Abstract
Background
Of all human cancers, gastric carcinoma is the one of the leading causes of death. Helicobacter pylori is considered a major etiologic agent of this disease. Spontaneously occurring gastric carcinoma is a rare diagnosis in nonhuman primates. A 2011 case report documented a high incidence of gastric adenocarcinoma in a closed colony of captive sooty mangabeys (Cercebus atys). However, H. pylori infection was not detected in these animals.
Materials and Methods
In this study, using archived formalin-fixed, paraffin-embedded stomach sections of these animals alternative methodologies were used to identify H. pylori and other non-H. pylori Helicobacter species. In addition, two additional cases of sooty mangabeys with metastatic gastric carcinoma are characterized.
Results
Using fluorescent in situ hybridization, we identified gastric H. suis in 75% of archived and new gastric carcinoma cases. In the two newly reported cases, H. suis and a novel Helicobacter species were detected via PCR and sequence analysis of the 16S rRNA gene. H. pylori was not identified in any of the gastric carcinoma cases via FISH and/or PCR and sequence analysis of Helicobacter spp. in DNA from of available tissues.
Conclusions
This report is the first to characterize Helicobacter species infection in spontaneous gastric carcinoma with metastatic potential in nonhuman primates.
Keywords: Helicobacter, metastatic gastric adenocarcinoma, sooty mangabeys
A recent case report described spontaneous gastric adenocarcinomas in captive sooty mangabeys (Cercocebus atys) [1]. This report was the first to document this disease in this species and was remarkable because of the high incidence of this rare cancer in a nonhuman primate (NHP) species [2–4]. The report described eight cases of sooty mangabeys with gastric carcinoma that occurred over the span of 9 years [1]. Human serum antigen tests and human immunohistochemistry assays (antihuman Helicobacter antibody, clone BC7, Biocare Medical, Concord, MA, USA) were negative for H. pylori.
Helicobacter pylori is not commonly identified in the stomachs of animals; however, it is known that H. pylori colonizes the stomach of macaques [5]. Several non-H. pylori Helicobacter (NHPH) species have also been identified in primates and subclassified into “H. heilmanni” type 1 and “H. heilmannii” type 2 [6,7]. “H. heilmanni” type 1 has been further classified as H. suis [8,9]. H. suis has been characterized in a the stomachs of a several nonhuman primates, including mandrill monkeys (Mandrillus sphinx), crab-eating (Macaca fascicularis), and rhesus macaques (Macaca mulatta) [6,10].
Given an etiologic agent of gastric adenocarcinoma in sooty mangabey was not identified and that H. pylori is the causative agent of this gastric cancer in humans, we performed follow-up diagnostic tests with the goal of detecting H. pylori or NHPH in the affected animals described in the 2011 case report. In addition, we characterized two additional cases of gastric carcinoma with metastasis.
Materials and Methods
Animals
All animals surveyed in this study were born at the Field Station of the Yerkes National Primate Research Center and housed in accordance with the 8th Edition of the Guide for the Care and Use of Laboratory Animals. All animals were fed a standard monkey chow diet (Purina Lab-Diet 5037, St Louis, MO, USA) with daily approved enrichment. Animals were housed in either large indoor/outdoor compounds or indoor/outdoor runs in various social groups. The animals were either part of the breeding colony or assigned to research protocols that involved periodic collection of minimally invasive biological samples such as blood. All procedures were performed under an approved Emory IACUC protocol.
A total of eight animals with gastric adenocarcinoma were examined in this study. Two males (age range 11 years 5 months to 16 years 9 months) and four females (age range 10 years to 21 years 5 months) were included from the previous 2011 study (animal ID #3 to #8). Two additional animals with gastric adenocarcinoma not previously published are described in this report (study animals #9 and #10). All study animals are described in Table 1. Typical case presentation for these animals was chronic weight loss and intermittent vomiting. Gross necropsy findings indicate moderate to severe stomach distension with gastric wall thickening and ulceration in all cases but one. In four animals, transmural extension of gastric tumors resulted in abdominal adhesions between gastric serosa and other viscera. Neoplastic lesions were located in the pyloric region with extension into gastric-duodenal junction. Histopathology of pyloric masses showed focal extensive ulceration of gastric mucosa, which was intermixed with noncircumscribed neoplastic infiltrated of epithelial cells arranged as acini or solid mass of cells. Epithelial cells exhibited cellular polymorphism, anisocytosis, and anisokaryosis.
Table 1.
