Abstract
The brain comprises an excitatory/inhibitory neuronal network that maintains a finely tuned balance of activity critical for normal functioning. Excitatory activity in the basolateral amygdala (BLA), a brain region that plays a central role in emotion and motivational processing, is tightly regulated by a relatively small population of gamma-aminobutyric acid (GABA) inhibitory neurons. Disruption in GABAergic inhibition in the BLA can occur when there is a loss of local GABAergic interneurons, alterations in GABAA receptor activation, or dysregulation of mechanisms that modulate BLA GABAergic inhibition. Disruptions in GABAergic control of the BLA emerge during development, in aging populations, or after a trauma, ultimately resulting in hyperexcitability. BLA hyperexcitability manifests behaviorally as an increase in anxiety, emotional dysregulation, or the development of seizure activity. This article reviews the anatomy, development, and physiology of the GABAergic system in the BLA, and circuits that modulate GABAergic inhibition, including the dopaminergic, serotonergic, noradrenergic, and cholinergic systems. We highlight how alterations in various neurotransmitter receptors, including the acid sensing ion channel 1a (ASIC1a), cannabinoid receptor 1 (CB1), and glutamate receptor subtypes, expressed on BLA interneurons, modulate GABAergic transmission and how defects of these systems affects inhibitory tonus within the BLA. Finally, we discuss alterations in the BLA GABAergic system in neurodevelopmental (autism/Fragile X syndrome) and neurodegenerative (Alzheimer’s disease) diseases, and after the development of epilepsy, anxiety, and traumatic brain injury. A more complete understanding of the intrinsic excitatory/inhibitory circuit balance of the amygdala and how imbalances in inhibitory control contribute to excessive BLA excitability will guide the development of novel therapeutic approaches in neuropsychiatric diseases.
Keywords: Basolateral Amygdala, GABA, Autism, Alzheimer’s disease, Anxiety, Epilepsy
Introduction
The brain is made up of a highly complex network of excitatory and inhibitory circuits that maintains exquisite balance in network activity. Hyperexcitability arises when there is an imbalance between excitation and inhibition (E/I) often as a result of deficiencies or disruption in gamma-aminobutyric acid (GABA) inhibitory system control. Hyperexcitability of the amygdala, in particular, can be strongly associated with anxiety, hypervigilance, and an inability to regulate emotions. Acquired deficiencies in the GABAergic inhibitory system have been observed after traumatic brain injury (TBI; Almeida-Suhett et al. 2014; Depue et al. 2014; Guerriero et al. 2015; Reger et al. 2012) and status epilepticus (SE; Fritsch et al. 2009; Gean et al. 1989; Prager et al. 2014b). In addition, amygdala hyperexcitability resulting in anxiety has been observed in neuropsychiatric disorders, such as posttraumatic stress disorder (PTSD; (Nuss 2015; Truitt et al. 2009), as well as in neurodevelopmental disorders, including autism/Fragile X syndrome (El-Ansary and Al-Ayadhi 2014; Martin et al. 2014; Olmos-Serrano et al. 2010), and neurodegenerative disorders, such as Alzheimer’s disease (AD; Klein et al. 2014; Palop and Mucke 2010).
Hyperexcitability of the basolateral nucleus of the amygdala (BLA) is associated with increased anxiety and often occurs in parallel with various neurodevelopmental, neurodegenerative, and neuropsychiatric disorders. The GABAergic inhibitory system is one target of therapeutic treatments to reduce anxiety and maintain homeostasis. For example, benzodiazepines, which allosterically enhance postsynaptic actions of GABA at the inhibitory type A GABA receptor (GABAA receptor), are one first line treatment for anxiety (Farb and Ratner 2014) and seizure disorders. However, in many cases, benzodiazepines are ineffective and/or exacerbate symptoms, as has been observed in seizure models, for example, where administration of diazepam initially suppress seizures, but lead to rebound seizures that are similar to or longer in duration than that of animals that do not receive the anticonvulsant (Apland et al. 2014). Thus, the efficaciousness of current treatments targeting the GABAergic system has been called into question and new therapeutic targets merit preclinical investigation.
The purpose of this article is to review the BLA GABAergic system in health and disease. First, we review the anatomy and development of the GABAergic system in the BLA and the different ways that GABAergic inhibitory synaptic transmission is modulated. Second, we review how local GABAergic inhibitory neurotransmission in the BLA is altered in disease. This review focuses on five diseases: Autism/Fragile X, AD, epilepsy, TBI, and anxiety and trauma or stressor related disorders (such as PTSD), as these disorders are prime examples of acquired amygdala dysfunction that occur during development, aging, or after injury. By studying how deficiencies in the GABAergic inhibitory system in the amygdala contribute to disease outcomes, future research may be directed at developing new therapies to reduce excitability or increase inhibition.
The GABAergic System in the Basolateral Amygdala
GABAergic Interneurons
The amygdala is located in the medial temporal lobe and is comprised of 13 subnuclei (for a comprehensive review of the anatomical connections of the rat and human amygdala, see Pitkanen 2000; Sah et al. 2003; Whalen and Phelps 2009). The BLA makes up a large component of this network, receiving input from cortical and subcortical structures. The BLA, which is generally comprised of the lateral and basal portions, contains two main types of neurons: glutamatergic (pyramidal) principal neurons and GABAergic interneurons (McDonald 1992; Pare and Smith 1998). Principal neurons constitute the majority of the neurons in the BLA (80–85%), whereas GABAergic interneurons form approximately 15–20% of the neuronal population (Sah et al. 2003; Spampanato et al. 2011). GABAergic interneurons can be subdivided into those that express calbindin (CB) or calretinin (CR) and can be further subdivided into groups by neuropeptide expression (i.e., vasoactive intestinal peptide; VIP and/or cholecystokinin; CCK) or by the expression of the calcium binding protein parvalbumin (PV; Table 1) (Davila et al. 2008; Kemppainen and Pitkanen 2000; Mascagni and McDonald 2003; McDonald and Mascagni 2001a; McDonald and Mascagni 2002). Importantly, PV-immunopositive neurons make up about 40% of GABAergic interneurons and are the main source of the perisomatic innervation of principal cells, suggesting that their primary role is in feedback inhibition. CR interneurons make up about 25–30% of BLA GABAergic interneurons and primarily innervate other interneurons (Capogna 2014; McDonald and Mascagni 2001a; Muller et al. 2003; Muller et al. 2006).
Table 1.
Summary of Systems Modulating BLA GABAergic Inhibition. Note that no study has differentiated receptor localization to VIP +/−. Therefore, we have placed the receptor modulating VIP +/− in each category. For citations, see text.
| Immunohistochemistry | Firing Patterns | Innervation of GABAergic Interneurons | Receptor Subtypes and role in Modulating GABAergic Transmission |
|
|---|---|---|---|---|
| General | Specific | |||
| CB | PV | Fast spiking, stuttering, non- adapting, adapting | ~50% cortical, <1% thalamic to CB interneurons, VTA, SN, dorsal raphé nucleus, substantia innominate, ventral pallidum of basal forebrain (cholinergic and GABAergic) | D1 - ↑ firing, induce rhythmic oscillations D2 - ↓ presynaptic GABA release 5-HT2A - ↑ excitability GABAB - ↓ excitability |
| CCK (VIP-) | Non-adapting, burst adapting | 5-HT3A - ↑ excitability; but rapidly desensitizing α1-AR, α2-AR - ↑ AP firing and IPSCs CB1 - ↓ excitability GABAB - ↓ excitability |
||
| SOM/NPY/NK1r | dorsal raphé nucleus | 5-HT1A (NPY, NK1r) - ↓ presynaptic GABA release 5-HT2C (NPY) - ↑ excitability α1-AR, α2-AR - ↑ AP firing and IPSCs GABAB - ↓ excitability |
||
| CR | CCK (VIP+) | Adapting | VTA and SN (< PV interneurons) | 5-HT3A - ↑ excitability; but rapidly desensitizing α1-AR, α2-AR - ↑ AP firing and IPSCs GABAB - ↓ excitability |
| Not localized to Specific GABAer gic Interneuronal Subpopulations | LC, NTS | M1-mAChR –↑ excitability M2-mAChR - ↓ excitability α7-, α4β2-nAChR ↑ excitability ASICs 1A - ↑ excitability AMPA lacking GluR2, NMDA - ↑ excitability GluK1 - ↑ presynaptic GABA release (dose dependent) |
||
GABAA Receptor Structure and Function
GABAergic inhibitory synaptic transmission plays a central role in the regulation of amygdala excitability. Pathological disruption of GABAA receptors causes a disruption of the E/I balance and has been increasingly implicated in neurological and neurodegenerative diseases (Deidda et al. 2014). Fast inhibitory synaptic transmission within the central nervous system is mediated by the GABAA receptor, a heteropentameric chloride permeable, GABA gated member of the cys-loop superfamily of ligand-gated ion channels. GABAA receptors are formed from limited combinations of subunits that have diverse structural and functional properties (α1–6, β1–3, γ1–3, δ, ε, θ, and π; Olsen and Sieghart 2009).
Proper maturation of the GABAergic system in the BLA is essential in neurodevelopment. Dysfunction in the development of the GABAergic inhibitory system within the BLA may be associated with neurodevelopmental diseases, such as autism or Fragile X. In rat, the development of the mature GABAergic system in the BLA takes place between postnatal (P) day P14 and P30 with the emergence of PV-interneurons (Berdel and Morys 2000; Davila et al. 2008), an increase in the density of GABAergic fibers, and a decrease in the density of GABAergic cell bodies (Brummelte et al. 2007). Concurrently, GABAA receptor mediated inhibitory postsynaptic currents (IPSCs) reach maturity between P21 and P28. Simultaneously, the reversal potential of GABAA receptors expressed in principal neurons shifts from −55 mV at P7 to −70 mV by P21. This increase in hyperpolarization may be due, in part, to a switch from a greater expression of sodium-potassium-chloride cotransporter 1, which accumulates intracellular chloride and renders GABAA receptors excitatory, to an increase in the potassium-chloride cotransporter 2, which extrudes chloride from the cell, rendering GABAA receptors inhibitory (Ben-Ari et al. 2012; Ehrlich et al. 2013). In addition, a decrease in rise time and decay time constant occurs due to a change in the GABAA receptor subunit composition (from primarily α2- to the α1-subunit; Ehrlich et al. 2013). This shift results in a GABAergic shunt that limits the extent of BLA activation (see below; Rainnie et al. 1991b).
The composition of GABAA receptors have been found to be quite diverse because of their subunit assembly making their roles significantly different depending on the timing of activation and subcellular localization (Marowsky et al. 2004; Pouille and Scanziani 2001). The BLA of mature animals contains α1- and α2-subunits of the GABAA receptor; α1-subunit containing GABAA receptors are primarily expressed at the somal level of PV-GABAergic interneurons, but also exhibit co-immunoreactivity with the β2/3 subunits (McDonald and Mascagni 2004). Alternatively, GABAA receptors on principal neurons primarily contain the α2-subunit, which is predominately responsible for the benzodiazepine allosteric potentiation of inhibitory currents (Marowsky et al. 2004). In addition, principal neurons in the BLA contain γ2 subunits, which likely contribute to the formation of α2βxγ2 pentameric GABAA receptors that contribute to fast inhibitory synaptic transmission (Esmaeili et al. 2009). Extrasynaptically, the GABAA receptor in the BLA is primarily made up of the α3 subunit, which strongly mediates tonic GABAergic currents (Marowsky et al. 2012). However, the α5 subunit, which is diazepam-sensitive and shapes the decay phase of the inhibitory postsynaptic currents (Marowsky et al. 2004) as well as the δ subunit, both of which are hallmark subunits that contribute to tonic inhibition (Farrant and Nusser 2005) are also expressed in the BLA, though not as strongly as the α3 (Marowsky et al. 2012).
Temporal dynamics and intra-amygdala regulation of excitatory activity
GABAergic interneurons can be differentiated by their firing properties. PV interneurons primarily fire short duration, non-adapting action potentials (Rainnie et al. 2006; Woodruff and Sah 2007b), whereas CB-expressing GABAergic interneurons fire broad action potentials, display firing adaptation, and primarily synapse with somata (see Table 1; Jasnow et al. 2009). Other interneurons expressing somatostatin (SOM), VIP, CR, and CCK also target dendrites or somata (Mascagni and McDonald 2003; Muller et al. 2007a). Though GABAergic interneurons only constitute a fraction of the total neuronal population, they tightly regulate network excitability, and lead to a low resting firing rate of principal neurons (Lang and Pare 1997; Pare and Gaudreau 1996; Woodruff and Sah 2007a).
The regulation of excitatory activity by local GABAergic interneurons is influenced by its firing properties (Lang and Pare 1997; Rainnie et al. 1991b). Most BLA GABAergic interneurons fire short-duration action potentials with little spike frequency adaptation in response to prolong depolarization, though specific subpopulations of GABAergic interneurons have different firing patterns (see Table 1). Principal neurons, by comparison, show spike frequency adaptation and prolonged after hyperpolarization in response to prolonged depolarizing currents (Pare et al. 1995; Rainnie et al. 1991a; Rainnie et al. 1991b; Sah et al. 2003). The axonal morphology of BLA GABAergic interneurons also allows for tight inhibitory control over principal neurons. GABAergic interneuron axons branch on average two to six times forming relatively dense terminal and collateral fields with principal neurons (Millhouse and DeOlmos 1983; Smith et al. 1998). BLA GABAergic projections participate in either feedback inhibition or transient dis-inhibition of principal neurons. Indeed, PV interneurons receiving strong excitatory local inputs from BLA projection neurons appear to be involved in feedback inhibition (Smith et al. 2000; Unal et al. 2014), whereas intercalated interneurons, which have recently been found to project to the BLA (Manko et al. 2011), appear to target PV- and CB-immunoreactive GABAergic interneurons and are likely to transiently disinhibit principal cells (Bienvenu et al. 2015).
The regulation of the firing rate by GABAergic interneurons controls the flow of information from the BLA, and evidence indicates that local inhibitory circuits in the amygdala mediate its functioning. Activation of the GABAergic system appears to play a central role in the synchronization of spiking activity, which can coordinate and enhance the effects of input signals, which precisely enables the activation of glutamatergic activity to drive behavioral responses (Courtin et al. 2014; Herry and Johansen 2014). Indeed, the initiation and expression of fear, for example, requires synchronization of amygdala activity, among other regions (Stujenske et al. 2014). Ongoing research has revealed that theta and the faster gamma oscillations may be fundamental to circuits underlying sensory processing and cognitive functions and that changes in emotional states may be mediated by alterations in BLA gamma coupling to theta frequency inputs (Fries 2009; Stujenske et al. 2014). Importantly, the activity of PV interneurons has been implicated in theta synchrony within the medial prefrontal cortex (PFC) (Courtin et al. 2014), as suppression of PV interneuronal activity in the PFC is necessary to disinhibit prefrontal projection neurons to the BLA, thereby synchronizing their firing by resetting local theta oscillations. While this work has not yet also been confirmed in the amygdala, it has been hypothesized that inhibiting PV interneurons in the BLA may also synchronize activity and enhance fear responses (Stujenske et al. 2014).
