Abstract
Opioid antagonists (e.g., naltrexone) and positive modulators of γ-aminobutyric acid type A (GABAA) receptors (e.g., alprazolam) each modestly attenuate the abuse-related effects of stimulants. A previous study demonstrated that acute pretreatment with the combination of naltrexone and alprazolam attenuated a greater number of the subject-rated effects of d-amphetamine than the constituent drugs alone. This study tested the hypothesis that maintenance on the combination of naltrexone and alprazolam XR would attenuate the reinforcing and “positive” subject-rated effects of methamphetamine to a greater extent than the constituent drugs alone.
Eight non-treatment-seeking, stimulant-using individuals completed a placebo-controlled, crossover, double-blind inpatient protocol. Participants were maintained on naltrexone (0 and 50 mg), alprazolam XR (0 and 1 mg), and the combination of naltrexone and alprazolam XR (50 mg and 1 mg, respectively) for 6–7 days. Under each maintenance condition, participants sampled intranasal doses of methamphetamine (0, 10, and 30 mg), and were then offered the opportunity to work for the sampled dose on a modified progressive-ratio procedure. Subject-rated drug effect questionnaires, psychomotor, and physiology assessments were collected.
Intranasal methamphetamine functioned as a reinforcer and produced prototypical stimulant-like “positive” subject-rated and physiological effects. Maintenance on naltrexone significantly decreased the reinforcing, but not subject-rated drug effects of 10 mg methamphetamine. Alprazolam XR and the combination of naltrexone and alprazolam XR did not impact methamphetamine self-administration or subject-rated drug effects. The results support the continued evaluation of naltrexone for methamphetamine dependence, as well as the identification of other drugs that enhance its ability to reduce drug-taking behavior.
Keywords: methamphetamine, naltrexone, alprazolam XR, self-administration
INTRODUCTION
Methamphetamine use is a significant public-health concern. The number of individuals reporting current methamphetamine use in the United States increased by 30% from 2011 to 2014.1 In addition, methamphetamine abuse and dependence in the United States accounted for 7% of drug treatment admissions.2 Estimates from the most recently available data indicate that the total cost for methamphetamine abuse in the United States was over $23 billion in 2005.3 Despite intense research efforts, a widely effective monotherapy has not yet been identified for methamphetamine use disorders.4–7
Combination therapy could address some limitations of monotherapy by targeting a more diverse range of neurotransmitters involved in methamphetamine use disorder, and also because lower doses of the constituent drugs can be used. Combining medications with modest efficacy could result in greater reductions in stimulant self-administration while reducing the risk of side effects associated with higher doses of the treatment drugs.8 For example, a series of preclinical experiments demonstrated that combining low doses of two different drugs that each modestly attenuate the abuse-related effects of methamphetamine is an effective strategy to manage stimulant use disorders.8–9 More specifically, combining low doses of oxazepam and metyrapone that were ineffective when tested alone resulted in reduced cocaine self-administration in rats.9 In agreement with the preclinical data, oxazepam-metyrapone combinations were well tolerated and significantly reduced cocaine use in a small clinical trial.10
In a previous study that tested the hypothesis that a treatment drug combination might more effectively attenuate the abuse-related effects (e.g., reinforcing effects and “positive” subject-rated effects) of a stimulant drug, we evaluated the mu opioid antagonist, naltrexone, and the γ-aminobutyric acid type A (GABAA) receptor positive allosteric modulator, alprazolam in individuals with stimulant use disorder.11 Acute pretreatment with each drug significantly attenuated some of the “positive” subject-rated effects of d-amphetamine and combining naltrexone and alprazolam attenuated a greater number of subject-rated effects than the constituent drugs alone. Furthermore, acute pretreatment with the combination of these drugs did not produce clinically problematic acute physiological effects or “negative” subject-rated effects.
Acute pretreatment with the combination of naltrexone and alprazolam was selected for testing in our previous study because neuroanatomical, neurochemical, and behavioral data support functional links between opioid and GABA systems with the dopamine system, a significant contributor to the abuse-related behavioral effects of psychostimulants.12 For example, mu opioid receptors are co-localized with dopamine D2 receptors on mesolimbic dopamine neurons and administration of mu opioid antagonists reduces amphetamine-induced dopamine release in the striatum and nucleus accumbens.13–16 Similarly, GABAA receptors are co-localized on dopamine cell bodies and GABAA receptor positive modulators attenuate methamphetamine-induced increases in dopamine levels in the striatum and nucleus accumbens.17,18
Prior preclinical and clinical laboratory studies also guided the decision to test the combination of naltrexone and alprazolam in the earlier study. In animal models, various behavioral effects of amphetamines are attenuated by opioid antagonists19–22 and GABAA receptor positive modulators.23–25 Especially germane to the present study are results from experiments demonstrating that naltrexone pretreatment decreased intravenous d-amphetamine self-administration in rhesus monkeys26 and similarly, alprazolam dose-dependently reduced cocaine self-administration in baboons.27 In human laboratory experiments, acute pretreatment with naltrexone11,28,29 and alprazolam11,30 reduced the subject-rated effects of d-amphetamine in separate studies. Maintenance on 1 mg/day alprazolam XR also produced a small, but orderly attenuation of some subject-rated effects of methamphetamine31, and likewise, maintenance on 50 mg/day naltrexone modestly decreased a subset of “positive” subject-rated drug effects of intravenous methamphetamine32, in individuals who met criteria for stimulant-use disorders (but see33).
As noted, the attenuation of the abuse-related behavioral effects of amphetamines by maintenance on either naltrexone or alprazolam could generally be considered modest. Higher doses of these drugs might more completely attenuate the response to amphetamine, but untoward side effects limit their use.34–36 The promising findings that the combination of acutely administered naltrexone and alprazolam was well tolerated and attenuated a greater number of subject-rated drug effects following administration of oral d-amphetamine than the constituent drugs alone11 supported further investigation of this combination using more clinically relevant study procedures.
The present experiment therefore tested the ability of 6–7 days of maintenance on oral naltrexone (50 mg/day) and extended release alprazolam (i.e., alprazolam XR; 1 mg/day), separately and in combination, to reduce the reinforcing and positive subject-rated effects of intranasal methamphetamine (0, 10, and 30 mg) in eight participants who met criteria for stimulant-use disorder. Although alprazolam has known abuse potential,35–37 the prior research described above demonstrated its ability to reduce the abuse-related effects of stimulants,11,24,27,30,31,38 so it was selected for initial proof-of-concept testing in an attempt to demonstrate the initial efficacy of maintenance on naltrexone and a potent GABAA positive allosteric modulator to attenuate the response to methamphetamine.
