Abstract
Cytokinesis, the final step of cell division, is a great example of robust cell shape regulation. A wide variety of cells ranging from the unicellular Dictyostelium to human cells in tissues proceed through highly similar, stereotypical cell shape changes during cell division. Typically, cells first round up forming a cleavage furrow in the middle, which constricts resulting in the formation of two daughter cells. Tight control of cytokinesis is essential for proper segregation of genetic and cellular materials, and its failure is deleterious to cell viability. Thus, biological systems have developed elaborate mechanisms to ensure high fidelity of cytokinesis, including the existence of multiple biochemical and mechanical pathways regulated through feedback. In this review, we focus on the built-in redundancy of the cytoskeletal machinery that allows cells to divide successfully in a variety of biological and mechanical contexts. Using Dictyostelium cytokinesis as an example, we demonstrate that the crosstalk between biochemical and mechanical signaling through feedback ensures correct assembly and function of the cell division machinery.
Keywords: Cytokinesis, feedback, cell mechanics, actomyosin contractility, control system
Introduction
Cell division is essential for cell survival and proliferation. Cells must interface with diverse chemical and mechanical stimuli to ensure successful division in a variety of cellular and tissue contexts. For example, a stem cell needs to decide if and how to divide asymmetrically while a dividing epithelial cell must choose the right division plane to maintain the tissue architecture. Thus, it makes sense that biological systems have developed intricate and robust mechanisms for cytokinesis, the final stage of cell division where the daughter cells separate. Thus, the father of modern cytokinesis research, Ray Rappaport, aptly said “When I began working on cytokinesis, I thought I was tinkering with a beautifully made Swiss watch, but what I was really working on was an old Maine fishing boat engine: overbuilt, inefficient, never-failed, and repaired by simple measures.” [1]. The reliability of the boat engine is an essential attribute for the fisherman dependent on it to make a living. Similarly, numerous feedback loops as well as crosstalk between several biochemical and mechanical pathways ensure robust cytokinesis [2-4].
Cells can be thought of as machines that use chemical and mechanical inputs to make decisions on cell proliferation and fate. For the early forms of life, it is likely that their behavior was largely governed by physical cues such as confinement, pressure and temperature, and cellular systems have since evolved to respond to their environment. For example, barophilic organisms living deep under the sea use different machinery to divide from brain cells in an extremely soft environment. Even within metazoans, though core cytokinesis machinery exists, it is regulated differently in various cell types depending on functionality and context. Thus, in addition to biochemical signaling pathways, the impact of mechanical forces on cell behavior must also be evaluated. This is especially important for cytokinesis, which is largely a physical process involving cell shape changes through cellular contractility. In this review, we will discuss the mechanisms for multi-level regulation of the cytokinesis machinery, using Dictyostelium cytokinesis as an example. Its genetic homology and mechanical similarity to many mammalian cells, as well as its amenability for genetic, biochemical and mechanical perturbations, make the social amoeba Dictyostelium discoideum an excellent model for cytokinesis research. Aspects of biochemical and mechanical feedback regulation of cytokinesis and cell shape changes observed in Dictyostelium have also been demonstrated in other types of cell processes, such as embryonic development, myoblast fusion, immune cell maturation, and cell entosis [5-8].
Molecular and physical framework for cytokinesis
The network of actin and associated proteins beneath the cell surface constitutes the cell cortex, and gives the cell its shape and mechanical properties. The cortex is a highly dynamic organelle that undergoes constant remodeling as the cell changes shape during cytokinesis. A diverse array of actin crosslinkers confers structural and mechanical properties to the cortex by interacting dynamically with the actin filament framework, while the myosin motors generate contractile forces in the cortex. Myosin II and many crosslinkers accumulate in the equatorial region or cleavage furrow of a dividing cell, promoting local contractility [4, 9] (Fig. 1A). However, cytokinesis is sufficiently robust that it can proceed without myosin II [10], guided by the Laplace pressures that arise from the viscoelastic properties of the cortex and cytoplasm and from the local curvature of the cell surface [3, 11, 12]. Many cleavage furrow-enriched proteins including myosin II and cortexillin I, an actin crosslinker, also accumulate to sites of applied mechanical stress such as by micropipette aspiration or agarose overlay in a stress-dependent manner (Fig. 1A, 1B) [13-15]. Both myosin II and cortexillin I show cooperative accumulation kinetics (Fig 1C), the mechanisms for which are discussed later. Further, the cortexillin I-binding regulatory protein IQGAP2 also shows mechanosensitive accumulation [13].
Figure 1. Myosin II localization is guided by mechanical stress.
