleagues (Doughty, 2002).
The vertical eye-blink power spectrum is concentrated in the range 0.5 to 3 Hz. There the power of blinks is in the order of ten times larger in amplitude than the average cortical signals, and lasts for approximately 300ms (Nazarpour, Wongsawat, Sanei, Chambers, & Oraintara, 2008). These particular characteristics enable reliable statistical separation of eye-blink-related signals from brain-related or EMG-related signal from the EEG signals. The amplitude threshold for peak-detection was verified manually for every participant and manually adapted if needed to assure correct quantification of eye blink rates.
Statistics
Normality of the distribution of the data was verified using a Shapiro Wilk test for normality and outliers were removed from the sample before analysis (studentized residual >3). In order to address our first question of whether long-term meditation is associated with changes in eye blink activity we evaluated the difference in eye-blink activity (sEBR and IEBI) between LTM and MNP (combined HEP, MBSR and WL, pre-intervention) using independent t-tests. We also explored if any observed differences in sEBR and IEBI in LTM are caused by differences in total lifetime hours of practice of LTM, while accounting for the effect of age, using an Analysis of Covariance (ANCOVA). To address our second question of whether short-term meditation induces changes in eye blink activity, we examined if eye blink activity changed after the MBSR intervention in the MNP group using a repeated measures ANOVA with Time (Time 2 vs. Time 1) as a within-subjects factor and Group (MBSR vs. HEP vs. WLC) as a between-subjects factor. To answer our third question as to whether eye blink activity is affected by the kind of meditation just practiced, we assessed the effect of a day of Compassion or Mindfulness practice on sEBR and IEBI using a 3 × 2 repeated measures ANOVA with condition as within-subject factor (pre-intervention Time 1, Compassion DOP, Mindfulness DOP) and Group as between-subjects factor (LTM vs. WL). For all analyses of differences in IEBI, sEBR was regressed out, since sEBR and IEBI are shown to be interdependent (Doughty, 2002). Statistical significance was assumed if the null hypothesis could be rejected at the p = .05 level.
Confounding factors, including age, gender, positive affect, and contact lens wear were taken into account in the analyses. For example, eye-blink activity can be affected by age, with progressive increments during childhood and adolescence (Bacher & Smotherman, 2004; Bentivoglio et al., 1997; Sforza, Rango, Galante, Bresolin, & Ferrario, 2008). Although evidence about effect of gender on sEBR is not consistent (Bentivoglio et al., 1997; Chen, Chiang, Hsu, & Liu, 2003; Declerck, Boone, & De Brabander, 2006; Deuschl & Goddemeier, 1998; Doughty, 2002; Sforza et al., 2008), statistical analysis was performed to rule out a possible gender-related confound. All statistical analyses were performed using SPSS for Windows 21.0 (SPSS Inc.) and R Version 1.35-dev (http://www.r-project.org/).
Results
Trait Effect - Pre-intervention (Time 1) Difference in Eye Blink Activity LTM vs. MNP
Our first prediction was that LTM would show lower sEBR and higher IEBI variance compared to MNP at Time 1, before any intervention. In other words, we expected that long term meditators would not only blink less frequently than MNP, but also that the blinks would be distributed in a different way, with longer periods without eye-blinks, interrupted by short periods of frequent blinks. Indeed, a one-way ANOVA showed that the sEBR of LTM during baseline recording was significantly lower than MNP’s sEBR F(1,146) = 8.1, p < .01 (Figure 2). In addition, highly significant differences were found in IEBI average (F(1,131) = 17.6, p < .001), standard deviation (F(1,127) = 18.0, p < .001), and maximum (F(1,140) = 19.9, p < .001) (Figure 2).
Figure 2.
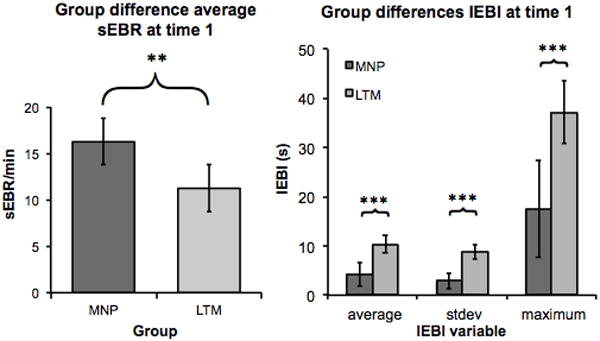
Since meditation naive participants were randomly assigned to the HEP, MBSR and waitlist (WL) group, no difference was expected between the MNP participants in the different groups at Time 1, before any intervention had taken place. This assumption was confirmed with a one-way ANOVA for sEBR (F(2,112) = .70, p = .51) and IEBI average (F(2,98) = 0.3, p = .77), standard deviation (F(2,94) = .60, p = .57), and maximum (F(2,103) = 1.60, p = .20).