Summary of clinical and gross findings of animals with gastric carcinoma (modified from Sharma et al. [1])
| Animal no. | Signalment | Clinical history | Gross lesions |
|---|---|---|---|
| 3 | Male, 12 year | Abdominal distension, weight loss, and bloody diarrhea. | The gastric pylorus had severe diffuse thickening of the wall (1 cm thick). Ulcerated and hemorrhagic mucosa (5 × 4 cm). Partially occluded lumen. |
| 4 | Male, 12 year 5 month | Abdominal distension and weight loss. Gastric fluid contained blood. | The pyloric wall was severely thickened constricting the gastric lumen. The gastric mucosa was focally ulcerated and hemorrhagic. |
| 5 | Female, 10 year | Weight loss, severe anemia, hypoproteinemia, hypoalbuminemia, with elevated liver enzymes. | A large irregular ulcer (approximately 5.0 × 6.0 cm) with deeply umbilicated edges extending from the esophageal junction to the pylorus. |
| 6 | Female, 21 year 5 month | Diabetes with associated weight loss; spondyloarthropathy. | The mucosa had a focal 2.0-cm-long erosion along with moderate thickening of the gastric wall. |
| 7 | Female, 16 year | Gastric distension, weight loss, hepatomegaly, azotemia, hypoalbuminemia, and elevated liver enzymes. | Stomach dilated and enlarged to approximately 5 times normal size. The pyloris region was thickened and ulcerated. The gastric mass appeared to have infiltrated into duodenum and pancreas |
| 8 | Female, 17 year | Weight loss, lethargy, abdominal pain. | Distended stomach, thickened pyloric wall, focal extensive mucosal ulceration. Adherence of gastric serosa to surrounding mesentery |
| 9 | Female, 20 year 2 month | Weight loss, vomiting, and abdominal distension. | Gastric and uterine mass with severe lymphadenopathy including the mesenteric and colonic lymph nodes |
| 10 | Male, 21 y 10 month | Vomiting, lethargy, and abdominal distension. | Stomach contains small amounts of gray viscous fluid with severe thickening of cardiac sphincter. The fundus and antrum are severely ulcerated and have dark coloration. Abdominal mesentary has diffuse miliary white nodules with extensive adhesions from the liver capsule and spleen to diaphragm. |
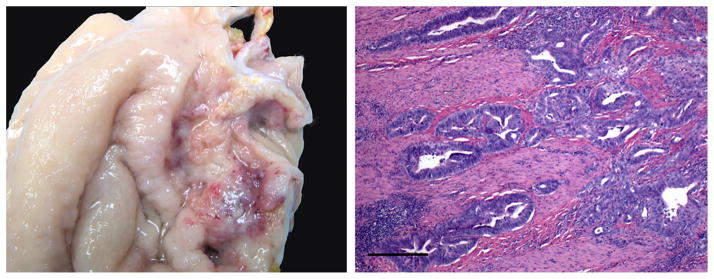
Animal #9 was a 20-year 2-month-old female sooty mangabey who presented with inappetence, weight loss, and vomiting. Radiographs revealed an enlarged stomach with generalized ileus. The monkey was treated with antacids, pain medication, and antibiotics along with supportive care. Gastric enlargement and clinical signs persisted despite treatments, and the animal was euthanized due to suspected gastric neoplasia. Approximately 2 years prior to this incident, the animal had been diagnosed with endometriosis, treated, and remained on medroxyprogesterone for long-term treatment. On gross necropsy, the gastric pyloric region was markedly thickened by a 5.0 × 4.0 cm raised mass that extended to the pancreas. The mucosa in this area had multifocal ulcerated areas (Fig. 1). The colonic and mesenteric lymph nodes were severely enlarged.
Figure 1.
Animal 9 gastric adenocarcinoma. Left panel indicates gross image of stomach from animal #9 depicting an ulcerated raised mass. Notice multifocal areas of hemorrhage and necrosis. Right panel depicts photomicrograph of a section of gastric mucosa and submucosa. The neoplastic epithelial cells extend deep into the submucosa and muscular layer (bar = 100 microns).
Animal #10 was 21-year 9-month-old male cryptorchid sooty mangabey that presented with vomiting and lethargy. The animal was treated with anti-emetics, but the vomiting did not resolve. Abdominal radiographs and ultrasound revealed an upper abdominal mass suspected to be stomach. This animal was euthanized due to a poor prognosis based on suspected gastric neoplasia. A review of the animal’s clinical history revealed that one and a half year prior to its recent clinical presentation, the animal was treated successfully with antibiotics and supportive care for severe regenerative anemia and enterocolitis with melena. Grossly, the cardia sphincter of the stomach was severely thickened and the mucosa of the fundus and antrum was severely ulcerated. There were extensive adhesions extending from the capsule of the liver and spleen to the diaphragm. The spleen was severely enlarged. The liver had occasional small white foci randomly distributed.
Tissue and Gastric Fluid Collection
Archived samples of the six animals that were collected in the previous case study were used for analysis. Animals #9 and #10 were euthanized after the 2011 case study. Samples were collected from these two animals before and after euthanasia for the purpose of diagnosing gastric helicobacteriosis. Tissue analyses were performed, in part, on formalin-fixed paraffin-embedded stomach tissues, prepared as previously described [1]. Additional samples were obtained from animals #9 and #10. During necropsy, gastric fundus and antrum were collected in 10% formalin or in 20% glycerol Brucella broth, and in sterile tubes.
Gastric washes were obtained from animals #9 and #10. Gastric wash samples were obtained from animal #9 at three time points prior to euthanasia, 15 months, 2 weeks, and immediately prior to euthanasia. Gastric wash samples were obtained from animal #10 immediately prior to euthanasia. Gastric washes were obtained from anesthetized animals using a measured 5–8″ French orogastric feeding tube placed in the stomach. If gastric juices could not be withdrawn from the orogastric tube, saline was instilled and retrieved. Gastric wash was then placed in 20% glycerol Brucella broth and frozen at −70 °C, and an additional gastric wash sample was placed in a sterile 15-mL conical tube and frozen at −20 °C. These samples were maintained at these temperatures and were shipped on dry ice for analysis.
PCR Amplification and Sequence of Helicobacter DNA
Gastric Helicobacter spp. infection status was determined in sooty mangabeys by the use of 16S rRNA Helicobacter genus-specific PCR amplification of bacterial DNA extracted from gastric tissue and wash. The High Pure PCR Product Template Preparation Kit (Roche Life Science, Indianapolis, IN, USA) was used according to the manufacturer’s protocol. Prior to DNA extraction, gastric wash samples were thawed and solid material was allowed to settle at the tube bottom. Approximately 1.5 mL of liquid sample was removed, placed in an Eppendorf test tube, and centrifuged at 18,000 × g for 5 minutes. Supernatant was removed and the pellet was subjected to DNA extraction following commercial instructions. Sample DNA was used as template in polymerase chain reaction using the primers indicated in (Table 2). The thermocycler protocol for C97/C05 (specific for Helicobacter genus) and CHP1/CHP2 (specific for H. pylori) primers was 94 °C for 4 minutes, 35 cycles of 94 °C for 1 minutes, 58 °C for 1.5 minutes, 72 °C for 2 minutes, and 72 °C for 8 minutes [11,12]. The protocol using V832f/V1261r (specific for H. suis) primers was identical except the annealing temperature was 60 °C [13]. Gel electrophoresis was performed on PCR products. Helicobacter genus-specific 16S rRNA products from either gastric tissue or wash samples were subject to 16S rRNA sequencing using methods previously described [14,15].
Table 2.
