Given the high prevalence rates of comorbidity of anxiety and depressive disorders, identifying a common neural pathway to both disorders is important not only for better diagnosis and treatment, but also for a more complete conceptualization of each disease. Hippocampal abnormalities have been implicated in anxiety and depression, separately; however, it remains unknown whether these abnormalities are also implicated in their comorbidity. Here we address this question by testing 32 adults with generalized anxiety disorder (15 GAD only and 17 comorbid MDD) and 25 healthy controls (HC) using multimodal MRI (structure, diffusion and functional) and automated hippocampal segmentation. We demonstrate that (i) abnormal microstructure of the CA1 and CA2-3 is associated with GAD/MDD comorbidity and (ii) decreased anterior hippocampal reactivity in response to repetition of the threat cue is associated with GAD (with or without MDD comorbidity). In addition, mediation-structural equation modeling (SEM) reveals that our hippocampal and dimensional symptom data are best explained by a model describing a significant influence of abnormal hippocampal microstructure on both anxiety and depression—mediated through their relationship to abnormal hippocampal function. Collectively, our findings show a strong association between changes in hippocampal microstructure and threat processing, which together may present a common neural pathway to comorbidity of anxiety and depression.
Abnormalities of the hippocampus play an important role in development and/or maintenance of pathological anxiety and depression. Behavioral studies using hippocampus-dependent learning paradigms report deficits in associative learning in clinical anxiety (Grillon, 2002); these may indirectly suggest a role of disrupted hippocampal threat processing in anxiety (Britton et al., 2011). Likewise, hippocampal structural deficits, such as decreased volume observed with structural magnetic resonance imaging (sMRI) or decreased N-Acetylaspartic acid (NAA) in magnetic resonance spectroscopy (MRS) have been frequently reported in clinical anxiety, such as post-traumatic stress disorder (PTSD) (Shin et al., 2006). In the depression literature, impaired hippocampal structure and function have been frequently linked to pathophysiology as well (Brown et al., 1999; Nestler et al., 2002; Sapolsky, 2000). Based upon this literature and the high rate of comorbidity between anxiety and depressive disorders [e.g., prevalence rates of generalized anxiety disorder and major depressive disorder range up to 60% (Carter et al., 2001; Fava et al., 2000)], it is likely that abnormalities of the hippocampus may play a role in the comorbidity between anxiety and depression.
To test this hypothesis, we recruited 57 female participants, of which 32 were diagnosed with generalized anxiety disorder (of these, 17 had comorbid major depression disorder (MDD) while 15 had no comorbidity), and 25 were age-matched as healthy controls (Table 1). Psychiatric diagnoses were based on the Structured Clinical Interview for DSM-IV Axis I Disorders. Patients with any psychiatric or neurological diagnosis aside from GAD and MDD were excluded. Participants were free from psychiatric medication for at least six months before the time of the experiment.
Table 1.
Descriptive Statistics
| GAD (n = 15) (s.e.) |
GAD/MDD comorbid (n = 17) (s.e.) |
HC (n= 25) (s.e.) |
Statistics (Significance) |
|
|---|---|---|---|---|
| STAI-trait | 53.1 (2.86) | 53.7 (1.88) | 39.5 (1.33) |
ta = 6.4 (<0.001)*** tb = 0.2 (0.8) |
| BDI | 19.1 (3.53) | 25.9 (2.80) | 4.4 (0.65) |
ta = 6.8 (<0.001)*** tb = 1.7 (0.09)† |
| MASQ-GDA | 26.6 (2.60) | 26.5 (1.26) | 16.8 (0.84) |
ta = 5.7 (<0.001)*** tb = 0.4 (0.9) |
| MASQ-AA | 31.8 (2.80) | 26.4 (1.81) | 20.8 (0.80) |
ta = 4.0 (<0.001)*** tb = 1.7 (0.11) |
| MASQ-GDD | 31.4 (3.37) | 38.6 (2.70) | 17.9 (0.74) |
ta = 6.8 (<0.001)*** tb = 1.7 (0.10)† |
| MASQ-AD | 67.7 (4.34) | 82.8 (2.70) | 53.3 (2.36) |
ta = 6.0 (<0.001)*** tb = 3.1 (0.005)** |
| age | 22.1 (1.20) | 22.5 (1.12) | 21.5 (1.07) |
ta = 0.7 (0.5) tb = 0.3 (0.8) |
HC vs. all participants with GAD (two-sided t-tests)
GAD vs. GAD/MDD groups (two-sided t-tests).
Abbreviations: AA, anxious arousal; AD, anhedonic depression; BDI, Beck depression inventory; GDA, general distress anxiety; GDD, general distress depression; MASQ, mood and anxiety symptom questionnaire; STAI, state-trait anxiety inventory.
