Abstract
Global treatment of drug addiction costs society billions of dollars annually, but current psychopharmacological therapies have not been successful at desired rates. The increasing number of individuals suffering from substance abuse has turned attention to what makes some people more vulnerable to drug addiction than others. One personality trait that stands out as a contributing factor is novelty seeking. Novelty seeking, affected by both genetic and environmental factors, is defined as the tendency to desire novel stimuli and environments. It can be measured in humans through questionnaires and in rodents using behavioral tasks. On the behavioral level, both human and rodent studies demonstrate that high novelty seeking can predict the initiation of drug use and a transition to compulsive drug use and create a propensity to relapse. These predictions are valid for several drugs of abuse, such as alcohol, nicotine, cocaine, amphetamine, and opiates. On the molecular level, both novelty seeking and addiction are modulated by the central reward system in the brain. Dopamine is the primary neurotransmitter involved in the overlapping neural substrates of both parameters. In sum, the novelty-seeking trait can be valuable for predicting individual vulnerability to drug addiction and for generating successful treatment for patients with substance abuse disorders.
Keywords: Addiction, drugs of abuse, heritability, molecular connections, novelty seeking
Introduction
Globally, 243 million individuals suffer from drug addiction, costing society through increased health care costs, lost productivity, and crime (Burns, 2014). Worldwide treatment of drug addiction costs more than $250 billion per year, but current psychopharmacological therapies have not reached the desired success rates; an estimated 5% of the world’s population remains plagued with addiction, and this number is increasing at an alarming rate (Burns, 2014). We argue that failed efforts to combat substance abuse are attributable to the inability to define what makes some individuals vulnerable to addiction. Of various vulnerability factors, individual differences in personality traits are an important risk factor for addiction. Although many personality traits have been implicated in this trait, novelty seeking stands out as key.
Research findings from studies on both human and animal models of drug addiction indicate that novelty seeking can predict the risk of compulsive use of addictive drugs. Human studies have shown that novelty seeking is associated with vulnerability to and practice and relapse of drug abuse (Zuckerman and Neeb, 1979; Cloninger, 1987). High novelty seeking increases one’s risk of using addictive drugs (Piazza et al., 1989; Belin and Deroche-Gamonet, 2012), and multiple studies have found that individuals who display high novelty-seeking traits use more drugs of abuse than do low novelty seekers (Piazza et al., 1989; Koob, 2000; Mahoney et al., 2015). Further, research has demonstrated a positive correlation between novelty seeking and relapse rates for several drugs of abuse among addicts (Meszaros et al., 1999; Kahler et al., 2009; Ismael and Baltieri, 2014).
Novelty seeking as a predisposing personality trait has been validated in animal models of multiple drugs of abuse and reflects a heritable tendency toward exploratory behavior and desire for novel sensations (Cain et al., 2005). In rodents, reactivity to novel stimuli correlates with drug self-administration and drug-seeking behaviors (Piazza et al., 1989; Blanchard et al., 2009; Flagel et al., 2010). After rats are categorized as high or low responders on the basis of behavioral reactivity to a novel environment, high-responder rats consistently self-administer drugs such as amphetamine, cocaine, nicotine, and alcohol at higher rates than do low responders (Piazza et al., 1989; Hooks et al., 1991; Ambrosio et al., 1995; Suto et al., 2001; Nadal et al., 2002; Belin and Deroche-Gamonet, 2012; Manzo et al., 2014).
Molecularly, addiction and novelty seeking show an overlap in the mesocorticolimbic reward pathway of the brain, which are modulated by similar neurotransmitter systems and neural circuits (Krebs et al., 2011). Exposure to novel stimuli activates the neural substrates in the brain that also mediate the rewarding and reinforcing effects of drugs of abuse. Response to novelty is modulated by the dopaminergic and non-dopaminergic (e.g., serotonergic, GABAergic, glutamatergic, and opioid) systems in the brain (Koob, 2000). These systems are involved in acquisition and maintenance of drug addiction (Kalivas and Volkow, 2011). However, it is less clear whether novelty seeking mediates the development of compulsive drug use, or if this trait reflects a behavioral adaptation induced by drugs of abuse. Further, individuals who display high novelty seeking may be more prone to experimenting with drugs. Elucidating the molecular mechanisms underlying novelty-seeking behavior in drug-addicted patients will help us to determine the unknowns in this debate.
In this review, we focus on the findings from both human and animal studies and discuss the behavioral and molecular connections between novelty seeking and drug addiction. The questions we address are: (1) what exactly is novelty seeking as it is currently understood in the field? (2) what behaviors related to drug addiction can be predicted using novelty seeking? (3) is the use of all addictive drugs correlated with novelty seeking, or does only a particular group of drugs stand out with a strong correlation to this behavioral trait? (4) because novelty seeking can predict vulnerability to drug addiction, how can we test and quantify this behavior in humans and animals? (5) what are the molecular bases of novelty seeking and its connection to drug addiction? Finally, (6) what future directions would generate valuable research for the field?
Definition of Novelty Seeking
Novelty seeking is one of the defining characteristics of a sensation-seeking personality in humans (Cloninger, 1987; Hiroi and Agatsuma, 2005). As defined by Zuckerman and colleagues, sensation seeking is a “trait defined by the seeking of novel sensations, and the willingness to take physical, social, legal and financial risks for the sake of such experiences” (Zuckerman et al., 1993). Novelty serves as a signal that provides motivation to pursue rewards. Individuals who seek novel experiences display overpowering motivational strength that leads to a decreased ability to control their actions, such as compulsive drug use. Individuals who are characterized as high sensation seekers often have high impulsiveness, exploratory excitability, extravagance, and disorderliness (Zuckerman and Neeb, 1979; Cloninger, 1987; Hiroi and Agatsuma, 2005). High novelty seekers often display high experience-seeking behaviors, boredom susceptibility, and greater rates of bipolar disorder (Zuckerman and Neeb, 1979; Zuckerman et al., 1993; Howard et al., 1997). These individuals exhibit higher than average rates of sociopathy, such as criminality and substance abuse (Zuckerman et al., 1993; Hiroi and Agatsuma, 2005; Flagel et al., 2014).
Novelty-seeking behavior has been correlated with vulnerability to psychopathological disorders and drug addiction in humans (Zuckerman and Neeb, 1979). Considerable evidence has implicated novelty seeking as a behavioral feature in drug addicts that could be innate (Zuckerman et al., 1993) or result from drug abuse (Ersche et al., 2010). Defining the novelty-seeking phenotype in humans with psychiatric disorders, including addiction, will give us more detailed information regarding potential treatment. Consequently, researchers attempt to measure novelty seeking in order to predict vulnerability to these psychiatric diseases including addictions.
A parallel between the sensation-seeking trait in humans is novelty-seeking behavior among animals. In rodents, novelty seeking has been defined as a preference for novel objects or environments; high novelty-seeking rodents display enhanced exploratory behavior toward novel situations, objects, or stimuli (Piazza et al., 1989). In rats and mice, preference for novelty has been correlated with higher sensitivity to the reinforcing effects of many drugs of abuse, including amphetamine, cocaine, nicotine, and alcohol (Piazza et al., 1989; Hooks et al., 1991; Ambrosio et al., 1995; Suto et al., 2001; Nadal et al., 2002; Belin and Deroche-Gamonet, 2012; Manzo et al., 2014). Measuring novelty-seeking behavior in rodent models that mimic human psychiatric disorders including addiction will help to elucidate the neurobehavioral details of psychopathological conditions.
Measurement of Novelty Seeking in Humans
Novelty seeking is typically measured using questionnaires in humans and behavioral tests in animals. In humans, novelty seeking is often measured alongside harm avoidance and reward dependence, which are three heritable personality traits implicated in the vulnerability to addiction (Zuckerman and Neeb, 1979). Harm avoidance is a tendency to respond intensely to aversive stimuli and to learn to avoid punishment and novelty. Reward dependence is a propensity to respond intensely to stimuli that produce a reward. In particular, individuals who display high novelty seeking, low harm avoidance, and high reward dependence may be at greater risk for substance abuse (Zuckerman and Neeb, 1979). Cloninger and associates suggest that the three dimensions of harm avoidance, novelty seeking, and reward dependence are correlated with high serotonergic activity, low dopaminergic activity, and low noradrenergic activity, respectively (Cloninger et al., 1991), although more recent studies have proposed different neurobiological explanations for these aspects of personality (Hooks et al., 1994; Tournier et al., 2013; Flagel et al., 2014).
Three of the most commonly used assessments of novelty seeking in humans are shown in Table 1. Briefly, the Tridimensional Personality Questionnaire (TPQ) measures personality dimensions (harm avoidance, novelty seeking, and reward dependence) in a 100-item, self-administered, true/false test format (Cloninger, 1987; Cloninger et al., 1993). The TPQ is intended to correspond closely to the underlying genetic structure of personality rather than to individual adaptations to experiences (Cloninger et al., 1991). A similar test, the Zuckerman Sensation Seeking Scale (SSS), specifically measures sensation seeking (Zuckerman, 2007). The SSS contains 40 items in a forced-choice format and has four subscales. Cloninger’s Temperament and Character Inventory (TCI) measures a constellation of personality traits (Cloninger, 1987; Cloninger et al., 1993). The TCI contains 226 items in a true/false format, encompassing seven subscales in two major categories.
Table 1.