Summary of the PCR primers used in this study for speciation of gastric Helicobacter infection
| Identification | Gene | Designation | Direction | Amplicon size (bp) | Sequence (5′ to 3′) | Reference |
|---|---|---|---|---|---|---|
| Helicobacter spp. | 16S rRNA | C97 C05 |
Forward Reverse |
1200 | GCTATGACGGGTATCC ACTTCACCCCAGTCGCTG |
Fox et al. [11] |
| H. pylori | Urease C | Chp1 Chp2 |
Forward Reverse |
294 | AAGCTTTTAGGGGTGTTAGGGGTT AAGCTTACTTTCTAACACTAACGC |
Bickley et al. [12] |
| H. pylori | HSP60 | H60F H60R |
Forward Reverse |
600 | GGNGAYGGNCANCANCANGCNC TCNCCRAANCCNGGNGCYTTNCA |
Mikkonen et al. [51] |
| H. suis | 16S rRNA | V832f V1261r |
Forward Reverse |
433 | TTG GGA GGC TTT GTC TTT CCA GAT TAG CTC TGC CTC GCG GCT |
De Groote et al. [13] |
Histological Evaluation
Archival gastric tissues from six cases and gastric tissues from the two most recent cases were assessed histologically. Gastric fundus was evaluated in all eight animals. In addition, gastric antrum was available from animals #7 and #9 (Table 1). At necropsy, linear strips of stomach were removed for histopathological evaluation and the mucosa was rinsed with sterile saline. Sections were fixed in 10% neutral-buffered formalin, and were routinely processed, and embedded in paraffin according to accepted histological techniques. Five-micron-thick sections were stained with hematoxylin and eosin (H&E) for histopathological evaluation by a veterinary pathologist. Warthin–Starry-stained sections were examined for the presence of curved or spiral organisms.
Flourescent in situ Hybridization
FISH was initially developed in human gastric biopsies [16] to detect helicobacter organisms at the genus and species levels, but has since been used to detect organisms in the gastrointestinal tract of mice [17], cats [18], dogs [19], horses [20], brushtail possum [21], and from samples of raw bovine milk [22]. FISH techniques and 16S rRNA gene probes used in this study are identical to those previously described by our laboratory [18]. Briefly, gastric tissue was deparaffinized. The hybridization buffer with a particular probe was preheated and applied to each slide. Slides were covered in parafilm, protected from light, and placed in a humidification chamber overnight. They were rinsed gently in double-distilled water to remove the parafilm and serially washed in prewarmed rinsing buffers. Slides were rinsed again with double-distilled water, allowed to air dry, and then mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA) and coverslipped. Slides for each probe were examined under a Zeiss Axioskop 2 fluorescent microscope. A slide was designated as positive for a particular strain of “H. heilmannii” if the distinctive spiral organisms could be viewed as fluorescent under the Rhod filter. A total of 7 probes, as previously described in Trebesius et al. [16] and Chan et al. [17], were used on each of the animal tissues available in this study: HEL274 and HEL717 (both probes combined to detect Helicobacter organisms at genus level), Hpy-1 (H. pylori), Hhe-1 (“H. heilmannii” type 1; referred to as H. suis), Hhe-2 (“H. heilmannii” type 2), Hhe-3 (Novel HHLO type), Hhe-4 (Novel HHLO type), HHe-5 (H. felis, H. bizzozeroni, H. salomonis).
Microbiological Cultivation
Gastric tissue and wash from animals #9 and #10 were placed in 20% glycerol Brucella broth and stored at −70 °C until shipped on dry ice and immediate placed in −80 °C. Animal specimens were grown in conditions favorable for the growth of H. pylori and H. suis [9,23,24]. Briefly, gastric tissue or wash was placed in a sterile tissue grinder and 1.0 mL of sterile phosphate-buffered saline (PBS, pH 7.4) and homogenized with under sterile conditions. For H. pylori isolation, homogenate was passed through a 0.65-micron filter onto a tryptic soy agar with 5% sheep blood (BBL, Cockeysville, MD, USA) and also was directly inoculated onto Brucella blood agar plates antibiotics (vancomycin, bacitracin, nalidixic acid, and amphotericin B; Remel, Lenexa, KS) and Campy CVA agar (BBL, Cockeysville, MD, USA). For H. suis isolation, tissue homogenate was directly inoculated in biphasic brucella agar and broth media as previously described [9]. The plates created in duplicate and were incubated at 37C in microaerobic conditions N2, H2, and CO2 (80:10:10) for up to 3 weeks.
Results
Helicobacter Species Infection Status of Previously Reported Animals
Animals that were previously reported as H. pylori negative were also negative for H. pylori on FISH analysis. However, four of the six animals were positive on Helicobacter genus level, three of which were identified as H. suis. Animal #4 had additional co-infections with HHe-3- and HHe-5-positive helicobacter organisms. Animals #3 and #7 tested negative using Helicobacter genus-level probes and were not tested using other helicobacter probes (Table 3). Animal #7 was negative for Helicobacter species in both gastric antrum and fundus sections.
Table 3.
Summary of FISH using probes defined in Trebesius et al. [16]
| Animal no. | Signalment | Warthin–starry | Fluorescent in situ hybridization
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| H. spp | H. suis | H. pylori | Hhe-2 | Hhe-3 | Hhe-4 | Hhe-5 | |||
| 3 | Male, 12 year | − | − | Not tested | |||||
| 4 | Male, 12 year 5 month | − | + | + | − | − | + | − | + |
| 5 | Female, 10 year | + | + | + | − | − | − | − | − |
| 6 | Female, 21 year 5 month | + | + | − | − | − | − | − | − |
| 7 | Female, 16 year | − | − | Not tested | |||||
| 8 | Female, 17 year | + | + | + | − | − | − | − | − |
| 9 | Female, 20 year 2 month | + | + | + | − | − | − | − | − |
| 10 | Male, 21 year 10 month | + | + | + | − | − | − | − | − |
Animals that tested negative for Helicobacter at the genus level were not tested with other FISH probes. H. suis referred to as Hhe-1 – “H. heilmannii” type 1 in Trebesius et al. [16].