Modulation of GABAA Receptor Mediated Inhibitory Synaptic Transmission in the BLA
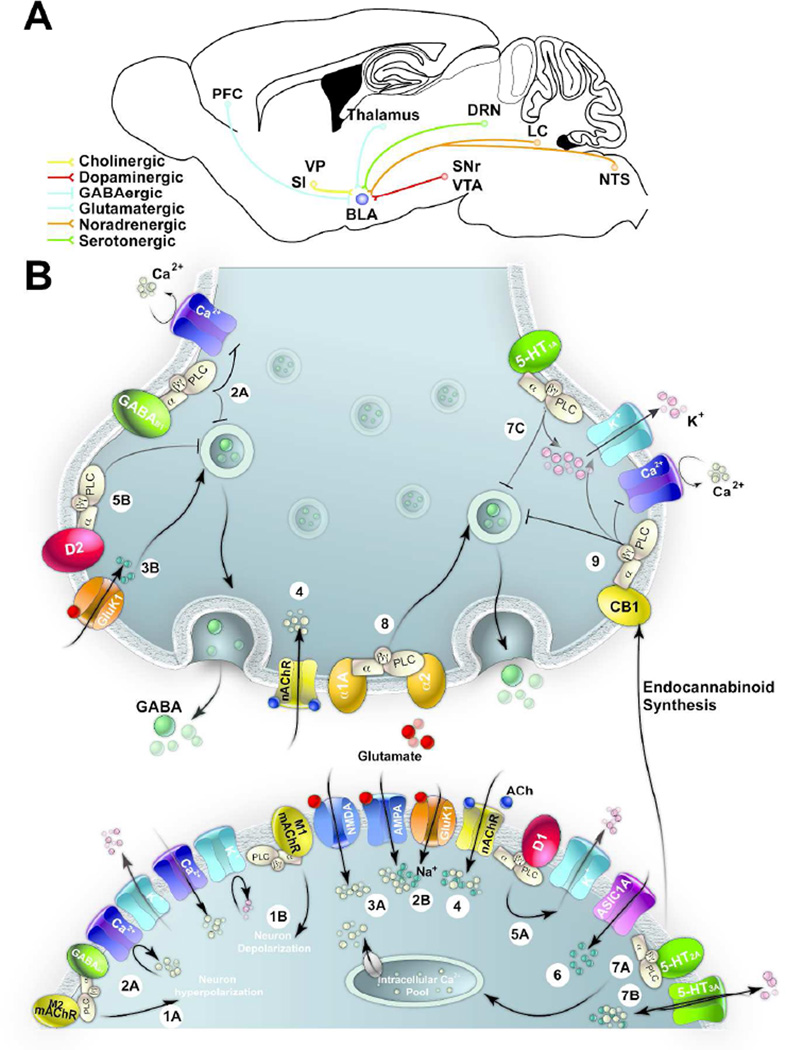
GABAergic inhibition in the BLA is modulated by afferents from both cortical and subcortical brain regions (Figure 1A). In most cases afferents from these regions project to both principal neurons and GABAergic interneurons. In some cases, it has been determined that projections are directed to particular subpopulations of neurons. This section reviews the afferent projections that modulate and facilitate GABAergic inhibitory synaptic transmission in the BLA. More specifically, we discuss how activation of different receptor types modulate the release of GABA from the presynaptic terminal or alter the excitation of GABAergic interneurons (Figure 1B).
Figure 1. Modulation of GABAergic Inhibitory Synaptic Transmission in the BLA.
(A) Schematic representation of GABAergic projections from the prefrontal cortex (PFC) and glutamatergic projections from the thalamus. In addition, GABAergic interneurons in the BLA receive cholinergic projections from the substantia innominate (SN) and ventral pallidum (VP), dopaminergic projections from the ventral tegmental area (VTN) and substantia nigra (SN), noradrenergic projections from the locus coeruleus (LC) and nucleus of the solitary tract (NTS), and serotonergic projections from the dorsal raphé nucleus (DRN). (B) Schematic representation of receptors modulating GABAergic inhibitory synaptic transmission in the BLA. Postsynaptic (1A) M2-mAChRs and (2A) GABAB receptors hyperpolarize GABAergic interneurons by reducing voltage gated Ca2+ channels and increasing K+ channel conductance. (1B) M1-mAChRs increase excitability (though primarily expressed on principal neurons) by suppressing several K+ currents and increasing voltage gated Ca2+ conductance. (2B) Activation of presynaptic GABAB receptors inhibit neurotransmitter release on both excitatory and inhibitory synapses by inhibiting voltage-gated Ca2+ channels and possibly by interacting with vesicular release machinery.(3A) Activation of postsynaptic NMDA or AMPA receptors on interneurons increases excitability. (3B) Activation of GluK1-containing kainate receptors depolarize interneurons by increasing the presynaptic release of GABA or increasing excitability via activation of postsynaptic GluK1 receptors. (4) Activation of α7-nAChRs and/or α4β2 nAChRs presynaptically modulate GABA release or regulate neuronal activity by their position on interneurons. (5A) Dopaminergic projections activate postsynaptic D1 receptors, which increase excitability by reducing slowly inactivating K+ currents while D2 (5B) receptors reduce presynaptic release of GABA. (6) Activation of ASIC1A receptors increase interneuronal excitability. Postsynaptically, activation of 5-HT2(7A) and 5-HT3A(7B) receptors increase interneuronal excitability via an increase in intracellular Ca2+ concentrations or increasing the interneuronal excitability, respectively. (7C) Activation of presynaptic 5-HT1A receptors reduce quantal release and increases hyperpolarization. (8) Activation of α1 and α2 receptors depolarize interneurons, subsequently increasing action potential firing and enhancing inhibitory synaptic transmission. (9) Activation of CB1 receptors on CCK interneurons reduces presynaptic release by inhibiting voltage gated Ca2+ channels and activating voltage gated K+ channel.
Cortical and thalamic regulation of BLA GABAergic interneurons
The BLA receives extensive cortical and thalamic projections, which synapse onto both principal neurons and GABAergic interneurons. Stimulating afferents from either the cortical or thalamic pathways has been found to monosynaptically activate BLA and lateral amygdala (LA) GABAergic interneurons, primarily in a feedforward manner (Lang and Pare 1998; Rainnie et al. 1991b; Szinyei et al. 2000; Washburn and Moises 1992). Recent studies have identified to what type of interneuron cortical and thalamic inputs project. Unal and colleagues (Unal et al. 2014) found that BLA interneurons expressing CB receive about half of the cortical inputs to local-circuit cells of the BLA and constitute a major source of feedforward inhibition, while thalamic inputs form less than one percent of synapses on interneurons (Carlsen and Heimer 1988; LeDoux et al. 1991). Importantly, there appears to be a possible discrepancy in the regulation of GABAergic interneurons in the LA versus the BLA. Cortical inputs to the BLA primarily regulate CB-expressing interneurons, while GABAergic interneurons in the LA equally respond to both cortical and thalamic pathways (Szinyei et al. 2000; Unal et al. 2014).
Dopaminergic Afferents
The BLA receives dense dopaminergic innervation from the ventral tegmental area (VTA) and substantia nigra (SN; Asan 1997; Fallon and Ciofi 1992). VTA and SN projections synapse on BLA principal (projection) neurons and PV- and CR-immunopositive GABAergic interneurons (Brinley-Reed and McDonald 1999; Pinard et al. 2008). However, compared to CR-immunopositive interneurons, PV-interneurons appear to be the preferential target of dopaminergic synapses in the BLA (Pinard et al. 2008). By projecting to principal neurons and GABAergic interneurons, dopamine (DA) influences the activity of both excitatory and inhibitory cell types within the BLA (Kroner et al. 2005; Rosenkranz and Grace 1999). Via activation of D1 receptors, DA increases excitability and evoked firing of principal neurons by reducing slowly inactivating K+ currents while activation of D2 receptors increases input resistance. Moreover, D1 receptor activation increases evoked firing in fast-spiking BLA interneurons and the frequency of spontaneous IPSCs (sIPSCs) (Kroner et al. 2005). Activation of DA receptors has also been found to induce rhythmic inhibitory oscillations (Loretan et al. 2004; Ohshiro et al. 2011), though increases in excitatory transmission is required to precede GABAergic interneuronal burst firing (Ohshiro et al. 2011). Though DA fibers synapse onto both GABAergic interneurons and principal neurons, there appears to be a net increase in excitatory activity within the BLA in response to DA application. This increase in excitatory activity may be the result of: 1) reduced activation of GABAergic interneurons, which occurs when activation of D2 receptors on GABAergic interneurons causes a reduction in the probability of presynaptic quantal release (Seamans et al. 2001); 2) amygdala disinhibition and the subsequent increase in excitatory activity may occur when DA suppresses GABA release from PV-interneurons onto principal neurons but not interneurons (Chu et al. 2012); or 3) DA might also increase the excitatory drive onto disinhibitory interneurons, which would subsequently increase excitatory activity (Bissiere et al. 2003; Kemppainen and Pitkanen 2000).
Serotonergic Afferents
Serotonergic projections originating from the dorsal raphé nucleus (DRN) primarily innervate BLA principal neurons, PV interneurons and interneurons containing neuropeptide Y (NPY), a subgroup of CB and SOM interneurons (Ma et al. 1991; Muller et al. 2007b). Postsynaptically, serotonin (5-HT) neurotransmission leads to the activation of 5-HT receptors, which are grouped into seven families (5-HT1 to 5-HT7). 5-HT1A receptors, which are Gi/o- protein coupled, have been localized to principal neurons (Stein et al. 2000) and the presynaptic terminal of GABAergic interneurons within the BLA (Kishimoto et al. 2000). 5-HT1A receptor activation inhibits the discharge rate of principal neurons by inducing hyperpolarization and reduce GABA release from presynaptic terminals by activating potassium channels (Kishimoto et al. 2000; Stein et al. 2000). Alternatively, 5-HT2 receptors, and more specifically the 5-HT2A, 5-HT2C receptors, are Gq/11-coupled membrane receptors that increase intracellular Ca2+ levels and increase interneuronal excitability (Bonn et al. 2012; Jiang et al. 2009). The 5-HT3A receptor is a ligand-gated sodium, potassium, and calcium channel that also increases interneuronal excitability but leads to a rapidly desensitizing depolarization (Gharedaghi et al. 2014; Mascagni and McDonald 2007; Rainnie 1999).
While all 5-HT receptors have been documented in the amygdala, the majority (~65–70%) of GABA-immunoreactive neurons in the BLA exhibit 5-HT2A immunoreactivity; a smaller percentage of 5-HT2A receptors are present on principal neurons (Bombardi 2011). 5-HT2A and 5-HT3A receptors have been localized to specific interneuronal types in the BLA. 5-HT2A receptors are found primarily on PV interneurons within the BLA (Bombardi 2011; McDonald and Mascagni 2007), and tightly control glutamatergic output by perisomatic inhibition (Holmes 2008; Muller et al. 2005), whereas 5-HT3A receptors are primarily expressed on the CCK interneuronal subpopulation (Mascagni and McDonald 2007), which constitute only a small proportion of GABAergic interneurons in the BLA (Mascagni and McDonald 2003). In contrast, 5-HT1A, which is expressed in low to moderate concentrations in the BLA (Asan et al. 2013 737), co-express with approximately 50% of NPY interneurons (Bonn et al. 2013), and about a third of neurokinin-1 receptor (NK1r) interneurons (Hafizi et al. 2012), while approximately 30–40% of NPY interneurons express the excitatory 5-HT2C receptor subtype (Bonn et al. 2012; Bonn et al. 2013). Importantly, 5-HT1A and 5-HT3 receptors have been localized on GABAergic nerve terminals in the BLA (Koyama et al. 1999; Koyama et al. 2000), where activation of these receptors inhibits or facilitates mIPSC frequency without effects on miniature IPSC (mIPSC) amplitude, respectively (Koyama et al. 2002).
Noradrenergic afferents
The BLA receives extensive noradrenergic (NA; norepinephrine, NE) innervation from the locus coeruleus (LC) and nucleus of the solitary tract (NTS) (Pitkanen 2000; Williams et al. 2000), which synapse onto GABAergic interneurons (Li et al. 2002). NA released from LC terminals activate three distinct classes of adrenoreceptors (AR; α1-, α2-, and β-AR), which have multiple subtypes and appear to be a more potent modulator of GABAergic inhibitory synaptic transmission than DA (Miyajima et al. 2010). While no study has yet anatomically identified to what type of interneuron the receptor subunits are localized in the BLA, electrophysiological evidence indicates that α1- and α2-AR activation depolarize SOM- and CCK-positive interneurons, resulting in action potential firing and enhanced inhibitory synaptic transmission (Braga et al. 2004b; Buffalari and Grace 2007; Kaneko et al. 2008). In addition to direct enhancement of inhibitory activity by LC afferents, NA enhancement of inhibitory activity in the BLA occurs indirectly. Indeed, activation of β1- and β3-AR in lateral paracapsular (LPCS) interneurons, which are a distinct class of GABAergic interneurons that border the BLA and external capsule and are thought to provide cortical feed-forward inhibition to the BLA (Marowsky et al. 2005), enhance LPCS GABAergic inhibition of the BLA (Silberman et al. 2010; Silberman et al. 2012).
Cholinergic afferents
The BLA is extensively innervated by fibers from the substantia innominate (SI) (nucleus basalis magnocellularis) and ventral pallidum (VP) of the basal forebrain (Carlsen et al. 1985; Emson et al. 1979; Woolf et al. 1984; Zaborszky et al. 1986). The extensive innervation leads to some of the highest levels of choline acetyltransferase (ChAT), the synthesizing enzyme for acetylcholine (ACh), and acetylcholinesterase (AChE), the hydrolyzing enzyme for ACh, in the BLA compared to other brain regions (Ben-Ari et al. 1977; Prager et al. 2013). The basal forebrain projects both cholinergic and non-cholinergic neurons to the BLA. Recent evidence indicates that approximately 10–15% of basal forebrain neurons projecting to the BLA are PV-immunopositive GABAergic interneurons (Mascagni and McDonald 2009), which primarily target PV interneurons in the BLA, but also target principal neurons (McDonald et al. 2011). By comparison, approximately 75–80% of the basal forebrain projection neurons are cholinergic (Carlsen et al. 1985; Mascagni and McDonald 2009; Zaborszky et al. 1986) and project primarily to dendritic shafts and spines of BLA principal neurons, but also innervate, to a small extent (approximately 7% of postsynaptic targets) PV GABAergic interneurons (Muller et al. 2011).
Stimulation of afferents from basal forebrain cholinergic neurons lead to the release of ACh, which extensively regulates neuronal excitability by acting on muscarinic (mAChR) and nicotinic (nAChR) acetylcholine receptors, both of which are abundantly present in the BLA (Hill et al. 1993; Mash and Potter 1986; Pidoplichko et al. 2013; Swanson et al. 1987; Zhu et al. 2005). mAChRs are G-protein coupled receptors that have five subtypes, designated M1-M5. M1, M3, and M5 receptors preferentially couple to Gq/11 proteins, which subsequently initiate signaling cascades that mobilize intracellular Ca2+, whereas M2 and M4 receptors couple to Gi/o proteins, which subsequently hyperpolarize neurons or inhibit neurotransmitter release (for a review, see Alger et al. 2014). While the BLA express M1-M4 mAChRs (Cortes et al. 1987; Mash and Potter 1986; McDonald and Mascagni 2010), M1 mAChR appears to be the predominant mAChR subtype in the amygdala. M1 mAChRs are primarily localized to principal neurons, and appear to increase excitability of BLA principal cells due to the suppression of several potassium currents, but M1 immunoreactivity has been observed in low levels on GABAergic interneurons (McDonald and Mascagni 2010; Muller et al. 2013). In contrast, M2 mAChRs are expressed on interneurons within the BLA, and predominately lead to hyperpolarization of GABAergic interneurons (McDonald and Mascagni 2011).