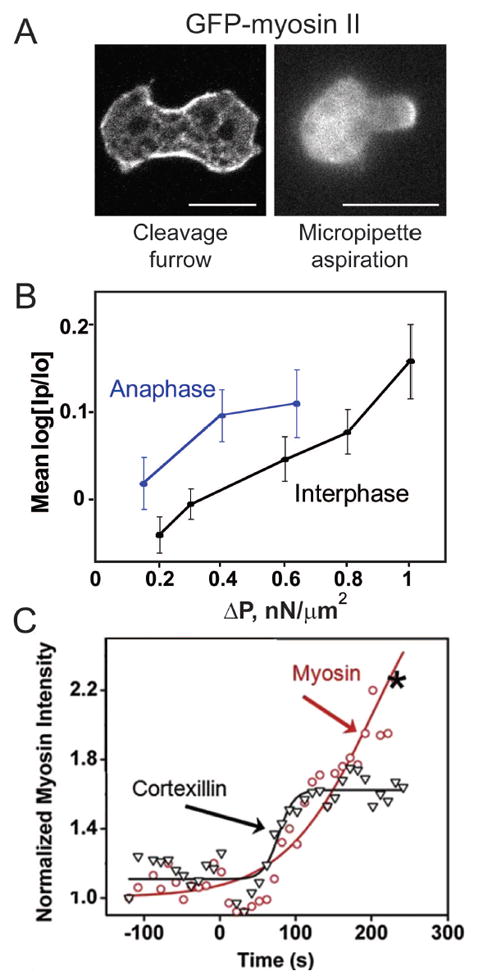
A. Myosin II is enriched at the cleavage furrow of a dividing cell and at the aspirated tip during micropipette aspiration (MPA). B. Myosin II enrichment during MPA depends on the applied pressure. Anaphase Dictyostelium cells are more mechanoresponsive than interphase cells. C. Myosin II and cortexillin I show cooperative accumulation kinetics during MPA (Reproduced from Luo et al. 2012 [38] with permission from Elsevier).
In addition to the actin cytoskeleton, microtubules and their associated proteins are essential for ensuring correct spatial and temporal positioning of the cytokinesis machinery. The mitotic spindle provides the necessary framework for the initial symmetry breaking and assembly of the cytokinesis apparatus [16-19]. Cues from both spindle and astral microtubules provide spatial regulation, while signaling pathways involving spindle-associated proteins regulate the timing of cytokinesis [2, 19, 20]. Many proteins that control the cell cycle also regulate cytokinesis. For example, Cdk1 inhibition upon anaphase onset triggers a signaling cascade through RhoA, resulting in major cytoskeletal reorganization associated with cytokinesis [21, 22]. Members of the chromosome passenger complex (CPC) localize to the cleavage furrow cortex from the mitotic spindle, and modulate shape change during cytokinesis [23-25]. Though signals from the mitotic spindle define contractility at the cleavage furrow, cellular contractility can also affect the localization of spindle signaling proteins through feedback (Fig. 2). For example, the mitotic kinesin-like protein (MKLP)-homolog kif12 and the CPC protein INCENP both accumulate to sites of high mechanical stress in Dictyostelium[ 25].
Figure 2. A combination of biochemical and mechanical cues from the mitotic spindle, and polar and equatorial cortices guide cytokinesis progression.
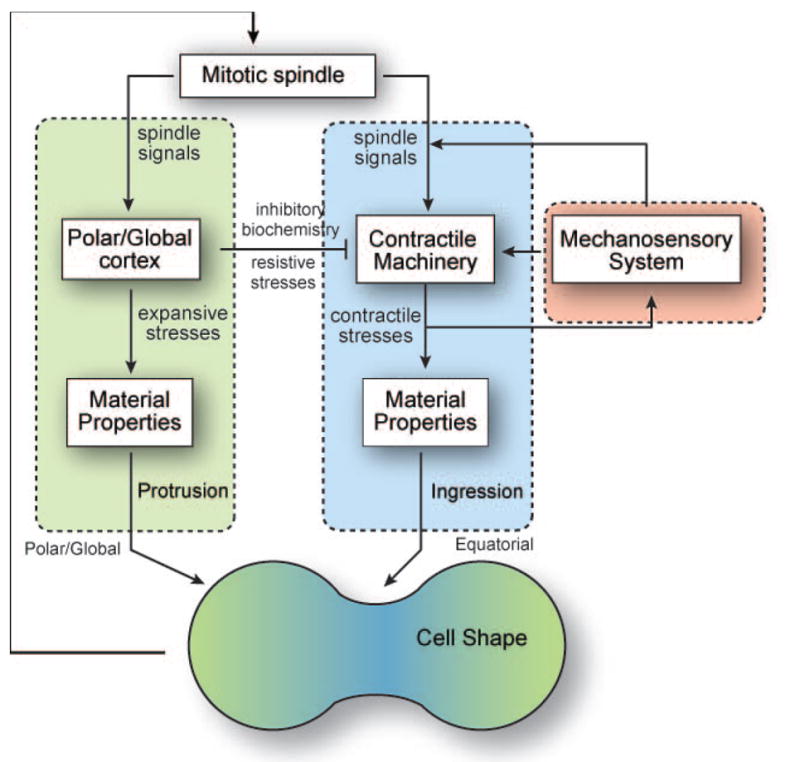
The contractile machinery, comprising of myosin II and cortexillin I, is recruited at the cleavage furrow and is regulated by a mechanosensory system. Polar actin crosslinkers define global mechanics, inhibiting contractility and promoting protrusions in the polar cortex.
Once we have assembled a parts list for cytokinesis, the question then becomes how do the various cellular modules communicate and collaborate to ensure cytokinesis proceeds with high fidelity. The signaling pathways regulating cytokinesis in different organisms have been extensively studied [2, 16, 26-28]. However, mechanical signaling must also be integrated with these pathways to completely understand cytokinesis regulation.