Common tests used to measure novelty seeking in humans
| Measure | Sub-measure | Sub-measure component | Number of questions |
|---|---|---|---|
| Tridimensional Personality Questionnaire (TPQ) | Novelty seeking (NS) scale | Exploratory excitability versus stoic rigidity | 9 |
| Impulsiveness versus reflection | 8 | ||
| Extravagance versus reserve | 7 | ||
| Disorderliness versus regimentation | 10 | ||
| Harm avoidance (HA) scale | Anticipatory worry and pessimism | 10 | |
| Fear of uncertainty | 7 | ||
| Shyness with strangers | 7 | ||
| Fatigability and asthenia | 10 | ||
| Reward dependence (RD) scale | Sentimentality | 5 | |
| Persistence | 9 | ||
| Attachment | 11 | ||
| Dependence | 5 | ||
| Zuckerman Sensation Seeking Scale (SSS) | Thrill and adventure seeking (TAS) | Desire to engage in sports or activities involving speed and danger | 10 |
| Disinhibition (Dis) | Desire for social and sexual disinhibition | 10 | |
| Experience seeking (ES) | Desire for experience through the mind and senses, travel, and a non-conforming lifestyle | 10 | |
| Boredom susceptibility (BS) | Aversion to repetition, routine, and dull people | 10 | |
| Temperament and Character Inventory (TCI) | Novelty seeking (Temperament) | Exploratory excitability vs rigidity | 11 |
| Impulsiveness vs reflection | 10 | ||
| Extravagance vs reserve | 9 | ||
| Disorderliness vs regimentation | 10 | ||
| Harm avoidance (Temperament) | Anticipatory worry vs optimism | 11 | |
| Fear of uncertainty vs confidence | 7 | ||
| Shyness vs gregariousness | 8 | ||
| Fatigability and asthenia vs vigor | 9 | ||
| Reward dependence (Temperament) | Sentimentality vs insensitiveness | 10 | |
| Attachment vs detachment | 8 | ||
| Dependence vs independence | 6 | ||
| Persistence (Temperament) | Single scale | 8 | |
| Self-directedness (Character) | Responsibility vs blaming | 8 | |
| Purposeful vs lack of goal direction | 8 | ||
| Resourcefulness vs apathy | 5 | ||
| Self-acceptance vs self-striving | 11 | ||
| Congruent second nature | 12 | ||
| Cooperativeness (Character) | Social acceptance vs intolerance | 8 | |
| Social disinterest | 7 | ||
| Helpfulness vs unhelpfulness | 8 | ||
| Compassion vs revengefulness | 10 | ||
| Pure-hearted vs self-serving | 9 | ||
| Self-transcendence (Character) | Self-forgetful vs self-conscious | 11 | |
| Transpersonal identification | 9 | ||
| Spiritual acceptance vs materialism | 13 |
Other assessments of novelty-seeking behavior in humans include the Zuckerman-Kuhlman Personality Questionnaire (ZKPQ), a true/false test that measures impulsivity and sensation seeking (Zuckerman and Neeb, 1979), as well as other self-reporting questionnaires and surveys that quantify novelty-seeking behavior in various formats. The ZKPQ is designed to measure a variety of personality traits in addition to risk-taking behaviors (Zuckerman et al., 1993). This questionnaire consists of 99 items in a true/false format, including five subscales plus items to detect inattention and socially desirable responding (Zuckerman et al., 1993). Finally, the Arnett Inventory of Sensation Seeking (Ahituv et al.) is designed to assess sensation seeking, defined specifically by Arnett as “the need for novel and intense stimulation” (Arnett, 1994). The AIS contains 20 items on 4-point scales to which participants respond on the basis of agreement (Arnett, 1994). The two subscales in this inventory are novelty and intensity (Arnett, 1994). There have been studies investigating correlations among assessments of novelty-seeking behavior in humans. For example, Zuckerman and Cloninger (1996) reported a high correlation between Zuckerman’s impulsive sensation-seeking scale and TCI’s novelty-seeking scale. Moreover, the novelty-seeking scale from the TPQ is positively correlated with the behavioral task of risk preference, as measured by a probabilistic decision-making task and negatively correlated with the brain activation elicited by risk prediction in the insula, striatum, and supplementary motor area (Wang et al., 2015).
Measurement of Novelty Seeking in Animals
Several behavioral tests have been proposed for measurement of novelty-seeking behavior in rodents (Table 2). The open field arena test is the most commonly used behavioral paradigm. Depicted in Figure 1, this test measures locomotor activity by providing an inescapable environment in which rodents are placed in an apparatus. The following behavioral parameters are recorded: time spent in the center versus the sides and movements against a floor grid. However, the open field arena test forces animals to be in an enclosed environment, provoking a strong fear response (Cain et al., 2005). Rodents display elevated serum concentrations of corticosterone when exposed to inescapable novelty tasks such as the open field test (Cain et al., 2005). Anxiety-related behaviors outweigh exploratory behaviors during the test session. Therefore, this test may provide detailed information about anxiolytic drugs and their effects on novelty-seeking behavior. Another disadvantage of this test is that locomotion can differ independently from neophilia (Brown and Nemes, 2008). One test that overcomes the aforementioned problems is the hole-board test, which more directly measures novelty-seeking behavior by providing free-choice novelty. As shown in Figure 2, this test requires that animals be placed in a hole-board, an enclosed box with equally spaced holes (Boissier and Simon, 1962). Researchers measure dependent variables that include nose poking and head dipping, which directly represent exploratory behavior independent of locomotor activity. Other behavioral tests used are the elevated plus maze and the light-dark box test. These two tests are based on the innate aversion of rodents to brightly lit environments; high novelty seekers would spend more time in the open or light regions of each test than would low novelty seekers (Hascoet et al., 2001; Crawford et al., 2013). However, these two tests directly measure anxiety- and stress-related behaviors and subsequent effects on novelty-seeking behaviors. In the elevated plus maze test, a maze is elevated above the surface and generally consists of four arms; two arms are considered “open” because of full exposure, while the other two arms are considered “closed” because of the wall enclosure (Crawford et al., 2013). The light-dark box test consists of an apparatus that contains a dark, enclosed compartment and a brightly illuminated “aversive” partition (Hascoet et al., 2001). In fact, researchers who selectively breed rats for novelty-seeking behavior often use these two tests. In short, the hole-board test may be more advantageous to researchers who are examining novelty-seeking behavior among naïve rodents. In contrast, researchers may choose to utilize the open field arena test, the elevated plus maze test, and the light-dark box test when studying selectively bred rodent lines.
Table 2.
Common tests used to measure novelty seeking in rodents
| Test | Apparatus | Measure | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| Hole-board test | Board that contains equally spaced holes | Head dipping, nose poking, grooming, rearing | Measures emotionality in addition to novelty seeking | Repeated exposure to novel environment can result in fewer head-dipping behaviors | (Boissier and Simon, 1962) |
| Open-field arena test | Arena with walls to prevent escape | Vertical, horizontal movement, time mobile in center versus time mobile in sides | Locomotor response to a drug of can predict individual vulnerability to addiction | Behavior recorded can reflect anxiety instead of novelty seeking behavior | (Welker, 1957) |
| Elevated plus maze | Plus-shaped apparatus containing 4 arms at right angles to each other; (2 open arms, 2 closed arms) | Time spent in open arms versus time spent in closed arms | Locomotor activity can be determined in addition to novelty seeking behavior | Behavior recorded can reflect anxiety in contrast to novelty seeking/exploratory behavior | (Crawford et al., 2013) |
| Light-dark box test | Apparatus containing a small dark box (1/3rd of apparatus) and large, brightly illuminated box (2/3rd of apparatus) | Time spent in light box versus time spent in dark box | Relatively inexpensive and rapid test to determine novelty seeking behavior | Behavior recorded can reflect anxiety or anxiogenic properties of drugs in contrast to novelty seeking/exploratory behavior | (Hascoet et al., 2001) |
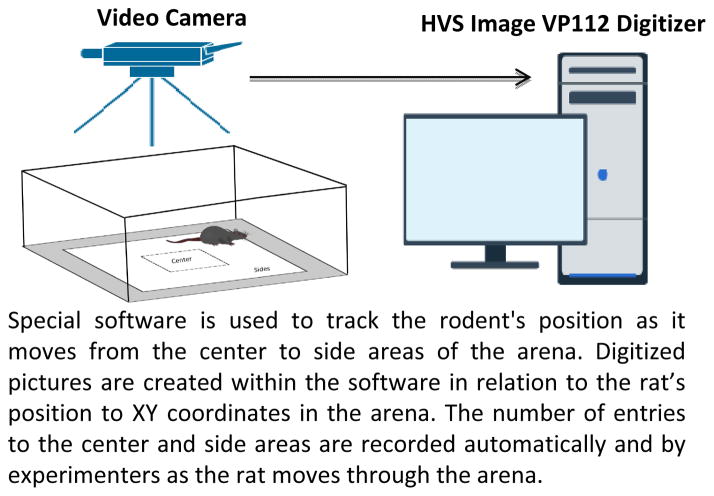
Figure 1.
Illustration of open field arena test, one of the most commonly used behavioral paradigms to test novelty-seeking behavior in rodents. This test provides an inescapable environment in which rodents are placed in an apparatus, with the following parameters being recorded for each animal: time spent in the center versus the sides and movements against a floor grid. Because of the enclosed environment, the open field test provokes a strong fear response in rodents, as measured by elevated corticosterone concentrations. This test directly measures locomotion, which can vary independently of neophilia. As such, this test is utilized by researchers who are testing anxiety in addition to novelty-seeking behavior in rodents.