P < 0.001
P < 0.01
P < 0.1.
Participants were scanned with a 3T Siemens Trio scanner; structure and diffusion MRI and echo planar images for the task fMRI were acquired. Our analytic strategy was threefold. First, we assessed hippocampal grey matter structure (atrophy-volumes and microstructure-grey matter diffusivity) using a combination of structural, diffusion MRI and a hippocampal segmentation analysis. Then, we investigated blood oxygenation level-dependent (BOLD) signal changes in cue reactivity as a function of repetition in a threat-associative learning task using functional magnetic resonance imaging (fMRI). Following univariate analyses to investigate effects of categorical diagnosis on the hippocampal structure and function, finally we investigated relationships among hippocampal structure, function, and dimensional symptom scales of anxiety and depression using SEM.
To investigate the microstructure of the hippocampus, we computed mean diffusivity (MD) of the hippocampal grey matter, elevation of which has been implicated in hippocampal-related pathology such as mild cognitive impairment (Muller et al., 2005). We calculated MD of the core hippocampal sub-regions (CA1, CA2/3, CA4/dentate gyrus), obtained from automated hippocampal subdivision segmentation (Van Leemput et al., 2009) (Detailed Methods). For the hippocampal functional measure, we used a hippocampal-dependent threat-associative learning task to investigate hippocampal threat processing (Detailed Methods). To measure hippocampal reactivity to condition (e.g., conditioned stimulus, a red rectangle, paired with unconditioned stimulus, an electric shock on a wrist; generalization stimuli, red rectangles with varying widths, without unconditioned stimulus) after preprocessing in SPM (Friston et al., 1994), we performed a parametric (time) modulation analysis (Detailed Methods). We performed a group-level random effect analysis on BOLD reactivity that was modulated by cue repetition within HC first and then across groups. For multiple comparison correction, we used an anatomical mask of the hippocampus using automated anatomical labeling (Tzourio-Mazoyer et al., 2002) for family-wise error correction.
Our diffusion MRI analyses showed significant group differences in the hippocampus. Effects of GAD (GAD only + GAD/MDD), compared with HC, were significant in the CA2-3 (t = 3.09; PFDR corrected = 0.009; robust linear regression)–indicating an increase in MD in GAD, marginally significant in CA4-DG (t = 2.00; PFDR corrected = 0.0765), and non-significant in CA1 (PFDR corrected = 0.285) (Fig 1). Within the GAD group, a contrast of GAD vs. GAD+MDD revealed significant effects of comorbid MDD in the CA1 (t = 2.37; PFDR corrected = 0.039) and CA2-3 (t = 2.71; PFDR corrected = 0.036), but not in CA4-DG (t = 1.66; PFDR corrected = 0.11). However, hippocampal volume measures showed non-significant group effects (P’s > 0.4). No group differences were found with respect to in-scanner head motion, whole brain MD, or whole brain volumes (intra-cranial volume) (P’s > 0.3). These findings suggest abnormal hippocampal microstructure (MD) in both GAD as well as comorbid GAD+MDD.
Figure 1. Disrupted threat learning in the hippocampus and grey matter microstructure in clinical anxiety, both with and without depression.
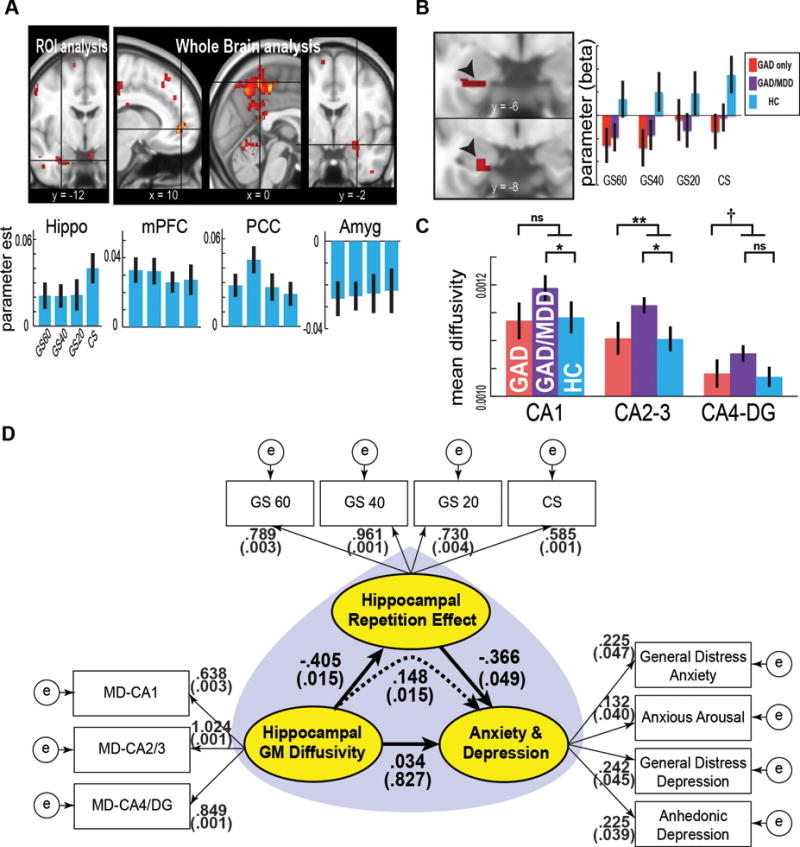
A, Effects of cue repetition on brain activation during a threat associative learning task in HC. An upper panel shows significant positive effects in the ventral hippocampus, the medial prefrontal cortex, and the posterior cingulate cortex. A significant negative effect was observed in the amygdala. Bar graphs represent the parameters estimates. B, Group comparison of hippocampal cue repetition effects. The GAD group in relative to HC showed significantly reduced effects in the ventral hippocampus. Non-significant differences were observed on an orthogonal contrast of GAD only vs. GAD+MDD comorbidity group. C, Bar graph showing a significant impact of GAD or GAD+MDD comorbidity on MD of the hippocampal subdivisions. D, SEM demonstrating the influence of hippocampal grey matter