Maintenance dosing was used because it more closely models a clinical treatment scenario. The extended release formulation of alprazolam was selected because it permitted twice per day maintenance dosing, rather than (>2) daily doses of the immediate release formulation and because it could then be administered at the same time as naltrexone. Further, alprazolam XR also has less abuse potential than immediate-related alprazolam.35 The present study also builds on our previous results by evaluating the impact of maintenance of naltrexone, alprazolam XR, and the combination on intranasal methamphetamine self-administration on a progressive-ratio procedure. Methamphetamine was tested because rates of methamphetamine dependence are higher and it has greater relative reinforcing strength compared to d-amphetamine.39,40 Self-administration procedures were used because they provide a direct measure of drug taking and are predictive of pharmacotherapeutic effectiveness.41,42 In particular, progressive-ratio schedules provide a means to assess changes in the relative reinforcing strength of abused drugs across different maintenance conditions.43–45
MATERIALS AND METHODS
Study Population, Inclusion/Exclusion Criteria, and Screening
The Institutional Review Board of the University of Kentucky Medical Center approved this study and the participants gave their sober, written informed consent prior to enrolling. In the consent process, participants were informed that during their participation they would receive various drugs, administered orally or intranasally, that could include placebo, methamphetamine, naltrexone, and alprazolam XR. Participants were instructed that these medications could be administered alone or in combination and potential side effects were described. Other than receiving this general information, participants were blind to the dose range and dosing schedule. Participants were told that the purpose of the study was to determine how different drugs affect physiology, mood, and behavior. Other than this general explanation, they were given no instruction of what they were “supposed” to do or of what outcomes might be expected. Participants were compensated for their time and effort.
Prior to enrollment, all potential participants underwent a comprehensive physical and mental health screening.46 A psychiatrist (L.R.H., P.E.A.G., or their designee) interviewed and examined each potential participant. Routine clinical laboratory blood chemistry testing, vital signs assessment, and electrocardiography were also conducted. Potential participants were excluded if they reported histories of serious physical disease or current physical disease, impaired cardiovascular functioning, chronic obstructive pulmonary disease, seizure, head trauma or central nervous system tumors, or current or past histories of serious psychiatric Axis I disorders according to the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) other than substance abuse or dependence. Participants had to meet the following criteria: (1) self-reported recent amphetamine or cocaine use, (2) confirmation of recent amphetamine or cocaine use by a stimulant-positive urine screen during the initial screening, and (3) fulfillment of the diagnostic criteria for amphetamine or cocaine dependence or abuse on a computerized version of the Structured Clinical Interview for DSM-IV. All participants were in good health with no contraindications to the study drugs.
General Procedures
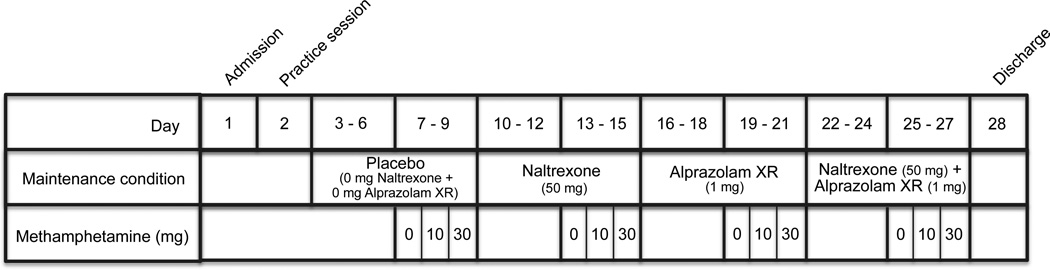
Participants were enrolled as inpatients at the University of Kentucky Chandler Medical Center Clinical Services Core (CSC) for 28 days and participated in 1 practice session and 12 experimental sessions (one session for each of the maintenance + methamphetamine dose combinations), with one exception. One participant was discharged from the study for personal reasons after completing 2 maintenance conditions and readmitted at a later date. This participant completed an additional practice session and spent 3 additional days on the inpatient unit. On the day of admission to the CSC, participants provided a urine sample negative for recent use of all drugs, other than cocaine, amphetamine and cannabis, as well as pregnancy. Participants also provided an expired air sample, which was assayed for the presence of alcohol using an Alco-Sensor breathalyzer (Intoximeters, St. Lous, MO, USA) and completed a field sobriety test to ensure that they were not currently intoxicated. Participants were then allowed to acclimate to the CSC for 1 day, during which they were observed for drug or alcohol withdrawal. No signs or symptoms of withdrawal were detected. The overall timeline for the experiment is presented in Figure 1.
Fig. 1.
Example of the experimental timeline. The order of methamphetamine sampling doses was randomized and the order of maintenance drug administration (i.e., placebo, naltrexone, alprazolam XR, naltrexone + alprazolam XR) was counterbalanced across participants with the exception that the combination of naltrexone and alprazolam XR was never administered as the first maintenance condition.
Practice Session
Following the acclimation period, participants completed a practice session to become familiarized with the modified progressive-ratio procedure, subject-rated drug-effect questionnaires, and daily laboratory routine, as described below. Study drugs were not administered during these sessions.
Drug Maintenance Days
Drug maintenance began the day after the practice session and continued throughout the protocol. Oral doses of naltrexone (25 mg naltrexone, b.i.d.) and/or alprazolam XR (0.5 mg alprazolam XR, b.i.d.) were administered at 0700 and 1900 h. These doses were selected based on clinical dosing recommendations and concerns over the use of side effects associated with higher doses.
Four maintenance conditions were tested in counterbalanced order across participants, and included maintenance on (1) placebo (i.e., 0 mg/day naltrexone and 0 mg/day alprazolam XR), (2) naltrexone (i.e., 50 mg/day naltrexone and 0 mg/day alprazolam XR), (3) alprazolam XR (i.e., 0 mg/day naltrexone and 1 mg/day alprazolam XR), and (4) the combination (i.e., 50 mg/day naltrexone and 1 mg/day alprazolam XR). Each maintenance condition lasted for 6 to 7 days. After the first 3–4 days of each maintenance condition, participants completed 3 experimental sessions. The initial 3–4 day maintenance period prior to experimental testing permitted blood levels to reach steady state, calculated according to the pharmacokinetic profile for naltrexone (T1./2 = 4 h)47 and alprazolam XR (T1./2 =15.6 h).48
Placebo refers to the condition in which 0 mg methamphetamine, naltrexone, and alprazolam XR were administered. Maintenance on placebo refers to maintenance on 0 mg naltrexone and 0 mg alprazolam XR. References to methamphetamine alone pertain to those instances in which 10 or 30 mg methamphetamine was administered during maintenance on placebo. References to naltrexone alone, alprazolam XR alone, and the combination of naltrexone and alprazolam XR alone pertain to those instances in which 0 mg methamphetamine was administered during each respective maintenance condition. Each combination of maintenance dose + methamphetamine dose was tested in a single session, with 12 total dose conditions administered.
Experimental Sessions
Three experimental sessions, conducted on consecutive days, were completed during each maintenance condition to test each methamphetamine dose (i.e., 0, 10, and 30 mg). Experimental sessions started at 0830 h and lasted approximately 7.5 h. The intranasal methamphetamine dose available for self-administration later that day was sampled at 0930 h. Participants completed all physiological and subject-rated drug-effect questionnaires, as well as the Digit Symbol Substitution Test (DSST),49 1 h prior to drug administration and 0, 15, 30, 45, 60, 90, and 120 min after drug sampling. Participants completed a modified progressive-ratio procedure at 1330 h and then self-administered the earned dose (see below).
Urine and expired breath samples were collected prior to each session to confirm drug and alcohol abstinence, respectively. Participants occasionally tested positive for amphetamine and benzodiazepines, which coincided with experimental administration. Participants tested negative for all other drug and alcohol use. The female participant received daily urine pregnancy tests, which were negative throughout her enrollment.