Mechanics of cytokinesis
Actin crosslinkers and the motor protein myosin II collectively bear the force (mechanical load) in the cortex. By altering the composition of the cortex, the physical properties of this composite material and protein behavior are affected [14]. Therefore, asymmetric localization of polar and equatorial crosslinkers during cytokinesis results in a mechanical gradient, where the furrow is less deformable than the poles [9]. Further, myosin II and the equatorial crosslinking protein cortexillin I are mechanoresponsive and accumulate to regions of high stress [14, 15, 25, 29]. Thus, the higher tension at the furrow helps stabilize the cleavage furrow localization of these proteins. Consistently, the cleavage furrow immobile fractions for cleavage furrow proteins, including myosin II, cortexillin I and IQGAP2, are significantly higher than those in the interphase cortex [30]. Importantly, the immobilization of cleavage furrow proteins during cytokinesis is due to changes in network properties and mechanical forces at the furrow, and is independent of many genetic perturbations [30]. In contrast, the polar crosslinkers, with faster, cytokinesis-independent protein dynamics, contribute to resistive stresses at the poles and modulate cortical tension [9, 31, 32]. Thus, spatial segregation of permissive and inhibitory signals helps stabilize the furrow positioning and ingression (Fig. 2).
The mechanoresponsiveness of the cell cortex and its components is dependent on how forces are shared between myosin II and actin crosslinkers. For example, in the absence of myosin II, certain crosslinkers such as α-actinin become more responsive to mechanical deformations. Moreover, mechanosensitive accumulation of myosin II is greater in mutants depleted of the polar crosslinker dynacortin or the small GTPase racE, which affects several polar crosslinkers [14]. Consistently, myosin II can accumulate to aspirated sites at much lower pressures in mitotic cells compared to interphase cells [14, 29]. From mechanical studies, a general pattern emerges: whereas loss of cleavage furrow crosslinker cortexillin I and its regulator IQGAP2 leads to diminished myosin II mechanosensitive accumulation, loss of any of the polar crosslinkers leads to increased myosin II mechanosensitive accumulation. This basic trend reflects the force-sharing between the polar crosslinkers and myosin II as well as the cooperative interactions between myosin II and cortexillin I. This paradigm will be expanded upon in the subsequent sections.
Myosin II mechanochemistry and cellular contractility
As myosin II is the major driver of furrow contractility, a detailed evaluation of the molecular mechanisms giving rise to myosin II’s mechanoresponsiveness and its interactions with other cortical proteins is necessary for understanding how different components discussed above work together to regulate cytokinesis. Myosin II can assemble into bipolar thick filaments, which generate contractile forces by simultaneously pulling on several actin filaments. Both myosin II-actin binding and thick filament dynamics are force-dependent, and control myosin II’s mechanoresponsiveness and cellular contractility.
A functional myosin II monomer is a hexamer comprised of two myosin heavy chains containing the motor and BTF assembly domains, two essential light chains and two regulatory light chains. Myosin II filament assembly and contractility are highly regulated by phosphorylation of both the heavy chain and the regulatory light chain [33, 34]. Phosphorylation of the regulatory light chain is controlled by the myosin light chain kinase (MLCK) and Rho-associated protein kinase (ROCK, in mammals; not found in Dictyostelium), and activates myosin II [35]. Phosphorylation of the myosin heavy chain regulates its ability to get incorporated into BTFs.
Heavy chain dephosphorylation by PP2A promotes BTF assembly while myosin heavy chain kinases inhibit BTF formation [36, 37]. The BTF assembly and disassembly dynamics are required for mechanosensitive remodeling of the cell cortex, as constitutively assembled or unassembled myosin II mutants do not accumulate to sites of high stress [15]. Hence, the regulation by phosphorylation sets the threshold for assembly, allowing mechanical stress to trigger the cooperativity of myosin motor heads (discussed in the next section) thereby dictating the precise location of where that BTF assembly will occur and on which actin filaments it will assemble [38, 39].
The enrichment of myosin II BTFs results in increased cellular contractility in regions of high stress. Consequently, the cell retracts from the aspirated site once myosin II is maximally recruited during micropipette aspiration [14, 29]. As the cell retracts, the cortex becomes less strained and myosin II unbinds from the cortex. In some mutants, like racE cells where myosin II is highly mechanoresponsive, myosin II accumulation and cellular retraction show out of phase oscillatory behavior [14]. Myosin II can also localize asymmetrically during early cytokinesis when the spindle is not centered to correct for shape asymmetries. Myosin II gets enriched furthest from the spindle and redistributes as the spindle elongates [29]. Myosin II induced contractility is also critical for tumor invasion and metastasis, where highly deformable and contractile cells have a higher metastatic potential [6, 40]. Thus, modulation of the contractile machinery is an important therapeutic strategy for cancer prevention and cure.
Cooperativity between myosin II and actin crosslinkers
Though myosin II is the force generating enzyme, actin crosslinkers are necessary for propagation of forces within the cortex. Both myosin II and cortexillin I accumulate at the cleavage furrow, and exhibit cooperative accumulation under micropipette aspiration (Fig. 1C), where the deletion of one disrupts the stress-dependent accumulation of the other [15]. Their synchronous accumulation suggests cooperative interactions between myosin II and cortexillin I.