In the brain nAChRs are ligand-gated ion channels permeable to cations, including Ca2+, that produce membrane depolarization and post-synaptic excitation or the stimulation of neurotransmitter release (Dani and Bertrand 2007). nAChRs are comprised of nine different subunits (α2–7 and β2–4), which combine as either homomeric or heteromeric pentameric receptors (Dani and Bertrand 2007; Yakel 2013). The homomeric α7 and the heteromeric α4β2 are the two major subtypes of nAChRs found in the mammalian brain (Gotti et al. 2009), have previously been found to be expressed in the BLA (Hill et al. 1993; Seguela et al. 1993), and appear to regulate neuronal excitability by presynaptically modulating neurotransmitter release, or directly regulate neuronal activity by their position on somatodendritic sites of interneurons or principal neurons (Klein and Yakel 2006; Pidoplichko et al. 2013). In addition, α3β4-nAChRs are also present on GABAergic interneurons and enhance GABAergic inhibitory synaptic transmission (Zhu et al. 2005).
The functional activity and subsequent modulation of either inhibition or excitation by mAChRs and nAChRs in the BLA appears to diverge from anatomical evidence. Indeed, while α7-nAChRs are present on GABAergic interneurons and principal neurons and enhance both excitatory and inhibitory synaptic transmission, their activation powerfully modulates GABAergic inhibition, resulting in a net reduction in BLA excitability (Pidoplichko et al. 2013). What’s more, optogenetic activation of basal forebrain inputs to the BLA during periods of neuronal quiescence does not trigger excitatory responses; rather, muscarinic activation increased the inhibitory response, which may be a result of the contrasting spatiotemporal profile of cholinergic receptor activation (Unal et al. 2015). Importantly, light-induced activation of basal forebrain inputs transiently silence cells, which was followed by a longer duration inhibitory postsynaptic potential (IPSP) (Unal et al. 2015). This suggests the early IPSP is due to activation of nicotinic receptors and, based upon the results by Pidoplichko and colleagues (Pidoplichko et al. 2013), presumptively α7-nAChR activation, though the subunit configuration was not tested by Unal and colleagues. By contrast, the late IPSP was mediated by M1 and not M2 mAChR activation. It is important to emphasize that this inhibitory effect only occurred in quiescent principal neurons. During periods of strong activation, mAChR inhibition appeared overwhelmed and M1 mediated excitation predominated (Unal et al. 2015).
Glutamate Receptors
GABAergic interneuronal excitability in the BLA is regulated, in part, by principal neurons within the BLA. Glutamatergic inputs make dual component synapses with both fast α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) and slower N-methyl-D-aspartate receptors (NMDAR), which are present on the postsynaptic membrane of interneurons and principal neurons (Mahanty and Sah 1998; McDonald 1992; Sah et al. 2003; Smith et al. 1998; Weisskopf and LeDoux 1999). Glutamatergic inputs to interneurons express AMPARs that have rapid kinetics and strong inward rectification, indicating calcium permeability and a lack of the GluA2 subunit (Mahanty and Sah 1998; Polepalli et al. 2010). Interneurons also express a heterogeneous population of NMDARs. These cells can be separated into groups that lack NR2B NMDAR subunits (Mahanty and Sah 1998; Polepalli et al. 2010), express NMDARs that contain mostly NR2B subunits and have fast decay kinetics (Polepalli et al. 2010; Williams 1993), or express NMDARs that have slow kinetics and largely contain GluN1/GluN2B heterodimers (Polepalli et al. 2010; Spampanato et al. 2011; Szinyei et al. 2003).
The heterogeneity of the subunits of AMPA and NMDA receptors within specific populations of interneurons is essential to the regulation of feedforward inhibition to principal cells. Polepalli and colleagues demonstrated, for example, that long-term potentiation (LTP) to interneurons is restricted to interneurons that contain GluR2-lacking AMPAR at the postsynaptic membrane. Importantly, it was only these neurons that provided feedforward inhibition to principal cells (Polepalli et al. 2010) and while it has not been specifically tested, it is likely that the CB-immunopositive interneurons may be the subpopulation of interneurons that provide the feedforward inhibition to principal cells (Unal et al. 2014).
In addition to AMPA and NMDA receptors, kainate receptors represent a distinct class of ionotropic glutamate receptors that are preferentially activated by kainic acid. Kainate receptors consist of five different subunits, namely GluK1–3 and KA1–2 (Chittajallu et al. 1999); the GluK1–3 subunits form functional homomeric and heteromeric channels, whereas the KA subunits only generate functional receptors with distinct physiological properties when combined with the GluK1–3 subunits (Herb et al. 1992; Schiffer et al. 1997). While kainate receptors are not widely distributed in the brain, the BLA has a markedly high expression of GluK1R subunit (Braga et al. 2003). Kainate receptors, and in particular the GluK1 subunit containing kainate receptor, have been found to enhance excitatory synaptic transmission in the BLA (Li and Rogawski 1998) by modulating pre- and postsynaptic release of glutamate (Braga et al. 2004a; Jiang et al. 2001). Moreover, presynaptic GluK1 containing kainate receptors have also been found to modulate GABA release in the BLA in a bidirectional manner. At low concentrations, activation of high affinity GluK1-containing kainate receptors depolarize both principal neurons and GABAergic interneurons, which leads to a substantial increase in GABA release. However, high concentrations of agonists activate low-affinity presynaptic GluK1-containing kainate receptors, which again depolarize both GABAergic interneurons and principal neurons, but suppress evoked GABA release, leading to an enhancement in BLA network excitability (Aroniadou-Anderjaska et al. 2012; Aroniadou-Anderjaska et al. 2007; Braga et al. 2003).
GABAB Receptors
The late or slow component of inhibitory synaptic transmission is mediated by activation of GABAB receptors, which are comprised of the GABAB1 and GABAB2 subunits (Craig and McBain 2014). Both GABAB1 and GABAB2 mRNA are expressed in the BLA (Bischoff et al. 1999; Clark et al. 2000; Durkin et al. 1999). Postsynaptically, the Gi/o-protein-coupled GABAB receptors (primarily the GABAB1B isoform) mediate hyperpolarization of postsynaptic membranes by inhibiting voltage-gated Ca2+ activation and activating inwardly rectifying potassium channels (Couve et al. 2000; Rainnie et al. 1991b; Sugita et al. 1993). Presynaptic GABAB receptors, and primarily the GABAB1A isoform, on the other hand, has been found to inhibit neurotransmitter release on both excitatory and inhibitory synapses by inhibiting voltage-gated Ca2+ channels and possibly by interacting with vesicular release machinery (Gassmann and Bettler 2012; Szinyei et al. 2000; Yamada et al. 1999).
Anatomically, GABAB1 receptors are found in many amygdala nuclei. However, in the BLA, GABAB receptor immunoreactivity is primarily found on GABAergic interneurons; very few principal neuronal somata in the BLA exhibit immunoreactivity for GABAB1 receptors (McDonald et al. 2004). However, the light staining found in the neuropil is likely due to the staining of dendritic shafts and spines belonging to pyramidal cells (McDonald et al. 2004). Of the subpopulations of GABAergic interneurons, GABAB1 receptor immunoreactivity is found primarily on large CCK+ neurons, but is also found, to a lesser extent, on small CCK+ interneurons as well. In addition, GABAB1 receptor immunoreactivity is also found on the remaining subpopulations of GABAergic interneurons in the BLA (e.g., SOM, PV, and VIP neurons) (McDonald et al. 2004). More recently, GABAB1 immunoreactivity has been examined at the synapse in the LA. Evidence indicates that GABAB1 receptors are localized to the extrasynaptic terminal of both interneurons and principal neurons. Though the majority of receptors are found in the sensory afferent terminals of principal neurons (Pan et al. 2009) where they act to reduce glutamate release, GABAB receptors are also present on inhibitory inputs to principal neurons where they act as autoreceptors (Szinyei et al. 2000).
Alterations in the modulation of inhibitory and excitatory synaptic transmission during development might be due, in part, to the activation of GABAB receptors, which are functionally expressed early in development (Bosch and Ehrlich 2015). Indeed, the GABAB receptor, which mediates paired-pulse depression (PPD) of sensory evoked IPSCs (Szinyei et al. 2000), is differentially regulated during development; intra-BLA inhibitory synapses show pronounced PPD in the first two weeks of development, but is reduced by the third week (Ehrlich et al. 2013). In other words, the contribution of PPD by GABAB receptors may be primarily due to activation of GABAB receptors in infancy, while PPD in old animals is only partially controlled by GABAB receptors. Moreover, Bosch & Ehrlich (Bosch and Ehrlich 2015) found that presynaptic GABAB receptors, which are present on sensory inputs to LA principal neurons, are activated as early as P8. Finally, tonic presynaptic control of ISPCs and EPSCs in the LA appears to be mediated by GABAB receptors, and is likely enabled by ambient GABA that also emerged in adolescence (Bosch and Ehrlich 2015).
Acid Sensing Ion Channels (ASICs)
ASICs channels, and in particular the ASIC1a splice variant, is highly expressed in the BLA (Biagini et al. 2001; Waldmann et al. 1997). ASICs are heterotrimeric or homotrimeric proton-gated channels activated by extracellular acidosis, intracellular pH, and other factors (Wemmie et al. 2013). Until recently the precise role of ASIC1a activation in the BLA remained unknown. ASIC1a was thought to promote hyperexcitability as it was found to reduce fear and have antidepressant and anxiolytic effects (Coryell et al. 2009; Coryell et al. 2007; Dwyer et al. 2009; Ziemann et al. 2009). Indeed, electrophysiological evidence indicates that ASIC1a channels are present on principal neurons within the BLA and are activated by ammonium or by lowering extracellular pH, which increases glutamatergic activity (Pidoplichko et al. 2014). However, ASIC1a are also found on GABAergic interneurons within the BLA and their activation increase GABAergic activity. The increase in GABAA receptor-mediated inhibitory synaptic transmission seems to predominate, suppressing overall excitability (Pidoplichko et al. 2014), which may be a result of the intrinsic organization of the BLA. Much of the excitatory input within the BLA is directed onto interneurons, which subsequently project back onto principal cells (Lang and Pare 1997; Smith et al. 1998).
Cannabinoid Receptors
Cannabinoids exert their effects by the activation of two known cannabinoid receptor subtypes: cannabinoid-type 1 (CB1) receptor, and the cannabinoid-type 2 receptor (CB2). The CB1 receptor, which is primarily expressed presynaptically and activated by retrograde transmission of endogenous cannabinoids, is coupled to Gi/o proteins. Activation of CB1 receptors decreases excitability of the presynaptic terminal by closing calcium (n- and P/Q type) channels, increasing G-protein coupled inwardly rectifying potassium channels, and decreasing cyclic adenosine monophosphate-dependent sodium conductance (Pertwee 1997; Schlicker and Kathmann 2001). The CB2 receptor is also coupled to Gi/o proteins, but its expression is primarily restricted to immunological tissues peripherally and is implicated in immunological functions (Schatz et al. 1997).
CB1 receptors are widely distributed in the brain, but are present in relatively high concentrations in the BLA and, in particular, are present on the presynaptic terminal of CCK interneurons (Katona et al. 2001; McDonald and Mascagni 2001b), which densely innervate principal neurons (McDonald and Pearson 1989). Activation of the presynaptic CB1 receptors on CCK GABAergic interneurons reduce the amplitude of sIPSCs, but do not affect mIPSCs (Katona et al. 2001), as CB1 receptors reduce GABA release via blockade of presynaptic N-type Ca2+ channels (Wilson et al. 2001). Activation of CB1 receptors has also been found to reduce excitatory synaptic transmission in the LA and decrease basal synaptic transmission, indicating that in the LA, CB1 modulation of neuronal activity was determined by CB1 receptors expressed on principal neurons (Azad et al. 2003). In addition to regulating GABA release, CB1 receptor activation appears essential for the expression of postsynaptic GABAA receptors. The expression of α1 and α2 subunits of the GABAA receptor are reduced in the amygdala of CB1−/− mice. This reduction in subunit expression may be the result of a developmental neuroadaptation that compensates for the overstimulation of postsynaptic receptors due to the lack of inhibitory presynaptic activity exerted by CB1 receptors (Diana and Bregestovski 2005; Uriguen et al. 2011).
GABAergic Circuit Dysfunction within the BLA
A functional BLA GABAergic system is essential throughout one’s life. Deficiencies in GABAergic inhibitory synaptic transmission are associated with neurodevelopmental diseases, such as autism or Fragile X syndrome, but also appear in neurodegenerative diseases such as Alzheimer’s disease. In addition, deficiencies in the GABAergic system can appear as a result of brain trauma, such as after TBI, or may be acquired after SE. In this section, we will first provide an overview as to how a reduction in GABAergic inhibitory synaptic transmission within the BLA is associated with the development of anxiety. We will then provide an example of a neurodevelopmental and neurodegenerative disorder that result in deficiencies in the GABAergic system within the BLA (see Table 2). In addition, we will provide two examples of acquired GABAergic deficiencies. It must be noted that genetic variations may be an underlying factor in deficiencies of GABAergic inhibitory synaptic transmission. Unless genetic variations are directly involved in changing GABAergic function within the amygdala, we will not address these variations.
Table 2.
Summary of Alterations in the BLA GABAergic System in Disease. For citations, see text.
| Anxiety/Trauma | Autism/Fragile X | Alzheimer’s Disease | Epilepsy | TBI | |
|---|---|---|---|---|---|
| Behavioral Symptoms | Anxiety, hypervigilance, stress, fear | Impaired social interaction, language/communication deficits, repetitive/restricted interest, aggression, anxiety, epilepsy | Memory impairment, impaired fear conditioning, anxiety, epilepsy | Seizures, status epilepticus | Cognitive and emotional deficits, PTE, anxiety, increased fear conditioning, PTSD |
| GABA Interneurons | ↓ SOM/NK1r | ↓ synaptic number but no interneuronal loss | ↓ in total # interneurons | ↓ total # interneurons ↓ density of SOM interneurons |
↓ total # interneurons |
| GAD/GABA | ↓ tonic/phasic IPSC ↓ GABA release |
↓ GABA metabolism ↓ GAD65/67 ↓ tonic/phasic IPSC |
No change in IPSC of 1- or 7- m/o apoE4 mice (in LA) ↑ IPSC in 18–20 m/o apoE4 mice (in LA) but < ↑ in EPSC |
↓ sIPSC frequency and amplitude ↓ mIPSC amplitude |
↓ sIPSC frequency and amplitude ↓ mIPSC frequency and amplitude |
| GABAA Receptors | ↑ α2 in highly anxious rats | Delayed maturation of α1 and α2 ↓ α5, 8 |
↑ α1 | ↓ α1, β2, γ2 |
Anxiety and Posttraumatic Stress Disorders
Anxiety and/or stress related disorders, such as PTSD, develop when individuals are exposed to situations eliciting extreme stress or fear (Heim and Nemeroff 2001; van der Kolk 2003). These disorders are commonly associated with amygdala hyperactivity (Nuss 2015; Terburg et al. 2012) and are often treated by administering benzodiazepines, which mediate their actions via GABAA receptors (Smith 2001). However, in many cases the treatment of anxiety disorders with benzodiazepine-like compounds may be ineffective, potentially due to deficits in GABA release, GABAergic interneuronal loss in the BLA, or alterations in GABAA receptor functionality (Farb and Ratner 2014).
