Abstract
The alarmin high mobility group box-1 (HMGB1) has been implicated as a key factor mediating neuroinflammatory processes. Recent findings suggest that the redox state of HMGB1 is a critical molecular feature of HMGB1 such that the reduced form (fr-HMGB1) is chemotactic, while the disulfide form (ds-HMGB1) is pro-inflammatory. The present study examined the neuroinflammatory effects of these molecular forms as well as the ability of these forms to prime the neuroinflammatory and microglial response to an immune challenge. To examine the neuroinflammatory effects of these molecular forms in vivo, animals were administered intra-cisterna magna (ICM) a single dose of fr-HMGB1 (10 µg), ds-HMGB1 (10 µg) or vehicle and basal pro-inflammatory effects were measured 2 and 24h post-injection in hippocampus. Results of this initial experiment demonstrated that ds-HMGB1 increased hippocampal pro-inflammatory mediators at 2h (NF-κBIα mRNA, NLRP3 mRNA and IL-1β protein) and 24h (NF-κBIα mRNA, TNFα mRNA, and NLRP3 protein) after injection. fr-HMGB1 had no effect on these mediators. These neuroinflammatory effects of ds-HMGB1 suggested that ds-HMGB1 may function to prime the neuroinflammatory response to a subsequent immune challenge. To assess the neuroinflammatory priming effects of these molecular forms, animals were administered ICM a single dose of fr-HMGB1 (10 µg), ds-HMGB1 (10 µg) or vehicle and 24h after injection, animals were challenged with LPS (10 µg/kg IP) or vehicle. Neuroinflammatory mediators and the sickness response (3, 8 and 24h after injection) were measured 2h after immune challenge. We found that ds-HMGB1 potentiated the neuroinflammatory (NF-κBIα mRNA, TNFα mRNA, IL-1β mRNA, IL-6 mRNA, NLRP3 mRNA and IL-1β protein) and sickness response (reduced social exploration) to LPS challenge. fr-HMGB1 failed to potentiate the neuroinflammatory response to LPS. To examine whether these molecular forms of HMGB1 directly induce neuroinflammatory effects in isolated microglia, whole brain microglia were isolated and treated with fr-HMGB1 (0, 1, 10, 100, or 1000 ng/ml) or ds-HMGB1 (0, 1, 10, 100, or 1000 ng/ml) for 4h and pro-inflammatory mediators measured. To assess the effects of these molecular forms on microglia priming, whole brain microglia were pre-exposed to these forms of HMGB1 (0, 1, 10, 100, or 1000 ng/ml) and subsequently challenged with LPS (10 ng/ml). We found that ds-HMGB1 increased expression of NF-κBIα mRNA and NLRP3 mRNA in isolated microglia, and potentiated the microglial pro-inflammatory response (TNFα mRNA, IL-1β mRNA and IL-1β protein) to LPS. fr-HMGB1 failed to potentiate the microglial pro-inflammatory response to LPS. Consistent with prior reports, the present findings demonstrate that the disulfide form of HMGB1 not only potentiates the neuroinflammatory response to a subsequent immune challenge in vivo, but also potentiates the sickness response to that challenge. Moreover, the present findings demonstrate for the first time that ds-HMGB1 directly potentiates the microglia pro-inflammatory response to an immune challenge, a finding that parallels the effects of ds-HMGB1 in vivo. In addition, ds-HMGB1 induced expression of NLRP3 and NF-κBIα in vivo and in vitro suggesting that the NLRP3 inflammasome may play role in the priming effects of ds-HMGB1. Taken together, the present results suggest that the redox state of HMGB1 is a critical determinant of the priming properties of HMGB1 such that the disulfide form of HMGB1 induces a primed immunophenotype in the CNS, which may result in an exacerbated neuroinflammatory response upon exposure to a subsequent pro-inflammatory stimulus.
Keywords: HMGB1, redox state, microglia, priming, neuroinflammation
1. Introduction
The alarmin high mobility group box-1 (HMGB1) has been implicated as a key factor mediating neuroinflammatory processes in several pathophysiological conditions including seizure (Maroso et al., 2010), ischemia (Kim et al., 2006), chronic pain (Agalave et al., 2014) and alcohol-induced neuroinflammation (Zou and Crews, 2014). Recently, we have shown that HMGB1 is also a critical mediator of stress-induced priming of the microglial pro-inflammatory response to a subsequent immune challenge (Weber et al., 2015). However, the mechanism(s) by which HMGB1 exerts these neuroinflammatory effects has not been clarified.
HMGB1 is a nuclear protein that functions as a danger associated molecular pattern (DAMP), which is released into the extracellular milieu to signal cellular damage, cellular stress, or pathogen insult (Bianchi, 2007). The primary structure of HMGB1 is composed of an A box domain, which functions as an HMGB1 antagonist and a B box domain, which exhibits pro-inflammatory properties (Klune et al., 2008). In addition, HMGB1 can function either as a chemotactic or pro-inflammatory mediator depending on the redox state of three critical cysteines (Venereau et al., 2012). HMGB1 functions as a chemotactic factor if cysteines C23, C45, and C106 remain in a thiol state (fully reduced HMGB1; fr-HMGB1), but in this state lacks pro-inflammatory properties. Alternatively, HMGB1 exerts pro-inflammatory effects if cysteines C23 and C45 become oxidized, while C106 remains in a thiol state. Oxidation of C23 and C45 results in the formation of a disulfide bond (disulfide HMGB1; ds-HMGB1), which is a critical determinant of the cytokine stimulating capacity of HMGB1 (Yang et al., 2012). Further, ds-HMGB1 lacks chemotactic properties, which suggests that fr- and ds-HMGB1 are mutually exclusive molecular forms (Venereau et al., 2012). Notably, oxidation of all three cysteines abrogates both the chemotactic and pro-inflammatory activity of HMGB1 (Yang et al., 2012).
The fr-HMGB1 forms a complex with the chemokine C-X-C motif ligand 12 (CXCL12), which then signals through the chemokine receptor, C-X-C chemokine receptor type 4 (CXCR4), to mediate chemotaxis, while the pro-inflammatory effects of ds-HMGB1 are mediated by the pattern recognition receptor, Toll-like receptor 4 (TLR4) (Lu et al., 2013). TLR4-mediated effects of HMGB1 are dependent upon the thiol state of cysteine C106 (Yang et al., 2012). Interestingly, the box A domain of HMGB1 can by itself function to competitively antagonize the pro-inflammatory effects of HMGB1 (Yang et al., 2004), presumably through TLR4.