Myosin II undergoes an ATP hydrolysis-dependent power stroke to generate, during which it takes an 8 nm step along the actin filament [41, 42]. Myosin II binding to the actin filament causes localized conformational changes in the filament, resulting in strain energy for the filament, which promotes the binding of additional myosin heads. The force generated during the myosin II power stroke creates tension in crosslinked actin filaments, and this tension results in increased binding lifetime of myosin II by locking it in the isometric state [43], which is also the cooperative binding state [38]. Coarse-grained Monte Carlo simulations for force-dependent myosin II binding can recapitulate the homo-cooperativity between myosin heads in silico [38], which has also been demonstrated in vitro. Microscopy showed that myosin II motors bind in clusters to actin filaments in a manner dependent on the actin conformation [44] or on the myosin motor’s nucleotide state (ATP- or transition state analog-bound, but not ADP-bound) [45]. Intriguingly, a mutant myosin S1, which populates the transition state (also the cooperative binding state), localized to specific populations of actin in the cell, including the actin in the cleavage furrow [46]. Further, in a reconstituted muscle myosin II contractile system, the fraction of myosin bound to actin increased sigmoidally indicative of cooperative binding as myosin concentration increases [47]. Finally, the rate of myosin II assembly into bipolar thick filaments was enhanced nearly 10-fold by the addition of actin filaments, but only in the presence of ATP [48]. In contrast, the addition of ADP plus actin inhibited thick filament assembly. We now understand that this rate enhancement is most likely due to cluster formation of the myosin II heads on the actin due to cooperative binding, which in turn increases the proximity of the myosin II tails, promoting thick filament assembly [38].
Similar hetero-cooperativity may exist between myosin II and cortexillin, which could lead to formation of clusters containing both proteins, as was observed in silico [38]. Thus, these proteins form the core of a mechanoresponsive contractile unit, where myosin II is the force-generating element and cortexillin I anchors the actin network, and helps propagate forces through this network. In contrast, α-actinin, which is also an actin bundling protein found at the cleavage furrow, only accumulates in response to mechanical stress in Dictyostelium myoII null cells [14]. The polar crosslinkers also exhibit antagonistic behavior with myosin II. Myosin II accumulates at ~2-fold lower pressures in interphase cells depleted of polar crosslinker dynacortin or the small GTPase racE, which regulates many polar crosslinkers [14]. Thus, force-sharing between myosin II and actin crosslinkers defines the cellular mechanical landscape, and determines cleavage furrow contractility during cytokinesis. Due to its importance in ensuring cytokinesis fidelity, cellular mechanosensing is highly regulated through feedback. Some such regulatory mechanisms are discussed in the section below.
Feedback regulation of cytokinesis and mechanosensing
In engineering, feedback loops have long been appreciated as a means of ensuring robustness [49]. Mechanosensory feedback during cytokinesis has a similar effect [29]. During cytokinesis, signals from the mitotic spindle initiate symmetry breaking and recruitment of cleavage furrow proteins to the cell equator. Subsequently, mechanical feedback and cooperativity between myosin II and cortexillin I controls the amount of protein and contractility at the furrow. Furthermore, two cortexillin I-binding proteins, IQGAP1 and IQGAP2, help regulate this mechanoresponsive system [25]. In the absence of IQGAP2, myosin II and cortexillin I fail to accumulate in response to applied stress due to inhibition by IQGAP1. However, a double mutant lacking both IQGAPs is highly mechanoresponsive, indicating that the IQGAPs are not required for mechanoresponsiveness, and that they only play regulatory roles. Further, IQGAP2 transduces the readout from the mechanosensor back to the spindle signaling proteins. IQGAP2 is required for directing mechanical stress-dependent accumulation of the mitotic kinesin-like protein (MKLP) Kif12 and the chromosome passenger complex protein INCENP to the cell cortex [25]. Overall, these mechanical feedback loops spatially and temporally tune myosin II accumulation and contractility, and are structured similar to control systems ubiquitous in engineering (Fig. 3).
Figure 3. A mechanosensory control system regulates myosin II accumulation and contractility at the cleavage furrow.
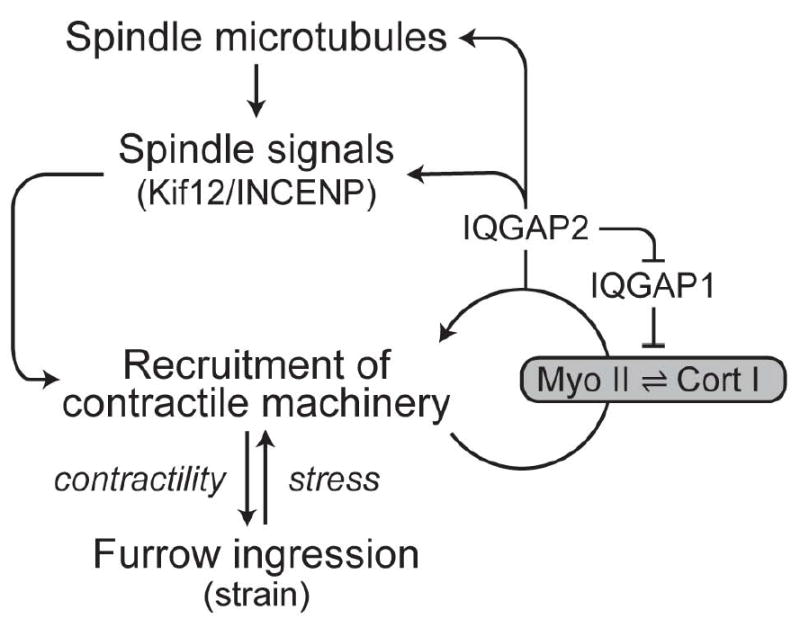
Signals from the mitotic spindle and mechanical stress recruit contractile machinery. Myosin II and cortexillin I form the core of this mechanosensory system. IQGAP-proteins regulate mechanosensitive accumulation of myosin II and cortexillin I. A feedback loop to spindle signaling proteins through IQGAP2 provides additional amplification of contractility at the furrow. Deletion of any of the factors of this feedback system causes increased cytokinesis failure, including daughter cell symmetry defects, which is indicative of each protein contributing to the robustness of the cell division system.