The loss of GABAergic interneurons or reductions in glutamate decarboxylase (GAD), an enzyme that catalyzes the decarboxylation of glutamate into GABA, may lead to deficits in the presynaptic release of GABA and contribute to increased anxiety and associated BLA hyperexcitability. Indeed, excess reductions in GAD, such as occurring when knocking out one of the GAD isoforms (GAD65), leads to reduced phasic and tonic inhibition, and subsequently results in BLA hyperexcitability, increased anxiety, and pathological fear memory reminiscent of PTSD (Lange et al. 2014; Muller et al. 2015; Walls et al. 2010).
Selectively targeting GABAergic interneurons in the BLA has been recently investigated for the development of anxiety-like behavior as well as in fear learning. Lesions to GABAergic interneurons that contain NK1r, which colocalize with interneurons containing NPY, SOM, and CB, has been found to increase anxiety-like behaviors in rats (Truitt et al. 2009; Truitt et al. 2007). Notably, NK1r containing interneurons account for only approximately 3% of the total population of GABAergic interneurons in the BLA. The loss of NK1r containing interneurons does not result in a significant reduction in the total number of interneurons (Truitt et al. 2009). However, selective ablation of these interneurons and in particular the SOM-GABAergic interneurons, which include approximately half of the NK1r-immunoreactive interneurons, likely disinhibit the synchronizing activity of projection neurons and may impair feedforward inhibition (Truitt et al. 2009). By comparison, increasing the number of GABAergic interneurons in the BLA will reduce anxiety and animals that had increased inhibitory neurons are less sensitive to unlearned fear, though they still could acquire conditioned fear responses (Cunningham et al. 2009). While anxiety appears to be regulated in part by NK1r-containing interneurons, PV- and SOM-GABAergic interneurons bidirectionally regulate the acquisition of a fear memory through two distinct mechanisms. During an auditory cue, PV-interneurons are excited through direct sensory input from the auditory thalamus and cortex, and indirectly disinhibit principal neurons via inhibition of SOM neurons. However, during an aversive footshock, both PV and SOM interneurons are inhibited, most likely via the activation of other interneuron subtypes that contact both PV and SOM interneurons, suggesting that the interneurons exhibit distinct temporal dynamics that correlate with specific behavioral differences (Wolff et al. 2014).
While impaired GABA release or disinhibition of GABAergic interneurons may result in anxiety-like behavior and increased fear responses, so too will deficiencies in the activation of postsynaptic GABAA receptors. Pharmacological alterations of GABAA receptor activity by microinjection of GABAA receptor agonists or antagonists induce anxiolytic or anxiogenic like effects, respectively (Barbalho et al. 2009; Da Cunha et al. 1992; Sanders and Shekhar 1995; Zangrossi et al. 1999). Moreover, highly anxious rats exhibit an increase in the expression of the α2 subunit of the GABAA receptor in the BLA (Lehner et al. 2010; Skorzewska et al. 2014). However, it remains unknown whether alterations in the expression of other GABAA subunits also contribute to increased anxiety. In all, these results indicate that deficiencies in GABAergic inhibitory synaptic transmission within the BLA contribute to BLA hyperexcitability and the subsequent development of anxiety and trauma-related disorders.
Autism Spectrum Disorders and Fragile X Syndrome
Autism spectrum disorders (ASDs), which include Fragile X syndrome, are a group of neurodevelopmental syndromes that are often associated with aggression, anxiety, and epilepsy (Parikh et al. 2008; Tuchman and Cuccaro 2011). Emerging evidence indicates a glutamatergic/GABAergic imbalance in multiple brain regions, including the amygdala, with greater levels of glutamatergic and reduced GABAergic activity, which results in the manifestation of symptoms associated with ASD (Coghlan et al. 2012; El-Ansary and Al-Ayadhi 2014). The amygdala has recently been implicated in ASD, including Fragile X (Suvrathan and Chattarji 2011), as increased activation of the left amygdala has been reported in functional magnetic resonance imaging studies of Fragile X patients (Watson et al. 2008).
Environmental models of autism or Fragile X knockout (KO) mice revealed deficiencies in the GABAergic system within the BLA. The reduced GABAergic inhibition appears to be a result of deficits in synaptic transmission and GABA metabolism, and not due to a loss of GABAergic interneurons (Kim et al. 2014). Indeed, in a Fragile X mental retardation 1 (FMR1) gene KO model of Fragile X syndrome, the total number of neurons, including GABA-immunopositive interneurons in the BLA is unaffected. Similarly, human studies of autistic children show little morphological alterations in the BLA as compared to developmentally typical children (see Blatt 2012 for a review). However, there appears to be a significant decrease in the total number of BLA inhibitory synaptic connections, indicating aberrant circuit development (Olmos-Serrano et al. 2010). Moreover, in the BLA of FMR1 KO mice, reductions in GAD1 mRNA and protein expression for GAD65/67 were observed and are associated with reduced presynaptic GABA release (Olmos-Serrano et al. 2010).
Though the overall number of amygdala GABAergic interneurons remains stable, mechanisms that modulate the activation of GABAergic interneurons may be impaired in ASD and Fragile X models. For example, FMR1 KO mice have reduced activation of SOM-expressing low-threshold-spiking interneurons in layer II/III of the somatosensory cortex causing reduced spike synchronization of BLA principal neurons (Paluszkiewicz et al. 2011b); reductions in spike synchronization from the cortex could subsequently impair neuronal activity in the BLA required in the expression of fear (Courtin et al. 2014) but also lead to hyperresponsivity within the amygdala (Rauch et al. 2006). In addition, in a rat model of ASD, reductions in dendritic morphology, including spine density, or distal connectivity between the PFC and BLA may lead to impaired cortical-BLA regulation (Bringas et al. 2013).
Deficits in GABAergic inhibitory synaptic transmission in ASD and Fragile X appear to also be a result of genetic variations in genes coding for particular subunits of the GABAA receptor, as has been documented throughout multiple brain regions in ASD (Coghlan et al. 2012; Fatemi et al. 2010) and in Fragile X (Deidda et al. 2014). In the amygdala of ASD and Fragile X models, delays in the maturation of postsynaptic GABAA receptors (Vislay et al. 2013) may lead to reductions in phasic and tonic GABAergic inhibitory synaptic transmission (D'Hulst et al. 2006; Olmos-Serrano et al. 2010). In the Fragile X model, the timing of the developmental expression of the α1 and α2 GABAA receptor subunits is delayed, which, in turn, may have impaired the switch in GABA polarity (He et al. 2014) and proper neuronal connections in wiring up local neuron networks in the BLA (Cossart 2011; Paluszkiewicz et al. 2011a).
In addition to the deficits in phasic (synaptic) inhibition, tonic inhibition, which is mediated by extrasynaptic GABAA receptors containing either the α5 or the δ subunits, is also compromised in the BLA of FMR1 KO mice (Martin et al. 2014) and in related ASDs (Zhang et al. 2008). Tonic inhibition, maintained by low levels of ambient GABA in the extrasynaptic space (Farrant and Nusser 2005), provides a persistent inhibitory conductance that regulates the E/I balance (Semyanov et al. 2004). The reduction in the expression of the α5 subunit of the GABAA receptor narrows the integration window necessary for feedforward inhibition. Moreover, because of the reduced GABA release, more synchronized afferent inputs must be generated to result in an action potential and modulate the integration of postsynaptic excitatory and inhibitory potentials (Gabernet et al. 2005; Pouille and Scanziani 2001).
Alzheimer’s Disease
AD is associated with severe neuronal loss, with a predilection for brain regions within the medial temporal lobe, including the amygdala (Arnold et al. 1991; Braak and Braak 1991). Recent studies have found that in symptomatic AD patients, the amygdala, and in particular the basomedial and lateral nuclei, display between 14% and 60% volumetric loss, compared to controls, as well as non-uniform shape changes (Cavedo et al. 2011; Cavedo et al. 2014; Miller et al. 2015; Poulin et al. 2011). In addition, postmortem studies reveal that although there is damage throughout the amygdala, the degree of atrophy and neurofibrillary tangles of amygdala nuclei is greater in the corticomedial group than in the BLA, suggesting that there perhaps is a selective loss of neurons that contribute to the loss in amygdala volume (Tsuchiya and Kosaka 1990).
While the overall loss of neurons, which contributes to volumetric changes, has been observed in the amygdala, it remains unknown whether GABAergic interneuronal subpopulations or specific subunits of the GABAA receptor are targeted in the BLA and contribute to the observed deficits in fear learning and increased anxiety. Indeed, a loss of GABAergic interneurons or alterations in the expression of GABAA receptor subunits in the amygdala is possible, as it has been observed that in the canine prefrontal cortex PV- or CR-immunoreactive interneurons are resistant to neuronal death, whereas CB-positive interneurons are depleted (Pugliese et al. 2004), and in the mouse dentate gyrus, significantly fewer SOM-immunopositive neurons are observed, whereas in the CA1 hippocampal region, there is a significant loss of PV and SOM interneurons (Levenga et al. 2013). In addition, as observed in the hippocampus (see Armstrong et al. 2003; Iwakiri et al. 2009; Mizukami et al. 1998), GABAA receptor subunit expression might also be reduced in the BLA, which could contribute to amygdala hyperactivity.
Though it may be hypothesized that there are alterations to the expression of GABAA receptors and the interneuronal population in the BLA, only one study has examined alterations to the E/I balance in an AD model, and focused this examination on the LA. Using the apolipoprotein E4 (apoE4) targeted replacement (TR) mouse to model AD (Wang et al. 2005), 1-or 7- month old mice expressing apoE4 display reduced excitatory synaptic transmission in the LA but no changes in inhibitory synaptic transmission (Klein et al. 2010). However, aged (18–20 months) apoE4 animals display significant increases in both inhibitory and excitatory synaptic transmission and an increased seizure phenotype (Hunter et al. 2012; Klein et al. 2014), suggesting that increased excitatory synaptic transmission predominates. Importantly, the results indicate that it is unlikely that in the LA there are alterations in the subunit composition of the GABAA receptor, but rather increased excitatory transmission may be the result of alterations in the presynaptic release of GABA. While it remains unknown why animals display increased excitatory activity in addition to the increased inhibitory activity, one hypothesis may be that the increased excitatory activity seen in apoE4 mice may be the result of a loss of inhibition from extrinsic afferent cortical inputs (Klein et al. 2014; Swanson and Petrovich 1998) or deficiencies in neuromodulatory mechanisms such as the loss of GABAergic interneurons but not cholinergic neurons in the basal forebrain (Loreth et al. 2012).
Epilepsy and Seizures
As a principal circuit projecting to many brain regions, hyperexcitability within the amygdala may be one source of seizure generation (Prager et al. 2013). For instance, spontaneous bursting activity has been found to first appear in the BLA of kindled animals (Smith and Dudek 1997; White and Price 1993) and seizure generation after a nerve agent exposure only occurs if AChE activity is significantly impaired in the amygdala compared to other seizurogenic brain regions (McDonough et al. 1987; Prager et al. 2013). The amygdala receives monosynaptic inputs from many frontal and temporal cortical areas that are known to generate and propagate seizure activity (Pitkanen et al. 1998). The convergence of input onto specific nuclei can then recruit a large number of neurons from interdivisional network connections, which may contribute to ictal-like activity within different amygdala nuclei. Efferents from amygdala nuclei may subsequently provide routes by which the amygdala can recruit other brain regions and lead to seizure propagation (Hirsch et al. 1997; Pitkanen 2000; Pitkanen et al. 1998).
The loss of neurons in the amygdala and subsequent reductions in amygdalar volume further implicates its role in the generation and propagation of seizures. The amygdala has previously been found to be severely damaged in patients with temporal lobe epilepsy and in both adults and children who experience SE (Pitkanen et al. 1998). While often occurring in combination with hippocampal damage, neuronal loss has been observed in the amygdale without any apparent damage to the hippocampus (Hudson et al. 1993; Miller et al. 1994; Pitkanen et al. 1998). The loss of neurons in the amygdala has also been observed in animal models with the BLA being among the highest damaged nuclei (Apland et al. 2010; Figueiredo et al. 2011; Prager et al. 2013; Prager et al. 2014a; Tuunanen et al. 1996). The loss of GABAergic interneurons in the amygdala in different seizure models has also been examined. Seizures or SE cause between 37% and 64% of GABAergic interneurons in the BLA to die, irrespective of the seizure model, though the loss of GABAergic interneurons was delayed by 7 days in animals that developed SE after a nerve agent exposure (Callahan et al. 1991; Figueiredo et al. 2011; Prager et al. 2014a); Tunnanen and colleagues found a 35% decrease in the density of SOM-immunoreactive neurons in a kindling model (Tuunanen et al. 1997).
Kindling or nerve agent induced SE causes long lasting changes in synaptic transmission in the BLA, including impaired feed-forward GABAergic inhibition and disinhibition of excitatory circuits (Rainnie et al. 1991a; Rainnie et al. 1992), and network reorganization resulting in BLA hyperexcitability (Prager et al. 2014a; Smith and Dudek 1997). The loss of feedforward inhibition has been observed indirectly as a significant increase in paired pulse facilitation beginning 24 h after SE and continuing up to 30 days after nerve agent exposure (Prager et al. 2014a; Zinebi et al. 2001), and directly as a prolonged reduction in GABAA receptor mediated inhibitory synaptic transmission, which was likely due to the loss of GABAergic interneurons in the BLA (Prager et al. 2014b). The loss of inhibitory synaptic transmission has been found to cause a concomitant increase in excitatory synaptic transmission (Prager et al. 2014b), which is associated with an increase in both NMDA and non-NMDA receptor mediated glutamatergic transmission (Gean et al. 1989; Rainnie et al. 1992; Shoji et al. 1998).
Although alterations in GABAergic synaptic transmission have been observed in the amygdala after nerve agent induced SE, reductions in GABAA receptor mediated IPSCs appears to be model specific. After nerve agent induced SE, there was a significant reduction in the frequency but not amplitude of GABAA receptor mediated mIPSCs (Prager et al. 2014b), indicating that the deficits in inhibitory synaptic transmission resulted from the loss of GABAergic interneurons. However, seven to 10 days after kainate acid induced SE, there was an increase in α1 subunit and GAD expression, but a reduction in tonic inhibition (Fritsch et al. 2009). While few studies have addressed how the stoichiometry of GABAA receptor subunits change in the BLA after prolonged SE, it is well known that the expression of GABAA subunits are altered in other brain regions such as the hippocampus (Ferando and Mody 2012; Mohler 2006). Thus, perhaps in some cases of epilepsy, alterations in the stoichiometry of GABAA receptor subunits may contribute to impaired inhibition in the BLA, whereas in other cases the loss of GABAergic inhibition may be the result of the death of interneurons.