integrity on anxiety and depression symptoms is mediated through an impact on hippocampal threat processing. First, abnormal hippocampal grey matter integrity (increased “Hippocampal GM Diffusivity”) significantly accounts for decreased hippocampal functional measure (“Hippocampal Repetition Effect”) (P = 0.015; Bias-corrected percentile method in bootstrap samples); second, decreased hippocampal functional measure (“Hippocampal Repetition Effect”) accounts for increased anxiety and depression symptoms (“Anxiety and Depression”) (P = 0.049); and, finally, the hippocampal functional measure (“Hippocampal Repetition Effect”) mediates the impact of decreased hippocampal grey matter integrity (increased “Hippocampal GM Diffusivity”) on increased negative affect symptoms (“Anxiety and Depression”) (mediation effect; P = 0.015). Direct effects of “Hippocampal GM Diffusivity” on “Anxiety and Depression” were non-significant (P = 0.827). Error bars denote 1 sem. PFDR corrected < 0.1; * PFDR corrected < 0.05; ** PFDR corrected < 0.01. Amyg, amygdala; CS, conditioned stimulus; DG, dentate gyrus; GAD, generalized anxiety disorder; GM, grey matter; GS, generalization stimulus; HC, healthy controls; MD, mean diffusivity; MDD, major depressive disorder; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex.
Our fMRI analyses showed a significant increase in left anterior hippocampus activation to cue repetition in HC (PROI FWE corrected = 0.011; MNI x y z = −18 −12 −26) (Fig 1). Effects of Condition were non-significant at P < 0.05. This increase may be consistent with enhanced hippocampal neuronal activity as a function of stimulus repetition in non-human primates—a potential signature of short-term memory (Miller and Desimone, 1994). In contrast, participants with GAD showed significantly decreased cue repetition effects in the left anterior hippocampus (PROI FWE corrected = 0.012; x y z = −18 −12 −26). The Condition × Group interaction was non-significant. Within the GAD group, effects of comorbid MDD were non-significant. In-scanner head motion did not differ across groups (P’s > 0.2). These indicate abnormal hippocampal threat processing, potentially indexing memory-related functioning, in both GAD as well as comorbid GAD+MDD.
Finally, the significant relationships between GAD+MDD comorbidity and hippocampal grey matter microstructure, as well as GAD and hippocampal cue-repetition, suggested that structural abnormality might be associated with increased anxiety and depression symptoms via its impact on functional abnormality. To test this hypothesis, we performed mediation-SEM. The SEM (Fig 1D) showed a good model fit (Comparative Fit Index or CFI = 0.983, Root Mean Square Error of Approximation or RMSEA = 0.047). In this model, disrupted hippocampal grey matter integrity (indexed by increased “Hippocampal GM Diffusivity”) significantly accounted for decreased “Hippocampal Repetition Effect” across cues (Standardized regression coefficient = −0.405, P = 0.015, Bias-corrected percentile method on bootstrap samples). Decreased “Hippocampal Repetition Effect” then significantly explained increased negative affect symptoms (“Anxiety and Depression”) (regression coefficient = −0.366, P = 0.049). Of note, increased “Hippocampal GM Diffusivity” significantly impacted increased “Anxiety and Depression”, an effect that appeared to be mediated by decreased “Hippocampal Repetition Effect” (standardized indirect effect = 0.148, P = 0.015). Direct effects of “Hippocampal Diffusivity” on “Anxiety and Depression” (partial effects) were non-significant (P = 0.827). In contrast, an alternative model that “Hippocampal Repetition Effect” mediates impact of “Anxiety and Depression” onto “Hippocampal Diffusivity” showed poorer fits, and an association between “Hippocampal Repetition Effect” and “Hippocampal Diffusivity” failed to reach significance (Supporting Information). These results suggest that our data are best explained by a causal model describing a significant impact of abnormal hippocampal microstructure on anxiety and depression that is mediated through an influence on impaired hippocampal functioning.