Very few studies have investigated the role of HMGB1 redox states in neuroinflammatory processes. Balosso and colleagues found that the ds-HMGB1 increased NMDA-induced neuronal cell death and potentiated kainate-induced seizures (Balosso et al., 2014). A recent study by Liesz and colleagues found that cerebral ischemia induced the release of the fr-HMGB1 from necrotic brain lesions and that HMGB1 in a disulfide redox state was released into serum (Liesz et al., 2015). Recently, we found that box A administered into the CNS blocks stress-induced sensitization of the microglial pro-inflammatory response to lipopolysaccharide (LPS) ex vivo, suggesting that stress induces the release of HMGB1 in the CNS, which then functions to sensitize neuroinflammatory processes (Weber et al., 2015). Although it is unclear which form of HMGB1 mediated stress-induced sensitization of microglia, we found that ds-HMGB1 administered into the CNS in vivo was sufficient to prime microglia, whereas fr-HMGB1 failed to induce priming. Several key questions arising from this study and addressed here pertain to 1) the ability of ds-HMGB1 to induce neuroinflammatory effects in vivo independent of priming, 2) the ability of ds-HMGB1 to prime neuroinflammatory processes in vivo to a subsequent immune challenge, 3) whether the neuroinflammatory priming effects of ds-HMGB1 are behaviorally relevant, 4) whether ds-HMGB1 directly acts upon microglia to induce a primed state, and 5) the mechanism by which ds-HMGB1 primes microglia and the neuroinflammatory response to a subsequent immune challenge.
Several lines of evidence raise the possibility that the nucleotide-binding domain and leucine-rich repeat containing family, pyrin domain containing 3 (NLRP3) inflammasome might mediate ds-HMGB1-induced neuroinflammatory priming. The NLRP3 inflammasome is a multiprotein complex that mediates the processing and maturation of the pro-inflammatory cytokine interleukin (IL)-1β (Lamkanfi and Kanneganti, 2010). We and others have shown that stress induces NLRP3 (Pan et al., 2014; Weber et al., 2015) and NLRP3 is considered a sensor of a diverse array of DAMPs (Leemans et al., 2011). Of particular relevance here, formation of the NLRP3 inflammasome requires both a priming step and an activating step for the processing of IL-1β to proceed (Hornung and Latz, 2010). Therefore, the present investigation explored the effects of ds-HMGB1 on NLRP3 in vivo and in vitro as a mechanistic basis of the neuroinflammatory priming effects of ds-HMGB1.
2. Methods
2.1. Animals
Male Sprague–Dawley rats (60–90 day-old; Harlan Sprague–Dawley, Inc., Indianapolis, IN, USA) were pair-housed with food and water available ad libitum. The colony was maintained at 25 °C on a 12-h light/dark cycle (lights on at 07:00 h). All rats were allowed 1 week of acclimatization to the colony rooms before experimentation. All experimental procedures were conducted in accordance with the University of Colorado Institutional Animal Care and Use Committee.
2.2. Reagents
Lyophilized fr-HMGB1 and ds-HMGB1 were obtained from HMGBiotech (Milan, IT), suspended in pyrogen-free sterile water and are certified LPS-free. LPS (E. coli serotype O111:B4) was obtained from Sigma (St. Louis, MO).
2.3. Intra-cisterna magna (ICM) injections of fr-HMGB1 and ds-HMGB1
Rats were anesthetized with isoflurane (~ 3 min). The dorsal aspect of the skull was shaved and swabbed with 70% EtOH, a 27-gauge needle, attached via PE50 tubing to a 25 µl Hamilton syringe, was inserted into the cisterna magna. To verify entry into the cisterna magna, cerebrospinal fluid (CSF) was withdrawn (~2 µl) and visually inspected for the presence of red blood cells. Clear CSF indicated entry in the cisterna magna. fr-HMGB1 (1 µg in 10 µl), ds-HMGB1 (1 µg in 10 µl) or vehicle (10 µl pyrogen-free sterile water) was injected ICM. ICM administration was used to avoid implanting cannulae, which itself produces enduring neuroinflammation (Holguin et al., 2007). Hippocampus was dissected 2 and 24h after ICM injection. Hippocampus was a focus here because we have found this brain region to be particularly sensitive to stress-induced sensitization of neuroinflammatory processes (Frank et al., 2007; Johnson et al., 2002; Weber et al., 2015).
2.4. fr-HMGB1- and ds-HMGB1-induced priming in vivo
LPS (10 µg/kg IP) or vehicle (0.9% pyrogen-free saline) was injected 24h post-ICM injection of fr-HMGB1 (1 µg in 10 µl), ds-HMGB1 (1 µg in 10 µl) or vehicle (10 µl pyrogen-free sterile water). 2h post-LPS or vehicle injection, hippocampus was dissected.
2.5. Tissue Collection
Rats were injected with a lethal dose of sodium pentobarbital. Upon deep anesthesia, rats underwent transcardial perfusion with ice-cold saline (0.9%) for 3 min to remove peripheral immune cells from the CNS vasculature. Brains were rapidly extracted and placed on ice. For in vivo experiments, hippocampus was dissected, flash frozen in liquid nitrogen and stored at −80 °C. For in vitro experiments, whole brain microglia were immediately isolated.
2.6. In vitro stimulation of microglia with fr-HMGB1 and ds-HMGB1
Whole brain microglia were isolated using a Percoll density gradient as previously described (Frank et al., 2006). We have previously shown (Frank et al., 2006) that this microglia isolation procedure yields highly pure (>95%) microglia (Iba-1+/MHCII+/CD163−/GFAP−). Immunophenotype and purity of microglia was assessed and verified using real time RT-PCR of MHCII, CD163, Iba-1 and GFAP gene expression. Microglia were routinely found to be MHCII+/Iba1+/CD163-/GFAP- (data not shown). Microglia were cultured in 100 µl DMEM + 10% FBS and microglia concentration determined by trypan blue exclusion. Microglia were plated in individual wells of a 96-well v-bottom plate and incubated at 37 °C, 5% CO2. Microglia were exposed to fr-HMGB1 (0, 1, 10, 100, and 1000 ng/ml) or ds-HMGB1 (0, 1, 10, 100, and 1000 ng/ml) for 4h. Plate was centrifuged at 1000 x g for 10 min, 4 °C. Supernatants were collected for assay of cytokine release and cells were lysed under differing conditions (see below) for assay of protein or mRNA. To determine priming effects in vitro, microglia were exposed to fr-HMGB1 (0, 1, 10, 100, and 1000 ng/ml) or ds-HMGB1 (0, 1, 10, 100, and 1000 ng/ml) for 4h. Cells were washed (2x in fresh media) free of HMGB1 by centrifuging at 1000 x g for 10 min, 4 °C. Cells were suspended in fresh media (100 µl) and treated with LPS (10 ng/ml) or media control for 18 h. Supernatants and cells were processed as described above.