Traumatic Brain Injury
Like many other disorders, TBI can affect many brain regions including the amygdala, and the disruption in neuronal excitability in surrounding regions may ultimately alter the homeostasis of the amygdala. The disruption in the E/I balance stems from an initial rise in glutamate release, which is responsible for excitotoxicity, but also a delayed disruption of excitatory glutamate circuits, which may underlie the cognitive and motor deficits observed after TBI (Guerriero et al. 2015). Alterations in both glutamatergic and GABAergic synaptic transmission and the expression of their corresponding receptors have been observed after TBI in many brain regions, including the BLA (Almeida-Suhett et al. 2014; Guerriero et al. 2015), though this work is in its infant stages. An increase in the NR1 subunit of the NMDA receptor has been observed in the amygdala two weeks after injury (Reger et al. 2012), while reductions in the α1, β2, and γ2 subunits of the GABAA receptor were observed seven days after a mild TBI (Almeida-Suhett et al. 2014). Moreover, even when there is no overt neuronal death in the BLA, a delayed loss of GABAergic interneurons is observed after a mild TBI, which may contribute to increased anxiety-like behavior (Almeida-Suhett et al. 2014) and enhanced fear conditioning (Reger et al. 2012).
Conclusions and Future Directions
While alterations in GABAergic inhibitory synaptic transmission in different diseases were reviewed separately, it cannot go unstated that in many cases, comorbidity occurs within many of these diseases. For example, estimates of comorbidity between PTSD and some types of TBI, including combat related TBI, are as high as 73% (Hoge et al. 2008; Taylor et al. 2012). Moreover, epilepsy is often found to occur with diseases including autism/Fragile X (Berry-Kravis et al. 2010; Khetrapal 2010), schizophrenia (Kandratavicius et al. 2012), AD (Chan et al. 2015; Palop et al. 2007), and anxiety disorders (Trimble and Van Elst 2003; Vazquez and Devinsky 2003). Indeed, many of these disorders have comorbidities and often these comorbidities involve deficiencies within the BLA GABAergic system.
We are not arguing in this review that amygdala hyperactivity results in the development of symptoms associated with the diseases discussed above. Rather, the purpose of this review is to provide evidence that reduced GABAergic inhibition and alterations in the mechanisms that modulate GABAergic inhibition, contribute, in part, to amygdalar hyperexcitability; BLA hyperexcitability is common amongst these disorders and may lead to comorbid behavioral deficits. The extensive innervation of the amygdala by multiple brain regions has revealed that specific pathways modulate GABAergic inhibitory synaptic transmission and that these pathways may be disrupted in different diseases.
GABAergic activity in the BLA is modulated by dopaminergic, serotonergic, noradrenergic, and cholinergic activation, as well as the activation of various glutamate receptor subtypes, and the CB1 and ASIC1a receptor. In the diseases discussed, deficiencies in the release of monoamines or ACh, or alterations in glutamatergic receptor activity, can lead to reduced modulation of GABAergic inhibition, and more locally, greater excitation via deficiencies in either feed-forward or feedback inhibitory mechanisms. What’s more, because many of these systems are interconnected, deficiencies in one system may result in a cascading effect, which could contribute to disinhibition of excitatory neurons in the BLA, and subsequently increased anxiety-like behavior or increased seizure activity. Yet, the data in many cases is not conclusive. Much remains unknown about how alterations in neurotransmitter release, receptor activation, and stoichiometry contributes to the behavioral deficits and increased anxiety that is often associated with these disorders.
E/I balance in the amygdala is dependent upon functional neuromodulatory mechanisms and local interneuronal regulation. Neuromodulation is ineffective when there is a substantial loss of GABAergic interneurons, as has been observed in the amygdala in various neurological and neuropsychiatric disorders. In some cases it is known that a specific class of interneurons are differentially affected, while in most cases it remains unknown what type of interneuron is most susceptible to cell death. Moreover, the loss of GABAergic interneurons may be delayed as compared to the death of principal neurons. The immediate death may be due to the excitotoxic effects that occur with glutamatergic excitotoxicity (Zhou et al. 2013); however, one hypothesis may be that the delayed loss of GABAergic interneurons is due to an upregulation of D-serine, an endogenous co-agonist for NMDA receptors (Liu et al. 2009). Alternatively, even if there is no interneuronal degeneration, deficits in GAD may reduce the synthesis of GABA, which could subsequently reduce the concentration of GABA released in the synapse and impair inhibitory synaptic transmission. Alternatively, in many of the diseases discussed, alterations in expression of GABAA receptor subunits have been observed in the BLA. While it is unknown whether these changes are transient or permanent, it can be assumed that alterations in the subunit stoichiometry may lead to reduced tonic and phasic inhibitory synaptic transmission.
This review comprised two major themes. First, we summarized the neuromodulatory systems that modulate GABAA receptor mediated inhibitory synaptic transmission. Second we discussed how reduced GABAergic inhibition in the BLA throughout the lifespan can contribute to the behavioral manifestation of symptoms associated with autism and Fragile X, AD, epilepsy, TBI, and anxiety or stress related disorders. In each case, results indicate that BLA hyperexcitability is associated with deficits in mechanisms that modulate GABAergic inhibitory synaptic transmission, the loss of GABAergic interneurons, or alterations in GABAA receptor subunit expression. However, in many of the diseases discussed above, much remains unknown about why the amygdala is hyperexcitable. By understanding how the GABAergic system is impaired, future research can target the functional aspects of the GABAA receptor for potential therapeutic options. Future research might also develop new therapies that induce the growth of interneurons in specific brain regions or target and reduce excitation of the glutamatergic system. The latter option has been implemented after a nerve agent induced seizure, for example, where administering a GluK1-kainate receptor antagonist prevented neurodegeneration and associated increases in anxiety or seizure activity (Figueiredo et al. 2011; Prager et al. 2015). In all, identifying the alterations to the inhibitory system and the mechanisms that modulate inhibitory synaptic transmission is a fundamental prerequisite in the design of effective and well-tolerated therapeutic treatments for these and other neurological and neuropsychiatric disorders.
Significance Statement.
Deficits in the brain inhibitory systems can occur at any stage of life. The resulting hyperexcitability leads to the development of neurological and/or neuropsychiatric diseases. We assessed how the loss of inhibitory synaptic transmission and mechanisms that modulate inhibition in the basolateral amygdala lead to increased anxiety. In addition, we examine how different diseases including autism/Fragile X syndrome, Alzheimer’s disease, traumatic brain injury and epilepsy result in amygdalar hyperexcitability. By evaluating how deficiencies in inhibition within the amygdala contributes to these diseases, future research may be directed at developing new therapies to reduce excitability, which may alleviate the behavioral symptomology of neurologic diseases.
Acknowledgements
This work was supported by the CounterACT program, National Institutes of Health, Office of the Director through the National Institute of Neurological Disorders and Stroke [Grant Number 5U01NS058162-07]
Abbreviations
- 5-HT
serotonin
- ACh
acetylcholine
- AChE
acetylcholinesterase
- AD
Alzheimer’s disease
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- apoE
apolipoprotein E
- AR
adrenoreceptor
- ASD
autism spectrum disorder
- ASIC
acid sensing ion channel
- Aβ
amyloid β protein
- BLA
basolateral nucleus of the amygdala
- CA1
cornus ammonis 1
- CB
calbindin
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CCK
cholecystokinin
- ChAT
choline acetyltransferase
- CR
calretinin
- DA
dopamine
- DRN
dorsal raphé nucleus
- E/I
excitation and inhibition
- FMR1
fragile X mental retardation 1 gene
- GABA
gamma-aminobutyric acid
- GAD
glutamate decarboxylase
- GluK
kainate receptor
- IPSC
inhibitory postsynaptic current
- IPSP
inhibitory postsynaptic potential
- KO
knockout
- LA
lateral amygdala
- LC
locus coeruleus
- LTP
long-term potentiation
- LPCS
lateral paracapsular interneuron
- mAChR
muscarinic acetylcholine receptor
- mIPSC
miniature inhibitory postsynaptic current
- NA
noradrenaline
- nAChR
neuronal nicotinic acetylcholine receptor
- NE
norepinephrine
- NKir
neurokinin 1 receptor
- NMDA
N-methyl-D-aspartate receptor
- NPY
neuropeptide Y
- NTS
nucleus of the solitary tract
- P
postnatal day
- PD
Parkinson’s disease
- PFC
prefrontal cortex
- PPD
paired pulse depression
- PTSD
posttraumatic stress disorder
- PV
parvalbumin
- SE
status epilepticus
- SI
substantia innominate
- sIPSC
spontaneous inhibitory postsynaptic current
- SN
substantia nigra
- SOM
somatostatin
- TBI
traumatic brain injury
- TR
targeted replacement
- VIP
vasoactive intestinal peptide
- VP
ventral pallidum
- VTA
ventral tegmental area
Footnotes
Disclaimer: The opinions or assertions contained herein are the private views of the author and are not to be construed as official or reflecting the views of the Uniformed Services University of the Health Sciences, the Department of the Army, or the Department of Defense.
Conflict of Interest: The authors declare that they have no competing interests
Role of Authors: All authors take responsibility for the integrity and accuracy of this manuscript: Drafting of the manuscript, critical revisions of the manuscript: EMP, HCB, GHW, MFMB
Contributor Information
Eric M Prager, Email: eprager@wiley.com.
Hadley C Bergstrom, Email: habergstrom@vassar.edu.
Gary H Wynn, Email: gary.wynn@usuhs.edu.
References
- Alger BE, Nagode DA, Tang AH. Muscarinic cholinergic receptors modulate inhibitory synaptic rhythms in hippocampus and neocortex. Front Synaptic Neurosci. 2014;6:18. doi: 10.3389/fnsyn.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Suhett CP, Prager EM, Pidoplichko V, Figueiredo TH, Marini AM, Li Z, Eiden LE, Braga MF. Reduced GABAergic inhibition in the basolateral amygdala and the development of anxiety-like behaviors after mild traumatic brain injury. PLoS One. 2014;9(7):e102627. doi: 10.1371/journal.pone.0102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Rossetti F, Miller SL, Braga MF. The limitations of diazepam as a treatment for nerve agent-induced seizures and neuropathology in rats: comparison with UBP302. J Pharmacol Exp Ther. 2014;351(2):359–372. doi: 10.1124/jpet.114.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Figueiredo TH, Qashu F, Aroniadou-Anderjaska V, Souza AP, Braga MF. Higher susceptibility of the ventral versus the dorsal hippocampus and the posteroventral versus anterodorsal amygdala to soman-induced neuropathology. Neurotoxicology. 2010;31(5):485–492. doi: 10.1016/j.neuro.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Sheffield R, Mishizen-Eberz AJ, Carter TL, Rissman RA, Mizukami K, Ikonomovic MD. Plasticity of glutamate and GABAA receptors in the hippocampus of patients with Alzheimer's disease. Cell Mol Neurobiol. 2003;23(4–5):491–505. doi: 10.1023/A:1025063811290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1(1):103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Pidoplichko VI, Figueiredo TH, Almeida-Suhett CP, Prager EM, Braga MF. Presynaptic facilitation of glutamate release in the basolateral amygdala: a mechanism for the anxiogenic and seizurogenic function of GluK1 receptors. Neuroscience. 2012;221:157–169. doi: 10.1016/j.neuroscience.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Qashu F, Braga MF. Mechanisms regulating GABAergic inhibitory transmission in the basolateral amygdala: implications for epilepsy and anxiety disorders. Amino Acids. 2007;32(3):305–315. doi: 10.1007/s00726-006-0415-x. [DOI] [PubMed] [Google Scholar]
- Asan E. Ultrastructural features of tyrosine-hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell Tissue Res. 1997;288(3):449–469. doi: 10.1007/s004410050832. [DOI] [PubMed] [Google Scholar]
- Asan E, Steinke M, Lesch KP. Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochem Cell Biol. 2013;139(6):785–813. doi: 10.1007/s00418-013-1081-1. [DOI] [PubMed] [Google Scholar]
- Azad SC, Eder M, Marsicano G, Lutz B, Zieglgansberger W, Rammes G. Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learn Mem. 2003;10(2):116–128. doi: 10.1101/lm.53303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbalho CA, Nunes-de-Souza RL, Canto-de-Souza A. Similar anxiolytic-like effects following intra-amygdala infusions of benzodiazepine receptor agonist and antagonist: evidence for the release of an endogenous benzodiazepine inverse agonist in mice exposed to elevated plus-maze test. Brain Res. 2009;1267:65–76. doi: 10.1016/j.brainres.2009.02.042. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18(5):467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Zigmond RE, Shute CC, Lewis PR. Regional distribution of choline acetyltransferase and acetylcholinesterase within the amygdaloid complex and stria terminalis system. Brain Res. 1977;120(3):435–444. doi: 10.1016/0006-8993(77)90397-3. [DOI] [PubMed] [Google Scholar]
- Berdel B, Morys J. Expression of calbindin-D28k and parvalbumin during development of rat's basolateral amygdaloid complex. Int J Dev Neurosci. 2000;18(6):501–513. doi: 10.1016/s0736-5748(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB. Seizures in fragile X syndrome: characteristics and comorbid diagnoses. Am J Intellect Dev Disabil. 2010;115(6):461–472. doi: 10.1352/1944-7558-115.6.461. [DOI] [PubMed] [Google Scholar]
- Biagini G, Babinski K, Avoli M, Marcinkiewicz M, Seguela P. Regional and subunit-specific downregulation of acid-sensing ion channels in the pilocarpine model of epilepsy. Neurobiol Dis. 2001;8(1):45–58. doi: 10.1006/nbdi.2000.0331. [DOI] [PubMed] [Google Scholar]
- Bienvenu TC, Busti D, Micklem BR, Mansouri M, Magill PJ, Ferraguti F, Capogna M. Large intercalated neurons of amygdala relay noxious sensory information. J Neurosci. 2015;35(5):2044–2057. doi: 10.1523/JNEUROSCI.1323-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S, Leonhard S, Reymann N, Schuler V, Shigemoto R, Kaupmann K, Bettler B. Spatial distribution of GABA(B)R1 receptor mRNA and binding sites in the rat brain. J Comp Neurol. 1999;412(1):1–16. [PubMed] [Google Scholar]
- Bissiere S, Humeau Y, Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6(6):587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Blatt GJ. The neuropathology of autism. Scientifica (Cairo) 2012;2012:703675. doi: 10.6064/2012/703675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardi C. Distribution of 5-HT2A receptor immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. Brain Res. 2011;1370:112–128. doi: 10.1016/j.brainres.2010.11.055. [DOI] [PubMed] [Google Scholar]