In addition to the equatorial mechanosensory control system, global cellular mechanics also contribute to cytokinesis fidelity. The small GTPase racE is a global regulator of cortical mechanics promoting resistive stresses [50], and is an upstream activator of many polar actin crosslinkers including dynacortin and coronin [3, 12, 51]. The overexpression of polar crosslinkers inhibits myosin II mechanoresponsiveness, while the absence of racE or dynacortin makes myosin II more responsive. During cytokinesis, the equatorial mechanoresponsive unit (myosin II and cortexillin I) and the polar resistive module (racE and other actin crosslinkers) exhibit inverse concentration gradients, promoting furrow ingression. Though the equatorial and polar modules have complementary roles during cytokinesis, crosstalk occurs between them. RacE also acts upstream of the regulatory protein 14-3-3, which is enriched in the polar cortex where it regulates cortical tension and steady state microtubule length [52]. 14-3-3 then binds to the myosin II heavy chain and promotes myosin II bipolar thick filament turnover at the furrow [52]. Collectively, these mechanisms demonstrate the interplay between polar and equatorial modules in regulating myosin II contractility, and their importance in maintaining cell shape during cytokinesis [4].
Though the roles of myosin II and many actin crosslinkers in cytokinesis and contractility have been examined carefully, the molecular mechanisms for the recruitment and retention of these proteins is not fully elucidated. For example, a myosin II mutant lacking the motor domain (headless myosin) can partially accumulate in the furrow region, though it does not incorporate into the cortex, highlighting the importance of the motor-actin interactions for integrating myosin II into the cortex [53]. However, the fact that the headless myosin can enrich in the furrow cytoplasm indicates that there are still more contributing mechanisms. Emphasizing this, a genetic selection has identified novel roles for many proteins, including RMD1 (regulator of microtubule dynamics-1) and mmsdh (methylmalonate semialdehyde dehydrogenase), in rescuing defects in myosin II BTF assembly and furrow enrichment [39]. Thus, multiple parallel pathways regulate myosin II furrow localization and cellular contractility, thereby providing robustness to the cellular cytokinesis machinery.
Cytokinesis regulation in other systems
In this article, we have mostly focused on the regulatory mechanisms for cytokinesis and cell shape control in the amoeba Dictyostelium. However, these concepts are not specific to one organism or process, but can be extended to a wide variety of organisms and cellular processes. The genetic simplicity and biochemical and mechanical amenability of Dictyostelium make it a powerful model to obtain mechanistic insights into cytokinesis regulation. Most proteins mentioned here have direct homologs, and their behavior has been replicated in other species. For example, myosin II is the major driver of contractility in most non-muscle cells, and is responsive to mechanical stress in other cell types including Drosophila S2 cells [5] and human hematopoietic stem cells [54]. In budding yeast, myo1 (the myosin II homolog) is immobilized during cytokinesis [55], similar to the stabilization of cleavage furrow proteins in Dictyostelium. Additionally, during mammalian cytokinesis, if the cortex becomes destabilized, the myosin II network can undergo fluctuating assembly and disassembly, resulting in a destabilized furrow and oscillating contractions across the cell and further highlighting the feedback control in contractile network [56]. Further, F-actin is not required for retaining myosin II at the equator once the ring-like structure has formed in fission yeast [57].
These results suggest that the cell cortex is highly mechanosensitive across species, and its ability to respond to mechanical forces is critical to ensuring robust cytokinesis. Perturbations to actomyosin contractility and cellular mechanics lead to cytokinesis defects in several species including vertebrates, nematodes and yeast [2, 26, 28]. In vertebrates, myosin II depletion results in highly multinucleated cells, and this defect can be restored by exogenous expression of any of the three myosin II isoforms [58]. Further, the ability of myosin II to generate tension in the network, rather than its ability to translocate actin filaments, is required for cytokinesis as shown using a myosin II mutant with no motility and an extremely long actin-attachment time [59, 60]. Similarly, in Dictyostelium, a myosin II mutant for which the Mg2+•ATPase activity is uncoupled to its power stroke responds to mechanical stress like wild type myosin II [17].
In addition to regulation of actomyosin contractility [61], feedback is also important in various other aspects of cell division. For example, the dynamic recruitment of the CPC to the central spindle is tightly controlled through many feedback loops involving Aurora B kinase [62, 63]. In fission yeast, the contractile network assembly is triggered by the septation initiation network (SIN) through the activation of Rho1 GTPase. Recent evidence has shown that Rho1 can also affect upstream regulators of SIN, thereby providing feedback to control actomyosin assembly and furrow ingression [64]. Thus, different systems have evolved intricate methods to ensure that cytokinesis proceeds with high fidelity.