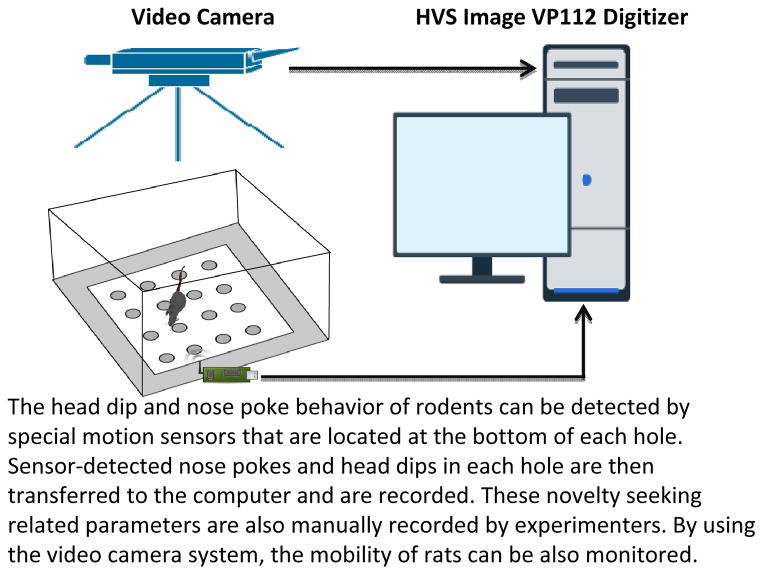
Figure 2.
Illustration of hole-board test, a free-choice novelty-seeking behavioral task test for rodents. The apparatus is an enclosed box with equally spaced holes; the following behavioral parameters are measured during the test session: head dipping, nose poking, rearing, and grooming. Head dipping and nose poking represent exploratory behaviors that are independent of locomotor activity. Compared with the open field arena test (see Figure 1), the hole-board test more directly measures novelty-seeking behavior, because it has not been shown to elevate the corticosterone concentration. The hole-board test is advantageous to researchers studying novelty-seeking behavior in naïve rodents.
Genetic Effects on Novelty Seeking and Addiction
Although most personality traits have a heritability (i.e., the proportion of phenotype variability that can be attributed to genetic variation) of only approximately 30%, the heritability of novelty seeking was estimated to reach nearly 60% in humans (Zuckerman et al., 1993). This estimate had been quantified in non-human primate studies as well (Fairbanks et al., 2011; Greenwood et al., 2013), with an estimated value ranging from 0.35 to 0.43 (Fairbanks et al., 2011). Further, novelty seeking as a heritable behavioral trait has received support from selective breeding of High Responder (HR) and Low Responder rats in the open field test (Stead et al., 2006; Flagel et al., 2014). Eight generations of selection for novelty-seeking behavior result in a consistent phenotype, such that the phenotypic differences in response to novelty can be predicted with >95% certainty (Cummings et al., 2011). An HR line exhibits relatively high exploratory behavior, whereas an LR line displays low affinity for a novel environment. Stead and colleagues used these selected lines to determine the heritability of the novelty-seeking trait in Sprague-Dawley rats; it proved to be 35.8% in the first generation. In other words, more than a third of the phenotypic variance within the founding generation’s offspring was attributable to heritable factors. After the fourth generation, the rate increased to more than 60% (Stead et al., 2006).
Further, human and animal studies have strongly indicated that the likelihood of abusing drugs is heritable (Han et al., 1999; Kendler et al., 2003; Agrawal and Lynskey, 2008). Heritabilities have been determined for addiction to several types of drugs, including alcohol, nicotine, amphetamine, cocaine, and opiates. Walters showed that the upper limit for heritability of alcohol misuse in humans was 0.36 (Walters, 2002). Li and colleagues determined that the heritability of smoking initiation among humans ranges from 0.37 to 0.55 and that of smoking persistence is 0.46 to 0.59 (Li et al., 2003). Nesil and colleagues demonstrated that the heritability for high nicotine intake was 0.26 in rats after nine generations of selective breeding (Nesil et al., 2013). Heritability for amphetamine self-administration was estimated to be 0.74 among inbred rats (Meyer et al., 2010) and that for cocaine abuse in humans ranges from 0.42 to 0.79 (Agrawal et al., 2012). The heritability for opiate dependence in humans is estimated to be 0.43 (Weyandt, 2005).
Taken together, these findings indicate that the propensity for substance abuse reflects in part a genetic predisposition that manifests as certain personality traits. These traits, such as novelty seeking, lead an individual to respond strongly to novel stimuli, including drugs of abuse (Flagel et al., 2014).
Novelty Seeking and Addiction
Vulnerability to substance abuse is multifaceted, modulated by genetic, environmental, and neural components (Figure 3). Even so, high novelty seekers have a greater propensity to first experiment with drugs. These individuals are thought to gravitate toward novel stimuli and respond rapidly to cues for reward despite potential punishment, an indicator of drug addiction (Flagel et al., 2014). To that end, novelty-seeking behavior is positively correlated with vulnerability to externalizing disorders such as drug dependence (Finn et al., 2002). High novelty seekers have a propensity for earlier and more varied use of drugs in comparison with the general population (Sutker et al., 1978; Cloninger, 1987). These individuals often report pleasure and curiosity as motivation for initial drug use and continue seeking and using drugs of abuse in order to maintain these satisfying feelings (Sutker et al., 1978). In addition, novelty-seeking, combined with impulsivity is related with craving and relapse in alcohol dependence (Evren et al., 2012). Furthermore, maladaptive levels of novelty-seeking behavior may mediate internalizing disorders, such as depression, anxiety, and panic disorders, which also can lead individuals to initiate drug use in order to self-medicate (Khan et al., 2005). High novelty seeking is not a broad predictor of all psychiatric disorders, but it is highly predictive of drug dependence (Zuckerman and Neeb, 1979; Khan et al., 2005; Flagel et al., 2014). Studies have shown that high novelty seekers use more alcohol and illicit substances than low novelty seekers (Zuckerman and Neeb, 1979; Cloninger, 1987). Taken together, novelty seeking provides a broad spectrum on which researchers can predict vulnerability to many types of psychiatric disorders. Animal models present a significant investigatory platform to determine the link between novelty-seeking behavior and addiction.
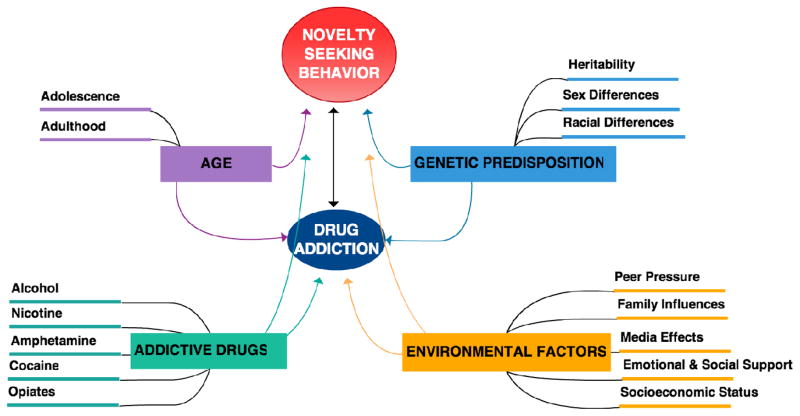
Figure 3.
Contributing factors to drug addiction, portraying the multifaceted vulnerability to substance abuse and its relation to novelty-seeking behavior. There is no clear cause-and-effect relation between novelty-seeking behavior and drug addiction. Rather, these two phenotypes synergistically influence each other, along with an intricate network of contributing factors including age, genetic predisposition, use of addictive drugs, and environmental factors.
Behavioral Connection between Novelty Seeking and Addiction
Animal Studies
The connection between novelty-seeking behavior and drug addiction has been widely studied in outbred and inbred rat strains using multiple drugs of abuse. Individual differences in vulnerability to addiction were investigated in animal studies beginning more than 20 years ago. Piazza and colleagues first showed the correlation between novelty-seeking behavior and drug addiction in outbred Sprague-Dawley rats (Piazza et al., 1989). Vulnerability to amphetamine intake was predicted by novelty seeking, measured by the open field arena test. Rats with high novelty-seeking scores were more likely to self-administer amphetamine and at higher doses than low novelty seekers (Piazza et al., 1989). A decade later, Bevins and colleagues demonstrated that there are individual differences in novelty-seeking behavior by using the novel place-preference test in outbred rats. In this task, rats were grouped into high and low responders to novelty and were subsequently tested for their responsively to amphetamine. The results showed that high responders had greater sensitivity to the activating effects of this drug (Bevins et al., 1997).
Considerable data have shown results consistent with those of these studies; cocaine, nicotine, morphine, and ethanol also display the positive correlation between novelty seeking and drug intake (Flagel et al., 2014). Belin and Deroche-Gamonet demonstrated that compulsive use of cocaine could be predicted by novelty seeking (Belin and Deroche-Gamonet, 2012). Piazza and colleagues showed that high novelty seekers of outbred Sprague-Dawley rats began cocaine self-administration after receiving low cocaine doses and a vertical shift in dose–response functions (Piazza et al., 2000). The study reported by Gulley and colleagues highlights the influence of a single cocaine exposure on subsequent behavioral responses to the drug. In their study, rats were grouped into high and low responders by response to initial cocaine exposure in a novel environment (Gulley et al., 2003). High novelty seekers of outbred Sprague-Dawley rats also self-administered amphetamine at doses that did not induce self-administration in low novelty seekers (Piazza et al., 1989). High novelty-seeking behavior also is predictive of the degree of nicotine self-administration among outbred Sprague-Dawley rats (Suto et al., 2001). High novelty seekers self-administered nicotine more frequently and worked more to acquire the drug than did low novelty seekers (Suto et al., 2001). Lewis, Fischer 344 (F344), NCI-Black Reiter (Cummings et al.), and August-Copenhagen Irish (ACI) rats exhibit a positive correlation between locomotor response to a novel environment and morphine self-administration (Ambrosio et al., 1995). Zheng and colleagues demonstrated that the response to novelty in both juvenile and adult Sprague-Dawley rats determined the magnitude of morphine’s rewarding effects (Zheng et al., 2003). Finally, studies show a positive correlation between ethanol self-administration and novelty-seeking behavior (Nadal et al., 2002; Manzo et al., 2014). Pelloux and colleagues recently demonstrated that novelty seeking strongly predicted the amount of ethanol consumption among Wistar rats (Pelloux et al., 2015). All in all, novelty-seeking behavior among outbred rat lines can predict with high consistency the intake rates of several drugs of abuse.