2.7. In vitro stimulation of peritoneal macrophages with fr-HMGB1 and ds-HMGB1
Peritoneal lavage was utilized to collect macrophages. An incision was made in the peritoneal cavity (~ 2 cm) and ice-cold Hank’s Balanced Salt Solution (30 ml) was pipetted into the abdominal cavity. The abdomen was massaged for 30 s after which the lavage fluid was removed (~20 ml) and centrifuged at 1000 rpm for 5 min at 4 °C. The cells were treated with red blood cell lysis buffer (160 mM NH4Cl, 12 mM NaHCO3, 100 µM EDTA, dissolved in dH2O, pH 7.3) for 2 min. The cells were suspended in 20 ml Iscove's medium supplemented with 10% fetal bovine serum, 1% penicillin– streptomycin and 2 µM L-glutamine and centrifuged at 1000 rpm for 5 min. Cells were then suspended in Iscove's media and plated at 200,000 cells per well/200 µl media in a 96-well tissue culture plate and incubated for 2 h. The cells were then washed with warm Dulbecco's Phosphate Buffered Saline, to remove any non-adherent cells and suspended in 200 µl media. The cells were cultured for 4 h ex vivo with 0, 1, 10, 100, 1000 ng/ml of either ds-HMGB-1 or fr-HMGB-1 at 37 °C with 5% CO2. Following incubation, cells were centrifuged, supernatant removed and Trizol added to lyse cells for mRNA analysis.
2.8. cDNA synthesis and real time RT-PCR
Total RNA was isolated from whole hippocampus and peritoneal macrophages utilizing a standard method of phenol:chloroform extraction (Chomczynski and Sacchi, 1987) and cDNA synthesis was performed using the SuperScript II cDNA synthesis kit (Invitrogen, Carlsbad, CA). For isolated microglia, cells were washed in 1 x PBS. Cells were lysed/homogenized and cDNA synthesis was performed according to the manufacturer’s protocol using the SuperScript III CellsDirect cDNA Synthesis System (Invitrogen). Gene expression was measured using real time RT-PCR. A detailed description of the PCR amplification protocol has been published previously (Frank et al., 2006). cDNA sequences were obtained from GenBank at the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov). Primer sequences were designed using the Eurofins MWG Operon Oligo Analysis & Plotting Tool (http://www.operon.com/technical/toolkit.aspx) and tested for sequence specificity using the Basic Local Alignment Search Tool at NCBI (Altschul et al., 1997). Primers were obtained from Invitrogen. Primer specificity was verified by melt curve analysis. All primers were designed to span exon/exon boundaries and thus exclude amplification of genomic DNA (see Table 1 for primer description and sequences). PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR Kit (Qiagen, Valencia, CA). Formation of PCR product was monitored in real time using the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Relative gene expression was determined by taking the expression ratio of the gene of interest to β-actin.
Table 1.
Primer Sequence and Gene Function
| Gene | Primer Sequence 5’ → 3’ |
Function |
|---|---|---|
| β-Actin | F: TTCCTTCCTGGGTATGGAAT R: GAGGAGCAATGATCTTGATC |
Cytoskeletal protein (Housekeeping gene) |
| CD163 | F: GTAGTAGTCATTCAACCCTCAC R: CGGCTTACAGTTTCCTCAAG |
Macrophage antigen not expressed by microglia |
| GFAP | F: AGATCCGAGAAACCAGCCTG R: CCTTAATGACCTCGCCATCC |
Astrocyte antigen |
| IL-1β | F: CCTTGTGCAAGTGTCTGAAG R: GGGCTTGGAAGCAATCCTTA |
Pro-inflammatory cytokine |
| IL-6 | F: AGAAAAGAGTTGTGCAATGGCA R: GGCAAATTTCCTGGTTATATCC |
Pro-inflammatory cytokine |
| Iba-1 | F: GGCAATGGAGATATCGATAT R: AGAATCATTCTCAAGATGGC |
Microglia/Macrophage antigen |
| MHCII | F: AGCACTGGGAGTTTGAAGAG R: AAGCCATCACCTCCTGGTAT |
Microglia/Macrophage antigen |
| NF-κBIα | F: CACCAACTACAACGGCCACA R: GCTCCTGAGCGTTGACATCA |
Induced by NFκB to inhibit NFκB function |
| NLRP3 | F: AGAAGCTGGGGTTGGTGAATT R: GTTGTCTAACTCCAGCATCTG |
Inflammasome component mediating caspase-1/IL-1β activation |
| TNFα | F: CAAGGAGGAGAAGTTCCCA R: TTGGTGGTTTGCTACGACG |
Pro-inflammatory cytokine |
2.9. Enzyme-linked immunosorbent assay (ELISA)
Hippocampus was sonicated using a tissue extraction reagent (Invitrogen) supplemented with a protease inhibitor cocktail (Sigma). Homogenate was centrifuged (10 min, 14,000 x g, 4 °C) and supernatant collected and stored at -20 °C. Total protein was quantified using a Bradford assay. IL-1β protein was measured using a commercially available ELISA (R & D Systems, Minneapolis, MN). For whole hippocampus, concentrations of IL-1β protein were scaled to total protein and expressed as pg/mg total protein. For cell culture supernatants, concentrations of IL-1β are expressed as pg/ml.
2.10. Western Blot
Hippocampus was processed and total protein determined as described under ELISA. Samples were heated to 75 °C for 10 min and loaded into a standard polyacrylamide Bis-Tris gel (Invitrogen). SDS-PAGE was performed in MOPS running buffer (Invitrogen) at 175 V for 1.25 h. Protein was transferred onto a nitrocellulose membrane using the iBlot dry transfer system (Invitrogen). The membrane was blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 h and incubated with a primary antibody in blocking buffer containing Tween 20 (0.2%) overnight at 4 °C. The following day, the membrane was washed in 1x PBS containing Tween 20 (0.1%) and then incubated in blocking buffer (0.2% Tween 20) containing either goat anti-rabbit or goat anti-mouse (LI-COR) IRDye 800CW secondary antibody at a concentration of 1:10,000 (LI-COR) for 1 h at RT. The membrane was washed in 1x PBS containing Tween 20 (0.1%). Protein expression was quantified using an Odyssey Infrared Imager (LI-COR) and expressed as a ratio to the housekeeping protein (β-actin). Primary antibodies included rabbit anti-rat NLRP3 monoclonal antibody (1:4000, Abcam, Cambridge, MA) and mouse anti-rat β-actin monoclonal antibody (1:200,000, Sigma).
2.11. Social Exploration Test
Each experimental subject was transferred to a novel cage with shaved wood bedding in a dimly lit room (40 lx). After a 15 min habituation period, a 28–32 day old juvenile male rat was introduced to the subject's cage for 5 min and exploratory behaviors (sniffing, pinning and allo-grooming) were timed by an observer blind to treatment condition. After the test, the juvenile was removed and the experimental adult rat was returned to its homecage. Although juvenile stimulus rats were reused for multiple tests, the adult was never re-tested with the same juvenile. Baseline social exploration was measured 24h prior to ICM injection of ds-HMGB1 (1 µg in 10 µl) or vehicle (10 µl pyrogen-free sterile water). 24 h post-ICM treatment, vehicle (0.9% pyrogen-free saline) or LPS (10 ug/kg) was injected IP. Social exploration was then measured 3, 8, and 24 h after IP injection.