Sampling Procedure
Sampling sessions were conducted in the morning to acquaint participants with the effects of the drug dose (i.e., 0, 10, and 30 mg methamphetamine) that would be available for self-administration in the afternoon session. Following the pre-drug assessments, participants insufflated the appropriate methamphetamine dose at 0930 h and were instructed to pay attention to the effects of the drug, because they would be offered the opportunity to work to receive that drug later that day. Participants then completed post-drug assessments as described above.
Self-Administration Procedure
Modified progressive-ratio procedures have been used previously to assess drug reinforcement in humans.43–45,50–52 Under the present procedure, participants were given 10 opportunities to respond on a computer mouse to earn all, or some, of the intranasal powder dose that was administered during the morning sampling session. Participants responded by clicking either a YES or NO presented on the computer screen when asked if they wanted to work for a portion (i.e., 1/10th) of the previously sampled dose. If the participant responded YES, they were then required to click the mouse a predetermined number of times to earn a portion of the sampled dose. To earn the first portion, participants were required to click the mouse 400 times. The number of clicks required to earn each additional portion increased by 100 (i.e., 500, 600, 700, 800, 900, 1000, 1100, 1200, and 1300 responses). To receive a full dose, participants had to click the mouse a total of 8500 times, lasting approximately 35 minutes. If the participant responded NO at any time they were asked if they wanted to work for a portion of the dose, the task was terminated. While completing each component of the progressive-ratio schedule, participants were also able to terminate the task by clicking a button labeled STOP. The dependent measure was the number of drug choices completed.
If the participants chose drug, intranasal drug administration occurred at 1430 h. After insufflating the amount of drug earned on the modified progressive-ratio procedure, participants completed the subject-rated drug-effect questionnaires, performance task, and the physiological assessments at the intervals described for the sampling dose. If a participant did not respond for any of the dose, he/she still completed the assessments as scheduled.
Experimental Measures
Subject-Rated Drug-Effect Questionnaires
Subject-rated drug-effect questionnaires and the performance task were administered on a laptop computer in fixed order. The subject-rated questionnaires included the Drug-Effect Questionnaire53 and the Adjective Rating Scale.54 The Drug-Effect Questionnaire used a 100-unit visual analog scale and the Adjective Rating Scale used a five-response Likert-type scale.
Performance Measure
The DSST is widely used in human behavioral pharmacology research to assess changes in psychomotor performance following drug administration.49 The dependent measure was the percent of geometric patterns entered correctly (i.e., Percent Trials Correct) in 90 s.
Physiological Measures
Blood pressure, heart rate, electrocardiography, and oral temperature were recorded with an automated monitor (Dinamap Pro 1000 Vital Signs monitor; Critikon Company LLC, Tampa, Fla, USA). If heart rate exceeded 100 beats per minute, systolic blood pressure exceeded 150 mm Hg, diastolic blood pressure exceeded 100 mm Hg, or clinically significant electrocardiographic changes occurred after administration of methamphetamine during the morning sampling procedure, the afternoon self-administration procedure was completed, but the earned dose was withheld. This occurred on two occasions for a single participant. A study investigator explained to the participant that the dose would not be administered for safety reasons, in an attempt to prevent the withheld dose from impacting future self-administration behavior. If heart rate exceeded 130 beats per minute, systolic blood pressure exceeded 180 mm Hg, diastolic blood pressure exceeded 120 mm Hg, or clinically significant electrocardiographic changes occurred after administration of methamphetamine at any point during the experiment, a participant would have been excluded from further participation, but this did not occur.
UKU Side Effects Scale
CSC nursing staff also completed the Udvalg for Kliniske UndersØgelser (UKU) side effects rating scale daily prior to the administration of maintenance doses.55 Side effects reported on these scales were not analyzed statistically, but were monitored regularly by unit physicians. The most common side effect reported was a burning feeling in the nose following drug administration. Although a few subjects reported insomnia after drug administration days, no participants were discharged due to side effects.
Cognitive and Performance Sessions
Participants also completed cognitive and performance tasks during drug maintenance days and experimental sessions, which included the Visual Dot Probe,56 Cued-Go/No Go,57 Cocaine Stroop,58 N-Back,59 BART,60 and Grooved Pegboard (Layfayette, Instruments, Layfayette, IN, USA). No significant impact of maintenance condition was observed and thus will not be discussed further.
Drug Administration
All drugs were administered in a double-blind fashion. Naltrexone doses (25 mg) were prepared by over-encapsulating commercially available drug (Barr Laboratories INC, Pomona, NY, USA). Extended-release alprazolam doses (0.5 mg) were prepared by over-encapsulating commercially available drug (Greenstone, Ltd., Peapack, NJ, USA). Cornstarch was used to fill the remainder of the capsules. Placebo capsules contained only cornstarch. Methamphetamine doses (0, 10, 30 mg) were prepared by combining the appropriate amount of methamphetamine HCI (National Institute on Drug Abuse, Research Triangle Park, NC, USA) with lactose to equal a total of 50 mg powder. None of the drugs tested here were used for their labeled indication.
During each methamphetamine administration, a research nurse presented the participant with the powder, a mirror, and a standard razor blade. The participant was then instructed to divide the powder into two even “lines” and to insufflate one line of powder through each nostril using a 65 mm plastic straw during a 2 min time period.
Data Analysis
Statistical analyses of group data were conducted to examine drug effects on the modified progressive-ratio procedure, subject-rated drug-effect questionnaires, performance task, and physiological measures. For all statistical analyses, effects were considered significant for p ≤ 0.05.
Data from the modified progressive-ratio procedure were analyzed as the number of drug choices using a three-factor, repeated-measures analysis of variance (ANOVA) with Naltrexone (0 and 50 mg), Alprazolam XR (0 and 1 mg), and Methamphetamine (0, 10, and 30 mg) as the factors (Statview, Cary, NC). If a statistically significant interaction (i.e., interaction of Methamphetamine and Naltrexone and/or Alprazolam XR) or a main effect of Methamphetamine and Naltrexone and/or Alprazolam XR was detected, the mean-square error term was used to conduct Fisher’s Least Significant Difference (LSD) post hoc test to compare the effects of each methamphetamine dose across maintenance conditions. Data from the subject-rated drug-effect questionnaires, performance measure, and physiological assessments collected during the sampling sessions were analyzed as peak effect (i.e., the maximum score observed following each methamphetamine administration) using the same method as the modified progressive-ratio data. Area under the curve (AUC) was also calculated and in cases where results diverged from the peak analysis, AUC is also reported. During self-administration sessions, participants ingested varying amounts of drug, so subject-rated drug-effect questionnaires and cardiovascular data from the self-administration sessions were not analyzed statistically.
RESULTS
Demographics
Eight non-treatment seeking adult participants completed this within-subjects, placebo-controlled study. Participants ranged in age from 25 to 53 years (mean, 41 years) and in weight from 63 to 101 kg (mean, 82 kg). Seven of these participants were male and one was female. Six participants reported their race as African American and two self reported as Caucasian. Participants scored between 4 and 12 (mean, 8) on the Drug Abuse Screening Test61 and reported using cocaine 1 to 15 days (mean, 10 days) in the month prior to screening. Seven participants reported consuming alcohol (range of 1–18 beverages per week; mean, 9), and scored between 0 and 20 (mean, 8) on the Michigan Alcoholism Screening Test.62 None of the participants met criteria for alcohol dependence. Seven participants reported using marijuana 0 to 30 days (mean, 12) in the month prior to screening, but none met diagnostic criteria for cannabis dependence. In addition, 4 participants reported that they consumed between 0 and 504 mg (mean, 235 mg) caffeine per day and 7 participants reported smoking tobacco cigarettes daily (range, 2–20 cigarettes per day; mean, 8 cigarettes). Two participants reported using opiates once in the month prior to screening.