Recent studies using outbred Sprague-Dawley rats have suggested new insights into the treatment of addiction. High novelty seekers preferentially self-administer natural rewards such as saccharin over addictive drugs such as cocaine (Vanhille et al., 2015). In turn, the vulnerability to addiction as a result of high novelty seeking may be offset by providing non-drug rewards at early stages of drug use in order to prevent the transition to compulsive drug abuse (Vanhille et al., 2015). For example, addicts may benefit from treatment that incorporates exercise as a non-drug reward.
Another approach to clarifying the behavioral connection between novelty seeking and addiction is to breed rats selectively on the basis of individual extents of locomotor response to a novel environment (Stead et al., 2006). Stead and colleagues first proposed a study to breed rats selectively in order to develop HR and LR lines and to study novelty seeking in the context of addiction (Stead et al., 2006; Davis et al., 2008). Davis and colleagues confirmed the reliability of using these rat lines to examine novelty seeking, as HR rats responded with significantly greater locomotor activity in a novel environment (Davis et al., 2008). The HR rats also displayed lower anxiety-like behaviors than did LR rats, indicating that HR rats have greater exploratory behavior as well as less anxiety (Davis et al., 2008). These results imply that these behavioral traits work synergistically to increase drug self-administration (Belin and Deroche-Gamonet, 2012). Flagel and colleagues found consistent results among selectively bred HR and LR lines, in that HRs displayed an increase in drug-seeking behavior, greater response to the rewarding effects of drugs such as cocaine, and a greater tendency to relapse after treatment (Flagel et al., 2010). Additionally, after cocaine administration, high novelty-seeking rats displayed greater locomotor activity in a novel environment than did low novelty seekers (Flagel et al., 2010). These researchers concluded that selectively bred HR rats exhibit “behavioral disinhibition” and may represent one end of the continuum of addiction-vulnerable populations (Flagel et al., 2010). Rodent behavioral experiments confirming human findings provide further evidence of novelty seeking as a contributing factor in drug abuse.
Human Studies
Human studies have validated animal study findings that maladaptive degrees of novelty-seeking behavior are correlated with substance abuse. These abnormalities are also correlated with age; a difference exists in novelty-seeking behavior between adolescents and adults. Not surprisingly, adolescents display higher levels of novelty-seeking behavior than do adults, so they are at higher risk for developing substance use disorders (Spear, 2000; Bernheim et al., 2013). During adolescence, novelty seeking as a personality trait is strongly predictive of drug use later in life, a fact that holds true for many types of drugs, such as depressants, psychostimulants, opiates, and hallucinogens (Andrucci et al., 1989). In a seminal study published by Andrucci and colleagues, adolescent sensation seeking was strongly correlated with drug use; specifically, high Sensation Seeking Scores strongly predicted drug use and not drug nonuse (Andrucci et al., 1989). This study indicated a positive correlation between sensation seeking and abuse of alcohol, amphetamines, barbiturates, caffeine, cocaine, hallucinogens, marijuana, and tobacco (Andrucci et al., 1989). Cloninger and colleagues showed that in 431 children, novelty seeking was strongly predictive of early-onset alcohol abuse (Cloninger, 1987). Comeau and colleagues found consistently that sensation seeking mediates a motivation for alcohol use among adolescents (Comeau et al., 2001). A study of 2733 high school students demonstrated that Sensation Seeking Scores positively correlated with recent use of alcohol and tobacco (Schepis et al., 2008). Further adolescent studies have highlighted the finding that sensation seeking is the best predictor of adolescents engaging in risky behaviors, including drug use (Stautz and Cooper, 2013). Sensation seeking peaks during the late adolescent years, so many individuals of this age initiate drug use; in turn, they experience enhanced responses to these drugs (Kandel et al., 1994; Spear, 2000; Blanchard et al., 2009). Because drug use has already commenced, during adulthood, although novelty-seeking behavior may decrease, use continues.
Among adults, novelty-seeking behavior as part of a human personality increases vulnerability to addiction and reflects in part a psychobiological adaptation in the response to chronic substance exposure (Koob, 2000; Belin and Deroche-Gamonet, 2012). In adults, novelty-seeking behavior can predict vulnerability to initiation of self-administration and to compulsive drug use (Belin and Deroche-Gamonet, 2012). Leyton and colleagues demonstrated a positive correlation between novelty seeking and amphetamine-induced drug wanting (Leyton et al., 2002a). Mahoney and colleagues found that individuals with cocaine or methamphetamine use disorders had significantly higher Sensation Seeking Scores across both drug groupings than did healthy controls (Mahoney et al., 2015). A recent study of 8646 adult individuals using the TCI showed that novelty seeking was the trait most associated with the use of alcohol, cannabis, and cocaine (Schneider et al., 2015). In addition, high novelty seeking or sensation seeking is associated with an increased frequency and amount of drug use, as well as an increased risk for using addictive drugs and developing addiction (Hawkins et al., 1992). Individuals with high sensation-seeking status showed greater sensitivity to the psychostimulant-like effects of d-amphetamine on self-report measures that were associated with the reinforcing effects of drugs (Kelly et al., 2006). Furthermore, novelty seeking is correlated with the magnitude of amphetamine-induced increases in extracellular dopamine in the ventral striatum. Leyton and colleagues reported that individuals with high novelty seeking showed greater amphetamine-induced dopamine release and amphetamine-induced drug wanting (Leyton et al., 2002b). Based on the aforementioned evidence, an individual displaying high novelty seeking can be vulnerable to developing addiction due to increased sensitivity to the reinforcing effects of psychostimulants. This information may provide insight into establishing prevention strategies aimed at reducing the incidence of high-risk behaviors.
In addition, high novelty seeking or sensation seeking is associated with a greater frequency and amount of drug use, as well as with a higher risk of using addictive drugs and developing addiction (Hawkins et al., 1992). Individuals with high sensation-seeking status showed greater sensitivity to the psychostimulant-like effects of d-amphetamine on self-report measures that were associated with the reinforcing effects of drugs (Kelly et al., 2006). Furthermore, novelty seeking is correlated with the magnitude of amphetamine-induced increases in extracellular dopamine in the ventral striatum. Leyton et al. (2002) reported that high novelty seeking predicted greater amphetamine-induced dopamine release and desire for the drug. In view of the aforementioned evidence, an individual displaying high novelty seeking appears vulnerable to developing addiction because of his or her higher sensitivity to the reinforcing effects of psychostimulants. This information may provide insight into effective prevention strategies aimed at reducing the incidence of high-risk behaviors, including drug addiction.
There is some disagreement regarding whether novelty seeking can predict cessation outcomes. Carton and colleagues found that, consistent with other human studies, smokers scored highly on Sensation Seeking Scores, but that sensation seeking predicted only initiation of smoking and was not related to cessation outcomes (Carton et al., 2000). Conversely, other studies in human adults have confirmed the idea that cessation outcome and relapse rates can be predicted by novelty seeking (Meszaros et al., 1999; Kahler et al., 2009; Ismael and Baltieri, 2014). For example, Meszaros and colleagues demonstrated that novelty seeking was a strong predictor of relapse among detoxified alcohol-dependent patients (Meszaros et al., 1999). Kahler and colleagues found that the level of sensation seeking was in fact negatively correlated with smoking cessation success; high sensation seekers faced reduced odds of cessation compliance and outcomes (Kahler et al., 2009). Ismael and Baltieri found that novelty seeking was positively associated with craving intensity among cocaine-dependent outpatients in therapy (Ismael and Baltieri, 2014). On the other hand, both impulsivity and novelty-seeking might be related with craving and relapse in alcohol dependence. In particular, extravagance of novelty-seeking using TCI was related with relapse both directly and indirectly via craving, whereas impulsivity was related with relapse only indirectly via craving (Evren et al., 2012).
Molecular Connection between Novelty Seeking and Addiction
The molecular connection between novelty seeking and addiction on the cellular and neural network level has not been fully elucidated. New findings indicate that the same neural networks and neurotransmitters at the synaptic connections modulate the response to both novel stimuli and addictive drugs. Few studies assess the effects of these elements on the gene sequence, extent of expression, protein structure, and the function of neurotransmitter systems within and between neural networks. In fact, the response to both novel stimuli and addictive drugs clearly is modulated by the mesocorticolimbic dopamine system of the brain. This pathway is mostly associated with stimulation of the reward system. Naturally rewarding stimuli can enhance the activity of dopaminergic neurons and lead to dopamine release in the neural networks of the mesocorticolimbic system. Dopaminergic transmission between the ventral tegmental area (VTA) and the nucleus accumbens (NAc) functions as the main reward site of this neural network. Different neurotransmitter projections arising from several cortical and limbic regions of the brain also modulate the activity of the central reward system as an integrated neural network. Both novelty and drugs of abuse activate the central and integrated natural reward system by increasing the activity of dopamine fibers in the VTA–NAc reward circuit (Bardo et al., 1996).