Short-term Meditation Training Effect –MNP Before and After MBSR/HEP Intervention
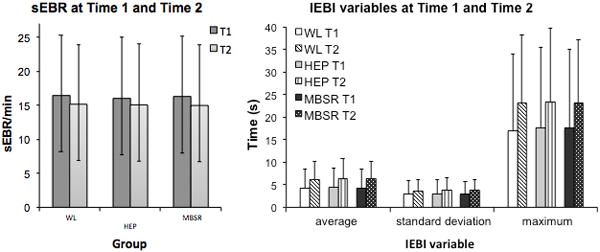
In order to assess whether MBSR training had an effect on sEBR and/or IEBI, a two-way repeated measures analysis of variance was performed, with Time as within-subject factor (pre-intervention Time 1 vs. post-intervention Time 2) and Group as between-subjects factor (WL, HEP, MBSR). As noted above, the 3 MNP groups did not significantly differ in EBR or IEBI at Time 1 (p > .20). No significant difference between groups was found in the change in sEBR over time, Wilks’ Lambda = .98, F(1, 59) = .50, p = .33 (Figure 3). In addition, no significant differences between groups were found in the change over time in IEBI average (Wilks’ Lambda = .99, F(1, 47) = 0.10, p = 0.78), IEBI standard deviation (Wilks’ Lambda = .99, F(1, 38) = .17, p = .68), or IEBI maximum (Wilks’ Lambda = .98, F(1, 44) = 1.00, p = .32) (Figure 3).
Figure 3.
Day of Practice (DOP), Mindfulness and Compassion
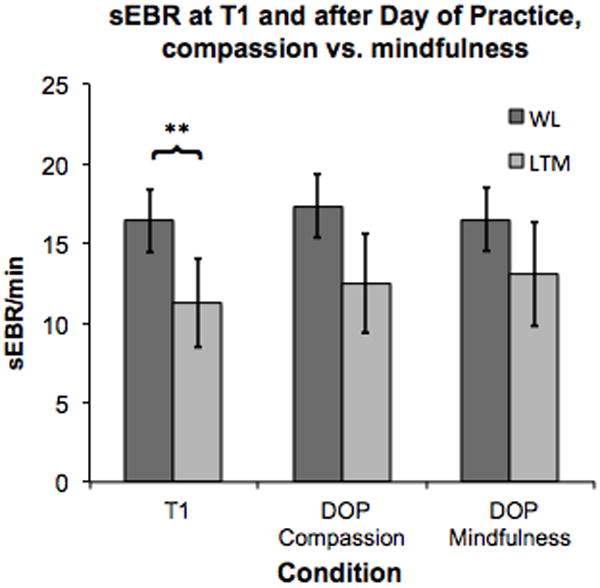
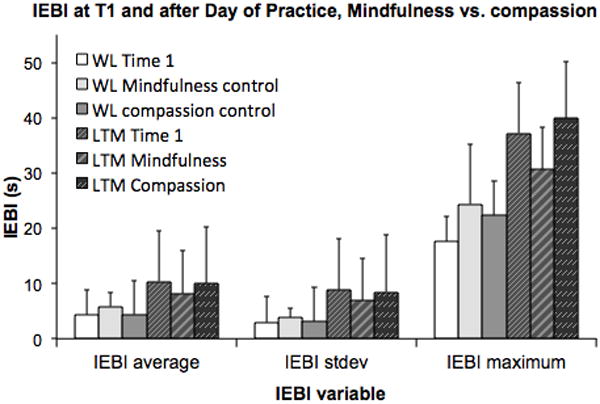
Our third research question was whether an intensive day of two types of meditation practice would have an effect on the sEBR and IEBI of LTM, compared to a waitlist control group having a day of leisure in a controlled laboratory environment. A two-way repeated measures ANOVA was performed, with condition as within-subject factor (pre-intervention Time 1, Compassion DOP, Mindfulness DOP) and Group as between-subjects factor (LTM vs. WL). A significant effect of group was found, reflecting lower sEBR in LTM compared to WL, (F(1,54) = 6.8, p = .01) (Figure 4). However, no significant Condition*Group interaction was found for baseline sEBR, Wilks’ Lambda = .95, (F(2,53) = 1.5, p = .23), indicating that LTM displayed reduced blink rates regardless of meditation practiced in the DOP. Indeed, as can be seen in Figure 4, LTM displayed consistently lower sEBR than WL controls at all three time-points. Overall, a good degree of consistency was observed over the three time-points for all the eye-blink variables, for both the LTM and MNP group. For the MNP group, Cronbach alpha values for sEBR, IEBIaverage, IEBI standard deviation, and IEBI maximum were a = 0.79, 0.71, 0.83, and 0.69, respectively. For LTM, Cronbach alpha values were a = 0.85, 0.69, 0.77, and 0.69, respectively.