Here we demonstrate evidence for the common role of the abnormal hippocampal structure and function in clinical anxiety, both with and without depression. First, the result of the positive hippocampal cue repetition effects in HC may be consistent with the signature of short-term memory; that is, the enhancement of the medial temporal lobe (MTL) neuronal activity as a function of stimulus repetition in non-human primates (Miller and Desimone, 1994). In rodents, the ventral hippocampus (the animal analogue of the human anterior hippocampus) is known to play a role in retrieval of threat memory (Hobin et al., 2006). Notably, our measure of the cue repetition effect in the hippocampal BOLD activity remains to be validated with behavioral measures of fear-associative learning or memory. Interpretation of this measure thus should be cautious. Nevertheless, the significant decrease in this measure in GAD appears to fit to the model of hippocampal dysfunction in pathophysiology of mood and anxiety disorders (Drevets, 2001; Price and Drevets, 2010). Indeed, the ventral hippocampus is well documented to play a key role in affective processing–via extensive projections to the brain regions associated with autonomic, neuroendocrine, and motivational responses to emotional stimuli, such as the amygdala, brain stem, and hypothalamus (Moser and Moser, 1998; Swanson and Cowan, 1977). Recent animal research also reports that stimulating the ventral hippocampus elicits anxiolytic effects (Kheirbek et al., 2013) (ref. conflicting findings in earlier intervention studies; Cimadevilla et al., 2001; Hunsaker and Kesner, 2008; Maren and Holt, 2004). Taken together, despite of limited mechanistic understanding of the hippocampal repetition effects, our results present novel evidence that may link the previous neurobiological evidence from animal studies indicating the role of the anterior hippocampus in negative affect processing and in pathophysiology of anxiety and depression.
Next, abnormal microstructure (i.e., increased MD), but normal volumes of the hippocampus (CA1, specifically) in the GAD+MDD comorbid group, compared with the GAD only and HC group, suggests important clinical implications. First, these results show significant effects of depression in the presence of anxiety on the hippocampal grey matter microstructure. These results are consistent with the previous literature on the role of the pathological remodeling within the hippocampus in pathophysiology of mood and anxiety disorders (Brown et al., 1999; Nestler et al., 2002; Sapolsky, 2000). Future studies may determine if increased hippocampal MD is related to detrimental effects of glucocorticoid (e.g., cortisol) along with activation of hypothalamic-pituitary-adrenal axis due to stress.
In the neurological literature, increased hippocampal MD has been widely implicated in hippocampus-related pathologies, such as mild cognitive impairment (Muller et al., 2005; Rose et al., 2006), Parkinson disease (Carlesimo et al., 2012), and Alzheimer’s disease (Yakushev et al., 2010). These studies indicate that hippocampal MD is more sensitive than volumes in predicting pathological conditions. Therefore, based on this literature and the present finding of non-significant group differences in hippocampal volumes, we suggest increased hippocampal MD as a potential biomarker for underlying structural anomalies in anxiety and depression.
Finally, the mediation-SEM suggests potential relationships among hippocampal structure and function, and symptoms of anxiety and depression. Our model supports that the impact of abnormal hippocampal structure on both anxiety and depression symptoms is mediated through its effect on hippocampal threat processing. This model outperforms an alternative model with opposite directions of impact: paths from negative affect symptoms to hippocampal structure and function. Of note, this model should not be interpreted as a firm conclusion about causality; instead, it may provide more in-depth information about the potential pathophysiology of anxiety and depression, beyond simple correlations. Moreover, it should be also noted that the present study did not include several factors known to mediate hippocampal structural disruption, such as chronic stress, cortisol levels, and genetic factors (Brown et al., 1999; Nestler et al., 2002; Sapolsky, 2000), which may be important to the underlying neurobiological mechanisms.
Limitations of the study merit consideration. First, validation with behavioral measures is required to better understand the hippocampal cue repetition effect in the context of threat-associated learning. Second, the present study does not test whether hippocampal MD is increased in MDD without comorbid GAD; testing this may elucidate specific associations of either MDD or GAD+MDD comorbidity with hippocampal degeneration. Third, our SEM findings regarding the impact of hippocampal disruption on clinical anxiety and depression are based on cross-sectional data. Therefore, no conclusions on causal inferences may be warranted. Fourth, to avoid sex-related heterogeneity in the sample we only recruited female participants; therefore, the generalizability of our findings to males remains to be tested. Furthermore, generalizability of the findings needs to be tested across different anxiety disorders or post traumatic stress disorder. Despite these limitations, our study presents novel evidence for a strong association between hippocampal (structural and functional) disruption and clinical anxiety and depression.