Concluding remarks
Biological systems are designed to survive in a variety of scenarios. Key to their survival is the ability of cells to divide robustly under challenging conditions. Thus, organisms have developed sophisticated machinery that, at first glance, appears to be over-built. However, these complex networks are needed to ensure robust tight spatial and temporal regulation of essential components. During cell division, many biochemical and mechanical feedback loops collectively serve as checkpoints that allow for the detection and correction of defects that could be deleterious. Here, we have detailed the elaborate mechanisms that tune the assembly and dynamics of the actomyosin contractile unit at the cleavage furrow in dividing cells. Cooperativity and force-sharing between molecular motors (myosin II) and structural proteins (actin crosslinkers) form the basis of this mechanically tunable system [14]. Hence, Rappaport appropriately concluded that cytokinesis is indeed a complex shape change process that is over-built and hard to fail.
Highlights.
Cytokinesis progression is guided by a combination of biochemical and mechanical cues
Multiple feedback loops regulate the position and timing of cytokinesis
Myosin II and actin crosslinkers collectively define cleavage furrow contractility
Acknowledgments
We thank members of the Robinson lab for their valuable comments during manuscript preparation. This work was funded by the Hay Graduate Fellowship Fund (Cell Biology, JHU) (to V.S.) and NIH grants GM66817 and GM109863 (to D.N.R.).
Abbreviations
- MPA
Micropipette aspiration
- CPC
Chromosome Passenger Complex
- SIN
Septation initiation network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Canman JC, Wells WA. Rappaport Furrows on Our Minds: The ASCB Cytokinesis Meeting Burlington, VT July 22–25, 2004. The Journal of Cell Biology. 2004;166:943–8. doi: 10.1083/jcb.200409019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eggert US, Mitchison TJ, Field CM. Animal Cytokinesis: From Parts List to Mechanisms. Annual Review of Biochemistry. 2006;75:543–66. doi: 10.1146/annurev.biochem.74.082803.133425. [DOI] [PubMed] [Google Scholar]
- 3.Robinson DN, Kee YS, Luo T, Surcel A. 7.5 Understanding How Dividing Cells Change Shape. In: Egelman EH, editor. Comprehensive Biophysics. Amsterdam: Elsevier; 2012. pp. 48–72. [Google Scholar]
- 4.West-Foyle H, Robinson DN. Cytokinesis mechanics and mechanosensing. Cytoskeleton. 2012:700–9. doi: 10.1002/cm.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JH, Ren Y, Ng W-P, Li S, Son S, Kee Y-S, et al. Mechanical tension drives cell membrane fusion. Dev Cell. 2015;32:561–73. doi: 10.1016/j.devcel.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Q, Luo T, Ren Y, Florey O, Shirasawa S, Sasazuki T, et al. Competition between human cells by entosis. Cell Res. 2014;24:1299–310. doi: 10.1038/cr.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Gonzalez R, de Matos Simoes S, Röper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–43. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin JW, Buxboim A, Spinler KR, Swift J, Christian DA, Hunter CA, et al. Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell Stem Cell. 2014;14:81–93. doi: 10.1016/j.stem.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichl EM, Ren Y, Morphew MK, Delannoy M, Effler JC, Girard KD, et al. Interactions between Myosin and Actin Crosslinkers Control Cytokinesis Contractility Dynamics and Mechanics. 2008;18:471–80. doi: 10.1016/j.cub.2008.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lozanne AD, Spudich JA. Disruption of the Dictyostelium Myosin Heavy Chain Gene by Homologous Recombination. Science. 1987;236:1086–91. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- 11.Poirier CC, Ng WP, Robinson DN, Iglesias PA. Deconvolution of the Cellular Force-Generating Subsystems that Govern Cytokinesis Furrow Ingression. PLoS Comput Biol. 2012;8:e1002467. doi: 10.1371/journal.pcbi.1002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Robinson DN. Balance of actively generated contractile and resistive forces controls cytokinesis dynamics. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7186–91. doi: 10.1073/pnas.0502545102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kee Y-S, Ren Y, Dorfman D, Iijima M, Firtel R, Iglesias PA, et al. A mechanosensory system governs myosin II accumulation in dividing cells. Molecular Biology of the Cell. 2012;23:1510–23. doi: 10.1091/mbc.E11-07-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo T, Mohan K, Iglesias PA, Robinson DN. Molecular mechanisms of cellular mechanosensing. Nat Mater. 2013;12:1064–71. doi: 10.1038/nmat3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren Y, Effler JC, Norstrom M, Luo T, Firtel RA, Iglesias PA, et al. Mechanosensing through Cooperative Interactions between Myosin II and the Actin Crosslinker Cortexillin I. 2009;19:1421–8. doi: 10.1016/j.cub.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glotzer M. The Molecular Requirements for Cytokinesis. Science. 2005;307:1735–9. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 17.Oliferenko S, Chew TG, Balasubramanian MK. Positioning cytokinesis. Genes & Development. 2009;23:660–74. doi: 10.1101/gad.1772009. [DOI] [PubMed] [Google Scholar]
- 18.Scholey JM, Brust-Mascher I, Mogilner A. Cell division. Nature. 2003;422:746–52. doi: 10.1038/nature01599. [DOI] [PubMed] [Google Scholar]