Figure 4.
As can be seen in Figure 5, average and median IEBI were generally longer in LTM compared to WL (main effect Group; respectively, F(1,40) = 3.70, p = .06) (trend), F(1,40) = 5.20, p = .03) and more variable as indexed by larger IEBI standard deviation F(1,40) = 4.30, p = .04. In addition, meditation condition affected this meditation-related increase in IEBI standard deviation (Condition*Group interaction for standard deviation; Wilks’ Lambda = .87, F(2, 44) = 3.20, p = .04). Other IEBI variables were not affected by DOP meditation type. A post-hoc paired sample t-test was conducted to evaluate whether IEBI standard deviation was affected differently by a full day of Compassion or Mindfulness meditation, when compared to Time 1. Post-hoc paired sampled t-test did not reveal a significant difference across sessions for each group, suggesting that this interaction was not specific (Figure 5). WL controls did not differ across sessions (p > .05).
Figure 5.
Lifetime Hours of Practice
While LTM displayed a differential pattern of eye blink activity, an 8-week MBSR intervention did not affect spontaneous eye blink activity, which may suggest that some amount of meditation practice is necessary to induce stable changes in baseline striatal dopaminergic functioning. To explore the relationship between the amount of meditation experience and spontaneous eye blink activity, a regression analysis was conducted to investigate whether sEBR and/or IEBI at Time 1 were dependent on the total number of hours of contemplative practice that LTM had completed before Time 1 measurements. Hours of daily practice, days of retreat and expertise in various types of mediation type (hours of Vipassana, concentration and Loving-Kindness practice) were taken into account - that is, after regressing out age as a confounding factor. No significant effect of either of the measures of hours of practice was correlated with sEBR or IEBI. Curve fitting models did not reveal any non-linear relationship between these two variables.
Verification of Potential Confounding Factors
Spontaneous eye blink rate can be influenced by age, gender, and eye-wear (contact lenses in particular) (Bacher & Smotherman, 2004; Barbato et al., 2000; Bentivoglio et al., 1997; Chen et al., 2003; Sforza et al., 2008). An analysis of covariance (ANCOVA) was performed in order to assess the effect of age on the sEBR difference, and showed that age did not significantly contribute to the found differences in eye-blink activity (F(1,59) = .90, p = .35). The ANCOVA also ruled out an effect of gender on sEBR (F(1,57) = 1.20, p = .28). All the reported significant effects still remained significant after regressing out age and gender (p < .05). In order to exclude the possibility that the results might be affected by contact lens wear, a one-way ANOVA was performed, comparing contact wearing to non-contact wearing participants. No effect of contact lens wear on sEBR was observed (F(2,143) = .50, p = .59).
Discussion
This study examined whether meditation practice can influence spontaneous eye-blink activity, a non-invasive peripheral measure that in part reflects striatal dopamine activity (e.g., Groman et al., 2014). We examined if eye blink activity differs between long-term meditation practitioners and meditation naïve participants. In addition, we investigated whether sEBR and IEBI are affected by an 8-week mindfulness based stress reduction course for MNP and a full day of compassion or mindfulness meditation. There were three main findings. First, at Time 1, before any intervention, a significant difference was found between LTM and MNP in measures of sEBR and IEBI. Interestingly, not only did LTM blink significantly less frequently, their blinks were also distributed differently over time compared to MNP. Whereas MNP showed a very regular blinking pattern, with little variance in the duration of the IEBI, LTM showed a large variance in IEBI duration, indicating long periods without blinks interrupted by brief periods with a high blinking frequency. These findings add to a growing body of studies indicating that meditation expertise is correlated to altered physiology. Considering the converging evidence supporting the relationship between sEBR and striatal dopaminergic functioning, this work supports the hypothesis that meditation might affect measures that reflect striatal dopaminergic activity. Our second main finding was that an 8-week meditation intervention did not alter eye blink activity. This may indicate that more meditation practice is necessary to alter eye blinking and, presumably, striatal dopaminergic activity. Prior work has demonstrated cognitive effects of an 8-week MBSR intervention, although these effects are typically small and sometimes absent when compared to active control interventions (MacCoon et al., 2014; Moynihan et al., 2013; Sedlmeier et al., 2012). The lack of an effect on sEBR by the 8-week intervention is thus not necessarily surprising, but certainly calls for follow-up research examining the effects of meditation interventions, preferably of longer duration, on both eye blinking and cognitive functioning.