Helicobacter Species Infection Status of Newly Reported Animals
FISH analysis
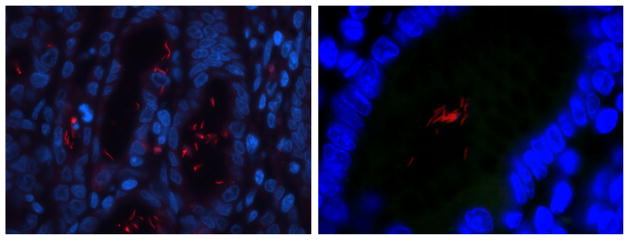
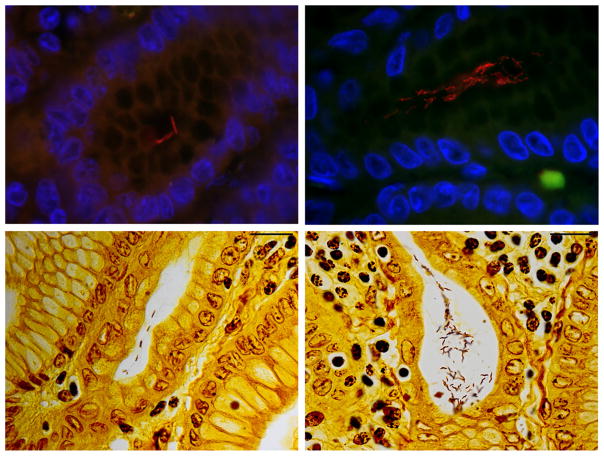
FISH analysis of animals #9 and #10 was positive for Helicobacter genus (Fig. 2) and H. suis (Table 3). Interestingly, animal #9’s gastric tissue contained microorganisms with two different morphologies, both being positive using Helicobacter genus FISH probes; Helicobacter spp. with different morphologies were also visualized using Warthin–Starry staining (Fig. 3). One morphology was typical to other Helicobacter genus FISH positive in this study, with tight corkscrew appearance with 4–10 complete turns, which positively stained positive with H. suis FISH assay. The other morphology was spiral with 1–2 complete turns and did not stain positive with any of the Helicobacter FISH probes used. All microorganisms colonized gastric pits; however, organisms with different morphologies did not colonize the same gastric crypts, but were adjacent to each other.
Figure 2.
FISH for Helicobacter genus of gastric tissue from study animals. Photomicrographs of positive hybridization of Helicobacter genus level probes to gastric tissue. Left and right panels are from animals #10 and #9, respectively. The left panel is at 630× magnification and the right panel is at 1000× magnification. Both hybridized probes demonstrate tightly coiled morphology typical of Helicobacter suis. (Red = FISH probe hybridization, Blue = DAPI, Green = Background).
Figure 3.
Animal #9 gastric Helicobacter spp. morphologies. Photomicrographs of the top panels indicate FISH assay and bottom panels indicate Warthin-Starry staining. Red color in the top left panel depicts Helicobacter suis. Red color in the top right panel indicates Helicobacter genus-level probe hybridization. Top and bottom left panels demonstrates typical, long, tightly coiled morphology of H. suis. Top and bottom right panels demonstrates a different morphology with spiral shaped microorganisms. (Red = FISH probe hybridization, Blue = DAPI, Green = Background). Top panels at 1000× magnification. Bottom panels, scale bar (top right) measures 20 microns.
PCR analysis
Helicobacter species infection of animals #9 and #10 was further characterized via molecular techniques. Both animals were positive for Helicobacter genus using PCR on DNA extracted from gastric wash, antrum, and fundus samples. In addition, all but one of these samples (gastric wash from animal #10) were positive for H. suis using species-specific PCR. As previously mentioned, multiple gastric washes were collected from animal #9 (gastric wash one at 15 months prior to euthanasia, gastric wash two at 2 weeks prior to euthanasia, and gastric wash three immediately prior to euthanasia). For gastric wash #1, sequence analysis of the 16s rRNA reveals 98% homology to H. suis isolated from rhesus (Macaca mulatta) and crab-eating (Macaca fascicularis) macaques (Fig. 4). Sequence analysis of Heliocbacter genus (1200 bp) PCR products of gastric washes #2 and #3, however, indicated a novel species with 95% homology to H. pullorum (Table 4). H. pylori DNA was not detected in any of these samples.
Figure 4.
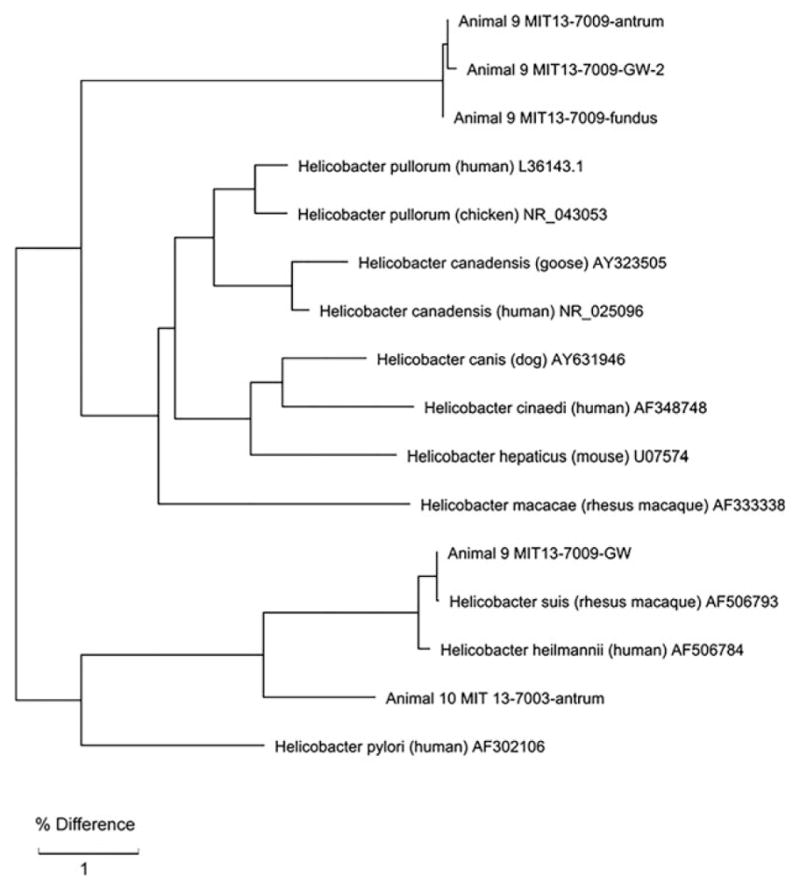
16s rRNA Phylogenetic tree of Helicobacter species in Sooty Mangabeys 9 and 10. Both animals 9 and 10 had gastric Helicobacter suis infection at one point in time. Animal 9, however, became colonized with a novel Helicobacter species infection, indicated in animal 9 gastric fundus and washes 2 and 3.