Detailed Methods
Participants Recruitment and Screening
A detailed description of participants of an umbrella protocol consisting of multiple neuroimaging experiments (MRI, Electroencephalography, and functional near-infrared spectroscopy) is provided elsewhere (Weinberg et al., 2012). Based on high prevalence rates of both GAD and MDD in females (Hyde et al., 2008), and to avoid gender-related brain differences (Cosgrove et al., 2007), only females were recruited for this study. Participants were recruited from the community via electronic and print advertisements. All potential participants were phone-screened prior to their visits to rule out current psychotropic medication usage and history of traumatic brain injury or systemic or neurological illness. The phone screen consisted of a modified version of the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998), a brief semi-structured diagnostic interview designed to screen for Axis I disorders. 236 potential participants underwent the full screening. 90 participants (38%) who were either (a) likely to meet criteria for current GAD and no other current Axis I diagnoses, or (b) likely to meet criteria for current GAD and current MDD but no other current Axis I diagnoses, or (c) unlikely to meet criteria for any Axis I diagnoses, past or present, were invited to come to the lab.
Once in the lab, all participants were administered the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (First et al., 2002) prior to MRI scanning to confirm diagnoses of GAD in the patient group and absence of Axis-I diagnoses in the HC group. The SCID-I was administered by one of three master’s-level clinicians. Each of the three clinicians was trained via SCID-I videos and supervision and feedback from senior authors (GHP). Inter-rater reliability was not assessed for the current study; however, kappas were calculated for a separate anxiety+depression study based upon eight interviews for each interviewer (who also interviewed in this study). Kappas in assessment of anxiety and mood disorders were high (0.88 – 0.92 range). All participants were free from psychiatric medication for at least six months prior to the time of the experiment. The Stony Brook University Institutional Review Board approved the study and all participants gave written informed consent.
Self-Report
In addition to the SCID-I, the Mood and Anxiety Symptom Questionnaire (MASQ; Clark and Watson, 1991) was administered to obtain a dimensional measure of symptom severity. We used a version with 90 items of mood and anxiety symptoms. Participants were asked to rate each item based on how much they had experienced it in the past week, using a scale from 1 = not at all to 5 = extremely. The MASQ has four subscales: General Distress Depressive Symptoms (GDD), General Distress Anxious Symptoms (GDA), Anhedonic Depression (AD), and Anxious Arousal (AA). Internal consistency, and convergent and discriminant validity of MASQ subscales has been shown (Watson et al., 1995).
MRI Data Acquisition
Participants were scanned with a 3T Siemens Trio scanner at the Stony Brook University Social, Cognitive and Affective Neuroscience center. We acquired 440 T2*-weighted echo planar images for the fear-generalization task. These were acquired with an oblique coronal angle and with the following parameters: TR, 2100 ms; TE, 23 ms; flip angle, 83°; matrix, 96 × 96; FOV, 224 × 224 mm; 37 slices; slice thickness, 3.5 mm. For structural scans, T1-weighted images were acquired with the following parameters: TR, 1900 ms; TE, 2.53 ms; flip angle, 9°; FOV, 176 × 250 × 250 mm; matrix, 176 × 256 × 256; voxel size, 1 × 0.98 × 0.98 mm. We collected dMRIs using the following parameters: TR, 5500 ms; TE, 93 ms; FOV, 220 × 220 mm; matrix, 120 × 220 × 220; voxel size, 1.7 × 1.7 × 3.0 mm; EPI factor, 128; 40 slices; slice thickness, 3 mm; bandwidth, 1396 Hz/pixel; Generalized Auto-calibrating Partially Parallel Acquisition acceleration factor, 2; the series included two initial images acquired without diffusion weighting and with diffusion weighting along 40 non-collinear directions (b = 800 s/m−2).
Measurement of Hippocampal Threat Processing
Threat-associative learning task (fear generalization)
Our rationale of using the fear-associative learning task (referred to a generalization task previously) in this study is different from the one in our previous reports using the very same task with a focus on generalization of fear acquisition (Cha et al., 2014; Greenberg et al., 2013; Greenberg, 2013). Here we aimed to test disrupted hippocampal threat processing in anxiety with and without comorbid depression. We investigated differential hippocampal reactivity to threat-associative cues as a function of cue repetition. This is based upon our reasoning that if the hippocampus is involved with fear-associative learning during the task, such as learning CS-GS discrimination or CS-US contingency, significant temporal patterns in its activation is to be expected: either a decrease (e.g., habituation) or an increase (e.g., potentiation).