Third, blink rates were unaffected by the type of meditation that was practiced during the day before the recording, supporting the notion that the observed differences in eye blink activity between Long Term Meditators (LTM) and Meditation Naïve Practitioners (MNP) reflect trait differences. Below, these findings are each discussed in more detail.
LTM blinked much less on average than MNP. As low blink rates are associated with a state of cognitive stability (Dreisbach et al., 2005; Müller et al., 2007), the observed reduction in sEBR might indicate that the baseline state of LTM is one of cognitive stability, at least when measured in our setting (i.e., quietly sitting in front of a monitor). In addition, blink patterns in LTM were characterized by longer periods without blinks, interrupted by short periods of blinking. MNP on the other hand displayed a more regular blinking pattern. It is unclear what this difference in blinking activity over time might reflect, as no studies have examined the relationship between IEBI measures and cognitive abilities. Based on the known positive relationship between EBR and cognitive flexibility, mind-wandering and distractibility (Chermahini & Hommel, 2010; Dreisbach et al., 2005; Müller et al., 2007; Oh et al., 2012), it is possible that a higher IEBI variance indicates an alternation between stable and flexible states. In meditative practice, although stability (i.e., attentional focus and resistance to distraction) is required to remain in an attentive state, flexibility (i.e., attention switching and flexible updating in response to novel information) is equally required, in order to return to the stable state if a distractor, such as mind wandering, arises. Indeed, a growing body of research indicates that meditation practice can improve performance on measures of both cognitive stability (Lutz et al., 2009) and flexibility (Moore & Malinowski, 2009). The observed consistent difference in IEBI between LTM and non-meditators over the three different time-points suggests that the relationship between IEBI and cognitive and/or dopaminergic functioning should be explored further.
Of note, Doughty (2002) showed that spontaneous eye-blink patterns can differ substantially between healthy individuals, even under a single experimental condition, and can be categorized in three types of eye-blink patterns, by taking both sEBR and IEBI into account. One of these blink patterns, named the irregular eye blink pattern, is characterized by low sEBR and high IEBI mean and variance, and is notably similar to the eye blink pattern that the LTM in the present study displayed. Yet LTM show a much larger variance of IEBI (standard deviation = 8.7) compared to Doughty’s participants (standard deviation = 2.5). This may suggest that the eye-blink pattern found in LTM is truly unique and is usually not found in a normal, healthy, non-meditating population. The behavioral significance of this pattern, for instance in regard to mind wandering, requires further study.
Baseline sEBR and IEBI were not significantly affected by 8 weeks of MBSR training compared to an active control intervention and a waitlist control group. It is possible that 8 weeks of mindfulness practice is not sufficient to measurably affect dopaminergic levels, at least as reflected in eye-blink rates. This is consistent with the findings that 3 month intensive meditation training (MacLean et al., 2010) but not an 8 week MBSR intervention (MacCoon et al., 2014) improves performance on the same sustained attention task. PET imaging might provide a more sensitive method for observing neurochemical effects of short-term training; a 6-week computerized training of cognitive updating has been shown to alter dopaminergic activity as measured using PET (Bäckman et al., 2011; Dahlin, Neely, Larsson, Bäckman, & Nyberg, 2008; McNab et al., 2009). To our knowledge, only one study has demonstrated that contemplative practice significantly alters striatal dopaminergic activity: Kjaer and colleagues (2002) showed with PET imaging that meditation (in this case Yoga Nidra meditation) increased (ventral) striatal dopamine release, which correlated with increased theta activity measured at the scalp.
In post-hoc analyses, we did not find a positive correlation between total hours of lifetime practice of long-term meditators and sEBR or any of the IEBI variables. Since no difference in eye-blink patterns were found after 8 weeks of meditation practice (which does not qualify yet as long term practice), but a highly significant and consistent difference was found between meditation naive participants and long term practitioners, the relationship between amount of meditation practice and eye blink rate may not be linear. This would be consistent with a study showing a non-linear but dose-dependent effect of hours of meditation expertise in long-term meditators (Brefczynski-Lewis, Lutz, Schaefer, Levinson, & Davidson, 2007). However, contradicting this hypothesis, we did not find a non-linear dose response between total lifetime hours of meditation practice and sEBR nor between total lifetime hours of meditation practice and IEBI in experts.