Modified Progressive Ratio
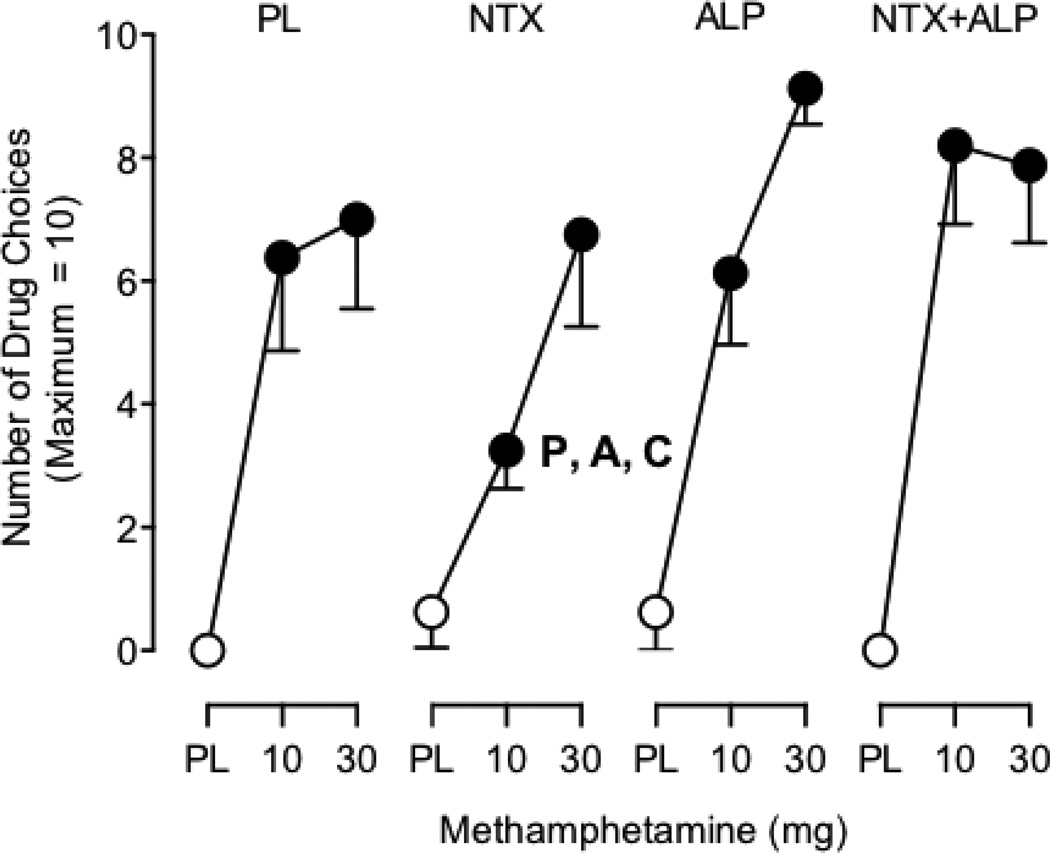
ANOVA revealed a significant interaction between Methamphetamine, Naltrexone, and Alprazolam XR (F2,14 = 5.9; p < 0.05) for the number of drug choices on the modified progressive-ratio procedure (Figure 2). Post hoc comparisons revealed that both active methamphetamine doses alone maintained significantly greater responding on the modified progressive-ratio procedure compared with placebo. Maintenance on naltrexone significantly decreased the number of drug choices for the 10 mg methamphetamine dose relative to each of the other three maintenance conditions. Maintenance on naltrexone also significantly decreased the number of drug choices for the 30 mg methamphetamine dose relative to maintenance on alprazolam XR.
Fig. 2.
Mean number of drug choices during maintenance on 1) Placebo naltrexone and placebo alprazolam XR (PL); 2) Naltrexone (NTX) and placebo; 3) Alprazolam XR (ALP) and placebo; and 4) Naltrexone and Alprazolam XR (NTX+ALP). Symbols represent the mean of eight (8) participants. Filled symbols indicate a significant difference from the placebo-placebo condition (i.e., the data point above the leftmost “PL” on the x-axis). ‘P’ indicates a significant difference from the corresponding methamphetamine dose under the placebo maintenance condition. ‘A’ indicates a significant difference from the corresponding methamphetamine dose under the alprazolam XR maintenance condition. ‘C’ indicates a significant difference from the corresponding methamphetamine dose under the combination of naltrexone and alprazolam XR maintenance condition. Error bars indicate 1 standard error of the mean (SEM).
Adjective Rating Scale
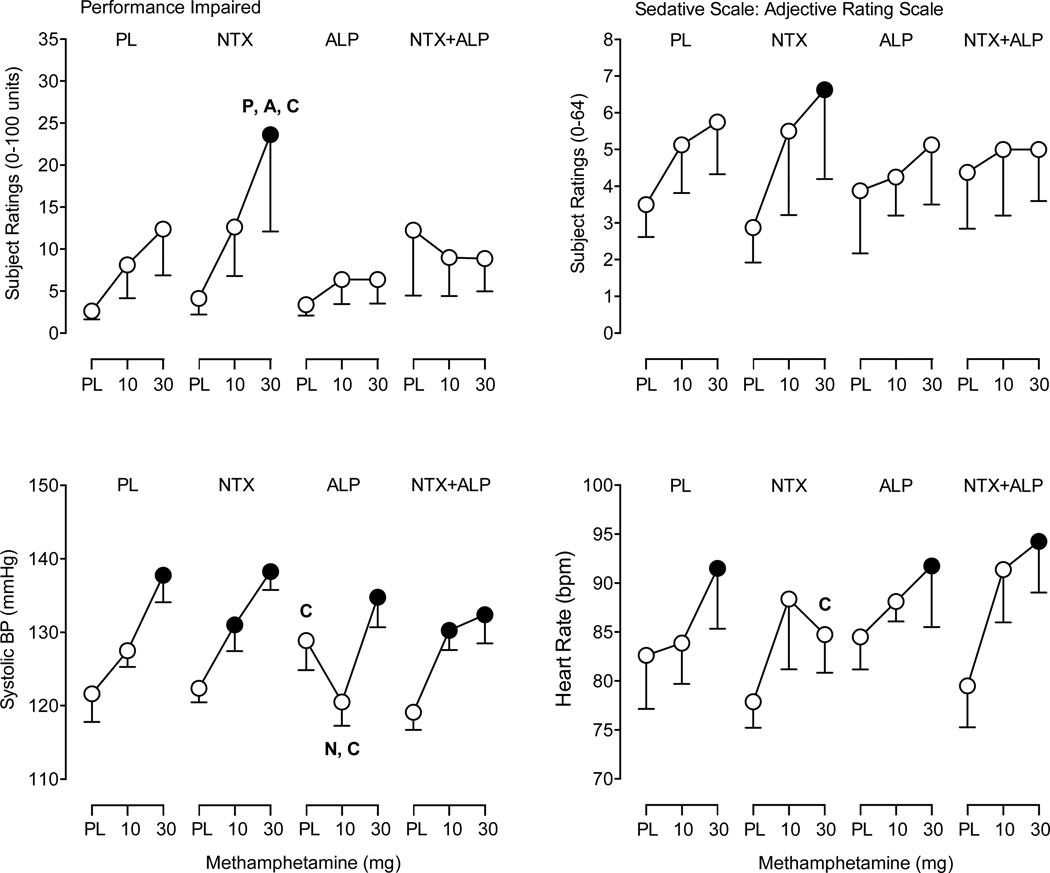
A significant interaction between Methamphetamine and Naltrexone (F2,14 = 4.4; p < 0.05) was observed for scores on the Sedative subscale of the Adjective Rating Scale (Figure 3). Post hoc comparisons revealed that active methamphetamine doses alone did not increase scores on the Sedative scale. Maintenance on naltrexone significantly increased scores on the Sedative scale following 30 mg methamphetamine relative to placebo. In contrast, the AUC analysis did not detect an interaction for the Sedative scale (p > 0.05). There were no main effects or interactions of methamphetamine and either maintenance drug on the Stimulant subscale (data not shown).