Dopamine stands out as the central neurotransmitter regulating this reward circuit, directly connecting the VTA and the NAc. Other neurotransmitters, such as serotonin, glutamate, GABA, opioid peptides, and norepinephrine, are secondary to dopamine, as they act in part to regulate the dopaminergic system. Novel stimuli and some drugs of abuse such as cocaine directly affect dopamine release in the brain. Other addictive drugs such as alcohol act on these secondary neurotransmitter systems, indirectly affecting dopamine release and altering the activity of the reward pathway in the brain (Cloninger, 1987).
Understanding how certain drugs of abuse and novel stimuli alter the neuronal and molecular circuitry of the natural reward system in the brain is critical for understanding the molecular link between novelty seeking and drug addiction. Koob summarized the effects of several drugs of abuse, particularly focusing on key neurotransmitters such as dopamine, opioid peptides, serotonin, GABA, and glutamate (Koob, 2000). Dopamine acutely mediates the rewarding effects of psychostimulants such as cocaine, nicotine, and amphetamines (Koob, 2000). Blum and colleagues stressed the importance of dopamine in addiction; in their words, “all roads lead to dopamine” (Blum et al., 2012). Opioid peptides are neuromodulators that can be produced by the body; opiate drugs mimic these peptides and are competitive antagonists that bind to the opioid receptors (Koob, 2000). The mu (μ) opioid receptor has been indicted in opiate addiction; for example, Matthes and colleagues showed that mice lacking this receptor subtype do not respond the rewarding effects of morphine (Matthes et al., 1996). Serotonin, GABA, and glutamate, in addition to dopamine, have been linked to the reinforcing effects of alcohol; Koob describes ethanol’s reinforcing effects as an “orchestra” of these combined neurotransmission systems (Koob, 2000). Also, GABA is a key modulator of both ethanol and benzodiazepine effects; this hypothesis is supported by the reversal of ethanol’s behavioral effects through GABAergic antagonists (Koob, 2000). Kalivas and Volkow describe the glutamatergic projections from the prefrontal cortex through the NAc as being key mediators in the development of addiction (Kalivas and Volkow, 2011). Drugs of abuse such as cocaine, nicotine, and heroin reduce the extracellular concentration of glutamate, the major excitatory transmitter in the brain. In addition, ethanol antagonizes glutamatergic transmission (Koob, 2000; Kalivas and Volkow, 2011). Studies with human and animal models of alcohol addiction show that a decrease in GABA inhibition in the central nervous system (CNS) can raise the extracellular glutamate concentration during withdrawal, resulting in hyperexcitability (Rossetti and Carboni, 1995; Faingold et al., 2000; Koob, 2000; Cagetti et al., 2003; Gomez et al., 2012).
These neurotransmission systems have also been proposed to mediate novelty-seeking behavior. Cocaine increases dopamine release in the NAc and, to a greater extent, in high novelty-seeking than in low novelty-seeking rodents (Hooks et al., 1991). Similarly, amphetamine induces dopamine release in human brains, and a high novelty-seeking score predicts greater amphetamine-induced dopamine release and further drug-seeking behavior (Leyton et al., 2002a). Serotonin is a mediator of novelty-seeking behavior; high novelty seekers generally exhibit low responsiveness of the serotonergic system (Netter et al., 1996). Evenden suggested that the association between low tissue serotonin concentrations and low impulsivity; serotonergic system underactivity may impact novelty-seeking behavior in humans secondary to serotonin-related impulsivity changes (Evenden, 1999). Parallel findings have shown the same behavioral and molecular pattern in rodent studies involving median forebrain lesions. Rats with decreased serotonergic activity show an increase in the rate of amphetamine self-administration (Leccese and Lyness, 1984).
In short, we know that drugs of abuse affect these neurotransmission systems and that novelty-seeking behavior has been linked to nearly all categories of addictive drug use. Thus, further studies that examine the role of neurotransmitters other than dopamine in mediating novelty-seeking behavior may provide greater insight into the underlying molecular connection between the novelty-seeking personality trait and vulnerability to addiction.
Because of its role as the primary neurotransmitter in the reward pathway of the brain, the dopaminergic system has been more fully examined as a mediator of novelty-seeking behavior and substance abuse than have other neurotransmitter systems. Cloninger argued for a baseline difference in mesolimbic dopaminergic signaling between high and low novelty seekers, that individual variations in the brain’s “behavioral activation system” lead high novelty seekers to exhibit greater rewards from drugs of abuse (Cloninger, 1987). He suggested that low basal firing of midbrain dopaminergic neurons that project from the VTA to the NAc may be observed in high novelty seekers; in turn, they are more sensitive to any release of dopamine that would result from using a drug of abuse (Cloninger, 1987). This hypothesis is supported by the fact that alcoholics typically have low levels of dopamine and its metabolites in cerebrospinal fluid samples (Cloninger, 1986).
Conversely, other studies have found that rats exhibiting novelty-seeking behavior are basally hyperdopaminergic (Marinelli and White, 2000; Nadal et al., 2002). Marinelli and White demonstrated that high novelty-seeking rats exhibited higher basal firing rates in the VTA [68]. Flagel and colleagues used fast-scan cyclic voltammetry on rats selectively bred for novelty-seeking behavior and found that high novelty seekers displayed a greater frequency of spontaneous dopamine release in the NAc (Flagel et al., 2010). High novelty-seeking rats also have been shown to display both a greater behavioral output response to an infusion of dopamine into the NAc and fewer dopamine receptor 2 (D2R) binding sites in this region and the striatum (Hooks et al., 1994; Tournier et al., 2013; Flagel et al., 2014). Tournier and colleagues demonstrated that high novelty-seeking rats had innately lower concentrations of D2R in the substantia nigra (SN) and VTA than did low novelty-seeking rats; these low concentrations may result in a specific pattern of dopaminergic signaling that underlies vulnerability to addiction (Tournier et al., 2013). However, these researchers conceded that the hyperdopaminergic activity could be the cause or the result of these low concentrations of D2 receptor function. Flagel and colleagues stated that there are more high-affinity D2 receptors in high than in low novelty-seeking rats but that the total number of dopaminergic receptors may be constant (Flagel et al., 2014). Those investigators concluded that the D2high receptor proportion represents individual differences in vulnerability to drug rewarding effects; a larger proportion represents the most addiction-vulnerable individuals because of the hypersensitivity to dopamine release induced by certain drugs (Flagel et al., 2014). The research on the mechanisms regulating dopamine uptake and release has implicated the dopamine transporter (Lim et al.) as having a key role in this action (Sabeti et al., 2002; Chefer et al., 2003; Gulley et al., 2003; Zhu et al., 2005). These studies have shown that there exists a negative correlation between DAT and novelty-seeking behavior, and this correlation can affect the response to drugs of abuse, particularly nicotine and cocaine.
A common approach to understanding the link between novelty seeking and addiction in animals is to identify brain regions that are well established to be associated with both novelty seeking and addiction and then to examine genetic similarities and differences in these regions between high and low novelty seekers. Brain regions linked to individual differences in novelty seeking and addiction include the NAc (Flagel et al., 2010), VTA (Marinelli and White, 2000), prefrontal cortex (PFC) (Bernheim et al., 2013), and hippocampus (Chefer et al.) (Fumagalli et al., 2003; Flagel et al., 2010). Addiction studies have further implicated the striatum (Toda, 2012), arcuate nucleus (Koob, 2006), ventral palladium (Mahler et al., 2014), amygdala (See et al., 2003), and raphe nuclei (Kirby et al., 2011). High-resolution functional magnetic resonance imaging (fMRI) has demonstrated that distinct clusters within these brain regions are modulated primarily by the anticipation of reward and novelty (Krebs et al., 2011). Figure 4 illustrates the neurotransmitter systems that modulate the response to rewarding stimuli.
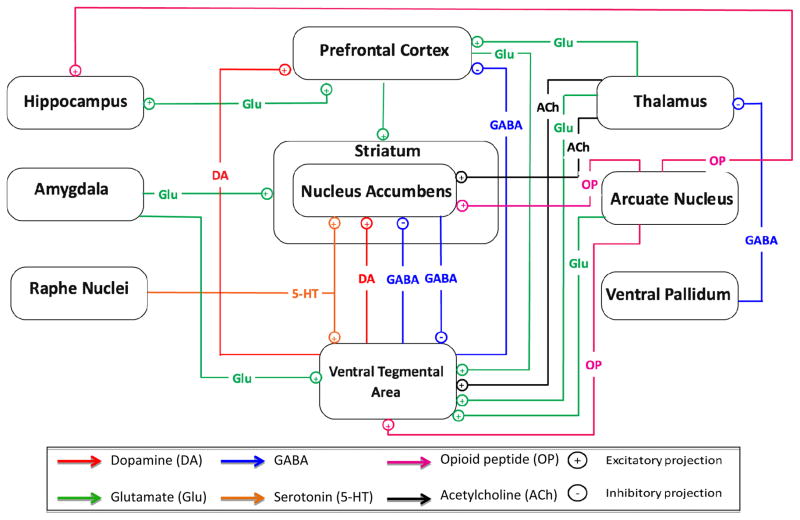
Figure 4.