2.12. Statistical analysis and data presentation
All data are presented as mean + SEM. Statistical analyses consisted of ANOVA followed by post-hoc tests (Newman-Keuls) using Prism 5 (Graphpad Software, Inc., La Jolla, CA). A one-way ANOVA was used to assess the effects of fr-HMGB1 and ds-HMGB1 in vivo and in vitro. A two-way ANOVA was used to assess the main effects and interaction of HMGB1 and LPS in vivo. All data met the assumptions of ANOVA including normality of data and homogeneity of variance. Omnibus F-values are reported for each ANOVA and serve as a criterion for performing post-hoc analyses. Post-hoc comparisons are provided in figures. Threshold for statistical significance was set at α = 0.05. 6-10 animals per experimental group were used in each experiment in vivo. 3-4 replications were performed for each in vitro experiment.
3. Results
3.1. Effect of fr-HMGB1 and ds-HMGB1 on pro-inflammatory mediators in hippocampus
To determine whether the redox state of HMGB1 differentially modulates the expression of pro-inflammatory mediators, fr-HMGB1, ds-HMGB1 and vehicle control were injected ICM. At 2 and 24h post-injection, the gene expression and protein levels of several pro-inflammatory mediators were measured in hippocampus (Fig. 1)
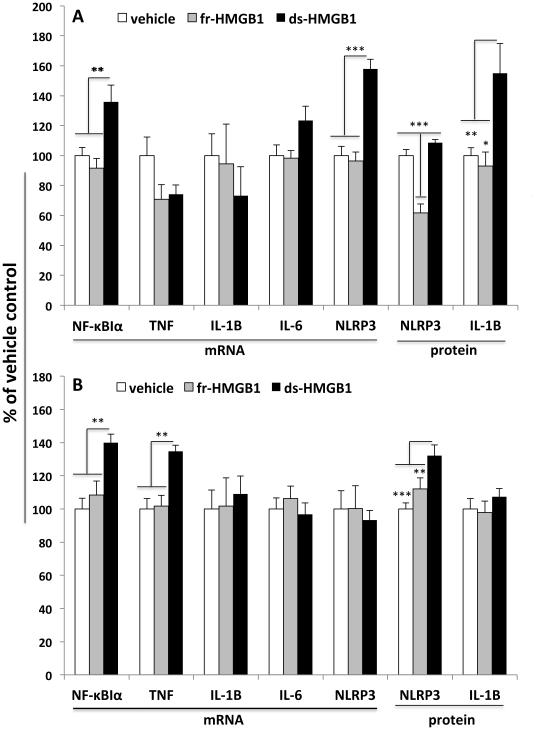
Fig. 1. Effect of fr-HMGB1 and ds-HMGB1 on pro-inflammatory mediators in hippocampus.
Vehicle, fr-HMGB1 (1 µg), or ds-HMGB1 (1 µg) was injected ICM and pro-inflammatory mediators measured 2h (A) and 24h (B) post-injection. A: fr-HMGB1 significantly reduced protein levels of NLRP3 protein compared to vehicle and ds-HMGB1 treatments, but failed to modulate the expression level of all other analytes. ds-HMGB1 induced a significant increase in NF-κBIα mRNA, NLRP3 mRNA and IL-1β protein compared to vehicle and fr-HMGB1 treatments. B: fr-HMGB1 failed to significantly modulate the expression level of all analytes tested. ds-HMGB1 significantly increased NF-κBIα mRNA, TNFα mRNA, and NLRP3 protein compared to vehicle and fr-HMGB1 treatments. N = 6 - 10 animals per experimental group. Data are presented as the mean + SEM. * p < 0.05, ** p < 0.01, *** p < 0.001.
2h post-ICM injection
HMGB1 treatment failed to significantly modulate the gene expression of IL-1β (df = 2, 15, F = 0.46, p = 0.64), TNFα (df = 2, 18, F = 3.17, p = 0.07), and IL-6 (df = 2, 17, F = 3.17, p = 0.07). However, ds-HMGB1 treatment significantly modulated the gene expression of NF-κBIα (df = 2, 18, F= 8.42, p = 0.003) and NLRP3 (df = 2, 18; F = 27.01, p < 0.0001)(Fig. 1A). ds-HMGB1 significantly increased NF-κBIα and NLRP3 mRNA compared to fr-HMGB1 and vehicle control treatment. NLRP3 protein was significantly changed by fr-HMGB1 treatment (df = 2, 18; F = 27.04, p < 0.0001), such that fr-HMGB1 treatment significantly decreased NLRP3 protein levels compared to vehicle and ds-HMGB1 treatment. ds-HMGB1 treatment also significantly altered IL-1β protein levels (df = 2, 17; F = 6.72, p = 0.01), whereby ds-HMGB1 significantly increased IL-1β protein compared to fr-HMGB1 and vehicle control groups (Fig. 1A).
24h post-ICM injection
IL-1β (df = 2, 16; F = 0.13, p = 0.87), IL-6 (df = 2, 17; F = 0.43, p = 0.66), and NLRP3 (df = 2, 18; F = 0.11, p = 0.89) gene expression was not significantly changed by HMGB1 treatment. ds-HMGB1 treatment did significantly modulate the expression of NF-κBIα (df = 2, 16; F = 9.31, p = 0.002) and TNFα (df = 2, 18; F = 9.85, p = 0.001)(Fig. 1B). ds-HMGB1 increased the expression of NF-κBIα and TNFα compared to fr-HMGB1 and vehicle control. IL-1β protein was unchanged by HMGB1 treatment (df = 2, 19; F = 0.67, p = 0.52), whereas NLRP3 protein was significantly changed (df = 2, 17; F = 10.23, p = 0.001)(See Fig. S1 in supplementary data for Western blot data). ds-HMGB1 treatment resulted in a significant increase in NLRP3 protein compared to fr-HMGB1 and vehicle control treatments (Fig. 1B).
3.2. Effect of fr-HMGB1 and ds-HMGB1 on priming of the hippocampal neuroinflammatory response
During an inflammatory response, changes in NF-κBIα expression reflect the transcriptional activity of NF-κB (Sun et al., 1993), which induces NLRP3 protein as part of the priming step of the NLRP3 inflammasome (Bauernfeind et al., 2009). Therefore, the observation that ds-HMGB1 increased the expression of NF-κBIα mRNA and NLRP3 protein at 24h post-treatment suggests that ds-HMGB1 may prime the neuroinflammatory response to a subsequent immune challenge. To test this possibility, fr-HMGB1 and ds-HMGB1 were injected ICM. 24h post-injection, LPS or vehicle were injected peripherally and pro-inflammatory mediators measured in hippocampus 2h post-LPS injection.