Fig. 3.
Subject-rated and cardiovascular measures. Mean peak participant ratings for Performance Impaired from the Drug-Effect Questionnaire, the Sedative scale from the Adjective Rating Scale (top panels) and mean peak systolic blood pressure and heart rate (bottom panels) following administration of methamphetamine and the four maintenance conditions. Other details are as in Fig. 2.
Drug-Effect Questionnaire
A significant interaction of Methamphetamine and Naltrexone was observed for the item Performance Impaired on the Drug-Effect Questionnaire (F2,14 = 4.1; p < 0.05; Figure 3). Post hoc comparisons revealed that active methamphetamine doses alone did not increase ratings of Performance Impaired, but that ratings on this item were increased following administration of 30 mg methamphetamine during maintenance on naltrexone relative to placebo as well as all other maintenance conditions.
A main effect of Methamphetamine (F2,14 = 4.0; p < 0.05) and Alprazolam XR (F2,14 = 7.5; p < 0.05) was observed for the item, Talkative/Friendly. Post hoc comparisons revealed that active methamphetamine doses alone did not increase ratings of Talkative/Friendly, but that ratings on this item were increased following administration of 30 mg methamphetamine during maintenance on naltrexone, alprazolam XR, and the combination of naltrexone and alprazolam XR.
A main effect of Methamphetamine was observed for eleven items on the Drug-Effect Questionnaire: Active/Alert/Energetic, Any Effect, Good Effect, High, Heart Racing, Like Drug, Nauseated, Rush, Stimulated, Willing to Pay For, and Willing to Take Again (F2,14 values = 4.0–10.0; p ≤ 0.05). Table 1 provides mean peak ratings for these items during maintenance on placebo following administration of 0, 10, and 30 mg methamphetamine. There were no main effects or interactions of methamphetamine and either maintenance drug on ratings for the items Bad Effects, Euphoric, Nervous/Anxious, Performance Improved, Restless, Shaky/Jittery, and Sluggish.
Table 1.
Mean (standard deviation) peak ratings during maintenance on placebo for items on the Drug Effect Questionnaire with a significant main effect of Methamphetamine only.
| Methamphetamine Dose (mg) | ||||
|---|---|---|---|---|
| PL | 10 | 30 | F-Value | |
| Active/Alert/Energetic | 5.9 (9.1) | 17.1 (18.9) | 17.6 (13.4) | 5.9 |
| Any Effect | 6.5 (7.8) | 18.9 (17.1) | 23.9 (19.7) | 10.0 |
| Good Effects | 6.9 (7.8) | 19.1 (16.3) | 23.4 (16.0) | 7.4 |
| High | 4.9 (7.9) | 19.3 (18.5) | 23.9 (19.4) | 9.3 |
| Irregular/Racing Heartbeat | 4.0 (6.7) | 11.8 (22.8) | 13.1 (11.3) | 4.5 |
| Like Drug | 4.3 (4.4) | 16.9 (16.1) | 23.0 (17.5) | 7.5 |
| Nauseated | 2.5 (2.7) | 13.0 (14.2) | 11.5 (12.0) | 4.0 |
| Pay For | 3.8 (4.5) | 15.1 (18.6) | 19.5 (20.1) | 4.0 |
| Rush | 4.8 (8.4) | 18.0 (18.4) | 19.9 (17.1) | 7.8 |
| Stimulated | 6.8 (9.1) | 17.1 (18.1) | 22.4 (17.0) | 5.3 |
| Take Again | 5.0 (5.5) | 20.0 (20.4) | 29.8 (30.9) | 6.4 |
PL refers to placebo; For all items, df = 2,14; p < 0.05
AUC analysis differed from the peak effects analysis in the detection of a significant interaction of Methamphetamine, Naltrexone, and Alprazolam XR for the item Nauseated (F2,14 = 4.7, p < 0.05) and an interaction of Methamphetamine and Naltrexone for the items Nervous/Anxious (F2,14 = 4.1, p < 0.05) and Restless (F2,14 = 4.1, p < 0.05). The AUC analysis did not detect effects for the item Talkative/Friendly (p > 0.05).
DSST
Statistical analysis revealed no significant main effects or interactions of Methamphetamine and the maintenance drugs on Percent Trials Correct on the DSST.
Physiological Measures
A significant interaction of Methamphetamine and Alprazolam XR was detected for systolic (F2,14 = 5.2, p < 0.05) and diastolic blood pressure (F2,14 = 4.1, p < 0.05). A main effect of Methamphetamine (F2,14 = 5.8, p < 0.05) and Naltrexone (F2,14 = 12.0, p < 0.05) was detected for heart rate. Post hoc comparisons revealed that 30 mg methamphetamine alone elevated systolic blood pressure and heart rate relative to placebo (Figure 3), whereas active doses of methamphetamine alone did not increase diastolic blood pressure. Systolic blood pressure following 10 mg methamphetamine was significantly decreased during maintenance on alprazolam XR relative to maintenance on naltrexone or the combination of naltrexone and alprazolam XR. Diastolic blood pressure following 10 and 30 mg methamphetamine was significantly increased during maintenance on naltrexone. Heart rate following 30 mg methamphetamine was significantly decreased during maintenance on naltrexone relative to maintenance on the combination of naltrexone and alprazolam XR.
AUC analysis detected a main effect of Methamphetamine for diastolic (F2,14 = 7.4, p < 0.05) and systolic (F2,14 = 26.4, p < 0.05) blood pressure and an interaction of Methamphetamine, Naltrexone, and Alprazolam XR for heart rate (F2,14 = 4.1, p < 0.05). There were no main effects or interactions of methamphetamine and maintenance condition on oral temperature (data not shown).
DISCUSSION
Our previous study demonstrated that the combination of acutely administered naltrexone and alprazolam was well tolerated and reduced some of the subject-rated drug effects of d–amphetamine.11 The goal of the present experiment was to extend those findings using a laboratory procedure that more closely resembles clinical use of addiction pharmacotherapies by testing the hypothesis that maintenance on the combination of naltrexone and alprazolam XR would produce a greater reduction in the reinforcing and abuse-related effects of methamphetamine relative to the constituent compounds, and without significant side effects. Intranasal methamphetamine functioned as a reinforcer under a modified progressive-ratio procedure and produced prototypical stimulant-like “positive” subject-rated drug effects. The combination of naltrexone and alprazolam XR did not decrease the reinforcing, subject-rated, or cardiovascular effects of methamphetamine. Alprazolam XR did not impact methamphetamine self-administration, attenuate the subject-rated effects of methamphetamine or produce clinically significant cardiovascular effects. Overall, the most promising finding from the present study was that maintenance on naltrexone significantly decreased the reinforcing effects of 10 mg methamphetamine, increased some “negative” subject-rated drug effects of 30 mg methamphetamine, and attenuated methamphetamine-induced elevations in heart rate.