The experimental paradigm has been previously described in detail (Greenberg et al., 2013). The task consists of two phases: “acquisition” and “generalization”. Before the acquisition phase, we titrated an amplitude of electric shock (unconditioned stimulus or US) to a level that was “uncomfortable but not painful” for each subject. For the acquisition phase, we instructed participants that a middle-sized rectangle (serving as the CS) indicated a 50% probability that they would receive an electric shock, but that shocks would never follow rectangles of greater or lesser size, while presenting the CS on a MRI-compatible screen. Then, each stimulus was presented once pseudo-randomly for 2 s with a jittered inter-stimulus interval (4 –10 s), while CS was pseudo-randomly presented five times with a 0.5 s long electric shock that terminated with CS. Stimuli consisted of seven red rectangles with identical height and varying in width. A middle-sized rectangle served as the CS, while six remaining rectangles varying ±20%, ±40%, or ±60% in width relative to the CS served as the GS. For the generalization phase, the same stimuli were pseudo-randomly presented 15 times. Similar to other human fear generalization studies (Kaczkurkin and Lissek, 2013; Lissek et al., 2014), to avoid extinction, CS-US contingency was reinforced by pairing CS and US 50% (15 times). Efficacy of the task in terms of recruitment of mesocorticolimbic fear or aversive motivation system has been previously demonstrated (Cha et al., 2014; Greenberg et al., 2013; Greenberg, 2013).
Measurement of temporal changes in hippocampal reactivity in cue repetition
The fMRI data were preprocessed using SPM 8 (www.fil.ion.ucl.ac.uk/spm) (Friston, 2007). Images were slice time corrected, realigned for motion correction, spatially normalized to the template brain of Montreal Neurological Institute (MNI 152) available in SPM 8, resampled to a resolution of 2 × 2 × 2 mm, spatially smoothed with a 4 mm full-width-half-maximum Gaussian kernel. A temporal high-pass filter with a cutoff of 128 seconds was applied.
Next, we performed parametric (time) modulation analysis in SPM 8 to investigate hippocampal reactivity changes in repetition of threat-associated cues. Regressors of the general linear model consisted of: (i) condition, (ii) time modulation regression (e.g., trial numbers) of each condition as parametric modulators, and (iii) motion parameters as nuisance variables. We first examined repetition effects in our hippocampus ROI (anatomical mask of the hippocampus from automated anatomical labeling; Tzourio-Mazoyer et al., 2002) in HC. To this end, we built a random effect, group-level, factorial model containing Subject and Condition as regressors and the parameter estimates (of the four stimuli of GS 60, GS 40, GS20, and “CS-unpaired with US”; “CS paired with US” was excluded to avoid artifacts from electric pulse) as dependent variables. For group comparison, we considered two orthogonal contrasts of “ALL GAD vs. HC” and “GAD ONLY vs. GAD/MDD comorbidity group”. We accordingly created a random effect, group-level, factorial model for each contrast: Subject, Condition, and Group were entered as main regressors. The results were corrected for multiple comparisons using a Family-Wise Error (FWE) correction on SPM within the anatomical hippocampus ROI.
Measurement of Hippocampal Grey Matter Structural Integrity
We aimed to test if anxiety and depression are related to hippocampal structural deficits and, if so, whether impact of structural deficits is linked to functional deficits. We thus examined two complementary structural measures of hippocampal subdivisions: volumes (representing macrostructure) and diffusivity (representing microstructure). With use of a hippocampal subdivision segmentation analysis, this approach allows us pinpointing a particular subdivision whose macro- or microstructure is linked to functional deficits or pathology of anxiety and depression.
Morphometry of hippocampal subdivisions
The purpose of this analysis is to obtain individualized segmentation of hippocampal subdivisions. This approach is motivated by the extensive animal literature on heterogeneity of anatomy and functions of the subdivisions (Hobin et al., 2006; Hunsaker and Kesner, 2008; Kheirbek et al., 2013; Lee and Kesner, 2004). We performed a fully automated cortical parcellation and subcortical segmentation–including hippocampal subfields–analysis using Freesurfer v5.3 (http://surfer.nmr.mgh.harvard.edu/).
Grey matter diffusivity of hippocampal subdivisions
As preprocessing steps, we first stripped the skull in the diffusion-weighted images and then performed eddy-current and head-motion correction by registering them to reference volumes using the Functional MRI of the Brain Software Library (FSL) package (www.fmrib.ox.ac.uk/fsl) (Jenkinson et al., 2012). Mean diffusivity (MD) and other diffusion parameters were estimated for each voxel by fitting a tensor model in FSL. MD images were then registered to MNI152 using a non-linear registration method (FNIRT) in FSL. For this, a non-linear warp function was calculated from a fractional anisotropy image in each individual using a template fractional anisotropy image (MNI152-1mm); then the warp was applied to a MD image in the same individual. Co-registration of MD images and hippocampal segmentation in the MNI152 space has been visually confirmed across all participants. Finally, a mean MD value was calculated in each hippocampal subdivision in each participant.