The interaction between fr-HMGB1 treatment and LPS was not statistically significant for NF-κBIα (df = 1, 20; F = 1.26, p = 0.27), IL-1β (df = 1, 20; F = 0.013, p = 0.91), IL-6 (df = 1, 20; F = 0.30, p = 0.59), TNFα (df = 1, 20; F = 0.075, p = 0.79) or NLRP3 (df = 1, 20; F = 1.05, p = 0.32) gene expression (data not shown). However, LPS treatment, irrespective of fr-HMGB1 treatment, significantly increased NF-κBIα (df = 1, 20; F = 64.72, p < 0.0001), IL-1β (df = 1, 20; F = 20.97, p = 0.0002), IL-6 (df = 1, 20; F = 51.06, p < 0.0001) and TNFα (df = 1, 20; F = 5.7, p < 0.0001) gene expression (data not shown). Similarly, fr-HMGB1 failed to differentially modulate the LPS induction of IL-1β protein levels (df = 1, 20; F = 0.15, p = 0.70), however LPS significantly increased IL-1β protein (df = 1, 20; F = 8.31, p = 0.009)(data not shown).
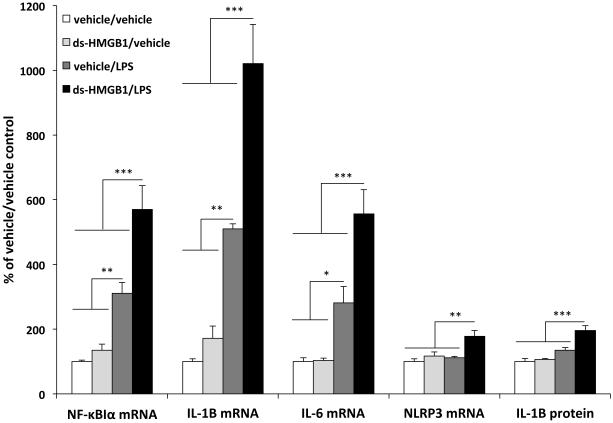
ds-HMGB1 treatment had no effect on any measures by itself, but it potentiated the pro-inflammatory effects of LPS on NF-κBIα (df = 1, 20; F = 7.27, p = 0.014), IL-1β (df = 1, 20; F = 11.58, p = 0.003), IL-6 (df = 1, 20; F = 8.8, p = 0.008), and NLRP3 (df = 1, 20; F = 4.77, p = 0.04) gene expression (Fig. 2). Likewise, the interaction between ds-HMGB1 and LPS on IL-1β protein was significant (df = 1, 20; F = 7.24, p = 0.014)(Fig. 2). ds-HMGB1 failed to differentially modulate the effect of LPS on TNFα gene expression (df = 1, 20; F = 1.51, p = 0.23), however LPS induced a significant increase in TNFα independent of ds-HMGB1 treatment (df = 1, 20; F = 9.36, p = 0.006)(data not shown).
Fig. 2. Effect of ds-HMGB1 on priming of the hippocampal neuroinflammatory response.
Vehicle or ds-HMGB1 (1 µg) was injected ICM. 24h post-ICM injection, vehicle or LPS (10 µg/kg) was injected IP and 2h post-LPS or vehicle treatment, pro-inflammatory cytokines were measured in hippocampus. ds-HMGB1 treatment potentiated the LPS induction of NF-κBIα mRNA (*** p < 0.001), IL-1β mRNA (*** p < 0.001), IL-6 mRNA (*** p < 0.001), NLRP3 mRNA (*** p < 0.001), and IL-1β protein (*** p < 0.001) compared to vehicle/LPS, ds-HMGB1/vehicle, and vehicle/vehicle treatment groups. Vehicle/LPS treatment significantly increased NF-κBIα mRNA (** p < 0.01), IL-1β mRNA (** p < 0.01), and IL-6 mRNA (* p < 0.05) compared to the ds-HMGB1/vehicle and vehicle/vehicle treatment groups. N = 6 animals per experimental group. Data are presented as the mean + SEM.
3.3. Effect of ds-HMGB1 on priming of the sickness response to LPS
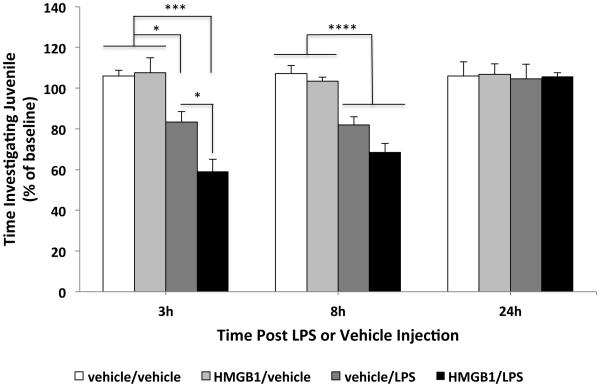
To explore whether the ds-HMGB1-induced potentiation of the neuroinflammatory response has behavioral consequences, we investigated the effect of ds-HMGB1 on LPS-induced decrements in social exploration of a conspecific juvenile. Juvenile social exploration is a widely used behavioral measure of the sickness response (Goshen and Yirmiya, 2009). The effect of fr-HMGB1 on the sickness response to LPS was not explored here given its failure to potentiate the LPS-induced neuroinflammatory response. ICM administration of ds-HMGB1 by itself had no effect on social exploration at any timepoint. LPS reduced social investigation, but importantly, prior ICM administration of ds-HMGB1 potentiated the LPS-induced reductions in social exploration at 3h post-LPS (LPS x ds-HMGB1 interaction; df = 1, 30; F = 5.15, p = 0.03), but failed to potentiate LPS induced sickness behavior at 8h and 24h post-LPS injection (Fig. 3). At 8h post-LPS, the main effect of LPS on social exploration was significant (df = 1, 30; F = 66.73, p < 0.0001) such that LPS decreased social exploration compared to the non-LPS treated animals. At 24h post-LPS, exploratory behavior returned to levels comparable to baseline levels.
Fig. 3. Effect of ds-HMGB1 on priming of the sickness response to LPS.
Vehicle or ds-HMGB1 (1 µg) was injected ICM and 24h post-ICM injection, vehicle or LPS (10 µg/kg) was injected IP. Social exploration of a con-specific juvenile by an adult treated animal was used as a behavioral measure of the sickness response to LPS. Social exploration (time investigating juvenile) was measured 24h prior to ICM injection (baseline) and 3, 8, and 24h post-LPS or vehicle injection. At 3h post-LPS treatment, ds-HMGB1 treatment significantly potentiated the LPS-induced decrease in social exploration compared to vehicle/LPS (* p < 0.05), ds-HMGB1/vehicle (*** p < 0.001), and vehicle/vehicle (*** p < 0.001) treatment groups. Vehicle/LPS treatment also significantly reduced social exploration compared to ds-HMGB1/vehicle (* p < 0.05) and vehicle/vehicle (* p < 0.05) treatment groups. At 8h post-LPS treatment, LPS independent of ds-HMGB1 treatment reduced social exploration compared to non-LPS treated animals (**** p < 0.0001). N = 7 - 8 animals per experimental group. Data are presented as the mean + SEM.