- Bonn M, Schmitt A, Asan E. Double and triple in situ hybridization for coexpression studies: combined fluorescent and chromogenic detection of neuropeptide Y (NPY) and serotonin receptor subtype mRNAs expressed at different abundance levels. Histochem Cell Biol. 2012;137(1):11–24. doi: 10.1007/s00418-011-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn M, Schmitt A, Lesch KP, Van Bockstaele EJ, Asan E. Serotonergic innervation and serotonin receptor expression of NPY-producing neurons in the rat lateral and basolateral amygdaloid nuclei. Brain Struct Funct. 2013;218(2):421–435. doi: 10.1007/s00429-012-0406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch D, Ehrlich I. Postnatal maturation of GABAergic modulation of sensory inputs onto lateral amygdala principal neurons. J Physiol. 2015 doi: 10.1113/JP270645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Li H. The physiological role of kainate receptors in the amygdala. Mol Neurobiol. 2004a;30(2):127–141. doi: 10.1385/MN:30:2:127. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology. 2004b;29(1):45–58. doi: 10.1038/sj.npp.1300297. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci. 2003;23(2):442–452. doi: 10.1523/JNEUROSCI.23-02-00442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringas ME, Carvajal-Flores FN, Lopez-Ramirez TA, Atzori M, Flores G. Rearrangement of the dendritic morphology in limbic regions and altered exploratory behavior in a rat model of autism spectrum disorder. Neuroscience. 2013;241:170–187. doi: 10.1016/j.neuroscience.2013.03.030. [DOI] [PubMed] [Google Scholar]
- Brinley-Reed M, McDonald AJ. Evidence that dopaminergic axons provide a dense innervation of specific neuronal subpopulations in the rat basolateral amygdala. Brain Res. 1999;850(1–2):127–135. doi: 10.1016/s0006-8993(99)02112-5. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Witte V, Teuchert-Noodt G. Postnatal development of GABA and calbindin cells and fibers in the prefrontal cortex and basolateral amygdala of gerbils (Meriones unguiculatus) Int J Dev Neurosci. 2007;25(3):191–200. doi: 10.1016/j.ijdevneu.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. J Neurosci. 2007;27(45):12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan PM, Paris JM, Cunningham KA, Shinnick-Gallagher P. Decrease of GABA-immunoreactive neurons in the amygdala after electrical kindling in the rat. Brain Res. 1991;555(2):335–339. doi: 10.1016/0006-8993(91)90361-x. [DOI] [PubMed] [Google Scholar]
- Capogna M. GABAergic cell type diversity in the basolateral amygdala. Curr Opin Neurobiol. 2014;26:110–116. doi: 10.1016/j.conb.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441(1–2):377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Zaborszky L, Heimer L. Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: a combined retrograde fluorescent and immunohistochemical study. J Comp Neurol. 1985;234(2):155–167. doi: 10.1002/cne.902340203. [DOI] [PubMed] [Google Scholar]
- Cavedo E, Boccardi M, Ganzola R, Canu E, Beltramello A, Caltagirone C, Thompson PM, Frisoni GB. Local amygdala structural differences with 3T MRI in patients with Alzheimer disease. Neurology. 2011;76(8):727–733. doi: 10.1212/WNL.0b013e31820d62d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavedo E, Pievani M, Boccardi M, Galluzzi S, Bocchetta M, Bonetti M, Thompson PM, Frisoni GB. Medial temporal atrophy in early and late-onset Alzheimer's disease. Neurobiol Aging. 2014;35(9):2004–2012. doi: 10.1016/j.neurobiolaging.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Jones NC, Bush AI, O'Brien TJ, Kwan P. A mouse model of Alzheimer's disease displays increased susceptibility to kindling and seizure-associated death. Epilepsia. 2015 doi: 10.1111/epi.12993. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Braithwaite SP, Clarke VR, Henley JM. Kainate receptors: subunits, synaptic localization and function. Trends Pharmacol Sci. 1999;20(1):26–35. doi: 10.1016/s0165-6147(98)01286-3. [DOI] [PubMed] [Google Scholar]
- Chu HY, Ito W, Li J, Morozov A. Target-specific suppression of GABA release from parvalbumin interneurons in the basolateral amygdala by dopamine. J Neurosci. 2012;32(42):14815–14820. doi: 10.1523/JNEUROSCI.2997-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JA, Mezey E, Lam AS, Bonner TI. Distribution of the GABA(B) receptor subunit gb2 in rat CNS. Brain Res. 2000;860(1–2):41–52. doi: 10.1016/s0006-8993(00)01958-2. [DOI] [PubMed] [Google Scholar]
- Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neurosci Biobehav Rev. 2012;36(9):2044–2055. doi: 10.1016/j.neubiorev.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes R, Probst A, Palacios JM. Quantitative light microscopic autoradiographic localization of cholinergic muscarinic receptors in the human brain: forebrain. Neuroscience. 1987;20(1):65–107. doi: 10.1016/0306-4522(87)90006-6. [DOI] [PubMed] [Google Scholar]
- Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci. 2009;29(17):5381–5388. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry. 2007;62(10):1140–1148. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Cossart R. The maturation of cortical interneuron diversity: how multiple developmental journeys shape the emergence of proper network function. Curr Opin Neurobiol. 2011;21(1):160–168. doi: 10.1016/j.conb.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505(7481):92–96. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- Couve A, Moss SJ, Pangalos MN. GABAB receptors: a new paradigm in G protein signaling. Mol Cell Neurosci. 2000;16(4):296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- Craig MT, McBain CJ. The emerging role of GABAB receptors as regulators of network dynamics: fast actions from a 'slow' receptor? Curr Opin Neurobiol. 2014;26:15–21. doi: 10.1016/j.conb.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Connor CM, Carlezon WA, Jr, Meloni E. Amygdalar GABAergic-rich neural grafts attenuate anxiety-like behavior in rats. Behav Brain Res. 2009;205(1):146–153. doi: 10.1016/j.bbr.2009.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121(1):238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Wolfman C, Levi de Stein M, Ruschel AC, Izquierdo I, Medina JH. Anxiogenic effects of the intraamygdala injection of flumazenil, a benzodiazepine receptor antagonist. Funct Neurol. 1992;7(5):401–405. [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Davila JC, Olmos L, Legaz I, Medina L, Guirado S, Real MA. Dynamic patterns of colocalization of calbindin, parvalbumin and GABA in subpopulations of mouse basolateral amygdalar cells during development. J Chem Neuroanat. 2008;35(1):67–76. doi: 10.1016/j.jchemneu.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Deidda G, Bozarth IF, Cancedda L. Modulation of GABAergic transmission in development and neurodevelopmental disorders: investigating physiology and pathology to gain therapeutic perspectives. Front Cell Neurosci. 2014;8:119. doi: 10.3389/fncel.2014.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue BE, Olson-Madden JH, Smolker HR, Rajamani M, Brenner LA, Banich MT. Reduced amygdala volume is associated with deficits in inhibitory control: a voxel- and surface-based morphometric analysis of comorbid PTSD/mild TBI. Biomed Res Int. 2014;2014:691505. doi: 10.1155/2014/691505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana MA, Bregestovski P. Calcium and endocannabinoids in the modulation of inhibitory synaptic transmission. Cell Calcium. 2005;37(5):497–505. doi: 10.1016/j.ceca.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Durkin MM, Gunwaldsen CA, Borowsky B, Jones KA, Branchek TA. An in situ hybridization study of the distribution of the GABA(B2) protein mRNA in the rat CNS. Brain Res Mol Brain Res. 1999;71(2):185–200. doi: 10.1016/s0169-328x(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Dwyer JM, Rizzo SJ, Neal SJ, Lin Q, Jow F, Arias RL, Rosenzweig-Lipson S, Dunlop J, Beyer CE. Acid sensing ion channel (ASIC) inhibitors exhibit anxiolytic-like activity in preclinical pharmacological models. Psychopharmacology (Berl) 2009;203(1):41–52. doi: 10.1007/s00213-008-1373-7. [DOI] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Hazra R, Guo JD, Rainnie DG. Postnatal maturation of GABAergic transmission in the rat basolateral amygdala. J Neurophysiol. 2013;110(4):926–941. doi: 10.1152/jn.01105.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ansary A, Al-Ayadhi L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J Neuroinflammation. 2014;11(1):189. doi: 10.1186/s12974-014-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emson PC, Paxinos G, Le Gal La Salle sG, Ben-Ari Y, Silver A. Choline acetyltransferase and acetylcholinesterase containing projections from the basal forebrain to the amygdaloid complex of the rat. Brain Res. 1979;165(2):271–282. doi: 10.1016/0006-8993(79)90559-6. [DOI] [PubMed] [Google Scholar]
- Esmaeili A, Lynch JW, Sah P. GABAA receptors containing gamma1 subunits contribute to inhibitory transmission in the central amygdala. J Neurophysiol. 2009;101(1):341–349. doi: 10.1152/jn.90991.2008. [DOI] [PubMed] [Google Scholar]
- Fallon J, Ciofi P. Distribution of monoamines within the amygdala. In: Aggleton J, editor. The Amygdala. New York, NY: Academic Press; 1992. [Google Scholar]
- Farb DH, Ratner MH. Targeting the modulation of neural circuitry for the treatment of anxiety disorders. Pharmacol Rev. 2014;66(4):1002–1032. doi: 10.1124/pr.114.009126. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6(3):215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Rooney RJ, Patel DH, Thuras PD. mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord. 2010;40(6):743–750. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferando I, Mody I. GABAA receptor modulation by neurosteroids in models of temporal lobe epilepsies. Epilepsia. 2012;(53 Suppl 9):89–101. doi: 10.1111/epi.12038. [DOI] [PubMed] [Google Scholar]
- Figueiredo TH, Qashu F, Apland JP, Aroniadou-Anderjaska V, Souza AP, Braga MF. The GluK1 (GluR5) Kainate/{alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist LY293558 reduces soman-induced seizures and neuropathology. J Pharmacol Exp Ther. 2011;336(2):303–312. doi: 10.1124/jpet.110.171835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Qashu F, Figueiredo TH, Aroniadou-Anderjaska V, Rogawski MA, Braga MF. Pathological alterations in GABAergic interneurons and reduced tonic inhibition in the basolateral amygdala during epileptogenesis. Neuroscience. 2009;163(1):415–429. doi: 10.1016/j.neuroscience.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48(2):315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Bettler B. Regulation of neuronal GABA(B) receptor functions by subunit composition. Nat Rev Neurosci. 2012;13(6):380–394. doi: 10.1038/nrn3249. [DOI] [PubMed] [Google Scholar]
- Gean PW, Shinnick-Gallagher P, Anderson AC. Spontaneous epileptiform activity and alteration of GABA- and of NMDA-mediated neurotransmission in amygdala neurons kindled in vivo. Brain Res. 1989;494(1):177–181. doi: 10.1016/0006-8993(89)90160-1. [DOI] [PubMed] [Google Scholar]
- Gharedaghi MH, Seyedabadi M, Ghia JE, Dehpour AR, Rahimian R. The role of different serotonin receptor subtypes in seizure susceptibility. Exp Brain Res. 2014;232(2):347–367. doi: 10.1007/s00221-013-3757-0. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78(7):703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Guerriero RM, Giza CC, Rotenberg A. Glutamate and GABA Imbalance Following Traumatic Brain Injury. Curr Neurol Neurosci Rep. 2015;15(5):545. doi: 10.1007/s11910-015-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S, Serres F, Pei Q, Totterdell S, Sharp T. Evidence for the differential co-localization of neurokinin-1 receptors with 5-HT receptor subtypes in rat forebrain. J Psychopharmacol. 2012;26(4):505–515. doi: 10.1177/0269881111425969. [DOI] [PubMed] [Google Scholar]
- He Q, Nomura T, Xu J, Contractor A. The developmental switch in GABA polarity is delayed in fragile X mice. J Neurosci. 2014;34(2):446–450. doi: 10.1523/JNEUROSCI.4447-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8(4):775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci. 2014;17(12):1644–1654. doi: 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- Hill JA, Jr, Zoli M, Bourgeois JP, Changeux JP. Immunocytochemical localization of a neuronal nicotinic receptor: the beta 2-subunit. J Neurosci. 1993;13(4):1551–1568. doi: 10.1523/JNEUROSCI.13-04-01551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Danober L, Simler S, Pereira de Vasconcelos A, Maton B, Nehlig A, Marescaux C, Vergnes M. The amygdala is critical for seizure propagation from brainstem to forebrain. Neuroscience. 1997;77(4):975–984. doi: 10.1016/s0306-4522(96)00503-9. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems and barriers to care. US Army Med Dep J. 2008:7–17. [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32(7):1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson LP, Munoz DG, Miller L, McLachlan RS, Girvin JP, Blume WT. Amygdaloid sclerosis in temporal lobe epilepsy. Ann Neurol. 1993;33(6):622–631. doi: 10.1002/ana.410330611. [DOI] [PubMed] [Google Scholar]
- Hunter JM, Cirrito JR, Restivo JL, Kinley RD, Sullivan PM, Holtzman DM, Koger D, Delong C, Lin S, Zhao L, Liu F, Bales K, Paul SM. Emergence of a seizure phenotype in aged apolipoprotein epsilon 4 targeted replacement mice. Brain Res. 2012;1467:120–132. doi: 10.1016/j.brainres.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Iwakiri M, Mizukami K, Ikonomovic MD, Ishikawa M, Abrahamson EE, DeKosky ST, Asada T. An immunohistochemical study of GABA A receptor gamma subunits in Alzheimer's disease hippocampus: relationship to neurofibrillary tangle progression. Neuropathology. 2009;29(3):263–269. doi: 10.1111/j.1440-1789.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Ressler KJ, Hammack SE, Chhatwal JP, Rainnie DG. Distinct subtypes of cholecystokinin (CCK)-containing interneurons of the basolateral amygdala identified using a CCK promoter-specific lentivirus. J Neurophysiol. 2009;101(3):1494–1506. doi: 10.1152/jn.91149.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Xu J, Nedergaard M, Kang J. A kainate receptor increases the efficacy of GABAergic synapses. Neuron. 2001;30(2):503–513. doi: 10.1016/s0896-6273(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xing G, Yang C, Verma A, Zhang L, Li H. Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology. 2009;34(2):410–423. doi: 10.1038/npp.2008.71. [DOI] [PubMed] [Google Scholar]
- Kandratavicius L, Lopes-Aguiar C, Bueno-Junior LS, Romcy-Pereira RN, Hallak JE, Leite JP. Psychiatric comorbidities in temporal lobe epilepsy: possible relationships between psychotic disorders and involvement of limbic circuits. Rev Bras Psiquiatr. 2012;34(4):454–466. doi: 10.1016/j.rbp.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Tamamaki N, Owada H, Kakizaki T, Kume N, Totsuka M, Yamamoto T, Yawo H, Yagi T, Obata K, Yanagawa Y. Noradrenergic excitation of a subpopulation of GABAergic cells in the basolateral amygdala via both activation of nonselective cationic conductance and suppression of resting K+ conductance: a study using glutamate decarboxylase 67-green fluorescent protein knock-in mice. Neuroscience. 2008;157(4):781–797. doi: 10.1016/j.neuroscience.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21(23):9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen S, Pitkanen A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comp Neurol. 2000;426(3):441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Khetrapal N. Overlap of autism and seizures: understanding cognitive comorbidity. Mens Sana Monogr. 2010;8(1):122–128. doi: 10.4103/0973-1229.58823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Burris J, Bassal F, Koldewyn K, Chattarji S, Tassone F, Hessl D, Rivera SM. Fear-specific amygdala function in children and adolescents on the fragile x spectrum: a dosage response of the FMR1 gene. Cereb Cortex. 2014;24(3):600–613. doi: 10.1093/cercor/bhs341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Koyama S, Akaike N. Presynaptic modulation of synaptic gamma-aminobutyric acid transmission by tandospirone in rat basolateral amygdala. Eur J Pharmacol. 2000;407(3):257–265. doi: 10.1016/s0014-2999(00)00673-7. [DOI] [PubMed] [Google Scholar]