Statistical analyses
We examined effects of group on volumes and MD of the hippocampal subdivisions. Our ROI consisted of the CA1, CA2-3, and CA4-Dentate Gyrus, based on the extensive animal literature on the role of these subdivisions in threat-associated learning and anxiety (Hobin et al., 2006; Hunsaker and Kesner, 2008; Kheirbek et al., 2013; Lee and Kesner, 2004; Leutgeb et al., 2007). Furthermore, given our interests in delineating the structure and function relationship of hippocampal disruption in the present study, anatomical proximity of the fMRI group comparison results to the hippocampal subdivisions also motivated us to focus on the left hemisphere. Likewise in the fMRI analyses, for the two orthogonal contrasts [“HC vs ALL GAD vs.” and “GAD ONLY vs GAD/MDD group”], we conducted robust linear regression analyses using volumes or MD of each of the subdivisions as the dependent variable; Group (“0” for HC or GAD only group; “1” for all GAD or GAD/MDD group) as the predictor; Age and intracranial volume or Whole Brain MD as nuisance variables. Results were corrected for multiple comparisons using False Discovery Rate (FDR) correction (Benjamini & Hochberg method; Benjamini and Hochberg, 1995).
Head motion during scanning
Motion parameters were estimated: for diffusion MRI, average translation and average rotation (Yendiki et al., 2014); for fMRI, Framewise Displacement (FD) and DVARS (D denotes temporal derivative of timeseries; VARS, root mean square variance over voxels).
Structural equation modeling
Our motivation for using SEM with mediation analysis was to understand underlying mechanisms across mode diverse samples in terms of neural measures and dimensional negative affect symptoms, rather than drawing inferences from analyses dependent on categorical diagnosis. SEM has several advantages in testing mediation among variables over conventional regression methods: latent variables (factors) in SEM allow easy interpretation of results; SEM can be used to test an extended mediation process with multiple variables or outcomes (Gunzler et al., 2013); measurement errors of observational data can be accounted for in SEM. In addition, although conventional SEM requires large observations, this is not always possible particularly in task-fMRI studies with clinical cohorts. Thus, to minimize potential bias to model fits or parameter estimates from small degrees of freedom, we used Bias-corrected bootstrap sampling for maximum likelihood estimation. We used AMOS 22 for SEM (SPSS Inc.) (Arbuckle, 1994).
Specification of SEM
We estimated three latent variables (factors)–“Hippocampal Repetition Effect (Hippo RE)”, “Hippocampal Grey Matter MD (Hippo MD)”, and “Anxiety & Depression (Anx-Dep)”: for Hippo RE, the parameter estimates of ventral hippocampal repetition effects (in CS) were used as the observed variables; for Hippo MD, the MD measures of the core hippocampal formation (CA1, CA2-3, CA4-Dentate Gyrus) were used; and for Anx-Dep, the four subscales within MASQ (General distress anxiety, anxious arousal, general distress depression, and anhedonic depression) were used. In order to make the model identifiable, we imposed a constraint that error terms of the observed variables for each latent variable have equal variances. Since this model with the minimal constraint showed a sub-optimal model fit (CFI = 0.807; RMSEA = 0.155), we imposed two additional constraints to the model referring to modification indices to improve the model fit: A covariance between MD-CA2/3 and MD-4/DG and a covariance between General Distress Anxiety and Anxious Arousal. This serves as the final model.
Bootstrap sampling
While traditional SEM that is dependent on the assumption of normality requires large samples for estimation, the use of sampling methods, such as bootstrap, to construct better confidence intervals of parameters has been advocated by a growing body of statistical simulation literature (Mackinnon et al., 2004). Here we used bootstrapping methods (5,000 bootstrap samples) to estimate significance of parameters under the maximum likelihood estimation.
Acknowledgments
This research was supported by NSF (CBET0954643; LRMP) and ONR (N0014-04-1-005; LRMP).