3.4. Effect of fr-HMGB1 and ds-HMGB1 on pro-inflammatory mediators in isolated microglia and peritoneal macrophages
The observation that ds-HMGB1 primed the neuroinflammatory response as well as the sickness response raised the question of whether this priming effect is due to the direct action of ds-HMGB1 on microglia. To examine this possibility, whole brain microglia were treated with fr-HMGB1 or ds-HMGB1 at varying concentrations for 4h and pro-inflammatory mediators measured.
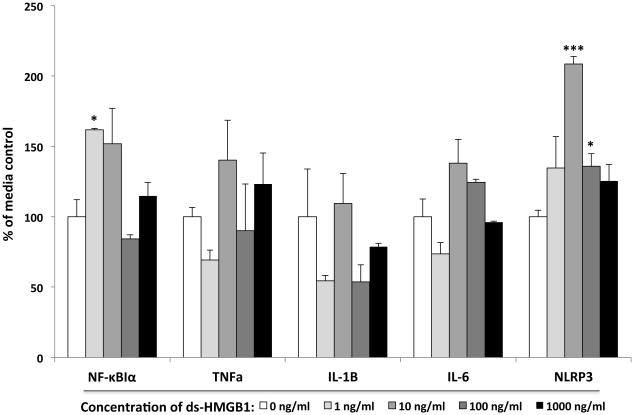
ds-HMGB1 failed to significantly alter TNFα (df = 4, 14; F = 1.55, p = 0.26) and IL-1β (df = 4, 14; F = 1.84, p = 0.2) gene expression. In addition, ds-HMGB1 failed to alter the concentration of IL-1β protein in the supernatant (df = 4, 14; F = 0.66, p = 0.63)(Fig. 4). However, the effect of ds-HMGB1 on microglia was significant for NF-κBIα (df = 4, 14; F = 6.17, p = 0.009), IL-6 (df = 4, 14; F = 6.3, p = 0.008) and NLRP3 (df = 4, 14; F = 10.81, p = 0.001) mRNA (Fig. 4) ds-HMGB1 significantly increased NF-κBIα (1 ng/ml ds-HMGB1) and NLRP3 (10 and 100 ng/ml ds-HMGB1) compared to media control. Though the one-way ANOVA for IL-6 was significant, post-hoc tests failed to show any group differences between media control and ds-HMGB1 treatment.
Fig. 4. Effect of ds-HMGB1 on pro-inflammatory mediators in isolated microglia.
Whole brain microglia were isolated and directly exposed to several concentrations of ds-HMGB1 for 4h and mRNA of pro-inflammatory mediators measured. ds-HMGB1 significantly increased NF-κBIα mRNA at 1 ng/ml (* p < 0.05) and NLRP3 mRNA at 10 (*** p < 0.001) and 100 ng/ml (* p < 0.05) compared to media control. N = 4 replications. Data are presented as the mean + SEM.
fr-HMGB1 failed to modulate the level of NF-κBIα (df = 4, 15; F = 0.49, p = 0.74), TNFα (df = 4, 15; F = 0.35, p = 0.84), IL-1β (df = 4, 15; F = 0.35, p = 0.84), IL-6 (df = 4, 15; F = 1.01, p = 0.43) and NLRP3 (df = 4, 15; F = 0.44, p = 0.78) mRNA. IL-1β protein levels in supernatant were also unchanged by fr-HMGB1 treatment (df = 4, 15; F = 1.46, p = 0.26)(data not shown).
In light of these results, we examined the effect of ds-HMGB1 and fr-HMGB1 on pro-inflammatory mediators in peritoneal macrophages to determine whether this pattern of pro-inflammatory effects was unique to microglia. In peritoneal macrophages, ds-HMGB1 induced a pattern of changes in pro-inflammatory mediators similar to that observed in microglia. While ds-HMGB1 failed to significantly alter the gene expression of IL-1β (df = 4, 10; F = 0.41, p = 0.8) and TNFα (df = 4, 10; F = 0.22, p = 0.92), ds-HMGB1 treatment had a significant effect on NF-κBIα (df = 4, 10; F = 6.83, p = 0.0064) and NLRP3 (df = 4, 10; F = 4.42, p = 0.016) mRNA (see Fig. S2 in supplementary data). fr-HMGB1 failed to significantly modulate the gene expression of NF-κBIα (df = 4, 10; F = 0.12, p = 0.97), TNFα (df = 4, 10; F = 0.7, p = 0.61), IL-1β (df = 4, 10; F = 0.94, p = 0.48), and NLRP3 (df = 4, 10; F = 2.6, p = 0.1) mRNA (data not shown).
3.5. Effect of ds-HMGB1 on priming of the microglial pro-inflammatory response
The effect of ds-HMGB1 on NF-κBIα and NLRP3 in vitro paralleled the effects of ds-HMGB1 in vivo suggesting that ds-HMGB1 may directly induce a primed immunophenotype in microglia. fr-HMGB1 was not examined in this context given its inability to prime neuroinflammatory responses in vivo or induce NF-κBIα and NLRP3 in vitro. Therefore, we examined whether prior exposure to ds-HMGB1 potentiates the microglial pro-inflammatory response to LPS.
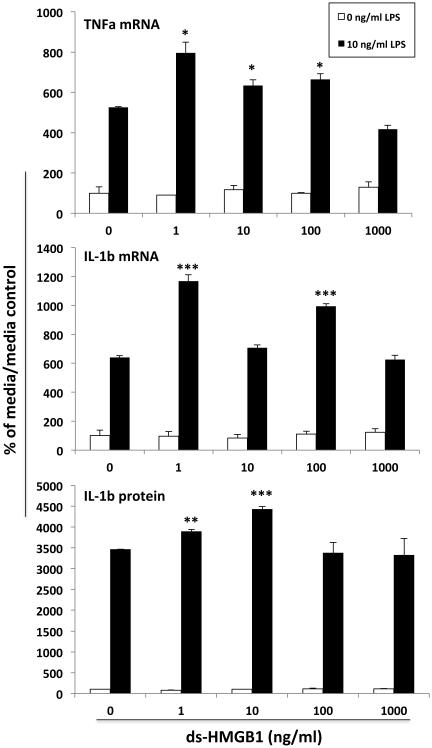
As before, microglia exposed to ds-HMGB1 alone did not alter TNFα mRNA, IL-1 β mRNA, IL-6 mRNA or IL-1β protein. However, prior exposure of microglia to ds-HMGB1 differentially modulated the TNFα mRNA (df = 4, 20; F = 16.85, p < 0.0001), IL-1β mRNA (df = 4, 20; F = 38.24, p < 0.0001) and IL-1β protein (df = 4, 20; F = 4.86, p = 0.007) response to LPS (Fig. 5). ds-HMGB1 potentiated the TNFα mRNA response (1, 10 and 100 ng/ml ds-HMGB1), IL-1β mRNA response (1 and 100 ng/ml ds-HMGB1) and IL-1β protein response (1 and 10 ng/ml ds-HMGB1) to LPS (Fig. 5). The IL-6 mRNA response to LPS was unchanged by ds-HMGB1 (df = 4, 20; F = 0.72, p = 0.59), however the main effect of LPS was significant (df = 4, 20; F = 725.2, p < 0.0001)(data not shown).