Naltrexone alone did not produce any physiological or subject-rated drug effects, which is consistent with some previous laboratory studies, although others have reported mild fatigue or gastrointestinal disturbance.11,28,29,32,63 Maintenance on 50 mg naltrexone significantly decreased the number of drug choices following 10 mg methamphetamine by an average of 49% (3.1 doses) relative to methamphetamine alone. Naltrexone did not significantly decrease methamphetamine self-administration in a previous human laboratory study that used similar methods.33 However, the present results are in agreement with larger clinical trials that demonstrated a reduction in amphetamine-positive urine samples during naltrexone treatment relative to placebo.64,65 Naltrexone did not alter self-administration of 30 mg methamphetamine, however, indicating that the ability of naltrexone to attenuate the reinforcing effects of methamphetamine is surmountable by higher doses. Together, these results are consistent with the notion that naltrexone reduces the reinforcing effects of methamphetamine, and supports the identification of a drug that could be administered in combination with naltrexone to enhance its therapeutic efficacy.
Methamphetamine produced both “positive” and “negative” subject-rated effects that varied as a function of dose. In contrast to the attenuation in the reinforcing effects of 10 mg methamphetamine, naltrexone maintenance did not significantly influence the “positive” ratings of methamphetamine. Self-administration and subject rating outcomes are not isomorphic, however, and the magnitude of their correlation appears weak.66 Naltrexone maintenance did enhance some of the “negative” (i.e., Performance Impaired and Sedative effects) subject-rated effects of 30 mg methamphetamine. These results are partially consistent with other human laboratory studies have demonstrated a modest decrease in “positive” subject-rated drug effects and an increase in “negative” subject-rated effects during naltrexone treatment relative to placebo. 11,28,29,32,67 For example, during maintenance on 50 mg naltrexone, 30 mg intravenous methamphetamine increased ratings of “Bad Drug Effects” relative to placebo maintenance.32
Maintenance on 1 mg alprazolam XR alone did not produce any performance or subject-rated effects, and also did not alter the effects of methamphetamine on these outcomes. In contrast, performance impairment and modest reductions in the subject-rated effects of methamphetamine during maintenance on alprazolam XR were observed in a previous study.31 Given that both Lile et al.31 and the present study enrolled participants with a similar stimulant use history and tested the same range of doses of methamphetamine and alprazolam XR, the reason for the discrepancy in results is uncertain. In the present study, participants were maintained on alprazolam XR alone and the combination of alprazolam XR and naltrexone for approximately 12 days total whereas in the prior study, participants were only maintained on alprazolam XR for approximately 6 days. As such, participants in the present study may have developed a tolerance to the effects of benzodiazepines (e.g., sedation and performance impairment).
In our previous study11 the acutely administered combination of naltrexone and alprazolam decreased the subject-rated effects of d-amphetamine on more questionnaire items than the constituent compounds, suggesting that maintaining individuals on the combination of these drugs would be effective at reducing the reinforcing and subject-rated effects of methamphetamine. In the present study, however, maintenance on the combination of naltrexone and alprazolam XR failed to significantly reduce the number of drug choices or subject-rated effects of methamphetamine (see discussion below). Moreover, naltrexone alone suppressed methamphetamine self-administration though not when combined with alprazolam XR. Although alprazolam XR alone did not significantly affect methamphetamine self-administration, visual inspection of the data suggests that alprazolam XR might have counteracted the naltrexone-attenuated methamphetamine response. Previous studies have demonstrated the abuse potential of alprazolam XR,36,37 which might have contributed to the reinforcing effects of methamphetamine, thereby offsetting any reduction by naltrexone. Future studies should determine whether other GABAA receptor positive modulators with less abuse potential (e.g., oxazepam)68,69 might reduce the reinforcing and subject-rated effects of methamphetamine.
The inconsistent findings between the present results and our prior study11 examining the separate and combined effects of acute naltrexone and alprazolam on d-amphetamine might also be attributed to differences in experimental designs. First, the present study utilized maintenance dosing instead of acute pretreatment, and maintenance-dosing regimens impact the efficacy of a potential therapeutic.30,42 Second, although methamphetamine and d-amphetamine produce a similar constellation of effects,70 there are neuropharmacological differences between these congeners (e.g., norepinephrine and serotonin release)71, which might explain the differential impact of naltrexone and alprazolam XR treatment between the two studies. Finally, the present study administered intranasal methamphetamine whereas the prior study administered d-amphetamine orally. At equivalent doses, intranasal drug administration more rapidly penetrates the central nervous system thereby decreasing the time to peak drug effects as compared to oral administration, and drug effects that onset rapidly might be more difficult to attenuate.72,73
In summary, maintenance on naltrexone and alprazolam XR, alone and in combination, did not produce clinically problematic physiological effects or “negative” subject-rated drug effects. The combination of naltrexone and alprazolam XR did not, however, decrease the abuse-related effects of methamphetamine. Interestingly, maintenance on naltrexone significantly decreased self-administration of the 10 mg methamphetamine dose, consistent with previous clinical trial results. Future studies should continue to explore the treatment efficacy of naltrexone for methamphetamine use disorders, with particular attention to the identification of other drugs with less abuse potential (e.g., oxazepam) that might enhance its ability to reduce drug-taking behavior and alternate delivery systems (e.g., depot naltrexone) that would enhance patient compliance.
Acknowledgments
Source of Funding: This research and the preparation of the manuscript were supported by NIDA Grants R01 DA025591 (CRR), T32 DA035200 (CRR, KRM), K02 DA031766 (JAL), CTSA Grants UL1 TR000117 (Philip A. Kern) and UL1 TR000115 (KRM). The content is solely the responsibility of the authors and dose not necessarily represent the official views of the National Institutes of Health. This study has been carried out in accordance with the Declaration of Helsinki.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest relevant to this research.
