Abstract
Greater physical activity and cardiorespiratory fitness are associated with reduced age-related cognitive decline and lower risk for dementia. However, significant gaps remain in the understanding of how physical activity and fitness protect the brain from adverse effects of brain aging. The primary goal of the current study was to empirically evaluate the independent relationships between physical activity and fitness with functional brain health among healthy older adults, as measured by the functional connectivity of cognitively and clinically relevant resting state networks. To build context for fitness and physical activity associations in older adults, we first demonstrate that young adults have greater within-network functional connectivity across a broad range of cortical association networks. Based on these results and previous research, we predicted that individual differences in fitness and physical activity would be most strongly associated with functional integrity of the networks most sensitive to aging. Consistent with this prediction, and extending on previous research, we showed that cardiorespiratory fitness has a positive relationship with functional connectivity of several cortical networks associated with age-related decline, and effects were strongest in the Default Mode Network (DMN). Furthermore, our results suggest that the positive association of fitness with brain function can occur independent of habitual physical activity. Overall, our findings provide further support that cardiorespiratory fitness is an important factor in moderating the adverse effects of aging on cognitively and clinically relevant functional brain networks.
Keywords: cardiorespiratory fitness, physical activity, aging, functional connectivity, resting state fMRI
1. Introduction
Research has demonstrated the protective and restorative potential of physical activity and fitness for age-related cognitive decline and neurodegenerative diseases including Alzheimer’s Disease (AD) (Buchman et al., 2012; Colcombe and Kramer, 2003; Hamer and Chida, 2009; Liu et al., 2012; Smith et al., 2010; Sofi et al., 2011). This is important because age-related neurodegenerative conditions like AD are associated with enormous costs from morbidity, mortality, and loss of independence (Barnes and Yaffe, 2011; Hurd et al., 2013). These public health challenges are likely to surge along with growth in the proportion of adults over age 65 in the United States and around the world (CDC, 2013; NIA, 2007). Physical exercise is a prime candidate for treating the aging brain because in addition to being effective, it is also low-cost, it can reduce or eliminate the need for costly drug treatments with negative side effects, and it is safe and accessible. However, we still do not understand how physical exercise protects the brain from the adverse effects of aging, and thus we are unable to maximize the benefits of physical activity for the brain across a broad population.
A critical aspect of understanding how physical activity is protective for brain aging is identifying the fundamental principles by which physical activity positively affects brain health. Physical activity is defined as bodily movement that increases energy expenditure relative to seated rest (Casperson et al., 1985), and is often categorized by intensity levels such as light, moderate, and vigorous, based on how much energy expenditure increases relative to rest. Some studies support that total daily physical activity is protective against age-related brain atrophy (Varma et al., 2014) and risk for cognitive decline and AD (Barnes et al., 2008; Buchman et al., 2012; Middleton et al., 2011). However, often other studies have relied on self-reported physical activity, which best captures moderate intensity exercise rather than total daily physical activity (Larson et al., 2006; Yaffe et al., 2001). Compared to physical activity, exercise is defined as a subset of physical activity that is planned, structured, and repetitive, and has an objective of improving or maintaining physical fitness (Casperson et al., 1985).
In the context of aging and AD, studies have also measured cardiorespiratory fitness (e.g., Barnes et al., 2003; Boots et al., 2014; Colcombe et al., 2004; Erickson et al., 2009; Hayes et al., 2013; Honea et al., 2009; Nyberg et al., 2014; Spirduso, 1980; Voss et al., 2010b), often intended as an objective biomarker of moderate intensity physical activity. Cardiorespiratory fitness is a physiological attribute defined as the ability for circulatory and respiratory systems to deliver oxygen to working muscles and the ability of the muscles to extract and use the oxygen to generate energy. The gold standard measurement of cardiorespiratory fitness is the highest rate of oxygen consumption during exhaustive exercise and is often expressed as oxygen capacity per kilogram of body weight over time (mL/kg/min) (ACSM, 2013; Casperson et al., 1985). Thus, while physical activity and cardiorespiratory fitness are related, they measure distinct outcomes related to a physically active lifestyle.