Fig. 5. Effect of ds-HMGB1 on priming of the microglial pro-inflammatory response.
Whole brain microglia were isolated and directly exposed to several concentrations of ds-HMGB1 for 4h. Cells were washed free of ds-HMGB1 and treated with LPS or media for 18h and pro-inflammatory mediators measured. ds-HMGB1 potentiated the LPS induction of TNFα mRNA (1, 10, and 100 ng/ml), IL-1β mRNA (1 and 100 ng/ml) and extra-cellular IL-1β protein (1 and 10 ng/ml) compared to LPS alone. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to the LPS/0 ng/ml ds-HMGB1 condition. N = 3 replications. Data are presented as the mean + SEM.
4. Discussion
The redox state of HMGB1 is a critical determinant of its immunomodulatory properties in peripheral innate immune cells (Venereau et al., 2012), such that the disulfide form is proinflammatory, whereas the reduced form is chemotactic. Our interest here was to further characterize how these different molecular forms of HMGB1 influence neuroinflammatory priming, and more specifically, microglia priming. Indeed, we found here that the redox state of HMGB1 is a critical molecular feature for determining the neuroinflammatory effects of HMGB1.
Prior studies have found that ds-HMGB1 is pro-inflammatory in both primary macrophages as well as macrophage cell lines (Venereau et al., 2012; Yang et al., 2012). Consistent with these findings, we found that ds-HMGB1, when injected ICM, induced pro-inflammatory effects in the hippocampus at 2h (NF-κBIα mRNA, NLRP3 mRNA and IL-1β protein) and 24h (NF-κBIα mRNA, TNFα mRNA, and NLRP3 protein) after injection. However, fr-HMGB1 failed to significantly increase the expression level of these pro-inflammatory mediators, which is consistent with prior findings demonstrating that fr-HMGB1 is a chemotactic factor and lacks pro-inflammatory properties (Venereau et al., 2012). It is important to note that the present findings do not exclude the possibility that fr-HMGB1 induced chemotactic processes in the CNS. However, the failure of fr-HMGB1 to induce neuroinflammatory priming suggests that such chemotactic processes, if induced under the experimental conditions employed here, were not sufficient to play a role in the priming of the neuroinflammatory response.
Interestingly, we found that ds-HMGB1 induced a persistent increase in NF-κBIα expression suggesting that ds-HMGB1 treatment induced activation of the NF-κB pathway, which is considered a pivotal regulator of pro-inflammatory gene transcription through TLRs (Kawai and Akira, 2007). Further, the induction of NLRP3 mRNA and protein by ds-HMGB1 also suggests that the NF-κB pathway was activated because NF-kB has been found to induce NLRP3 protein as part of the priming step of the NLRP3 inflammasome (Bauernfeind et al., 2009). Thus, the effects of ds-HMGB1 on NF-κBIα and NLRP3 suggested that ds-HMGB1 induces a primed neuroinflammatory state. To examine this possibility, animals were pretreated with ds-HMGB1 and subsequently exposed to a sub-threshold dose of the pro-inflammatory stimulus LPS. Indeed, prior exposure to ds-HMGB1 potentiated the neuroinflammatory response to LPS. However, fr-HMGB1 failed to potentiate the neuroinflammatory effects of LPS, which is consistent with our observation that fr-HMGB1 failed to induce a shift in basal expression of hippocampal NF-κBIα or NLRP3. To address the behavioral consequences of ds-HMGB1-induced neuroinflammatory priming, we examined whether ds-HMGB1 would potentiate the sickness response to LPS. Such an outcome was expected since ds-HMGB1 potentiated the IL-1β increase produced by LPS, and IL-1β is a pivotal mediator of the sickness response to pathogens and pro-inflammatory stimuli (Goshen and Yirmiya, 2009). Indeed, prior exposure to ds-HMGB1 potentiated the sickness response to LPS (i.e. reduced social exploration of a juvenile). Therefore, the present findings suggest that the neuroinflammatory priming effects of ds-HMGB1 play an important role in its potentiation of the sickness response to pro-inflammatory stimuli.
Microglia are the predominant innate immune cell in the CNS and are thus considered a pivotal mediator of neuroinflammatory processes (Gehrmann et al., 1995). Microglia express TLRs, in particular TLR4 (Ransohoff and Perry, 2009), which has been found to mediate the pro-inflammatory effects of HMGB1 in peripheral innate immune cells (Yang et al., 2010; Yang et al., 2015). Therefore, we directly exposed primary microglia to ds-HMGB1 or fr-HMGB1 and assessed the induction of a pro-inflammatory/primed immunophenotype. Similar to our observations of the effect of ds-HMGB1 in vivo, ds-HMGB1 induced an inflammatory phenotype (increased NF-κBIα and NLRP3 mRNA, but not TNFα, IL-1β, or IL-6 mRNA) in vitro remarkably similar to the primed neuroinflammatory phenotype induced in vivo. Interestingly, the effect of ds-HMGB1 on NF-κBIα and NLRP3 was not dose dependent, but rather exhibited an inverted-U function. It is unclear what may account for this effect, but one possibility is that at high concentrations of ds-HMGB1, increased oxidative stress may have resulted in complete oxidation of ds-HMGB1, which would abrogate the pro-inflammatory properties of ds-HMGB1. fr-HMGB1 failed to alter the pro-inflammatory phenotype of microglia in vitro, which is consistent with our observations of the null effects of fr-HMGB1 in vivo. Notably, the pro-inflammatory effects of ds-HMGB1 in vitro were not unique to microglia as we found that ds-HMGB1 increased NF-κBIα and NLRP3 mRNA in primary peritoneal macrophages as it did in primary microglia. The ds-HMGB1-induced shift in basal expression of NF-κBIα and NLRP3 suggests that ds-HMGB1 directly induces a primed immunophenotype. To determine whether ds-HMGB1 induces a primed phenotype, microglia were pre-exposed to ds-HMGB1 and subsequently challenged with the TLR4 agonist LPS. Consistent with our observations in vivo, prior exposure to ds-HMGB1 potentiated the pro-inflammatory cytokine response to LPS in primary microglia. Interestingly, the effects of ds-HMGB1 on LPS-induced cytokines were not dose dependent, but exhibited a pattern (inverted-U response) consistent with the effects of ds-HMGB1 on basal expression of NF-κBIα and NLRP3.