- 19.Straight AF, Field CM. Microtubules, membranes and cytokinesis. Current Biology. 2000;10:R760–R70. doi: 10.1016/s0960-9822(00)00746-6. [DOI] [PubMed] [Google Scholar]
- 20.Glotzer M. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat Rev Mol Cell Biol. 2009;10:9–20. doi: 10.1038/nrm2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maddox AS, Burridge K. RhoA is required for cortical retraction and rigidity during mitotic cell rounding. The Journal of Cell Biology. 2003;160:255–65. doi: 10.1083/jcb.200207130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heng Y-W, Koh C-G. Actin cytoskeleton dynamics and the cell division cycle. The International Journal of Biochemistry & Cell Biology. 2010;42:1622–33. doi: 10.1016/j.biocel.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Chen Q, Lakshmikanth GS, Spudich JA, De Lozanne A. The Localization of Inner Centromeric Protein (INCENP) at the Cleavage Furrow Is Dependent on Kif12 and Involves Interactions of the N Terminus of INCENP with the Actin Cytoskeleton. Molecular Biology of the Cell. 2007;18:3366–74. doi: 10.1091/mbc.E06-10-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Chen Q, Kaller M, Nellen W, Gräf R, De Lozanne A. Dictyostelium Aurora Kinase Has Properties of both Aurora A and Aurora B Kinases. Eukaryotic Cell. 2008;7:894–905. doi: 10.1128/EC.00422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kee YS, Ren Y, Dorfman D, Iijima M, Firtel RA, Iglesias PA, et al. A mechanosensory system governs myosin II accumulation in dividing cells. Mol Biol Cell. 2012;23:1510–23. doi: 10.1091/mbc.E11-07-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balasubramanian MK, Bi E, Glotzer M. Comparative Analysis of Cytokinesis in Budding Yeast, Fission Yeast and Animal Cells. Current Biology. 2004;14:R806–R18. doi: 10.1016/j.cub.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Bowerman B. Cell division: Timing the machine. Nature. 2004;430:840–2. doi: 10.1038/430840a. [DOI] [PubMed] [Google Scholar]
- 28.Pollard TD. Mechanics of cytokinesis in eukaryotes. Current Opinion in Cell Biology. 2010;22:50–6. doi: 10.1016/j.ceb.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Effler JC, Kee Y-S, Berk JM, Tran MN, Iglesias PA, Robinson DN. Mitosis-Specific Mechanosensing and Contractile-Protein Redistribution Control Cell Shape. Current Biology. 2006;16:1962–7. doi: 10.1016/j.cub.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava V, Robinson Douglas N. Mechanical Stress and Network Structure Drive Protein Dynamics during Cytokinesis. Current Biology. 2015;25:663–70. doi: 10.1016/j.cub.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girard KD, Chaney C, Delannoy M, Kuo SC, Robinson DN. Dynacortin contributes to cortical viscoelasticity and helps define the shape changes of cytokinesis. EMBO J. 2004;23:1536–46. doi: 10.1038/sj.emboj.7600167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Octtaviani E, Effler JC, Robinson DN. Enlazin, a Natural Fusion of Two Classes of Canonical Cytoskeletal Proteins, Contributes to Cytokinesis Dynamics. Mol Biol Cell. 2006;17:5275–86. doi: 10.1091/mbc.E06-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosgraaf L, van Haastert PJM. The regulation of myosin II in Dictyostelium. European Journal of Cell Biology. 2006;85:969–79. doi: 10.1016/j.ejcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Egelhoff TT, Lee RJ, Spudich JA. Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell. 1993;75:363–71. doi: 10.1016/0092-8674(93)80077-r. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends in Cell Biology. 2005;15:371–7. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Yumura S, Yoshida M, Betapudi V, Licate LS, Iwadate Y, Nagasaki A, et al. Multiple myosin II heavy chain kinases: roles in filament assembly control and proper cytokinesis in Dictyostelium. Mol Biol Cell. 2005;16:4256–66. doi: 10.1091/mbc.E05-03-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Steimle PA, Ren Y, Ross CA, Robinson DN, Egelhoff TT, et al. Dictyostelium huntingtin controls chemotaxis and cytokinesis through the regulation of myosin II phosphorylation. Mol Biol Cell. 2011;22:2270–81. doi: 10.1091/mbc.E10-11-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo T, Mohan K, Srivastava V, Ren Y, Iglesias PA, Robinson DN. Understanding the Cooperative Interaction between Myosin II and Actin Cross-Linkers Mediated by Actin Filaments during Mechanosensation. Biophysical Journal. 2012;102:238–47. doi: 10.1016/j.bpj.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren Y, West-Foyle H, Surcel A, Miller C, Robinson DN. Genetic suppression of a phosphomimic myosin II identifies system-level factors that promote myosin II cleavage furrow accumulation. Molecular Biology of the Cell. 2014;25:4150–65. doi: 10.1091/mbc.E14-08-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surcel A, Ng WP, West-Foyle H, Zhu Q, Ren Y, Avery LB, et al. Pharmacological activation of myosin II paralogs to correct cell mechanics defects. Proceedings of the National Academy of Sciences. 2015 doi: 10.1073/pnas.1412592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finer JT, Simmons RM, Spudich JA. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994;368:113–9. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- 42.Murphy CT, Rock RS, Spudich JA. A myosin II mutation uncouples ATPase activity from motility and shortens step size. Nat Cell Biol. 2001;3:311–5. doi: 10.1038/35060110. [DOI] [PubMed] [Google Scholar]