- Klein RC, Acheson SK, Mace BE, Sullivan PM, Moore SD. Altered neurotransmission in the lateral amygdala in aged human apoE4 targeted replacement mice. Neurobiol Aging. 2014;35(9):2046–2052. doi: 10.1016/j.neurobiolaging.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RC, Mace BE, Moore SD, Sullivan PM. Progressive loss of synaptic integrity in human apolipoprotein E4 targeted replacement mice and attenuation by apolipoprotein E2. Neuroscience. 2010;171(4):1265–1272. doi: 10.1016/j.neuroscience.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RC, Yakel JL. Functional somato-dendritic alpha7-containing nicotinic acetylcholine receptors in the rat basolateral amygdala complex. J Physiol. 2006;576(Pt 3):865–872. doi: 10.1113/jphysiol.2006.118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Kubo C, Rhee JS, Akaike N. Presynaptic serotonergic inhibition of GABAergic synaptic transmission in mechanically dissociated rat basolateral amygdale neurons. J Physiol 518. 1999;(Pt 2):525–538. doi: 10.1111/j.1469-7793.1999.0525p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Matsumoto N, Kubo C, Akaike N. Presynaptic 5-HT3 receptor-mediated modulation of synaptic GABA release in the mechanically dissociated rat amygdale neurons. J Physiol. 2000;529(Pt 2):373–383. doi: 10.1111/j.1469-7793.2000.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Matsumoto N, Murakami N, Kubo C, Nabekura J, Akaike N. Role of presynaptic 5-HT1A and 5-HT3 receptors in modulation of synaptic GABA transmission in dissociated rat basolateral amygdala neurons. Life Sci. 2002;72(4–5):375–387. doi: 10.1016/s0024-3205(02)02280-4. [DOI] [PubMed] [Google Scholar]
- Kroner S, Rosenkranz JA, Grace AA, Barrionuevo G. Dopamine modulates excitability of basolateral amygdala neurons in vitro. J Neurophysiol. 2005;93(3):1598–1610. doi: 10.1152/jn.00843.2004. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Pare D. Similar inhibitory processes dominate the responses of cat lateral amygdaloid projection neurons to their various afferents. J Neurophysiol. 1997;77(1):341–352. doi: 10.1152/jn.1997.77.1.341. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Pare D. Synaptic responsiveness of interneurons of the cat lateral amygdaloid nucleus. Neuroscience. 1998;83(3):877–889. doi: 10.1016/s0306-4522(97)00420-x. [DOI] [PubMed] [Google Scholar]
- Lange MD, Jungling K, Paulukat L, Vieler M, Gaburro S, Sosulina L, Blaesse P, Sreepathi HK, Ferraguti F, Pape HC. Glutamic acid decarboxylase 65: a link between GABAergic synaptic plasticity in the lateral amygdala and conditioned fear generalization. Neuropsychopharmacology. 2014;39(9):2211–2220. doi: 10.1038/npp.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, Milner TA. Ultrastructure and synaptic associations of auditory thalamo-amygdala projections in the rat. Exp Brain Res. 1991;85(3):577–586. doi: 10.1007/BF00231742. [DOI] [PubMed] [Google Scholar]
- Lehner M, Wislowska-Stanek A, Skorzewska A, Maciejak P, Szyndler J, Turzynska D, Sobolewska A, Plaznik A. Differences in the density of GABA-A receptor alpha-2 subunits and gephyrin in brain structures of rats selected for low and high anxiety in basal and fear-stimulated conditions, in a model of contextual fear conditioning. Neurobiol Learn Mem. 2010;94(4):499–508. doi: 10.1016/j.nlm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Levenga J, Krishnamurthy P, Rajamohamedsait H, Wong H, Franke TF, Cain P, Sigurdsson EM, Hoeffer CA. Tau pathology induces loss of GABAergic interneurons leading to altered synaptic plasticity and behavioral impairments. Acta Neuropathol Commun. 2013;1(1):34. doi: 10.1186/2051-5960-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Rogawski MA. GluR5 kainate receptor mediated synaptic transmission in rat basolateral amygdala in vitro. Neuropharmacology. 1998;37(10–11):1279–1286. doi: 10.1016/s0028-3908(98)00109-9. [DOI] [PubMed] [Google Scholar]
- Li R, Nishijo H, Ono T, Ohtani Y, Ohtani O. Synapses on GABAergic neurons in the basolateral nucleus of the rat amygdala: double-labeling immunoelectron microscopy. Synapse. 2002;43(1):42–50. doi: 10.1002/syn.10017. [DOI] [PubMed] [Google Scholar]
- Liu YH, Wang L, Wei LC, Huang YG, Chen LW. Up-regulation of D-serine might induce GABAergic neuronal degeneration in the cerebral cortex and hippocampus in the mouse pilocarpine model of epilepsy. Neurochem Res. 2009;34(7):1209–1218. doi: 10.1007/s11064-008-9897-0. [DOI] [PubMed] [Google Scholar]
- Loretan K, Bissiere S, Luthi A. Dopaminergic modulation of spontaneous inhibitory network activity in the lateral amygdala. Neuropharmacology. 2004;47(5):631–639. doi: 10.1016/j.neuropharm.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Loreth D, Ozmen L, Revel FG, Knoflach F, Wetzel P, Frotscher M, Metzger F, Kretz O. Selective degeneration of septal and hippocampal GABAergic neurons in a mouse model of amyloidosis and tauopathy. Neurobiol Dis. 2012;47(1):1–12. doi: 10.1016/j.nbd.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Ma QP, Yin GF, Ai MK, Han JS. Serotonergic projections from the nucleus raphe dorsalis to the amygdala in the rat. Neurosci Lett. 1991;134(1):21–24. doi: 10.1016/0304-3940(91)90499-j. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394(6694):683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Manko M, Geracitano R, Capogna M. Functional connectivity of the main intercalated nucleus of the mouse amygdala. J Physiol. 2011;589(Pt 8):1911–1925. doi: 10.1113/jphysiol.2010.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Fritschy JM, Vogt KE. Functional mapping of GABA A receptor subtypes in the amygdala. Eur J Neurosci. 2004;20(5):1281–1289. doi: 10.1111/j.1460-9568.2004.03574.x. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Rudolph U, Fritschy JM, Arand M. Tonic inhibition in principal cells of the amygdala: a central role for alpha3 subunit-containing GABAA receptors. J Neurosci. 2012;32(25):8611–8619. doi: 10.1523/JNEUROSCI.4404-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48(6):1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Martin BS, Corbin JG, Huntsman MM. Deficient tonic GABAergic conductance and synaptic balance in the fragile X syndrome amygdala. J Neurophysiol. 2014;112(4):890–902. doi: 10.1152/jn.00597.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976(2):171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. A novel subpopulation of 5-HT type 3A receptor subunit immunoreactive interneurons in the rat basolateral amygdala. Neuroscience. 2007;144(3):1015–1024. doi: 10.1016/j.neuroscience.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Parvalbumin-immunoreactive neurons and GABAergic neurons of the basal forebrain project to the rat basolateral amygdala. Neuroscience. 2009;160(4):805–812. doi: 10.1016/j.neuroscience.2009.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Potter LT. Autoradiographic localization of M1 and M2 muscarine receptors in the rat brain. Neuroscience. 1986;19(2):551–564. doi: 10.1016/0306-4522(86)90280-0. [DOI] [PubMed] [Google Scholar]
- McDonald A. Cell types and intrinsic connections of the amygdala. In: Aggleton J, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York, NY: Wiley-Liss, Inc; 1992. [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 2001a;105(3):681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 2001b;107(4):641–652. doi: 10.1016/s0306-4522(01)00380-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943(2):237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Parvalbumin-containing interneurons in the basolateral amygdala express high levels of the alpha1 subunit of the GABAA receptor. J Comp Neurol. 2004;473(1):137–146. doi: 10.1002/cne.20101. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience. 2007;146(1):306–320. doi: 10.1016/j.neuroscience.2007.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of m1 muscarinic receptor immunoreactivity in the rat basolateral amygdala. Brain Struct Funct. 2010;215(1):37–48. doi: 10.1007/s00429-010-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of M2 muscarinic receptor immunoreactivity in the rat amygdala. Neuroscience. 2011;196:49–65. doi: 10.1016/j.neuroscience.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Muller JF. Immunocytochemical localization of GABABR1 receptor subunits in the basolateral amygdala. Brain Res. 2004;1018(2):147–158. doi: 10.1016/j.brainres.2004.05.053. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. Postsynaptic targets of GABAergic basal forebrain projections to the basolateral amygdala. Neuroscience. 2011;183:144–159. doi: 10.1016/j.neuroscience.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci Lett. 1989;100(1–3):53–58. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, McLeod CG, Jr, Nipwoda MT. Direct microinjection of soman or VX into the amygdala produces repetitive limbic convulsions and neuropathology. Brain Res. 1987;435(1–2):123–137. doi: 10.1016/0006-8993(87)91593-9. [DOI] [PubMed] [Google Scholar]
- Miller LA, McLachlan RS, Bouwer MS, Hudson LP, Munoz DG. Amygdalar sclerosis: preoperative indicators and outcome after temporal lobectomy. J Neurol Neurosurg Psychiatry. 1994;57(9):1099–1105. doi: 10.1136/jnnp.57.9.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Younes L, Ratnanather JT, Brown T, Trinh H, Lee DS, Tward D, Mahon PB, Mori S, Albert M, Team BR. Amygdalar atrophy in symptomatic Alzheimer's disease based on diffeomorphometry: the BIOCARD cohort. Neurobiol Aging. 2015;(36 Suppl 1):S3–S10. doi: 10.1016/j.neurobiolaging.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhouse OE, DeOlmos J. Neuronal configurations in lateral and basolateral amygdala. Neuroscience. 1983;10(4):1269–1300. doi: 10.1016/0306-4522(83)90112-4. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Ozaki M, Wada K, Sekiguchi M. Noradrenaline-induced spontaneous inhibitory postsynaptic currents in mouse basolateral nucleus of amygdala pyramidal neurons: comparison with dopamine-induced currents. Neurosci Lett. 2010;480(3):167–172. doi: 10.1016/j.neulet.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Mizukami K, Ikonomovic MD, Grayson DR, Sheffield R, Armstrong DM. Immunohistochemical study of GABAA receptor alpha1 subunit in the hippocampal formation of aged brains with Alzheimer-related neuropathologic changes. Brain Res. 1998;799(1):148–155. doi: 10.1016/s0006-8993(98)00437-5. [DOI] [PubMed] [Google Scholar]
- Mohler H. GABAA receptors in central nervous system disease: anxiety, epilepsy, and insomnia. J Recept Signal Transduct Res. 2006;26(5–6):731–740. doi: 10.1080/10799890600920035. [DOI] [PubMed] [Google Scholar]
- Muller I, Caliskan G, Stork O. The GAD65 knock out mouse - a model for GABAergic processes in fear- and stress-induced psychopathology. Genes Brain Behav. 2015;14(1):37–45. doi: 10.1111/gbb.12188. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol. 2003;456(3):217–236. doi: 10.1002/cne.10435. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Coupled networks of parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Neurosci. 2005;25(32):7366–7376. doi: 10.1523/JNEUROSCI.0899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494(4):635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007a;500(3):513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J Comp Neurol. 2007b;505(3):314–335. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Cholinergic innervation of pyramidal cells and parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Comp Neurol. 2011;519(4):790–805. doi: 10.1002/cne.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, Zaric V, McDonald AJ. Muscarinic cholinergic receptor M1 in the rat basolateral amygdala: ultrastructural localization and synaptic relationships to cholinergic axons. J Comp Neurol. 2013;521(8):1743–1759. doi: 10.1002/cne.23254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat. 2015;11:165–175. doi: 10.2147/NDT.S58841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshiro H, Kubota S, Murakoshi T. Dopaminergic modulation of oscillatory network inhibition in the rat basolateral amygdala depends on initial activity state. Neuropharmacology. 2011;61(4):857–866. doi: 10.1016/j.neuropharm.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci. 2010;30(29):9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56(1):141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55(5):697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Synaptic depression and aberrant excitatory network activity in Alzheimer's disease: two faces of the same coin? Neuromolecular Med. 2010;12(1):48–55. doi: 10.1007/s12017-009-8097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluszkiewicz SM, Martin BS, Huntsman MM. Fragile X syndrome: the GABAergic system and circuit dysfunction. Dev Neurosci. 2011a;33(5):349–364. doi: 10.1159/000329420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluszkiewicz SM, Olmos-Serrano JL, Corbin JG, Huntsman MM. Impaired inhibitory control of cortical synchronization in fragile X syndrome. J Neurophysiol. 2011b;106(5):2264–2272. doi: 10.1152/jn.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BX, Dong Y, Ito W, Yanagawa Y, Shigemoto R, Morozov A. Selective gating of glutamatergic inputs to excitatory neurons of amygdala by presynaptic GABAb receptor. Neuron. 2009;61(6):917–929. doi: 10.1016/j.neuron.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci. 1996;16(10):3334–3350. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Pape HC, Dong J. Bursting and oscillating neurons of the cat basolateral amygdaloid complex in vivo: electrophysiological properties and morphological features. J Neurophysiol. 1995;74(3):1179–1191. doi: 10.1152/jn.1995.74.3.1179. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. Intrinsic circuitry of the amygdaloid complex: common principles of organization in rats and cats. Trends Neurosci. 1998;21(6):240–241. doi: 10.1016/s0166-2236(98)01240-5. [DOI] [PubMed] [Google Scholar]
- Parikh MS, Kolevzon A, Hollander E. Psychopharmacology of aggression in children and adolescents with autism: a critical review of efficacy and tolerability. J Child Adolesc Psychopharmacol. 2008;18(2):157–178. doi: 10.1089/cap.2007.0041. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74(2):129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Aroniadou-Anderjaska V, Prager EM, Figueiredo TH, Almeida-Suhett CP, Miller SL, Braga MF. ASIC1a activation enhances inhibition in the basolateral amygdala and reduces anxiety. J Neurosci. 2014;34(9):3130–3141. doi: 10.1523/JNEUROSCI.4009-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, Prager EM, Aroniadou-Anderjaska V, Braga MF. alpha7-Containing nicotinic acetylcholine receptors on interneurons of the basolateral amygdala and their role in the regulation of the network excitability. J Neurophysiol. 2013;110(10):2358–2369. doi: 10.1152/jn.01030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinard CR, Muller JF, Mascagni F, McDonald AJ. Dopaminergic innervation of interneurons in the rat basolateral amygdala. Neuroscience. 2008;157(4):850–863. doi: 10.1016/j.neuroscience.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A. Connectivity of the rat amygdaloid complex. In: Aggleton J, editor. The amygdala: A functional analysis. Oxford: OUP; 2000. [Google Scholar]