There are few studies examining a role for HMGB1 in neuroinflammatory priming. We recently conducted an initial study showing that HMGB1 mediates stress-induced priming of microglia ex vivo (Weber et al., 2015). In this study, animals were administered (ICM) the HMGB1 antagonist box A and then exposed to a severe acute stressor. 24h after termination of the stressor, hippocampal microglia were isolated and challenged with LPS. Consistent with our prior studies (Frank et al., 2007; Frank et al., 2012; Weber et al., 2013), pre-exposure to stress potentiated the proinflammatory response of microglia to LPS. However, box A treatment abrogated this effect of stress on microglia priming. Furthermore, ds-HMGB1 administered ICM was sufficient to prime the pro-inflammatory response of microglia ex vivo suggesting that ds-HMGB1 might directly prime microglia. Two key questions arose from this study: 1) does ds-HMGB1 prime the neuroinflammatory and sickness response to an immune challenge and more importantly 2) does ds-HMGB1 directly prime the microglial proinflammatory response. Indeed, the present findings demonstrate that ds-HMGB1 is sufficient to prime the neuroinflammatory and sickness response to a pro-inflammatory challenge. Further, ds-HMGB1 was capable of directly priming the microglia pro-inflammatory response to an immune challenge suggesting that the neuroinflammatory effects of ds-HMGB1 are mediated, in part, through direct actions on microglia. It is likely that the priming effects of ds-HMGB1 on microglia are mediated by the TLR4 signalosome given the findings of Yang and colleagues, who found that the TLR4 adaptor MD-2 is necessary for the pro-inflammatory effects of ds-HMGB1 (Yang et al., 2015). In parallel with its priming effects, ds-HMGB1 consistently induced an immunophenotype characterized by increased NF-κBIα and NLRP3 expression, which play pivotal roles in immune priming (Bauernfeind et al., 2009). These findings suggest that NF-kB induction of NLRP3 may play a key role in the neuroinflammatory priming effects of ds-HMGB1. However, it is important to note that our findings are correlative in nature and do not address a mediating role of the NLRP3 inflammasome in ds-HMGB1-induced priming. Also, it is important to note that our data suggests that ds-HMGB1 exhibits relatively minor pro-inflammatory effects per se compared to the observed priming effects of this molecular form. ds-HMGB1 induced small effects on IL-1β protein (2h post injection) and TNFα mRNA (24h post-injection). Further, these proinflammatory changes were not sufficient to elicit a sickness response in the social exploration test. Rather, ds-HMGB1 was only capable of potentiating the sickness response after a proinflammatory challenge with LPS. This finding again highlights the priming qualities of ds-HMGB1 under these experimental conditions, which involved a single ICM administration of one dose of ds-HMGB1. Clearly, an increase in dose and/or chronicity of ds-HMGB1 treatment might demonstrate the pro-inflammatory properties per se of ds-HMGB1. On this note, under more chronic neuroinflammatory conditions, HMGB1 has been found to play a mediating role in the neuroinflammatory response associated with seizure (Maroso et al., 2010), ischemia (Kim et al., 2006), chronic pain (Agalave et al., 2014), and alcohol-induced neuroinflammation (Zou and Crews, 2014) suggesting that prolonged HMGB1 signaling is sufficient to elicit a pro-inflammatory response.
Taken together, the present findings suggest that acute increases or exposure to ds-HMGB1, as may occur during acute stress or trauma, may induce a primed immunophenotype in the CNS, which might lead to an exacerbated neuroinflammatory response if exposure to a subsequent pro-inflammatory stimulus occurs.
Supplementary Material
ds-HMGB1 induced NF-κBIα and NLRP3 expression in in hippocampus and microglia.
ds-HMGB1 potentiated the neuroinflammatory and sickness response to LPS.
ds-HMGB1 primed the microglial proinflammatory response to an immune challenge.
fr-HMGB1 had no effect on neuroinflammatory or microglial priming.
Acknowledgements
The present work was supported by an NIH grant (R21MH096224) to MGF and SFM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundback P, Palmblad K, Andersson U, Harris H, Svensson CI. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain. 2014;155:1802–1813. doi: 10.1016/j.pain.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balosso S, Liu J, Bianchi ME, Vezzani A. Disulfide-containing high mobility group box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxidants & redox signaling. 2014;21:1726–1740. doi: 10.1089/ars.2013.5349. [DOI] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain, behavior, and immunity. 2012;26:337–345. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: Immunophenotypic and functional characteristics. Journal of Neuroscience Methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res. Brain Res. Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Holguin A, Frank MG, Biedenkapp JC, Nelson K, Lippert D, Watkins LR, Rudy JW, Maier SF. Characterization of the temporo-spatial effects of chronic bilateral intrahippocampal cannulae on interleukin-1beta. J Neurosci Methods. 2007;161:265–272. doi: 10.1016/j.jneumeth.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol. 2010;40:620–623. doi: 10.1002/eji.200940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti TD. Nlrp3: an immune sensor of cellular stress and infection. Int J Biochem Cell Biol. 2010;42:792–795. doi: 10.1016/j.biocel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunological reviews. 2011;243:152–162. doi: 10.1111/j.1600-065X.2011.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Dalpke A, Mracsko E, Antoine DJ, Roth S, Zhou W, Yang H, Na SY, Akhisaroglu M, Fleming T, Eigenbrod T, Nawroth PP, Tracey KJ, Veltkamp R. DAMP Signaling is a Key Pathway Inducing Immune Modulation after Brain Injury. J Neurosci. 2015;35:583–598. doi: 10.1523/JNEUROSCI.2439-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Wang H, Andersson U, Tracey KJ. Regulation of HMGB1 release by inflammasomes. Protein & cell. 2013;4:163–167. doi: 10.1007/s13238-012-2118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nature medicine. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- Pan Y, Chen XY, Zhang QY, Kong LD. Microglial NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 2014;41:90–100. doi: 10.1016/j.bbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L, Shams SS, Yang H, Varani L, Andersson U, Tracey KJ, Bachi A, Uguccioni M, Bianchi ME. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. The Journal of experimental medicine. 2012;209:1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Sobesky JL, Watkins LR, Maier SF. Blocking toll-like receptor 2 and 4 signaling during a stressor prevents stress-induced priming of neuroinflammatory responses to a subsequent immune challenge. Brain, behavior, and immunity. 2013 doi: 10.1016/j.bbi.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF. Stress Induces the Danger-Associated Molecular Pattern HMGB-1 in the Hippocampus of Male Sprague Dawley Rats: A Priming Stimulus of Microglia and the NLRP3 Inflammasome. J Neurosci. 2015;35:316–324. doi: 10.1523/JNEUROSCI.3561-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lundback P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, Al-Abed Y, Andersson U, Tracey KJ, Antoine DJ. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1) Mol Med. 2012;18:250–259. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Ju Z, Ragab AA, Lundback P, Long W, Valdes-Ferrer SI, He M, Pribis JP, Li J, Lu B, Gero D, Szabo C, Antoine DJ, Harris HE, Golenbock DT, Meng J, Roth J, Chavan SS, Andersson U, Billiar TR, Tracey KJ, Al-Abed Y. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. The Journal of experimental medicine. 2015;212:5–14. doi: 10.1084/jem.20141318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JY, Crews FT. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PloS one. 2014;9:e87915. doi: 10.1371/journal.pone.0087915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.