Table 4.
Molecular characterization of gastric Helicobacter species in animals 9 and 10
| Animal no. | Signalment | PCR
|
16s rRNA sequence
|
||||
|---|---|---|---|---|---|---|---|
| H. spp | H. suis | H. pylori | % Identity | BLAST Description | |||
| 9 | Female, 20 year 2 month | Gastric Wash | + | + | − | 99% | AF506793.1 - Helicobacter suis strain RM1 |
| Gastric Wash 2 | + | + | − | 95% | L36143.1 - Helicobacter pullorum NCTC 12826 | ||
| Gastric Wash 3 | + | + | − | 95% | L36143.1 - Helicobacter pullorum NCTC 12826 | ||
| Antrum | + | + | − | 95% | L36143.1 - Helicobacter pullorum NCTC 12826 | ||
| Fundus | + | + | − | 95% | L36143.1 - Helicobacter pullorum NCTC 12826 | ||
| 10 | Male, 21 year 10 month | Gastric Wash | + | − | − | ||
| Antrum | + | + | − | 97% | AF506793.1 - Helicobacter suis strain RM1 | ||
| Fundus | + | + | − | ||||
DNA isolated from fresh frozen gastric wash and tissue tested positive for Helicobacter spp. infection via PCR and confirmed with 16S rRNA sequence analysis. Novel Helicobacter species indicated by those matching closest (95%) homology to H. pullorum.
Histopathology of Gastric Lesions of Animals #9 and #10
Animal #9
The gastric mass had an ulcerated surface and was composed of neoplastic epithelial cells that extended from the mucosa to the submucosa, muscularis, and serosa (Fig. 1). The neoplastic cells ranged from tall columnar to cuboidal epithelial cells arranged in a tubular patter and separated by fibrous connective tissue septa. Mitotic figures were uncommon: 0–1 per high power field. The mass was covered by large numbers of neutrophils that extended to the adjacent lamina propria. Similar neoplastic cells arranged in a tubular pattern were observed occasionally in the mesenteric lymph nodes, indicating metastatic spread of the tumor.
Animal #10
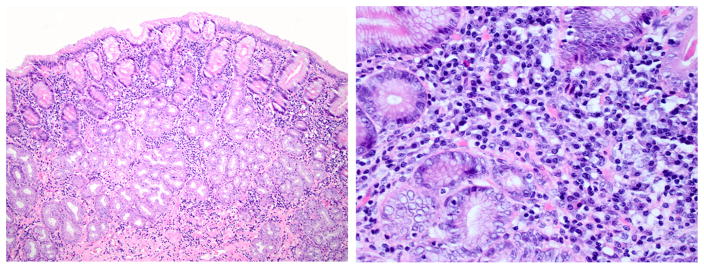
The gastric mass, covered by ulcerated mucosa, was composed of round to oval cells with round to oval vesicular nuclei and abundant eosinophilic cytoplasm arranged in a tubular pattern. The neoplastic cells had marked anisocytosis and anisokaryosis. Mitotic index was high: 3–5 per high power field. The ulcerated surface was covered by necrotic material, hemorrhage, and severe infiltration of degenerate neutrophils intermixed with large numbers of bacterial rods. The adjacent gastric lamina propria was lined by severely hyperplastic epithelium. The glands were hyperplastic and often dilated containing eosinophilic material. The lamina propria of the fundus and antrum had a diffuse moderate infiltration of plasma cells and lymphocytes intermixed with areas of edema (Fig. 5). The muscularis had multifocal areas of infiltration of neoplastic epithelium. Metastasis was noted in the liver and spleen which contained multifocal aggregates of similar neoplastic cells often arranged in an acinar pattern.
Figure 5.
Lymphoplasmacytic inflammation from Animal #10. Gastric fundus with representative moderate lymphoplasmacytic inflammation. H&E stain. Left panel 10×. Right panel 40×.
Microbiological Cultivation of Animals #9 and #10
Helicobacter species were not cultivated from animal #9 or #10. With exception to 0.65-micron-filtered gastric samples grown on blood agar, many other cultivation methods were eventually overgrown with other bacteria, including Campylobacter species and Gram-positive rods.
Discussion
Of the eight sooty mangabeys confirmed with gastric carcinoma, six were Helicobacter species positive by FISH and/or PCR. The previous report, which reported negative H. pylori results, used human-based reagents for the detection of H. pylori, which were ineffective in detecting NHPH. Given the inherent sampling error associated with testing FFPE tissues in the detection of gastric Helicobacter species infection, lack of antrum tissue in 6 animals, coupled with possible loss of Helicobacter spp. in advanced cases of gastric tumors, the two animals that did not have detectable Helicobacter organisms may be falsely negative.
Analysis of gastric tissues in the archive and two new cases of gastric cancer reveals that animals that were infected NHPH were infected with H. suis. H. suis is the most common NHPH of humans [25–27] and the most commonly identified naturally occurring gastric Helicobacter in nonhuman primates [6,7]. Clinical presentations of NHPH infections in humans and nonhuman primates typically, with the exception of MALT lymphoma in humans, are clinically less severe than those caused by H. pylori. In humans, NHPH infections have been associated with dyspepsia [28–30], gastric antrum erosions [31], duodenal ulcers [32,33], and low-grade MALT lymphoma of the stomach [34,35]. Natural gastric NHPH infections have been documented in baboons (Papio hamadryas) with gastritis [36] and in macaque species with various degrees of gastritis [7,37]. Of interest is the recent finding of chronic lymphoplasmacytic gastritis in two red-capped mangabeys (Cercocebus torquatus) maintained in a zoological facility. These lesions progressed to severe hypertrophic gastropathy similar to Ménétrier’s disease that affects humans. Both cases were positive for simian immunodeficiency virus and Helicobacter spp. were demonstrated by the presence of spiral organisms in gastric glands [38]. In mice and Mongolian gerbils, experimental infection of H. suis causes death of parietal cells, epithelial cell hyperproliferation, and severe inflammation. MALT-like lymphoma lesions are experimentally produced in the gerbil model [39]. Infection with other NHPH has been implicated in MALT lymphoma in BALB/c [8] and C57BL/6 [40] mice.