REFERENCES
- 1.Center for Behavioral Health Statistics and Quality. 2014 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015. [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Treatment Episode Data Set (TEDS): 2002–2012. National Admissions to Substance Abuse Treatment Services. BHSIS Series S-71, HHS Publication No. (SMA) 14-4850. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- 3.Nicosia N, Pacula RL, Kilmer B, et al. The economic cost of methamphetamine use in the United States, 2005. Santa Monica, CA, USA: Rand Corporation; 2009. [Google Scholar]
- 4.Elkashef A, Vocci F, Hanson G, et al. Pharmacotherapy of methamphetamine addiction: an update. Subst Abuse. 2008;29:31–49. doi: 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karila L, Weinstein A, Aubin HJ, et al. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rush CR, Vansickel AR, Lile JA, et al. Cohen L, Collins FL, Young AM, McChargue DE, Leffingwell TR, Cook KL, editors. Evidence-based treatment of amphetamine dependence: Pharmacological and behavioral approaches. Pharmacology and Treatment of Substance Abuse: Evidence and Outcomes Based Perspectives: Routledge, Taylor and Francis Group. 2009:335–358. [Google Scholar]
- 7.Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102:96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- 8.Stoops WW, Rush CR. Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research. Expert Rev Clin Pharmacol. 2014;7:363–374. doi: 10.1586/17512433.2014.909283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goeders NE, Guerin GF. Effects of the combination of metyrapone and oxazepam on cocaine and food self-administration in rats. Pharmacol Biochem Behav. 2008;91:181–189. doi: 10.1016/j.pbb.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kablinger AS, Lindner MA, Casso S, et al. Effects of the combination of metyrapone and oxazepam on cocaine craving and cocaine taking: a double-blind, randomized, placebo controlled pilot study. J Psychopharmacol. 2012;26:973–981. doi: 10.1177/0269881111430745. [DOI] [PubMed] [Google Scholar]
- 11.Marks KR, Lile JA, Stoops WW, et al. Separate and combined impact of acute naltrexone and alprazolam on subjective and physiological effects of oral d-amphetamine in stimulant users. Psychopharmacology. 2014;231:2741–2750. doi: 10.1007/s00213-014-3449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Ambrose LM, Unterwald EM, Van Bockstaele EJ. Ultrastructural evidence for co-localization of dopamine D2 and mu-opioid receptors in the rat dorsolateral striatum. Anat Rec. 2004;279:583–591. doi: 10.1002/ar.a.20054. [DOI] [PubMed] [Google Scholar]
- 14.Hooks MS, Jones DN, Justice JB, Jr, et al. Naloxone reduces amphetamine-induced stimulation of locomotor activity and in vivo dopamine release in the striatum and nucleus accumbens. Pharmacol Biochem Behav. 1992;42:765–770. doi: 10.1016/0091-3057(92)90027-d. [DOI] [PubMed] [Google Scholar]
- 15.Pollard H, Llorens C, Bonnet JJ, et al. Opiate receptors on mesolimbic dopaminergic neurons. Neurosci Lett. 1977;7:295–299. doi: 10.1016/0304-3940(78)90216-1. [DOI] [PubMed] [Google Scholar]
- 16.Schad CA, Justice JB, Jr, Holtzman SG. Naloxone reduces the neurochemical and behavioral effects of amphetamine but not those of cocaine. Eur J Pharmacol. 1995;275:9–16. doi: 10.1016/0014-2999(94)00726-n. [DOI] [PubMed] [Google Scholar]
- 17.Churchill L, Dilts RP, Kalivas PW. Autoradiographic localization of gamma-aminobutyric acidA receptors within the ventral tegmental area. Neurochem Res. 1992;17:101–106. doi: 10.1007/BF00966870. [DOI] [PubMed] [Google Scholar]
- 18.Dewey SL, Chaurasia CS, Chen CE, et al. GABAergic attenuation of cocaine-induced dopamine release and locomotor activity. Synapse. 1997;25:393–398. doi: 10.1002/(SICI)1098-2396(199704)25:4<393::AID-SYN11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 19.Dettmar PW, Cowan A, Walter DS. Naloxone antagonizes behavioural effects of d-amphetamine in mice and rats. Neuropharmacology. 1978;17:1041–1044. doi: 10.1016/0028-3908(78)90031-x. [DOI] [PubMed] [Google Scholar]
- 20.Häggkvist J, Lindholm S, Franck J. The effect of naltrexone on amphetamine-induced conditioned place preference and locomotor behaviour in the rat. Addict Bio. 2009;14:260–269. doi: 10.1111/j.1369-1600.2009.00150.x. [DOI] [PubMed] [Google Scholar]
- 21.Holtzman SG. Behavioral effects of separate and combined administration of naloxone and d-amphetamine. J Pharmacol Exp Ther. 1974;189:51–60. [PubMed] [Google Scholar]
- 22.Trujillo KA, Belluzzi JD, Stein L. Naloxone blockade of amphetamine place preference conditioning. Psychopharmacology. 1991;104:265–274. doi: 10.1007/BF02244190. [DOI] [PubMed] [Google Scholar]
- 23.Goeders NE, McNulty MA, Mirkis S, et al. Chlordiazepoxide alters intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1989;33:859–66. doi: 10.1016/0091-3057(89)90483-8. [DOI] [PubMed] [Google Scholar]
- 24.Goeders NE, McNulty MA, Guerin GF. Effects of alprazolam on intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1993;44:471–474. doi: 10.1016/0091-3057(93)90493-d. [DOI] [PubMed] [Google Scholar]
- 25.Barrett AC, Negus SS, Mello NK, et al. Effect of GABA agonists and GABA-A receptor modulators on cocaine- and food-maintained responding and cocaine discrimination in rats. J Pharmacol Exp Ther. 2005;315:858–871. doi: 10.1124/jpet.105.086033. [DOI] [PubMed] [Google Scholar]
- 26.Jimenez-Gomez C, Winger G, Dean RL, et al. Naltrexone decreases D-amphetamine and ethanol self-administration in rhesus monkeys. Behav Pharmacol. 2011;22:87–90. doi: 10.1097/FBP.0b013e3283423d55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weerts EM, Froestl W, Griffiths RR. Effects of GABAergic modulators on food and cocaine self-administration in baboons. Drug Alcohol Depend. 2005;80:369–376. doi: 10.1016/j.drugalcdep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Jayaram-Lindström N, Wennberg P, Hurd YL, et al. Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. J Clin Psychopharmacol. 2004;24:665–669. doi: 10.1097/01.jcp.0000144893.29987.e5. [DOI] [PubMed] [Google Scholar]
- 29.Jayaram-Lindström N, Konstenius M, Eksborg S, et al. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2008;33:1856–1863. doi: 10.1038/sj.npp.1301572. [DOI] [PubMed] [Google Scholar]
- 30.Rush CR, Stoops WW, Wagner FP, et al. Alprazolam attenuates the behavioral effects of d-amphetamine in humans. J Clin Psychopharmacol. 2004;24:410–420. doi: 10.1097/01.jcp.0000130553.55630.ad. [DOI] [PubMed] [Google Scholar]
- 31.Lile JA, Stoops WW, Glaser PEA, et al. Physiological and subjective effects of acute intranasal methamphetamine during extended-release alprazolam maintenance. Drug Alcohol Depend. 2011;119:187–193. doi: 10.1016/j.drugalcdep.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ray LA, Bujarski S, Courtney KE, et al. The effects of naltrexone on subjective response to methamphetamine in a clinical sample: a double-blind, placebo-controlled laboratory study. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.83. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoops WW, Pike E, Hays LR, et al. Naltrexone and bupropion, alone or combined, do not alter the reinforcing effects of intranasal methamphetamine. Pharmacol Biochem Behav. 2015;129:45–50. doi: 10.1016/j.pbb.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garbutt JC. Efficacy and tolerability of naltrexone in the management of alcohol dependence. Curr Pharm Des. 2010;16:2091–2097. doi: 10.2174/138161210791516459. [DOI] [PubMed] [Google Scholar]