Illustration of the neurocircuitry involved in the response to rewarding stimuli, including drugs of abuse and novelty. Dopamine (DA) plays a key role in this system, through excitatory projections directly connecting the ventral tegmental area (VTA) with the nucleus accumbens (NAc) and prefrontal cortex (PFC). Other excitatory neurotransmitters include glutamate (Ahmadi et al.), serotonin (5-HT), opioid peptides (OP), and acetylcholine (ACh). A major inhibitory neurotransmitter of this reward system is GABA, connecting the VTA, NAc, PFC, thalamus, and ventral palladium. Other brain regions implicated in the response to rewarding stimuli include the hippocampus, amygdala, raphe nuclei, striatum, and arcuate nucleus.
Several genes have been singled out as being associated with a novelty-seeking phenotype. Allelic heterogeneity in the dopamine D4 receptor gene (DRD4) has consistently been associated with novelty seeking; Okuyama and colleagues first demonstrated that polymorphisms in the human DRD4 exon III could contribute to individual differences in novelty-seeking behavior (Okuyama et al., 2000). Specifically, the −521 C/T polymorphism was significantly associated with novelty seeking among adult males (n = 86) using the TCI. Multiple studies have confirmed the view that this polymorphism mediates novelty-seeking behavior, including studies with subjects displaying extreme phenotypes, animal models including gene knockouts, and studies investigating the evolutionary history of the DRD4 exon III polymorphism (Ronai et al., 2001; Bookman et al., 2002; Eichhammer et al., 2005; Golimbet et al., 2005; Ebstein, 2006). Substance abuse has also been linked to DRD4; genome scans for genetic linkage to drug addiction have shown a region on chromosome 11p (D11S1984) that includes the DRD4 locus (Long et al., 1998). Recently, Thanos and colleagues found that DRD4 has an important role in novelty-seeking behavior in male mice and that the extent of such behavior positively correlated with alcohol consumption (Thanos et al., 2015).
The val158met polymorphism of the catechol-O-methyltransferase gene (COMT) (rs4680), whose protein is involved in the degradation of dopamine, has been implicated in the novelty-seeking trait (Hosak, 2007; Montag et al., 2012). Val158 allele carriers display more resilience in their temperament (Kang et al., 2013), whereas Met158 carriers have a higher risk of emotional dysregulation (Kempton et al., 2009). The link between COMT and novelty seeking was shown by Chen and colleagues, who demonstrated that males (n = 250) carrying the Val/Val genotype displayed lower novelty seeking (Chen et al., 2011). An earlier study by Hosak and colleagues found consistent results, in that 37 individuals who carried the Met/Met genotype displayed higher novelty seeking (Hosak et al., 2006).
The serotonin receptor 2A gene (HTR2A) also mediates novelty-seeking behavior (Heck et al., 2009; Nakamura et al., 2010). Heck and colleagues examined 17 genes involving serotonergic and dopaminergic signaling that had been associated with the novelty-seeking personality trait (Heck et al., 2009). This gene was associated with novelty seeking; specifically, two intronic SNPS, rs2770296 and rs927544, located within this gene correlated with novelty seeking (Heck et al., 2009).
The dopamine receptor 2 gene (DRD2) has been linked to both substance abuse and novelty-seeking behavior (Blum et al., 1990; Compton et al., 1996; Lawford et al., 2000). Subjects with the DRD2 TaqI A1 allele have fewer dopamine D2 receptors in the striatum and are basally hypodopaminergic (Compton et al., 1996). Alcoholism and opiate dependence also have been linked to polymorphisms in DRD2 (Blum et al., 1990; Lawford et al., 2000).
Taken together, these findings provide further evidence for the molecular link between novelty seeking and drug addiction. However, because various studies examining this gene in the context of both addiction and novelty seeking have provided contradictory evidence (Bolos et al., 1990; Lu et al., 1996; Crettol et al., 2008), much remains to be elucidated. Nonetheless, studies in both animals and humans looking at the behavioral and molecular connections between novelty seeking and drug addiction provide new insights into using this phenotype for creating preventative and curative measures for addiction-vulnerable individuals.
Conclusions and Future Directions
The body of research in the field has provided solid evidence for association but not for causality between novelty seeking and addiction. If the correlation is truly causal, then individuals who display underlying high novelty seeking will be more likely to experiment with drugs, transition to compulsive drug use, and develop addictive behaviors. However, if addiction precedes high novelty seeking, then individuals who compulsively abuse drugs may experience psychobiological adaptations in the brain that increase novelty-seeking behavior. Of course, the relation between novelty seeking and substance abuse could be a combination of input and output, whereby high novelty seekers are more likely to initiate drug use and then experience adaptations that lead to even higher measures of novelty seeking.
Future research should include assessing the effect of age on novelty seeking and addiction. Because it is well established that age affects novelty seeking in addition to substance abuse development, these studies would provide important insights into how to prevent and treat drug addiction. Human studies would provide good evidence for a relation; animal models may demonstrate in a controlled setting the causation between age and novelty seeking on drug addiction-related behaviors. Furthermore, because the causal relation between novelty seeking and drug addiction is still not entirely clear, researchers may wish to carry out animal studies that demonstrate how novelty seeking and addiction development are connected to each other temporally. It may also be of interest to examine the difference in self-administration between stimulants and sedatives in high and low novelty seekers, as high novelty seekers might prefer cocaine to benzodiazepines, for example, as reported by Blanchard and colleagues (Blanchard et al., 2009). Further studies elucidating the link between various mental illnesses, novelty seeking, and substance abuse may also enrich the field; for example, Dervaux and coworkers hypothesized that novelty seeking could be the glue between schizophrenia and alcohol dependence (Dervaux et al., 2010). Ballon and colleagues found a link between childhood attention deficit hyperactivity disorder (ADHD), later sensation seeking in adults, and eventual cocaine dependence (Ballon et al., 2015). Finally, as the population continues to age, focus within these paradigms may be turned onto the growing presence of debilitating diseases – such as HIV-1 infection, Parkinson’s disease, and Alzheimer’s disease. Assessing the connection between novelty seeking and substance abuse in patients exhibiting these conditions may provide valuable information regarding potential pharmacologic and behavioral treatments. Regardless of the approach used in future research, novelty seeking will continue to be a key personality trait in individuals when predicting vulnerability to addiction.
Acknowledgments
This work was supported, in part, by US National Institutes of Health grants DA-012844 and DA-026356.
Footnotes
Financial Disclosures: The authors claim no conflict interest regarding this report.
References
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Verweij KJ, Gillespie NA, Heath AC, Lessov-Schlaggar CN, Martin NG, Nelson EC, Slutske WS, Whitfield JB, Lynskey MT. The genetics of addiction-a translational perspective. Translational psychiatry. 2012;2:e140. doi: 10.1038/tp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahituv N, Kavaslar N, Schackwitz W, Ustaszewska A, Martin J, Hebert S, Doelle H, Ersoy B, Kryukov G, Schmidt S, Yosef N, Ruppin E, Sharan R, Vaisse C, Sunyaev S, Dent R, Cohen J, McPherson R, Pennacchio LA. Medical sequencing at the extremes of human body mass. Am J Hum Genet. 2007;80:779–791. doi: 10.1086/513471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi R, Ugurluoglu A, Schillinger M, Katzenschlager R, Sabeti S, Minar E. Duplex ultrasound-guided femoropopliteal angioplasty: Initial and 12-month results from a case controlled study. J Endovasc Ther. 2002;9:873–881. doi: 10.1177/152660280200900622. [DOI] [PubMed] [Google Scholar]
- Ambrosio E, Goldberg SR, Elmer GI. Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol. 1995;6:229–237. [PubMed] [Google Scholar]
- Andrucci GL, Archer RP, Pancoast DL, Gordon RA. The Relationship of Mmpi and Sensation Seeking Scales to Adolescent Drug-Use. Journal of personality assessment. 1989;53:253–266. doi: 10.1207/s15327752jpa5302_4. [DOI] [PubMed] [Google Scholar]
- Arnett J. Sensation Seeking - a New Conceptualization and a New Scale. Pers Individ Dif. 1994;16:289–296. [Google Scholar]
- Ballon N, Brunault P, Cortese S. Sensation Seeking and Cocaine Dependence in Adults With Reported Childhood ADHD. J Atten Disord. 2015;19:335–342. doi: 10.1177/1087054714543651. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behavioural brain research. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim A, Halfon O, Boutrel B. Controversies about the enhanced vulnerability of the adolescent brain to develop addiction. Frontiers in pharmacology. 2013;4:118. doi: 10.3389/fphar.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Klebaur JE, Bardo MT. Individual differences in response to novelty, amphetamine-induced activity and drug discrimination in rats. Behav Pharmacol. 1997;8:113–123. [PubMed] [Google Scholar]
- Blanchard MM, Mendelsohn D, Stamp JA. The HR/LR model: Further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev. 2009;33:1145–1154. doi: 10.1016/j.neubiorev.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, Nogami H, Briggs AH, Cohn JB. Allelic association of human dopamine D2 receptor gene in alcoholism. Jama. 1990;263:2055–2060. [PubMed] [Google Scholar]