NHPH have also been implicated in zoonotic transmission from pets to humans [25]. A recent case report identifies H. suis infection associated with a swine veterinarian with dyspepsia and chronic, diffuse lymphocytic inflammation in the antrum and corpus [41]. This report highlights the zoonotic potential of H. suis.
In this study, we have also identified a novel gastric Helicobacter species based on 16S rRNA sequence analysis. In the same gastric tissue sample, we identified two different morphologies via FISH probes specific for Helicobacter genus and Warthin–Starry stain. We cannot confirm these microorganisms are novel, which have a morphology similar to many enterohepatic Helicobacter spp. and different than H. suis; however, our 16S rRNA sequence analysis demonstrating 95% homology to H. pullorum supports this possibility. We have recently reported that another enterohepatic helicobacter, H. cinaedi, persistently colonizes the stomach of mice [42]. This finding also emphasizes previous studies where H. cinaedi was identified in the stomachs of humans, some with gastric cancer [43,44]. Additional analysis of this organism will require successful cultivation.
The presence of gastric NHPH infection in sooty mangabeys with gastric cancer is suggestive of a causal association similar to H. pylori-associated gastric cancer. There are several limitations in confirming this causal association. Sooty mangabeys are listed as a vulnerable species by the International Union for Conservation of Nature vulnerable species [45], which limits the ability to collect biopsy samples from antemortem gastroscopies. The overall prevalence of gastric helicobacteriosis in this colony of sooty mangabeys is not known, and furthermore, another colony of sooty mangabeys that are negative for NHPH to ascertain whether they develop gastric cancer is not available. Sharma et al. [1] postulated that a high degree of interrelatedness may play a role in the development of gastric adenocarcinoma. This colony was originally established with approximately 22 founder animals and has remained a closed colony since its establishment in 1966. Determining familial relationships of individuals with gastric adenocarcinoma is complicated by incomplete pedigrees given these animals are mostly co-housed in large cohorts consisting of both males and females. While parental lineage is confirmed by microsatellite analysis for some members of this colony, there are none for the reported cases of gastric adenocarcinoma. Additional analysis of various genes from gastric tissues of animals with and without gastric adenocarcinoma may show host genetic markers of disease, such as MUC1 [46], characteristic DNA damage patterns [47], polymorphisms of cytokine genes IL-1B, IL-1RN, TNF-A, and IL-10 [48], or amplification of JAK2, CD274, or PDCD1LG2 genes noted in human cases of gastric adenocarcinoma [49].
This study provides additional information on naturally occurring Helicobacter species associated with gastric cancer in a closed primate colony that has been isolated from the wild for almost 50 years. Importantly, for the first time H. suis infection has been diagnosed in a primate model with metastatic gastric adenocarcinoma. Further, due to its fastidious nature, H. suis to date, has been studied primarily in experimental models. However, H. suis has recently been cultured and its genome sequenced; this will allow more detailed analysis of the organism’s pathogenic potential in both humans and animals [50]. Continued monitoring of spontaneous gastric disease in this closed sooty mangabey colony should provide further insight regarding the association of gastric Helicobacter spp. infection and host factors contributing to this debilitating neoplasm.
Acknowledgments
This work was supported by the National Institutes of Health (T32-OD010978, P30-ES002109, P01-CA028842, and R01-CA0 93405).
Footnotes
Competing interests: The authors have no competing interests.
References
- 1.Sharma P, Cohen JK, Paul KS, Courtney CL, Johnson ZP, Anderson DC. Spontaneous gastric carcinomas in sooty mangabeys (Cercocebus atys) Comp Med. 2011;61:527–31. [PMC free article] [PubMed] [Google Scholar]
- 2.DePaoli A, McClure HM. Gastrointestinal neoplasms in nonhuman primates: a review and report of eleven new cases. Vet Pathol Suppl. 1982;7:104–25. [PubMed] [Google Scholar]
- 3.Lowenstine LJ. Neoplasms and proliferative disorders in nonhuman primates. In: Benirschke K, editor. Primates: The Road to Self-Sustaining Populations. New York: Springer-Verlag; 1986. pp. 781–814. [Google Scholar]
- 4.Kimbrough R. Spontaneous malignant gastric tumor in a Rhesus monkey (Macaca mulatta) Arch Pathol. 1966;81:343–51. [PubMed] [Google Scholar]
- 5.Drazek ES, Dubois A, Holmes RK. Characterization and presumptive identification of Helicobacter pylori isolates from rhesus monkeys. J Clin Microbiol. 1994;32:1799–804. doi: 10.1128/jcm.32.7.1799-1804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Rourke JL, Solnick JV, Neilan BA, Seidel K, Hayter R, Hansen LM. Description of ‘Candidatus Helicobacter heilmannii’ based on DNA sequence analysis of 16S rRNA and urease genes. Int J Syst Evol Microbiol. 2004;54:2203–11. doi: 10.1099/ijs.0.63117-0. [DOI] [PubMed] [Google Scholar]
- 7.Haesebrouck F, Pasmans F, Flahou B, Chiers K, Baele M, Meyns T, Decostere A, Ducatelle R. Gastric helicobacters in domestic animals and nonhuman primates and their significance for human health. Clin Microbiol Rev. 2009;22:202–23. doi: 10.1128/CMR.00041-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Rourke JL, Dixon MF, Jack A, Enno A, Lee A. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma in an animal model of ‘Helicobacter heilmannii’ infection. J Pathol. 2004;203:896–903. doi: 10.1002/path.1593. [DOI] [PubMed] [Google Scholar]