- 43.Veigel C, Molloy JE, Schmitz S, Kendrick-Jones J. Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat Cell Biol. 2003;5:980–6. doi: 10.1038/ncb1060. [DOI] [PubMed] [Google Scholar]
- 44.Orlova A, Egelman EH. Cooperative rigor binding of myosin to actin is a function of F-actin structure. Journal of Molecular Biology. 1997;265:469–74. doi: 10.1006/jmbi.1996.0761. [DOI] [PubMed] [Google Scholar]
- 45.Tokuraku K, Kurogi R, Toya R, Uyeda TQP. Novel mode of cooperative binding between myosin and Mg2+ -actin filaments in the presence of low concentrations of ATP. J Mol Biol. 2009;386:149–62. doi: 10.1016/j.jmb.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Uyeda TQ, Iwadate Y, Umeki N, Nagasaki A, Yumura S. Stretching actin filaments within cells enhances their affinity for the myosin II motor domain. PLoS ONE. 2011;6:e26200. doi: 10.1371/journal.pone.0026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trybus KM, Taylor EW. Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:7209–13. doi: 10.1073/pnas.77.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahajan RK, Vaughan KT, Johns JA, Pardee JD. Actin filaments mediate Dictyostelium myosin assembly in vitro. Proc Natl Acad Sci U S A. 1989;86:6161–5. doi: 10.1073/pnas.86.16.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stelling J, Sauer U, Szallasi Z, Doyle Iii FJ, Doyle J. Robustness of Cellular Functions. Cell. 2004;118:675–85. doi: 10.1016/j.cell.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Gerald N, Dai J, Ting-Beall HP, De Lozanne A. A Role for Dictyostelium RacE in Cortical Tension and Cleavage Furrow Progression. J Cell Biol. 1998;141:483–92. doi: 10.1083/jcb.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson DN, Spudich JA. Dynacortin, a Genetic Link between Equatorial Contractility and Global Shape Control Discovered by Library Complementation of a Dictyostelium discoideum Cytokinesis Mutant. J Cell Biol. 2000;150:823–38. doi: 10.1083/jcb.150.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Q, Kee Y-S, Poirier CC, Jelinek C, Osborne J, Divi S, et al. 14-3-3 Coordinates Microtubules, Rac, and Myosin II to Control Cell Mechanics and Cytokinesis. Current Biology. 2010;20:1881–9. doi: 10.1016/j.cub.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zang J-H, Spudich JA. Myosin II localization during cytokinesis occurs by a mechanism that does not require its motor domain. Proceedings of the National Academy of Sciences. 1998;95:13652–7. doi: 10.1073/pnas.95.23.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin J-W, Buxboim A, Spinler Kyle R, Swift J, Christian David A, Hunter Christopher A, et al. Contractile Forces Sustain and Polarize Hematopoiesis from Stem and Progenitor Cells. Cell Stem Cell. 2014;14:81–93. doi: 10.1016/j.stem.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wloka C, Vallen EA, Thé L, Fang X, Oh Y, Bi E. Immobile myosin-II plays a scaffolding role during cytokinesis in budding yeast. The Journal of Cell Biology. 2013 doi: 10.1083/jcb.201208030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sedzinski J, Biro M, Oswald A, Tinevez J-Y, Salbreux G, Paluch E. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature. 2011;476:462–6. doi: 10.1038/nature10286. [DOI] [PubMed] [Google Scholar]
- 57.Naqvi NI, Eng K, Gould KL, Balasubramanian MK. Evidence for F-actin-dependent and-independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast. The EMBO Journal. 1999;18:854–62. doi: 10.1093/emboj/18.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bao J, Jana SS, Adelstein RS. Vertebrate Nonmuscle Myosin II Isoforms Rescue Small Interfering RNA-induced Defects in COS-7 Cell Cytokinesis. Journal of Biological Chemistry. 2005;280:19594–9. doi: 10.1074/jbc.M501573200. [DOI] [PubMed] [Google Scholar]
- 59.Kanada M, Nagasaki A, Uyeda TQP. Adhesion-dependent and Contractile Ring-independent Equatorial Furrowing during Cytokinesis in Mammalian Cells. Molecular Biology of the Cell. 2005;16:3865–72. doi: 10.1091/mbc.E05-03-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma X, Kovács M, Conti MA, Wang A, Zhang Y, Sellers JR, et al. Nonmuscle myosin II exerts tension but does not translocate actin in vertebrate cytokinesis. Proceedings of the National Academy of Sciences. 2012;109:4509–14. doi: 10.1073/pnas.1116268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levayer R, Lecuit T. Biomechanical regulation of contractility: spatial control and dynamics. Trends in Cell Biology. 2012;22:61–81. doi: 10.1016/j.tcb.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 62.van der Horst A, Lens SMA. Cell division: control of the chromosomal passenger complex in time and space. Chromosoma. 2014;123:25–42. doi: 10.1007/s00412-013-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bastos RN, Cundell MJ, Barr FA. KIF4A and PP2A–B56 form a spatially restricted feedback loop opposing Aurora B at the anaphase central spindle. The Journal of Cell Biology. 2014;207:683–93. doi: 10.1083/jcb.201409129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alcaide-Gavilán M, Lahoz A, Daga RR, Jimenez J. Feedback Regulation of SIN by Etd1 and Rho1 in Fission Yeast. Genetics. 2014;196:455–70. doi: 10.1534/genetics.113.155218. [DOI] [PMC free article] [PubMed] [Google Scholar]