- 35.Mumford GK, Evans SM, Fleishaker JC, et al. Alprazolam absorption kinetics affects abuse liability. Clin Pharmacol Ther. 1995;57:356–365. doi: 10.1016/0009-9236(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 36.Mumford GK, Rush CR, Griffiths RR. Abecarnil and alprazolam in humans: behavioral, subjective and reinforcing effects. J Pharmacol Exp Ther. 1995;272:570–580. [PubMed] [Google Scholar]
- 37.Rush CR, Higgins ST, Bickel WK, et al. Abuse liability of alprazolam relative to other commonly used benzodiazepines: a review. Neurosci Biobehav Rev. 1993;17:277–285. doi: 10.1016/s0149-7634(05)80011-9. [DOI] [PubMed] [Google Scholar]
- 38.Goeders NE, Clampitt DM, Keller C, et al. Alprazolam and oxazepam block the cue-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology. 2009;201:581–588. doi: 10.1007/s00213-008-1326-1. [DOI] [PubMed] [Google Scholar]
- 39.Lile JA, Charnigo RJ, Nader MA. The relative reinforcing strength of methamphetamine and D-amphetamine in monkeys self-administering cocaine. Behav Pharmacol. 2013;24:482–485. doi: 10.1097/FBP.0b013e3283644d44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]; 2013. ICPSR35509-v1. 2014-11-18. http://doi.org/10.3886/ICPSR35509.v1. [Google Scholar]
- 41.Comer SD, Ashworth JB, Foltin FW, et al. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol. 1997;8:677–690. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Comer SD, Collins ED, Wilson ST, et al. Effects of an alternative reinforcer on intravenous heroin self-administration by humans. Eur J Pharmacol. 1998;345:13–26. doi: 10.1016/s0014-2999(97)01572-0. [DOI] [PubMed] [Google Scholar]
- 45.Rush CR, Essman WD, Simpson CA, et al. Reinforcing and subject-rated effects of methylphenidate and d-amphetamine in non-drug-abusing humans. J Clin Psychopharmacology. 2001;21:273–286. doi: 10.1097/00004714-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Sevak RJ, Stoops WW, Glaser PEA. Reinforcing effects of d-amphetamine: influence of novel ratios on a progressive-ratio schedule. Behav Pharmacol. 2010;21:745–753. doi: 10.1097/FBP.0b013e32833fa7b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer MC, Straughn AB, Lo MW, et al. Bioequivalence, dose-proportionality, and pharmacokinetics of naltrexone after oral administration. J Clin Psychiatry. 1984;45:15–19. [PubMed] [Google Scholar]
- 48.Glue P, Fang A, Gandelman K, et al. Pharmacokinetics of an extended release formulation of alprazolam (Xanax XR) in healthy normal adolescent and adult volunteers. Am J Ther. 2006;13:418–422. doi: 10.1097/01.mjt.0000182358.63457.48. [DOI] [PubMed] [Google Scholar]
- 49.McLeod DR, Griffiths RR, Bigelow GE, et al. An automated version of the digit symbol substitution test (DSST) Behav Res Meth Instrum. 1982;14:463–466. [Google Scholar]
- 50.Stoops WW, Glaser PEA, Fillmore MT, et al. Reinforcing, subject-rated, performance and physiological effects of methylphenidate and d-amphetamine in stimulant abusing humans. J Psychopharmacol. 2004;18:534–543. doi: 10.1177/0269881104047281. [DOI] [PubMed] [Google Scholar]
- 51.Stoops WW, Lile JA, Fillmore MT, et al. Reinforcing effects of methylphenidate: influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005;177:349–355. doi: 10.1007/s00213-004-1946-z. [DOI] [PubMed] [Google Scholar]
- 52.Stoops WW, Lile JA, Fillmore MT, et al. Reinforcing effects of modafinil: Influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005;182:186–193. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- 53.Rush CR, Stoops WW, Hays LR, et al. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- 54.Oliveto AH, Bickel WK, Hughes JR, et al. Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–894. [PubMed] [Google Scholar]
- 55.Lingjaerde O, Ahlfors UG, Bech P, et al. The UKU side effect rating scale. A new comprehensive rating scale for psychotrophic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 56.Ehrman RN, Robbins SJ, Bromwell MA, et al. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug Alcohol Depend. 2002;67:185–191. doi: 10.1016/s0376-8716(02)00065-0. [DOI] [PubMed] [Google Scholar]
- 57.Fillmore MT, Rush CR, Marczinski CA. Effects of d-amphetamine on behavioral control in stimulant abusers: the role of prepotent response tendencies. Drug Alcohol Depend. 2003;71:143–152. doi: 10.1016/s0376-8716(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 58.Liu S, Lane SD, Schmitz JM, et al. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Amer J Drug Alc Abuse. 2011;37:117–22. doi: 10.3109/00952990.2010.543204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conway ARA, Kane MJ, Bunting MF, et al. Working memory span tasks: a methodological review and user’s guide. Psychon Bull & Rev. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- 60.Lejuez CW, Read JP, Kahler CW, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Taking (BART) J Exp Psychol App. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 61.Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363–71. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 62.Selzer ML. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. Am J Psychiat. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 63.Hollister LE, Johnson K, Boukhabza D, et al. Aversive effects of naltrexone in subjects not dependent on opiates. Drug Alcohol Depend. 1981;8:37–41. doi: 10.1016/0376-8716(81)90084-3. [DOI] [PubMed] [Google Scholar]
- 64.Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165:1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- 65.Tiihonen J, Krupitsky E, Verbitskaya E, et al. Naltrexone implant for the treatment of polydrug dependence: a randomized controlled trial. Am J Psychiatry. 2012;169:531–536. doi: 10.1176/appi.ajp.2011.11071121. [DOI] [PubMed] [Google Scholar]
- 66.Bolin BL, Reynolds AR, Stoops WW, et al. Relationship between oral d-amphetamine self-administration and ratings of subjective effects: Do subjective-effect ratings correspond with a progressive-ratio measure of drug-taking behavior? Behav Pharmacol. 2013;24:533–542. doi: 10.1097/FBP.0b013e3283645047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Comer SD, Mogali S, Saccone PA, et al. Effects of acute oral naltrexone on the subjective and physiological effects of oral D-amphetamine and smoked cocaine in cocaine abusers. Neuropsychopharmacology. 2013;38:2427–2438. doi: 10.1038/npp.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Griffiths RR, McLeod DR, Bigelow GE, et al. Relative abuse liability of diazepam and oxazepam: behavioral and subjective dose effects. Psychopharmacology. 1984;84:147–154. doi: 10.1007/BF00427437. [DOI] [PubMed] [Google Scholar]
- 69.Griffiths RR, McLeod DR, Bigelow GE, et al. Comparison of diazepam and oxazepam: preference, liking and extent of abuse. J Pharmaco Exp Ther. 1984;229:501–508. [PubMed] [Google Scholar]
- 70.Sevak RJ, Stoops WW, Hays LR. Discriminative stimulus and subject-rated effects of methamphetamine, d-amphetamine, methylphenidate, and triazolam in methamphetamine-trained humans. J Pharmacol Exp Ther. 2009;328:1007–1018. doi: 10.1124/jpet.108.147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rothman RB, Baumann MH, Dersch CM. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 72.Lile JA, Babalonis S, Emurian C, et al. Comparison of the behavioral and cardiovascular effects of intranasal and oral d-amphetamine in healthy human subjects. J Clin Psychopharmacol. 2011;51:888–898. doi: 10.1177/0091270010375956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Volkow ND, Wang GJ, Fischman MW, et al. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000;67:1507–1515. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]