- Blum K, Chen AL, Giordano J, Borsten J, Chen TJ, Hauser M, Simpatico T, Femino J, Braverman ER, Barh D. The addictive brain: all roads lead to dopamine. J Psychoactive Drugs. 2012;44:134–143. doi: 10.1080/02791072.2012.685407. [DOI] [PubMed] [Google Scholar]
- Boissier JR, Simon P. The exploration reaction in the mouse. Preliminary note. Therapie. 1962;17:1225–1232. [PubMed] [Google Scholar]
- Bolos AM, Dean M, Lucasderse S, Ramsburg M, Brown GL, Goldman D. Population and Pedigree Studies Reveal a Lack of Association between the Dopamine-D2 Receptor Gene and Alcoholism. Jama-J Am Med Assoc. 1990;264:3156–3160. [PubMed] [Google Scholar]
- Bookman EB, Taylor RE, Adams-Campbell L, Kittles RA. DRD4 promoter SNPs and gender effects on Extraversion in African Americans. Mol Psychiatry. 2002;7:786–789. doi: 10.1038/sj.mp.4001075. [DOI] [PubMed] [Google Scholar]
- Brown GR, Nemes C. The exploratory behaviour of rats in the hole-board apparatus: is head-dipping a valid measure of neophilia? Behavioural processes. 2008;78:442–448. doi: 10.1016/j.beproc.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns L. World Drug Report 2013 United Nations Office on Drugs and Crime. Drug Alcohol Rev. 2014;33:216–216. [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Carton S, Le Houezec J, Lagrue G, Jouvent R. Relationships between sensation seeking and emotional symptomatology during smoking cessation with nicotine patch therapy. Addictive behaviors. 2000;25:653–662. doi: 10.1016/s0306-4603(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Zakharova I, Shippenberg TS. Enhanced responsiveness to novelty and cocaine is associated with decreased basal dopamine uptake and release in the nucleus accumbens: Quantitative microdialysis in rats under transient conditions. J Neurosci. 2003;23:3076–3084. doi: 10.1523/JNEUROSCI.23-07-03076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Moyzis R, Dong Q, He Q, Zhu B, Li J, Li H, Lessard J. Sex modulates the associations between the COMT gene and personality traits. Neuropsychopharmacol. 2011;36:1593–1598. doi: 10.1038/npp.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatric developments. 1986;4:167–226. [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM. The Tridimensional Personality Questionnaire: U.S. normative data. Psychol Rep. 1991;69:1047–1057. doi: 10.2466/pr0.1991.69.3.1047. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Archives of general psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Comeau N, Stewart SH, Loba P. The relations of trait anxiety, anxiety sensitivity, and sensation seeking to adolescents’ motivations for alcohol, cigarette, and marijuana use. Addictive behaviors. 2001;26:803–825. doi: 10.1016/s0306-4603(01)00238-6. [DOI] [PubMed] [Google Scholar]
- Compton PA, Anglin MD, Khalsa-Denison ME, Paredes A. The D2 dopamine receptor gene, addiction, and personality: clinical correlates in cocaine abusers. Biological psychiatry. 1996;39:302–304. doi: 10.1016/0006-3223(95)00476-9. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Der-Ghazarian T, Britt CE, Varela FA, Kozanian OO. Novelty-induced conditioned place preference, sucrose preference, and elevated plus maze behavior in adult rats after repeated exposure to methylphenidate during the preweanling period. Behavioural brain research. 2013;246:29–35. doi: 10.1016/j.bbr.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crettol S, Besson J, Croquette-Krokar M, Hammig R, Gothuey I, Monnat M, Deglon JJ, Preisig M, Eap CB. Association of dopamine and opioid receptor genetic polymorphisms with response to methadone maintenance treatment. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:1722–1727. doi: 10.1016/j.pnpbp.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ. 2011;2:3. doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacology, biochemistry, and behavior. 2008;90:331–338. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervaux A, Laqueille X, Bourdel MC, Olie JP, Krebs MO. Impulsivity and sensation seeking in alcohol abusing patients with schizophrenia. Front Psychiatry. 2010;1:135. doi: 10.3389/fpsyt.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP. The molecular genetic architecture of human personality: beyond self-report questionnaires. Mol Psychiatry. 2006;11:427–445. doi: 10.1038/sj.mp.4001814. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Sand PG, Stoertebecker P, Langguth B, Zowe M, Hajak G. Variation at the DRD4 promoter modulates extraversion in Caucasians. Mol Psychiatry. 2005;10:520–522. doi: 10.1038/sj.mp.4001658. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug Addiction Endophenotypes: Impulsive Versus Sensation-Seeking Personality Traits. Biol Psychiatry. 2010;68:770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties sf impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evren C, Durkaya M, Evren B, Dalbudak E, Cetin R. Relationship of relapse with impulsivity, novelty seeking and craving in male alcohol-dependent inpatients. Drug and alcohol review. 2012;31:81–90. doi: 10.1111/j.1465-3362.2011.00303.x. [DOI] [PubMed] [Google Scholar]
- Faingold C, Li Y, Evans MS. Decreased GABA and increased glutamate receptor-mediated activity on inferior colliculus neurons in vitro are associated with susceptibility to ethanol withdrawal seizures. Brain research. 2000;868:287–295. doi: 10.1016/s0006-8993(00)02342-8. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Bailey JN, Breidenthal SE, Laudenslager ML, Kaplan JR, Jorgensen MJ. Environmental stress alters genetic regulation of novelty seeking in vervet monkeys. Genes, brain, and behavior. 2011;10:683–688. doi: 10.1111/j.1601-183X.2011.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J. Early-onset alcoholism with conduct disorder: go/no go learning deficits, working memory capacity, and personality. Alcoholism, clinical and experimental research. 2002;26:186–206. [PubMed] [Google Scholar]
- Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology. 2014;76(Pt B):425–436. doi: 10.1016/j.neuropharm.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacol. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Maragnoli ME, Gennarelli M, Perez J, Racagni G, Riva MA. Dopaminergic D2 receptor activation modulates FGF-2 gene expression in rat prefrontal cortex and hippocampus. J Neurosci Res. 2003;74:74–80. doi: 10.1002/jnr.10733. [DOI] [PubMed] [Google Scholar]
- Golimbet VE, Gritsenko IK, Alfimova MV, Ebstein RP. Polymorphic markers of the dopamine D4 receptor gene promoter region and personality traits in mentally healthy individuals from the Russian population. Russian Journal of Genetics. 2005;41:789–793. [PubMed] [Google Scholar]
- Gomez H, Lluch JM, Masgrau L. Essential role of glutamate 317 in galactosyl transfer by alpha3GalT: a computational study. Carbohydrate research. 2012;356:204–208. doi: 10.1016/j.carres.2012.03.027. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Badner JA, Byerley W, Keck PE, McElroy SL, Remick RA, Dessa Sadovnick A, Kelsoe JR. Heritability and linkage analysis of personality in bipolar disorder. Journal of affective disorders. 2013;151:748–755. doi: 10.1016/j.jad.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JM, Hoover BR, Larson GA, Zahniser NR. Individual differences in cocaine-induced locomotor activity in rats: Behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacol. 2003;28:2089–2101. doi: 10.1038/sj.npp.1300279. [DOI] [PubMed] [Google Scholar]
- Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- Hascoet M, Bourin M, Dhonnchadha BAN. The mouse light-dark paradigm: A review. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:141–166. doi: 10.1016/s0278-5846(00)00151-2. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY. Risk and Protective Factors for Alcohol and Other Drug Problems in Adolescence and Early Adulthood - Implications for Substance-Abuse Prevention. Psychol Bull. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Heck A, Lieb R, Ellgas A, Pfister H, Lucae S, Roeske D, Putz B, Muller-Myhsok B, Uhr M, Holsboer F, Ising M. Investigation of 17 candidate genes for personality traits confirms effects of the HTR2A gene on novelty seeking. Genes Brain and Behavior. 2009;8:464–472. doi: 10.1111/j.1601-183X.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Agatsuma S. Genetic susceptibility to substance dependence. Mol Psychiatry. 2005;10:336–344. doi: 10.1038/sj.mp.4001622. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Juncos JL, Justice JB, Jr, Meiergerd SM, Povlock SL, Schenk JO, Kalivas PW. Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14:6144–6152. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosak L. Role of the COMT gene Val158Met polymorphism in mental disorders: a review. European psychiatry: the journal of the Association of European Psychiatrists. 2007;22:276–281. doi: 10.1016/j.eurpsy.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Hosak L, Libiger J, Cizek J, Beranek M, Cermakova E. The COMT Val158Met polymorphism is associated with novelty seeking in Czech methamphetamine abusers: preliminary results. Neuro endocrinology letters. 2006;27:799–802. [PubMed] [Google Scholar]
- Howard MO, Kivlahan D, Walker RD. Cloninger’s tridimensional theory of personality and psychopathology: applications to substance use disorders. J Stud Alcohol. 1997;58:48–66. doi: 10.15288/jsa.1997.58.48. [DOI] [PubMed] [Google Scholar]