- Pitkanen A, Tuunanen J, Kalviainen R, Partanen K, Salmenpera T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32(1–2):233–253. doi: 10.1016/s0920-1211(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Polepalli JS, Sullivan RK, Yanagawa Y, Sah P. A specific class of interneuron mediates inhibitory plasticity in the lateral amygdala. J Neurosci. 2010;30(44):14619–14629. doi: 10.1523/JNEUROSCI.3252-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293(5532):1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC Alzheimer's Disease Neuroimaging I. Amygdala atrophy is prominent in early Alzheimer's disease and relates to symptom severity. Psychiatry Res. 2011;194(1):7–13. doi: 10.1016/j.pscychresns.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, Figueiredo TH, Apland JP, Braga MF. Acetylcholinesterase inhibition in the basolateral amygdala plays a key role in the induction of status epilepticus after soman exposure. Neurotoxicology. 2013;38:84–90. doi: 10.1016/j.neuro.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, Figueiredo TH, Apland JP, Rossetti F, Olsen CH, Braga MF. The recovery of acetylcholinesterase activity and the progression of neuropathological and pathophysiological alterations in the rat basolateral amygdala after soman-induced status epilepticus: relation to anxiety-like behavior. Neuropharmacology. 2014a;81:64–74. doi: 10.1016/j.neuropharm.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Figueiredo TH, Long RP, 2nd, Aroniadou-Anderjaska V, Apland JP, Braga MF. LY293558 prevents soman-induced pathophysiological alterations in the basolateral amygdala and the development of anxiety. Neuropharmacology. 2015;89:11–18. doi: 10.1016/j.neuropharm.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Pidoplichko VI, Aroniadou-Anderjaska V, Apland JP, Braga MF. Pathophysiological mechanisms underlying increased anxiety after soman exposure: reduced GABAergic inhibition in the basolateral amygdala. Neurotoxicology. 2014b;44:335–343. doi: 10.1016/j.neuro.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese M, Carrasco JL, Geloso MC, Mascort J, Michetti F, Mahy N. Gamma-aminobutyric acidergic interneuron vulnerability to aging in canine prefrontal cortex. J Neurosci Res. 2004;77(6):913–920. doi: 10.1002/jnr.20223. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82(1):69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Excitatory transmission in the basolateral amygdala. J Neurophysiol. 1991a;66(3):986–998. doi: 10.1152/jn.1991.66.3.986. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. J Neurophysiol. 1991b;66(3):999–1009. doi: 10.1152/jn.1991.66.3.999. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Kindling-induced long-lasting changes in synaptic transmission in the basolateral amygdala. J Neurophysiol. 1992;67(2):443–454. doi: 10.1152/jn.1992.67.2.443. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Mania I, Mascagni F, McDonald AJ. Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. J Comp Neurol. 2006;498(1):142–161. doi: 10.1002/cne.21049. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Reger ML, Poulos AM, Buen F, Giza CC, Hovda DA, Fanselow MS. Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biol Psychiatry. 2012;71(4):335–343. doi: 10.1016/j.biopsych.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci. 1999;19(24):11027–11039. doi: 10.1523/JNEUROSCI.19-24-11027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83(3):803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52(4):701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Schatz AR, Lee M, Condie RB, Pulaski JT, Kaminski NE. Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol. 1997;142(2):278–287. doi: 10.1006/taap.1996.8034. [DOI] [PubMed] [Google Scholar]
- Schiffer HH, Swanson GT, Heinemann SF. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. 1997;19(5):1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22(11):565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21(10):3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13(2):596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27(5):262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shoji Y, Tanaka E, Yamamoto S, Maeda H, Higashi H. Mechanisms underlying the enhancement of excitatory synaptic transmission in basolateral amygdala neurons of the kindling rat. J Neurophysiol. 1998;80(2):638–646. doi: 10.1152/jn.1998.80.2.638. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Chappell AM, Yorgason JT, Weiner JL. Lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the anxiolytic effects of beta 3 adrenoceptor activation. Neuropsychopharmacology. 2010;35(9):1886–1896. doi: 10.1038/npp.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Weiner JL. beta1-adrenoceptor activation is required for ethanol enhancement of lateral paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2012;343(2):451–459. doi: 10.1124/jpet.112.196022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorzewska A, Lehner M, Wislowska-Stanek A, Krzascik P, Ziemba A, Plaznik A. The effect of chronic administration of corticosterone on anxiety- and depression-like behavior and the expression of GABA-A receptor alpha-2 subunits in brain structures of low- and high-anxiety rats. Horm Behav. 2014;65(1):6–13. doi: 10.1016/j.yhbeh.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Enhanced population responses in the basolateral amygdala of kainate-treated, epileptic rats in vitro. Neuroscience Letters. 1997;222(1):1–4. doi: 10.1016/s0304-3940(97)13326-2. [DOI] [PubMed] [Google Scholar]
- Smith TA. Type A gamma-aminobutyric acid (GABAA) receptor subunits and benzodiazepine binding: significance to clinical syndromes and their treatment. Br J Biomed Sci. 2001;58(2):111–121. [PubMed] [Google Scholar]
- Smith Y, Pare JF, Pare D. Cat intraamygdaloid inhibitory network: ultrastructural organization of parvalbumin-immunoreactive elements. J Comp Neurol. 1998;391(2):164–179. doi: 10.1002/(sici)1096-9861(19980209)391:2<164::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare JF, Pare D. Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J Comp Neurol. 2000;416(4):496–508. [PubMed] [Google Scholar]
- Spampanato J, Polepalli J, Sah P. Interneurons in the basolateral amygdala. Neuropharmacology. 2011;60(5):765–773. doi: 10.1016/j.neuropharm.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Stein C, Davidowa H, Albrecht D. 5-HT(1A) receptor-mediated inhibition and 5-HT(2) as well as 5-HT(3) receptor-mediated excitation in different subdivisions of the rat amygdala. Synapse. 2000;38(3):328–337. doi: 10.1002/1098-2396(20001201)38:3<328::AID-SYN12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Stujenske JM, Likhtik E, Topiwala MA, Gordon JA. Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron. 2014;83(4):919–933. doi: 10.1016/j.neuron.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Tanaka E, North RA. Membrane properties and synaptic potentials of three types of neurone in rat lateral amygdala. J Physiol. 1993;460:705–718. doi: 10.1113/jphysiol.1993.sp019495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvrathan A, Chattarji S. Fragile X syndrome and the amygdala. Curr Opin Neurobiol. 2011;21(3):509–515. doi: 10.1016/j.conb.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Swanson L, Petrovich G. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM, Whiting PJ, Lindstrom J. Immunohistochemical localization of neuronal nicotinic receptors in the rodent central nervous system. J Neurosci. 1987;7(10):3334–3342. doi: 10.1523/JNEUROSCI.07-10-03334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szinyei C, Heinbockel T, Montagne J, Pape HC. Putative cortical and thalamic inputs elicit convergent excitation in a population of GABAergic interneurons of the lateral amygdala. J Neurosci. 2000;20(23):8909–8915. doi: 10.1523/JNEUROSCI.20-23-08909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szinyei C, Stork O, Pape HC. Contribution of NR2B subunits to synaptic transmission in amygdaloid interneurons. J Neurosci. 2003;23(7):2549–2556. doi: 10.1523/JNEUROSCI.23-07-02549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BC, Hagel EM, Carlson KF, Cifu DX, Cutting A, Bidelspach DE, Sayer NA. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care. 2012;50(4):342–346. doi: 10.1097/MLR.0b013e318245a558. [DOI] [PubMed] [Google Scholar]
- Terburg D, Morgan BE, Montoya ER, Hooge IT, Thornton HB, Hariri AR, Panksepp J, Stein DJ, van Honk J. Hypervigilance for fear after basolateral amygdala damage in humans. Transl Psychiatry. 2012;2:e115. doi: 10.1038/tp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble MR, Van Elst LT. The amygdala and psychopathology studies in epilepsy. Ann N Y Acad Sci. 2003;985:461–468. doi: 10.1111/j.1749-6632.2003.tb07100.x. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;160(2):284–294. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt WA, Sajdyk TJ, Dietrich AD, Oberlin B, McDougle CJ, Shekhar A. From anxiety to autism: spectrum of abnormal social behaviors modeled by progressive disruption of inhibitory neuronal function in the basolateral amygdala in Wistar rats. Psychopharmacology (Berl) 2007;191(1):107–118. doi: 10.1007/s00213-006-0674-y. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Kosaka K. Neuropathological study of the amygdala in presenile Alzheimer's disease. J Neurol Sci. 1990;100(1–2):165–173. doi: 10.1016/0022-510x(90)90029-m. [DOI] [PubMed] [Google Scholar]
- Tuchman R, Cuccaro M. Epilepsy and autism: neurodevelopmental perspective. Curr Neurol Neurosci Rep. 2011;11(4):428–434. doi: 10.1007/s11910-011-0195-x. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Halonen T, Pitkanen A. Seizures cause selective regional damage and loss of GABAergic neurons in the rat amygdaloid complex. Eur J Neurosci. 1996;8:2711–2725. doi: 10.1111/j.1460-9568.1996.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Halonen T, Pitkanen A. Decrease in somatostatin-immunoreactive neurons in the rat amygdaloid complex in a kindling model of temporal lobe epilepsy. Epilepsy Res. 1997;26(2):315–327. doi: 10.1016/s0920-1211(96)00900-x. [DOI] [PubMed] [Google Scholar]
- Unal CT, Pare D, Zaborszky L. Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. J Neurosci. 2015;35(2):853–863. doi: 10.1523/JNEUROSCI.2706-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal G, Pare JF, Smith Y, Pare D. Cortical inputs innervate calbindin-immunoreactive interneurons of the rat basolateral amygdaloid complex. J Comp Neurol. 2014;522(8):1915–1928. doi: 10.1002/cne.23511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriguen L, Garcia-Gutierrez MS, Manzanares J. Decreased GABAA and GABAB receptor functional activity in cannabinoid CB1 receptor knockout mice. J Psychopharmacol. 2011;25(1):105–110. doi: 10.1177/0269881109358204. [DOI] [PubMed] [Google Scholar]
- van der Kolk BA. The neurobiology of childhood trauma and abuse. Child Adolesc Psychiatr Clin N Am. 2003;12(2):293–317. doi: 10.1016/s1056-4993(03)00003-8. [DOI] [PubMed] [Google Scholar]
- Vazquez B, Devinsky O. Epilepsy and anxiety. Epilepsy Behav. 2003;(4 Suppl 4):S20–S25. doi: 10.1016/j.yebeh.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Vislay RL, Martin BS, Olmos-Serrano JL, Kratovac S, Nelson DL, Corbin JG, Huntsman MM. Homeostatic responses fail to correct defective amygdala inhibitory circuit maturation in fragile X syndrome. J Neurosci. 2013;33(17):7548–7558. doi: 10.1523/JNEUROSCI.2764-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386(6621):173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Walls AB, Nilsen LH, Eyjolfsson EM, Vestergaard HT, Hansen SL, Schousboe A, Sonnewald U, Waagepetersen HS. GAD65 is essential for synthesis of GABA destined for tonic inhibition regulating epileptiform activity. J Neurochem. 2010;115(6):1398–1408. doi: 10.1111/j.1471-4159.2010.07043.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Wilson WA, Moore SD, Mace BE, Maeda N, Schmechel DE, Sullivan PM. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol Dis. 2005;18(2):390–398. doi: 10.1016/j.nbd.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Inhibitory responses of rat basolateral amygdaloid neurons recorded in vitro. Neuroscience. 1992;50(4):811–830. doi: 10.1016/0306-4522(92)90206-h. [DOI] [PubMed] [Google Scholar]
- Watson C, Hoeft F, Garrett AS, Hall SS, Reiss AL. Aberrant brain activation during gaze processing in boys with fragile X syndrome. Arch Gen Psychiatry. 2008;65(11):1315–1323. doi: 10.1001/archpsyc.65.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, LeDoux JE. Distinct populations of NMDA receptors at subcortical and cortical inputs to principal cells of the lateral amygdala. J Neurophysiol. 1999;81(2):930–934. doi: 10.1152/jn.1999.81.2.930. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14(7):461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen P, Phelps E. The Human Amygdala. New York, NY: The Guilford Press; 2009. [Google Scholar]
- White LE, Price JL. The Functional-Anatomy of Limbic Status Epilepticus in the Rat .1. Patterns of C-14 2-Deoxyglucose Uptake and Fos Immunocytochemistry. Journal of Neuroscience. 1993;13(11):4787–4809. doi: 10.1523/JNEUROSCI.13-11-04787.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Men D, Clayton EC. The effects of noradrenergic activation of the nucleus tractus solitarius on memory and in potentiating norepinephrine release in the amygdala. Behav Neurosci. 2000;114(6):1131–1144. doi: 10.1037//0735-7044.114.6.1131. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44(4):851–859. [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31(3):453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. J Neurophysiol. 2007a;98(5):2956–2961. doi: 10.1152/jn.00739.2007. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci. 2007b;27(3):553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic systems in the rat brain: I. projections to the limbic telencephalon. Brain Res Bull. 1984;13(6):751–784. doi: 10.1016/0361-9230(84)90236-3. [DOI] [PubMed] [Google Scholar]
- Yakel JL. Cholinergic receptors: functional role of nicotinic ACh receptors in brain circuits and disease. Pflugers Arch. 2013;465(4):441–450. doi: 10.1007/s00424-012-1200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Saitow F, Satake S, Kiyohara T, Konishi S. GABA(B) receptor-mediated presynaptic inhibition of glutamatergic and GABAergic transmission in the basolateral amygdala. Neuropharmacology. 1999;38(11):1743–1753. doi: 10.1016/s0028-3908(99)00126-4. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Heimer L, Eckenstein F, Leranth C. GABAergic input to cholinergic forebrain neurons: an ultrastructural study using retrograde tracing of HRP and double immunolabeling. J Comp Neurol. 1986;250(3):282–295. doi: 10.1002/cne.902500303. [DOI] [PubMed] [Google Scholar]
- Zangrossi H, Jr, Viana MB, Graeff FG. Anxiolytic effect of intra-amygdala injection of midazolam and 8-hydroxy-2-(di-n-propylamino)tetralin in the elevated T-maze. Eur J Pharmacol. 1999;369(3):267–270. doi: 10.1016/s0014-2999(99)00075-8. [DOI] [PubMed] [Google Scholar]
- Zhang L, He J, Jugloff DG, Eubanks JH. The MeCP2-null mouse hippocampus displays altered basal inhibitory rhythms and is prone to hyperexcitability. Hippocampus. 2008;18(3):294–309. doi: 10.1002/hipo.20389. [DOI] [PubMed] [Google Scholar]
- Zhou X, Hollern D, Liao J, Andrechek E, Wang H. NMDA receptor-mediated excitotoxicity depends on the coactivation of synaptic and extrasynaptic receptors. Cell Death Dis. 2013;4:e560. doi: 10.1038/cddis.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Stewart RR, McIntosh JM, Weight FF. Activation of nicotinic acetylcholine receptors increases the frequency of spontaneous GABAergic IPSCs in rat basolateral amygdala neurons. J Neurophysiol. 2005;94(5):3081–3091. doi: 10.1152/jn.00974.2004. [DOI] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139(5):1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinebi F, Russell RT, McKernan M, Shinnick-Gallagher P. Comparison of paired-pulse facilitation of AMPA and NMDA synaptic currents in the lateral amygdala. Synapse. 2001;42(2):115–127. doi: 10.1002/syn.1107. [DOI] [PubMed] [Google Scholar]