- 9.Baele M, Decostere A, Vandamme P, Ceelen L, Hellemans A, Mast J, Chiers K, Ducatelle R. Isolation and characterization of Helicobacter suis sp. nov. from pig stomachs. Int J Syst Evol Microbiol. 2008;58:1350–8. doi: 10.1099/ijs.0.65133-0. [DOI] [PubMed] [Google Scholar]
- 10.Martin ME, Bhatnagar S, George MD, Paster BJ, Canfield DR, Eisen JA, Solnick JV. The impact of Helicobacter pylori infection on the gastric microbiota of the rhesus macaque. PLoS ONE. 2013;8:e76375. doi: 10.1371/journal.pone.0076375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox JG, Dewhirst FE, Shen Z, Feng Y, Taylor NS, Paster BJ, et al. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–63. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 12.Bickley J, Owen RJ, Fraser AG, Pounder RE. Evaluation of the polymerase chain reaction for detecting the urease C gene of Helicobacter pylori in gastric biopsy samples and dental plaque. J Med Microbiol. 1993;39:338–44. doi: 10.1099/00222615-39-5-338. [DOI] [PubMed] [Google Scholar]
- 13.De Groote D, Ducatelle R, van Doorn LJ, Tilmant K, Verschuuren A, Haesebrouck F. Detection of “Candidatus Helicobacter suis” in gastric samples of pigs by PCR: comparison with other invasive diagnostic techniques. J Clin Microbiol. 2000;38:1131–5. doi: 10.1128/jcm.38.3.1131-1135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewhirst FE, Chien CC, Paster BJ, Ericson RL, Orcutt RP, Schauer DB, Fox JG. Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl Environ Microbiol. 1999;65:3287–92. doi: 10.1128/aem.65.8.3287-3292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Z, Xu S, Dewhirst FE, Paster BJ, Pena JA, Modlin IM, Kidd M, Fox JG. A novel enterohepatic Helicobacter species ‘Helicobacter mastomyrinus’ isolated from the liver and intestine of rodents. Helicobacter. 2005;10:59–70. doi: 10.1111/j.1523-5378.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 16.Trebesius K, Adler K, Vieth M, Stolte M, Haas R. Specific detection and prevalence of Helicobacter heilmannii-like organisms in the human gastric mucosa by fluorescent in situ hybridization and partial 16S ribosomal DNA sequencing. J Clin Microbiol. 2001;39:1510–6. doi: 10.1128/JCM.39.4.1510-1516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan V, Crocetti G, Grehan M, Zhang L, Danon S, Lee A, Mitchell H. Visualization of Helicobacter species within the murine cecal mucosa using specific fluorescence in situ hybridization. Helicobacter. 2005;10:114–24. doi: 10.1111/j.1523-5378.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 18.Bridgeford EC, Marini RP, Feng Y, Parry NM, Rickman B, Fox JG. Gastric Helicobacter species as a cause of feline gastric lymphoma: a viable hypothesis. Vet Immunol Immunopathol. 2008;123:106–13. doi: 10.1016/j.vetimm.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Recordati C, Gualdi V, Craven M, Sala L, Luini M, Lanzoni A, Rishniw M, Simpson KW, Scanziani E. Spatial distribution of Helicobacter spp. in the gastrointestinal tract of dogs. Helicobacter. 2009;14:180–91. doi: 10.1111/j.1523-5378.2009.00674.x. [DOI] [PubMed] [Google Scholar]
- 20.Perkins GA, den Bakker HC, Burton AJ, Erb HN, McDonough SP, McDonough PL, Parker J, Rosenthal RL, Wiedmann M, Dowd SE, Simpson KW. Equine stomachs harbor an abundant and diverse mucosal microbiota. Appl Environ Microbiol. 2012;78:2522–32. doi: 10.1128/AEM.06252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coldham T, Rose K, O’Rourke J, Neilan BA, Dalton H, Lee A, Mitchell H. Detection of Helicobacter species in the gastrointestinal tract of ringtail possum and koala: possible influence of diet, on the gut microbiota. Vet Microbiol. 2013;166:429–37. doi: 10.1016/j.vetmic.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Angelidis AS, Tirodimos I, Bobos M, Kalamaki MS, Papageorgiou DK, Arvanitidou M. Detection of Helicobacter pylori in raw bovine milk by fluorescence in situ hybridization (FISH) Int J Food Microbiol. 2011;151:252–6. doi: 10.1016/j.ijfoodmicro.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Perkins SE, Fox JG, Marini RP, Shen Z, Dangler CA, Ge Z. Experimental infection in cats with a cagA+ human isolate of Helicobacter pylori. Helicobacter. 1998;3:225–35. doi: 10.1046/j.1523-5378.1998.08037.x. [DOI] [PubMed] [Google Scholar]
- 24.de Sablet T, Piazuelo MB, Shaffer CL, Schneider BG, Asim M, Chaturvedi R, et al. Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut. 2011;60:1189–95. doi: 10.1136/gut.2010.234468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Groote D, Van Doorn LJ, Van den Bulck K, Vandamme P, Vieth M, Stolte M, Debongnie JC, Burette A, Haesebrouck F, Ducatelle R. Detection of non-pylori Helicobacter species in “Helicobacter heilmannii”-infected humans. Helicobacter. 2005;10:398–406. doi: 10.1111/j.1523-5378.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 26.Van den Bulck K, Baele M, Hermans K, Ducatelle R, Haesebrouck F, Decostere A. First report on the occurrence of ‘Helicobacter heilmannii’ in the stomach of rabbits. Vet Res Commun. 2005;29:271–9. doi: 10.1023/b:verc.0000048502.81661.64. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, He L, Haesebrouck F, Gong Y, Flahou B, Cao Q, Zhang J. Prevalence of coinfection with gastric non-Helicobacter pylori helicobacter (NHPH) species in Helicobacter pylori-infected patients suffering from gastric disease in Beijing, China. Helicobacter. 2015;20:284–290. doi: 10.1111/hel.12201. [DOI] [PubMed] [Google Scholar]