- Ismael F, Baltieri DA. Role of personality traits in cocaine craving throughout an outpatient psychosocial treatment program. Rev Bras Psiquiatr. 2014;36:24–31. doi: 10.1590/1516-4446-2013-1206. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Metrik J, Leventhal AM, Monti PM. Sensation seeking as a predictor of treatment compliance and smoking cessation treatment outcomes in heavy social drinkers. Pharmacology, biochemistry, and behavior. 2009;93:285–290. doi: 10.1016/j.pbb.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health. 1994;84:1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JI, Kim SJ, Song YY, Namkoong K, An SK. Genetic influence of COMT and BDNF gene polymorphisms on resilience in healthy college students. Neuropsychobiology. 2013;68:174–180. doi: 10.1159/000353257. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, Rush CR. Individual differences in drug abuse vulnerability: d-Amphetamine and sensation-seeking status. Psychopharmacology. 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Haldane M, Jogia J, Christodoulou T, Powell J, Collier D, Williams SC, Frangou S. The effects of gender and COMT Val158Met polymorphism on fearful facial affect recognition: a fMRI study. Int J Neuropsychopharmacol. 2009;12:371–381. doi: 10.1017/S1461145708009395. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS. Personality and comorbidity of common psychiatric disorders. Br J Psychiatry. 2005;186:190–196. doi: 10.1192/bjp.186.3.190. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Annals of the New York Academy of Sciences. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Heipertz D, Schuetze H, Duzel E. Novelty increases the mesolimbic functional connectivity of the substantia nigra/ventral tegmental area (SN/VTA) during reward anticipation: Evidence from high-resolution fMRI. NeuroImage. 2011;58:647–655. doi: 10.1016/j.neuroimage.2011.06.038. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Young RM, Noble EP, Sargent J, Rowell J, Shadforth S, Zhang XX, Ritchie T. The D-2 dopamine receptor al allele and opioid dependence: Association with heroin use and response to methadone treatment. Am J Med Genet. 2000;96:592–598. doi: 10.1002/1096-8628(20001009)96:5<592::aid-ajmg3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Leccese AP, Lyness WH. The Effects of Putative 5-Hydroxytryptamine Receptor Active Agents on D-Amphetamine Self-Administration in Controls and Rats with 5,7-Dihydroxytryptamine Median Forebrain-Bundle Lesions. Brain research. 1984;303:153–162. doi: 10.1016/0006-8993(84)90223-3. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002a;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: A PET/[C-11]raclopride study in healthy men. Neuropsychopharmacol. 2002b;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Lim SS, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E, Bennett PH, Goldman D. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet. 1998;81:216–221. doi: 10.1002/(sici)1096-8628(19980508)81:3<216::aid-ajmg2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Lu RB, Ko HC, Chang FM, Castiglione CM, Schoolfield G, Pakstis AJ, Kidd JR, Kidd KK. No association between alcoholism and multiple polymorphisms at the dopamine D2 receptor gene (DRD2) in three distinct Taiwanese populations. Biological psychiatry. 1996;39:419–429. doi: 10.1016/0006-3223(95)00182-4. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014;17:577–U136. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JJ, Thompson-Lake DGY, Cooper K, Verrico CD, Newton TF, De La Garza R. A comparison of impulsivity, depressive symptoms, lifetime stress and sensation seeking in healthy controls versus participants with cocaine or methamphetamine use disorders. Journal of Psychopharmacology. 2015;29:50–56. doi: 10.1177/0269881114560182. [DOI] [PubMed] [Google Scholar]
- Manzo L, Gomez MJ, Callejas-Aguilera JE, Donaire R, Sabariego M, Fernandez-Teruel A, Canete A, Blazquez G, Papini MR, Torres C. Relationship between ethanol preference and sensation/novelty seeking. Physiol Behav. 2014;133:53–60. doi: 10.1016/j.physbeh.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Meszaros K, Lenzinger E, Hornik K, Fureder T, Willinger U, Fischer G, Schonbeck G, Aschauer HN. The Tridimensional Personality Questionnaire as a predictor of relapse in detoxified alcohol dependents. The European Fluvoxamine in Alcoholism Study Group. Alcoholism, clinical and experimental research. 1999;23:483–486. [PubMed] [Google Scholar]
- Meyer AC, Rahman S, Charnigo RJ, Dwoskin LP, Crabbe JC, Bardo MT. Genetics of novelty seeking, amphetamine self-administration and reinstatement using inbred rats. Genes, brain, and behavior. 2010;9:790–798. doi: 10.1111/j.1601-183X.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Jurkiewicz M, Reuter M. The role of the catechol-O-methyltransferase (COMT) gene in personality and related psychopathological disorders. CNS & neurological disorders drug targets. 2012;11:236–250. doi: 10.2174/187152712800672382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162:333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Ito Y, Aleksic B, Kushima I, Yasui-Furukori N, Inada T, Ono Y, Ozaki N. Influence of HTR2A polymorphisms and parental rearing on personality traits in healthy Japanese subjects. Journal of human genetics. 2010;55:838–841. doi: 10.1038/jhg.2010.110. [DOI] [PubMed] [Google Scholar]
- Nesil T, Kanit L, Li MD, Pogun S. Nine generations of selection for high and low nicotine intake in outbred Sprague-Dawley rats. Behav Genet. 2013;43:436–444. doi: 10.1007/s10519-013-9605-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netter P, Hennig J, Roed IS. Serotonin and dopamine as mediators of sensation seeking behavior. Neuropsychobiology. 1996;34:155–165. doi: 10.1159/000119318. [DOI] [PubMed] [Google Scholar]
- Okuyama Y, Ishiguro H, Nankai M, Shibuya H, Watanabe A, Arinami T. Identification of a polymorphism in the promoter region of DRD4 associated with the human novelty seeking personality trait. Mol Psychiatry. 2000;5:64–69. doi: 10.1038/sj.mp.4000563. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Costentin J, Duterte-Boucher D. Differential involvement of anxiety and novelty preference levels on oral ethanol consumption in rats. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3910-5. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronai Z, Szekely A, Nemoda Z, Lakatos K, Gervai J, Staub M, Sasvari-Szekely M. Association between Novelty Seeking and the-521 C/T polymorphism in the promoter region of the DRD4 gene. Mol Psychiatry. 2001;6:35–38. doi: 10.1038/sj.mp.4000832. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol Withdrawal Is Associated with Increased Extracellular Glutamate in the Rat Striatum. Eur J Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: Behavioral and electrochemical recordings in freely moving rats. J Pharmacol Exp Ther. 2002;302:1201–1211. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- Schepis TS, Desai RA, Smith AE, Cavallo DA, Liss TB, McFetridge A, Potenza MN, Krishnan-Sarin S. Impulsive Sensation Seeking, Parental History of Alcohol Problems, and Current Alcohol and Tobacco Use in Adolescents. Journal of addiction medicine. 2008;2:185–193. doi: 10.1097/adm.0b013e31818d8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R, Jr, Ottoni GL, de Carvalho HW, Elisabetsky E, Lara DR. Temperament and character traits associated with the use of alcohol, cannabis, cocaine, benzodiazepines, and hallucinogens: evidence from a large Brazilian web survey. Rev Bras Psiquiatr. 2015;37:31–39. doi: 10.1590/1516-4446-2014-1352. [DOI] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Annals of the New York Academy of Sciences. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stautz K, Cooper A. Impulsivity-related personality traits and adolescent alcohol use: A meta-analytic review. Clinical Psychology Review. 2013;33:574–592. doi: 10.1016/j.cpr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Sutker PB, Archer RP, Allain AN. Drug abuse patterns, personality characteristics, and relationships with sex, race, and sensation seeking. J Consult Clin Psychol. 1978;46:1374–1378. doi: 10.1037//0022-006x.46.6.1374. [DOI] [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology (Berl) 2001;158:175–180. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Roushdy K, Sarwar Z, Rice O, Ashby CR, Jr, Grandy DK. The effect of dopamine D4 receptor density on novelty seeking, activity, social interaction, and alcohol binge drinking in adult mice. Synapse. 2015;69:356–364. doi: 10.1002/syn.21822. [DOI] [PubMed] [Google Scholar]
- Toda S. The role of the striatum in addiction. Brain and nerve = Shinkei kenkyu no shinpo. 2012;64:911–917. [PubMed] [Google Scholar]
- Tournier BB, Steimer T, Millet P, Moulin-Sallanon M, Vallet P, Ibanez V, Ginovart N. Innately low D2 receptor availability is associated with high novelty-seeking and enhanced behavioural sensitization to amphetamine. Int J Neuropsychopharmacol. 2013;16:1819–1834. doi: 10.1017/S1461145713000205. [DOI] [PubMed] [Google Scholar]
- Vanhille N, Belin-Rauscent A, Mar AC, Ducret E, Belin D. High locomotor reactivity to novelty is associated with an increased propensity to choose saccharin over cocaine: new insights into the vulnerability to addiction. Neuropsychopharmacol. 2015;40:577–589. doi: 10.1038/npp.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters GD. The heritability of alcohol abuse and dependence: a meta-analysis of behavior genetic research. The American journal of drug and alcohol abuse. 2002;28:557–584. doi: 10.1081/ada-120006742. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Yang LZ, Gu F, Li XM, Zha RJ, Wei ZD, Pei YK, Zhang P, Zhou YF, Zhang XC. Novelty seeking is related to individual risk preference and brain activation associated with risk prediction during decision making. Sci Rep-Uk. 2015:5. doi: 10.1038/srep10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyandt LL. The Physiological Bases of Cognitive and Behavioral Disorders. Routledge; 2005. [Google Scholar]
- Zheng X, Ke X, Tan B, Luo X, Xu W, Yang X, Sui N. Susceptibility to morphine place conditioning: relationship with stress-induced locomotion and novelty-seeking behavior in juvenile and adult rats. Pharmacology, biochemistry, and behavior. 2003;75:929–935. doi: 10.1016/s0091-3057(03)00172-2. [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. The sensation seeking scale V (SSS-V): Still reliable and valid. Pers Indiv Differ. 2007;43:1303–1305. [Google Scholar]
- Zuckerman M, Neeb M. Sensation seeking and psychopathology. Psychiatry research. 1979;1:255–264. doi: 10.1016/0165-1781(79)90007-6. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM, Joireman J, Teta P, Kraft M. A Comparison of 3 Structural Models for Personality - the Big 3, the Big 5, and the Alternative 5. J Pers Soc Psychol. 1993;65:757–768. [Google Scholar]