Since no correlation between hours of practice of LTM and eye-blink activity was found, nor an effect of MBSR training, it is also possible that the reported difference in eye-blink patterns is driven by a factor that is not related to meditation expertise. For example, individual differences in dopaminergic neurotransmission may comprise a self-selection factor for meditation practice. More research is needed to investigate what other possible factors unique to long term meditators could affect eye-blink activity, and to confirm that it is indeed meditation practice that causes the reported difference in eye-blink patterns between LTM and MNP. It would also be informative if meditators with a wider range of meditation expertise, covering the full range of experience, from no experience to >10,000 hours, are included in future studies. In the current study, LTM had a minimum of 1439 hours of meditation experience.
No effect of meditation type (mindfulness and compassion) was found in LTM after the day of practice. We did find an interaction between group and condition for the IEBI standard deviation measure, but this interaction was not specific to the type of meditation. This lack of specificity is consistent with the hypothesis that sEBR and IEBI reflect trait baseline dopaminergic activity.
Overall, a good to high degree of consistency was observed over the three time-points for all the eye-blink variables for both the LTM and MNP groups. This finding is important since a growing number of studies use sEBR to index baseline striatal dopaminergic activity, and the high consistency across time observed in the present study indicates that sEBR indeed provides a relatively stable measure at the individual level. The average eye-blink rate per minute found in the present study (14.9, taken over all time-points and groups) is higher than the mean eye-blink rate per minute found by Doughty (ranging from 7.5 – 12.3 eye-blinks per minute, across all eye-blink pattern categories), but similar to what many other studies have reported (e.g., 17.6 p/min. in Kaminer, Powers, Horn, Hui, & Evinger, 2011; 15,2 p/min. in Slagter, Georgopoulou, & Frank, 2015). It has been demonstrated that sEBR is significantly affected by the time of day, and rises after 5pm. In a study performed by Barbato et al. (Barbato et al., 2000), mean baseline sEBR between 10:00am and 5:00pm varied between 11–13 blinks per minutes, whereas the sEBR was found to be around 16 blinks per minute at 8:30pm. In the current study, all baseline recordings were performed around 7:00pm, which may have enhanced average blink rates somewhat. Importantly, however, since eye blinks were recorded in all participants at the same time of day, we do not expect that this effect is the underlying cause of the observed differences in eye-blink patterns between LTM and MNP.
In conclusion, we observed consistent differences in sEBR and IEBI between LTM and MNP, but no effects of an 8-week MBSR training. We speculate that these findings reflect that striatal dopaminergic activity is altered by long-term, but not short-term meditation practice, but this will have to be confirmed by future studies. Moreover, the two types of meditation practiced during one full day of meditation did not differently affect eye-blink activity, suggesting a stable change in baseline (i.e., a trait effect). They also indicate the potential usefulness of eye-blink activity for examining the neurochemical mechanisms underlying meditation-induced changes in cognitive functioning.
Table 1.
Effect sizes (Cohen’s d) of the difference in sEBR, IEBI average, standard deviation and maximum between Mediation Naïve Practitioners (MNP) and Long Term Meditators (LTM) at Time 1.
| sEBR | IEBI average | IEBI std. dev. | IEBI maximum | |
|---|---|---|---|---|
| Cohen’s d | 0.57 | 1.28 | 1.41 | 1.30 |
Table 2.
Effect sizes (Cohen’s d) of the difference in sEBR, IEBI average, standard deviation, and maximum between Time 1 and Time 2.
| sEBR | IEBI average | IEBI std. dev. | IEBI maximum | |
|---|---|---|---|---|
| WL | 0.17 | 0.15 | 0.49 | 0.62 |
| HEP | 0.03 | 0.11 | 0.18 | 0.45 |
| MBSR | 0.22 | 0.49 | 0.33 | 0.35 |
Acknowledgments
This work was supported by the National Center for Complementary and Alternative Medicine (NCCAM) P01AT004952 to RJD and AL, by an International Re-integration Grant (IRG), FP7-PEOPLE-2009-RG, by a gift from the Adam J. Weissman foundation for AL, and a VIDI grant by the Netherlands Organization for Scientific Research to HAS. We would like to thank Amelia Cayo for assistance with data collection.