- 28.Yakoob J, Abbas Z, Khan R, Naz S, Ahmad Z, Islam M, Awan S, Jafri F, Jafri W. Prevalence of non Helicobacter pylori species in patients presenting with dyspepsia. BMC Gastroenterol. 2012;12:3. doi: 10.1186/1471-230X-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kivisto R, Linros J, Rossi M, Rautelin H, Hanninen ML. Characterization of multiple Helicobacter bizzozeronii isolates from a Finnish patient with severe dyspeptic symptoms and chronic active gastritis. Helicobacter. 2010;15:58–66. doi: 10.1111/j.1523-5378.2009.00730.x. [DOI] [PubMed] [Google Scholar]
- 30.Iwanczak B, Biernat M, Iwanczak F, Grabinska J, Matusiewicz K, Gosciniak G. The clinical aspects of Helicobacter heilmannii infection in children with dyspeptic symptoms. J Physiol Pharmacol. 2012;63:133–6. [PubMed] [Google Scholar]
- 31.Sykora J, Hejda V, Varvarovska J, Stozicky F, Gottrand F, Siala K. Helicobacter heilmannii related gastric ulcer in childhood. J Pediatr Gastroenterol Nutr. 2003;36:410–3. doi: 10.1097/00005176-200303000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto T, Kawakubo M, Akamatsu T, Koide N, Ogiwara N, Kubota S, Sugano M, Kawakami Y, Katsuyama T, Ota H. Helicobacter heilmannii sensu stricto-related gastric ulcers: a case report. World J Gastroenterol. 2014;20:3376–82. doi: 10.3748/wjg.v20.i12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jhala D, Jhala N, Lechago J, Haber M. Helicobacter heilmannii gastritis: association with acid peptic diseases and comparison with Helicobacter pylori gastritis. Mod Pathol. 1999;12:534–8. [PubMed] [Google Scholar]
- 34.Okiyama Y, Matsuzawa K, Hidaka E, Sano K, Akamatsu T, Ota H. Helicobacter heilmannii infection: clinical, endoscopic and histopathological features in Japanese patients. Pathol Int. 2005;55:398–404. doi: 10.1111/j.1440-1827.2005.01844.x. [DOI] [PubMed] [Google Scholar]
- 35.Joo M, Kwak JE, Chang SH, Kim H, Chi JG, Kim KA, Jeon HY, June SL, Moon YS, Kim KM. Helicobacter heilmannii-associated gastritis: clinicopathologic findings and comparison with Helicobacter pylori-associated gastritis. J Korean Med Sci. 2007;22:63–9. doi: 10.3346/jkms.2007.22.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackie JT, O’Rourke JL. Gastritis associated with Helicobacter-like organisms in baboons. Vet Pathol. 2003;40:563–6. doi: 10.1354/vp.40-5-563. [DOI] [PubMed] [Google Scholar]
- 37.Whary MT, Fox JG. Natural and experimental Helicobacter infections. Comp Med. 2004;54:128–58. [PubMed] [Google Scholar]
- 38.Emerson JA, Adkesson MJ, Colegrove KM, Burdick SK, Langan JN. Menetrier’s disease-like hypertrophic gastritis in two red-capped mangabeys (Cercocebus torquatus) Vet Q. 2014;34:29–36. doi: 10.1080/01652176.2014.894263. [DOI] [PubMed] [Google Scholar]
- 39.Flahou B, Haesebrouck F, Pasmans F, D’Herde K, Driessen A, Van Deun K, Smet A, Duchateau L, Chiers K, Ducatelle R. Helicobacter suis causes severe gastric pathology in mouse and mongolian gerbil models of human gastric disease. PLoS ONE. 2010;5:e14083. doi: 10.1371/journal.pone.0014083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura M, Murayama SY, Serizawa H, Sekiya Y, Eguchi M, Takahashi S, et al. “Candidatus Helicobacter heilmannii” from a cynomolgus monkey induces gastric mucosa-associated lymphoid tissue lymphomas in C57BL/6 mice. Infect Immun. 2007;75:1214–22. doi: 10.1128/IAI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joosten M, Flahou B, Meyns T, Smet A, Arts J, De Cooman L, Pasmans F, Ducatelle R, Haesebrouck F. Case report: Helicobacter suis infection in a pig veterinarian. Helicobacter. 2013;18:392–6. doi: 10.1111/hel.12054. [DOI] [PubMed] [Google Scholar]
- 42.Shen Z, Feng Y, Rickman B, Fox JG. Helicobacter cinaedi induced typhlocolitis in Rag-2-deficient mice. Helicobacter. 2015;20:146–55. doi: 10.1111/hel.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han HS, Lee KY, Lim SD, Kim WS, Hwang TS. Molecular identification of Helicobacter DNA in human gastric adenocarcinoma tissues using Helicobacter species-specific 16S rRNA PCR amplification and pyrosequencing analysis. Oncol lett. 2010;1:555–8. doi: 10.3892/ol_00000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pena JA, McNeil K, Fox JG, Versalovic J. Molecular evidence of Helicobacter cinaedi organisms in human gastric biopsy specimens. J Clin Microbiol. 2002;40:1511–3. doi: 10.1128/JCM.40.4.1511-1513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oates JF, Gippoliti S, Groves CP. The IUCN Red List of Threatened Species. 2008. Cercocebus atys. (vol. Version 2015.1.). January 24, 2015 ed. [Google Scholar]
- 46.Liu X, Wang Z, Zhang X, Chang J, Tang W, Gan L, Wu Z, Li J. MUC1 gene polymorphism rs4072037 and susceptibility to gastric cancer: a meta-analysis. SpringerPlus. 2014;3:599. doi: 10.1186/2193-1801-3-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koeppel M, Garcia-Alcalde F, Glowinski F, Schlaermann P, Meyer TF. Helicobacter pylori infection causes characteristic DNA damage patterns in human cells. Cell Rep. 2015;11:1703–1713. doi: 10.1016/j.celrep.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 48.Ando T, Goto Y, Ishiguro K, Maeda O, Watanabe O, Ohmiya N, Niwa Y, Hamajima N, El-Omar E, Goto H. The interaction of host genetic factors and Helicobacter pylori infection. Inflammopharmacology. 2007;15:10–4. doi: 10.1007/s10787-006-1556-y. [DOI] [PubMed] [Google Scholar]
- 49.The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vermoote M, Vandekerckhove TT, Flahou B, Pasmans F, Smet A, De Groote D, Van Criekinge W, Ducatelle R, Haesebrouck F. Genome sequence of Helicobacter suis supports its role in gastric pathology. Vet Res. 2011;42:51. doi: 10.1186/1297-9716-42-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mikkonen TP. Phylogenetic analysis of gastric and enterohepatic Helicobacter species based on partial HSP60 gene sequences. Int J Syst Evol Microbiol. 2004;54:753–758. doi: 10.1099/ijs.0.02839-0. [DOI] [PubMed] [Google Scholar]