References
- Arbuckle JL. Amos - Analysis of Moment Structures. Psychometrika. 1994;59(1):135–137. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995:289–300. [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: the role of threat appraisal and fear learning. Depress Anxiety. 2011;28(1):5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Rush AJ, McEwen BS. Hippocampal remodeling and damage by corticosteroids: implications for mood disorders. Neuropsychopharmacology. 1999;21(4):474–84. doi: 10.1016/S0893-133X(99)00054-8. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Piras F, Assogna F, Pontieri FE, Caltagirone C, Spalletta G. Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology. 2012;78(24):1939–45. doi: 10.1212/WNL.0b013e318259e1c5. [DOI] [PubMed] [Google Scholar]
- Carter RM, Wittchen HU, Pfister H, Kessler RC. One-year prevalence of subthreshold and threshold DSM-IV generalized anxiety disorder in a nationally representative sample. Depress Anxiety. 2001;13(2):78–88. doi: 10.1002/da.1020. [DOI] [PubMed] [Google Scholar]
- Cha J, Carlson JM, Dedora DJ, Greenberg T, Proudfit GH, Mujica-Parodi LR. Hyper-reactive human ventral tegmental area and aberrant mesocorticolimbic connectivity in overgeneralization of fear in generalized anxiety disorder. J Neurosci. 2014;34(17):5855–60. doi: 10.1523/JNEUROSCI.4868-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadevilla JM, Wesierska M, Fenton AA, Bures J. Inactivating one hippocampus impairs avoidance of a stable room-defined place during dissociation of arena cues from room cues by rotation of the arena. Proc Natl Acad Sci U S A. 2001;98(6):3531–6. doi: 10.1073/pnas.051628398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of abnormal psychology. 1991;100(3):316. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62(8):847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240–9. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Fava M, Rankin MA, Wright EC, Alpert JE, Nierenberg AA, Pava J, Rosenbaum JF. Anxiety disorders in major depression. Compr Psychiatry. 2000;41(2):97–102. doi: 10.1016/s0010-440x(00)90140-8. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP) New York: New York State Psychiatric Institute. Biometrics Research; 2002. [Google Scholar]
- Friston K. Statistical parametric mapping. Statistical Parametric Mapping: The Analysis of Functional Brain Images. 2007:10–31. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Human brain mapping. 1994;2(4):189–210. [Google Scholar]
- Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR. Neural reactivity tracks fear generalization gradients. Biol Psychol. 2013;92(1):2–8. doi: 10.1016/j.biopsycho.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Greenberg T, C JM, Cha J, Hajcak G, Mujica-Parodi LR. Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress Anxiety. 2013;30(3):242–250. doi: 10.1002/da.22016. [DOI] [PubMed] [Google Scholar]
- Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biol Psychiatry. 2002;51(11):851–8. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- Gunzler D, Chen T, Wu P, Zhang H. Introduction to mediation analysis with structural equation modeling. Shanghai Arch Psychiatry. 2013;25(6):390–4. doi: 10.3969/j.issn.1002-0829.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16(2):174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem. 2008;89(1):61–9. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol Rev. 2008;115(2):291–313. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN, Lissek S. Generalization of Conditioned Fear and Obsessive-Compulsive Traits. J Psychol Psychother. 2013;7:3. [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA, Hen R. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77(5):955–68. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus. 2004;14(3):301–10. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315(5814):961–6. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC, Grillon C. Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc Cogn Affect Neurosci. 2014;9(8):1134–42. doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon DP, Lockwood CM, Williams J. Confidence Limits for the Indirect Effect: Distribution of the Product and Resampling Methods. Multivariate Behav Res. 2004;39(1):99. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci. 2004;118(1):97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Mialet JP, Pope HG, Yurgelun-Todd D. Impaired attention in depressive states: a non-specific deficit? Psychol Med. 1996;26(5):1009–20. doi: 10.1017/s0033291700035339. [DOI] [PubMed] [Google Scholar]
- Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263(5146):520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–19. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Muller MJ, Greverus D, Dellani PR, Weibrich C, Wille PR, Scheurich A, Stoeter P, Fellgiebel A. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. Neuroimage. 2005;28(4):1033–1042. doi: 10.1016/j.neuroimage.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SE, McMahon KL, Janke AL, O’Dowd B, de Zubicaray G, Strudwick MW, Chalk JB. Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2006;77(10):1122–8. doi: 10.1136/jnnp.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biological psychiatry. 2000;48(8):755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172(1):49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Leemput K, Bakkour A, Benner T, Wiggins G, Wald LL, Augustinack J, Dickerson BC, Golland P, Fischl B. Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus. 2009;19(6):549–57. doi: 10.1002/hipo.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104(1):3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, Hajcak G. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. J Abnorm Psychol. 2012;121(4):885–96. doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushev I, Muller MJ, Lorscheider M, Schermuly I, Weibrich C, Dellani PR, Hammers A, Stoeter P, Fellgiebel A. Increased hippocampal head diffusivity predicts impaired episodic memory performance in early Alzheimer’s disease. Neuropsychologia. 2010;48(5):1447–53. doi: 10.1016/j.neuropsychologia.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage. 2014;88:79–90. doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]