Footnotes
The authors declare no conflict of interest.
References
- Bacher LF, Smotherman WP. Spontaneous eye blinking in human infants: a review. Developmental Psychobiology. 2004;44(2):95–102. doi: 10.1002/dev.10162. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Soveri A, Johansson J, Andersson M, Dahlin E, Rinne JO. Effects of working-memory training on striatal dopamine release. Science (New York, NY) 2011;333(6043):718. doi: 10.1126/science.1204978. [DOI] [PubMed] [Google Scholar]
- Barbato G, Ficca G, Muscettola G, Fichele M, Beatrice M, Rinaldi F. Diurnal variation in spontaneous eye-blink rate. Psychiatry Research. 2000;93(2):145–151. doi: 10.1016/S0165-1781(00)00108-6. [DOI] [PubMed] [Google Scholar]
- Bentivoglio AR, Bressman SB, Cassetta E, Carretta D, Tonali P, Albanese A. Analysis of blink rate patterns in normal subjects. Movement Disorders: Official Journal of the Movement Disorder Society. 1997;12(6):1028–34. doi: 10.1002/mds.870120629. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. Dopamine, cognitive control, and schizophrenia: the gating model. Progress in Brain Research. 1999;121:327–49. doi: 10.1016/S0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(27):11483–8. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Masters SE, Bath K, Frank MJ. Conflict acts as an implicit cost in reinforcement learning. Nature Communications. 2014;5:5394. doi: 10.1038/ncomms6394. [DOI] [PubMed] [Google Scholar]
- Chambers R, Lo BCY, Allen NB. The Impact of Intensive Mindfulness Training on Attentional Control, Cognitive Style, and Affect. Cognitive Therapy and Research. 2007;32(3):303–322. doi: 10.1007/s10608-007-9119-0. [DOI] [Google Scholar]
- Chen WH, Chiang TJ, Hsu MC, Liu JS. The validity of eye blink rate in Chinese adults for the diagnosis of Parkinson’s disease. Clinical Neurology and Neurosurgery. 2003;105(2):90–92. doi: 10.1016/S0303-8467(02)00107-5. [DOI] [PubMed] [Google Scholar]
- Chermahini SA, Hommel B. The (b)link between creativity and dopamine: spontaneous eye blink rates predict and dissociate divergent and convergent thinking. Cognition. 2010;115(3):458–65. doi: 10.1016/j.cognition.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Slagter HA, Spapé MMA, Hommel B. Blinks of the eye predict blinks of the mind. Neuropsychologia. 2008;46(13):3179–83. doi: 10.1016/j.neuropsychologia.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Cools R. Enhanced or Impaired Cognitive Function in Parkinson’s Disease as a Function of Dopaminergic Medication and Task Demands. Cerebral Cortex. 2001;11(12):1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry. 2011;69(12):e113–25. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Bäckman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science (New York, NY) 2008;320(5882):1510–2. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Declerck CH, Boone C, De Brabander B. On feeling in control: a biological theory for individual differences in control perception. Brain and Cognition. 2006;62(2):143–76. doi: 10.1016/j.bandc.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Goddemeier C. Spontaneous and reflex activity of facial muscles in dystonia, Parkinson’s disease, and in normal subjects. Journal of Neurology, Neurosurgery & Psychiatry. 1998;64(3):320–324. doi: 10.1136/jnnp.64.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillbeck MC, Bronson EC. Short-term longitudinal effects of the transcendental meditation technique on EEG power and coherence. The International Journal of Neuroscience. 1981;14(3–4):147–51. doi: 10.3109/00207458108985827. [DOI] [PubMed] [Google Scholar]
- Doughty MJ. Further Assessment of Gender- and Blink Pattern-Related Differences in the Spontaneous Eyeblink Activity in Primary Gaze in Young Adult Humans. Optometry and Vision Science. 2002;79(7):439–447. doi: 10.1097/00006324-200207000-00013. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Müller J, Goschke T, Strobel A, Schulze K, Lesch KP, Brocke B. Dopamine and cognitive control: the influence of spontaneous eyeblink rate and dopamine gene polymorphisms on perseveration and distractibility. Behavioral Neuroscience. 2005;119(2):483–90. doi: 10.1037/0735-7044.119.2.483. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Lawrence MS, Roth RH, Taylor JR, Mailman RB, Nichols DE, Lewis MH, Redmond DE. D1 and D2 dopamine receptors independently regulate spontaneous blink rate in the vervet monkey. The Journal of Pharmacology and Experimental Therapeutics. 1991;259(2):595–600. [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2(4):313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KCR, Nijeboer S, Dixon ML, Floman JL, Ellamil M, Rumak SP, Christoff K. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neuroscience and Biobehavioral Reviews. 2014;43:48–73. doi: 10.1016/j.neubiorev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. Journal of Cognitive Neuroscience. 2005;17(1):51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Gaylord C, Orme-Johnson D, Travis F. The effects of the transcendental mediation technique and progressive muscle relaxation on EEG coherence, stress reactivity, and mental health in black adults. The International Journal of Neuroscience. 1989;46(1–2):77–86. doi: 10.3109/00207458908991618. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion (Washington, DC) 2010;10(1):83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. Insight Meditation: The Practice of Freedom: 9781590300169: Joseph Goldstein: Books: Shambhala Publications. Vol. 2003. Shambala; 2003. [Google Scholar]
- Groman SM, James AS, Seu E, Tran S, Clark TA, Harpster SN, Jentsch JD. In the blink of an eye: relating positive-feedback sensitivity to striatal dopamine D2-like receptors through blink rate. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2014;34(43):14443–54. doi: 10.1523/JNEUROSCI.3037-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland M, Tarlow G. Blinking and thinking. Perceptual and Motor Skills. 1975:403–406. [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(2):109–119. doi: 10.3758/CABN.7.2.109. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Bergman J. Effects of Dopamine D 1 Ligands on Eye Blinking in Monkeys : Efficacy, Antagonism, and D 1/D 2 Interactions. Journal of Pharmacology and Experimental Therapeutics. 2004;311(3):1008–1015. doi: 10.1124/jpet.104.071092.conditions. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York: Delacorte; 1990. [Google Scholar]
- Kaminer J, Powers AS, Horn KG, Hui C, Evinger C. Characterizing the spontaneous blink generator: an animal model. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(31):11256–67. doi: 10.1523/JNEUROSCI.6218-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminer J, Thakur P, Evinger C. Effects of subthalamic deep brain stimulation on blink abnormalities of 6-OHDA lesioned rats. Journal of Neurophysiology. 2015;113(9):3038–46. doi: 10.1152/jn.01072.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson CN. Spontaneous eye-blink rates and dopaminergic systems. Brain: A Journal of Neurology. 1983;106(Pt 3):643–53. doi: 10.1093/brain/106.3.643. [DOI] [PubMed] [Google Scholar]
- Karson CN. Physiology of normal and abnormal blinking. Advances in Neurology. 1988;49:25–37. [PubMed] [Google Scholar]
- Karson CN, Burns RS, LeWitt PA, Foster NL, Newman RP. Blink rates and disorders of movement. Neurology. 1984;34(5):677–677. doi: 10.1212/WNL.34.5.677. [DOI] [PubMed] [Google Scholar]
- Karson CN, LeWitt PA, Calne DB, Wyatt RJ. Blink rates in parkinsonism. Annals of Neurology. 1982;12(6):580–3. doi: 10.1002/ana.410120614. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Current Opinion in Neurobiology. 2010;20(2):199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Bertelsen C, Piccini P, Brooks D, Alving J, Lou HC. Increased dopamine tone during meditation-induced change of consciousness. Cognitive Brain Research. 2002;13(2):255–259. doi: 10.1016/S0926-6410(01)00106-9. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Differential effects of direct and indirect dopamine agonists on eye blink rate in cynomolgus monkeys. The Journal of Pharmacology and Experimental Therapeutics. 1996;279(3):1211–1219. [PubMed] [Google Scholar]
- Lattimore P, Fisher N, Malinowski P. A cross-sectional investigation of trait disinhibition and its association with mindfulness and impulsivity. Appetite. 2011;56(2):241–8. doi: 10.1016/j.appet.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Lawrence, Redmond MPTP lesions and dopaminergic drugs alter eye blink rate in African green monkeys. Pharmacology, Biochemistry, and Behavior. 1991;38(4):869–874. doi: 10.1016/0091-3057(91)90255-Z. [DOI] [PubMed] [Google Scholar]
- Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PloS One. 2008;3(3):e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(46):16369–73. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Jha A, Dunne JD, Saron CD. Investigating the phenomenological and neurocognitive matrix of mindfulness-related practices from a cognitive neuroscience perspective. American Psychologist. 2015;70(7):632–658. doi: 10.1037/a0039585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences. 2008;12(4):163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Rawlings NB, Francis AD, Greischar LL, Davidson RJ. Mental training enhances attentional stability: neural and behavioral evidence. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(42):13418–27. doi: 10.1523/JNEUROSCI.1614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCoon DG, Imel ZE, Rosenkranz MA, Sheftel JG, Weng HY, Sullivan JC, Lutz A. The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR) Behaviour Research and Therapy. 2012;50(1):3–12. doi: 10.1016/j.brat.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCoon DG, MacLean KA, Davidson RJ, Saron CD, Lutz A. No sustained attention differences in a longitudinal randomized trial comparing mindfulness based stress reduction versus active control. PLoS ONE. 2014;9:e97551. doi: 10.1371/journal.pone.0097551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackert A, Flechtner K, Woyth C, Frick K. Influences blink rates in schizophrenics of neuroleptics and psychopathology. Schizophrenia Research. 1991;4:41–47. doi: 10.1016/0920-9964(91)90008-F. [DOI] [PubMed] [Google Scholar]
- MacLean KA, Ferrer E, Aichele SR, Bridwell DA, Zanesco AP, Jacobs TL, Saron CD. Intensive meditation training improves perceptual discrimination and sustained attention. Psychological Science. 2010;21(6):829–39. doi: 10.1177/0956797610371339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science (New York, NY) 2009;323(5915):800–2. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Moore A, Malinowski P. Meditation, mindfulness and cognitive flexibility. Consciousness and Cognition. 2009;18(1):176–186. doi: 10.1016/j.concog.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Moynihan JA, Chapman BP, Klorman R, Krasner MS, Duberstein PR, Brown KW, Talbot NL. Mindfulness-Based Stress Reduction for Older Adults: Effects on Executive Function, Frontal Alpha Asymmetry and Immune Function. Neuropsychobiology. 2013;68(1):34–43. doi: 10.1159/000350949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Dreisbach G, Brocke B, Lesch KP, Strobel A, Goschke T. Dopamine and cognitive control: the influence of spontaneous eyeblink rate, DRD4 exon III polymorphism and gender on flexibility in set-shifting. Brain Research. 2007;1131(1):155–62. doi: 10.1016/j.brainres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Nazarpour K, Wongsawat Y, Sanei S, Chambers JA, Oraintara S. Removal of the eye-blink artifacts from EEGs via STF-TS modeling and robust minimum variance beamforming. IEEE Transactions on Bio-Medical Engineering. 2008;55(9):2221–31. doi: 10.1109/TBME.2008.919847. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Progress in Neurobiology. 2002;67(1):53–83. doi: 10.1016/S0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Oh J, Han M, Peterson BS, Jeong J. Spontaneous eyeblinks are correlated with responses during the Stroop task. PloS One. 2012;7(4):e34871. doi: 10.1371/journal.pone.0034871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlmeier P, Eberth J, Schwarz M, Zimmermann D, Haarig F, Jaeger S, Kunze S. The Psychological Effects of Meditation: A Meta-Analysis. Psychological Bulletin. 2012;138(6):1139–1171. doi: 10.1037/a0028168. [DOI] [PubMed] [Google Scholar]
- Sforza C, Rango M, Galante D, Bresolin N, Ferrario VF. Spontaneous blinking in healthy persons: an optoelectronic study of eyelid motion. Ophthalmic & Physiological Optics: The Journal of the British College of Ophthalmic Opticians (Optometrists) 2008;28(4):345–53. doi: 10.1111/j.1475-1313.2008.00577.x. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Georgopoulou K, Frank MJ. Spontaneous eye blink rate predicts learning from negative, but not positive, outcomes. Neuropsychologia. 2015;71:126–132. doi: 10.1016/j.neuropsychologia.2015.03.028. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Francis AD, Nieuwenhuis S, Davis JM, Davidson RJ. Mental training affects distribution of limited brain resources. PLoS Biology. 2007;5(6):e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nature Reviews Neuroscience. 2015;16(4):213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, Posner MI. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17152–6. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Lawrence MS, Sladek JR, Roth RH, Redmond DE. Spontaneous blink rates correlate with dopamine levels in the caudate nucleus of MPTP-treated monkeys. Experimental Neurology. 1999;158(1):214–20. doi: 10.1006/exnr.1999.7093. [DOI] [PubMed] [Google Scholar]
- Travis F, Wallace RK. Autonomic and EEG patterns during eyes-closed rest and transcendental meditation (TM) practice: the basis for a neural model of TM practice. Consciousness and Cognition. 1999;8(3):302–18. doi: 10.1006/ccog.1999.0403. [DOI] [PubMed] [Google Scholar]