The research linking individual differences in cardiorespiratory fitness and training-related changes in fitness to brain health has led to a hypothesis that cardiorespiratory fitness is a critical mediator of these benefits (Angevaren et al., 2008; Etnier et al., 2006; Smith, 2012; Spirduso, 1980). This fitness hypothesis can be differentiated from one about total physical activity (i.e., movement) because it implies that training-related improvements in fitness are needed to improve brain health (Voss et al., 2014), rather than simply increasing physical activity. Supporting the fitness hypothesis, studies have shown a cross-sectional association between fitness and brain structure in older adults (Erickson et al., 2009; Gordon et al., 2008; Weinstein et al., 2012), and also between fitness and brain function (Dupuy et al., 2015; Dustman et al., 1990; Gauthier et al., 2014; Prakash et al., 2011; Voss et al., 2010b). Prospective studies have shown that cardiorespiratory fitness predicts cognitive decline and risk of dementia-related death (Barnes et al., 2003; Liu et al., 2012; Wendell et al., 2014). In addition, intervention studies have shown that changes in exercise-induced fitness correlates with training-related change in brain structure (Erickson et al., 2011; Voss et al., 2013a) and hippocampal blood flow and volume (Maass et al., 2014; Pereira et al., 2007).
However, there are also studies that do not show an association between fitness and brain health (Angevaren et al., 2008; Brickman et al., 2014; Dustman et al., 1984; Etnier et al., 2006; Smiley-Oyen et al., 2008; Young et al., 2015). Indeed, as noted in Dustman et al., 1984, “Since VO2 max is not specific for brain oxygen consumption and since there is no reason to expect that exercise related increases in oxygen to the brain would closely parallel increases to muscle, a direct relationship between VO2 max and neuropsychological measures would not be predicted” (pp. 39–40). Therefore, there is mixed support for the fundamental significance of cardiorespiratory fitness in the relationship between physical activity and brain health. One step towards clarifying this relationship further is for more studies to directly contrast multiple features of a physically active lifestyle (e.g., intensity of daily physical activity, cardiorespiratory fitness) to determine their unique contributions to health benefits on the aging brain.
For instance, studies have shown that physical activity and fitness are separable at the level of individual differences (Jacobs et al., 1993; Laye et al., 2015; Williams, 2001), either because it is feasible that the same individual can achieve daily moderate-to-vigorous exercise while being sedentary the rest of the day (Craft et al., 2012; Pate et al., 2008), or because there are determinants of cardiorespiratory fitness that account for variance beyond habitual physical activity such as genetics, which may account for about half of the variance in individual differences in fitness (Bouchard et al., 1999; Bouchard et al., 2011). In addition, more nuanced approaches for objectively measuring physical activity are now available (Chen et al., 2012; Rowlands et al., 2015), and these provide an opportunity to more objectively test what features of daily physical activity (Copeland and Esliger, 2009; Tudor-Locke et al., 2013) are associated with brain health in aging populations (Makizako et al., 2014; Varma et al., 2014). Thus, similar to research on other health outcomes such as cardiovascular disease risk or mortality (e.g., Lee et al., 2011), it is important to determine the independent associations of physical activity and cardiorespiratory fitness with brain health in older adults.
One approach to measuring functional brain health is assessing the functional connectivity of brain networks observed during the resting state with functional magnetic resonance imaging (rsfMRI). Resting-state functional connectivity (FC) is a valuable measure of brain health because it is suggested to be a correlate for normal age-related decline in brain function (Andrews-Hanna et al., 2007; Dennis and Thompson, 2014) and a biomarker of AD (Brier et al., 2012; Greicius et al., 2004; He et al., 2014; Seeley et al., 2009; Sorg et al., 2007). Briefly, resting-state FC measures the correlation between regional fluctuations in the fMRI blood oxygenation level-dependent (BOLD) signal while a participant is resting quietly in the scanner without exposure to an experimentally controlled task. The motivation for this approach is the discovery that many of the brain systems that are evoked by experimentally controlled cognitive tasks are also fluctuating in synchrony during rest (e.g., Smith et al., 2009), that these resting state networks can be identified across the lifespan (Chan et al., 2014; Damoiseaux et al., 2008), and that their functional integration and segregation may be predictive of individual differences in cognitive performance and clinical status or trajectories (Brier et al., 2012; He et al., 2014; Shaw et al., 2015). Thus, rsfMRI networks provide a means to measure the functional integrity of a broad range of brain systems without confounds related to task performance, in a relatively shorter period of time than most task-based imaging, and without targeting a specific system with an experimental manipulation.
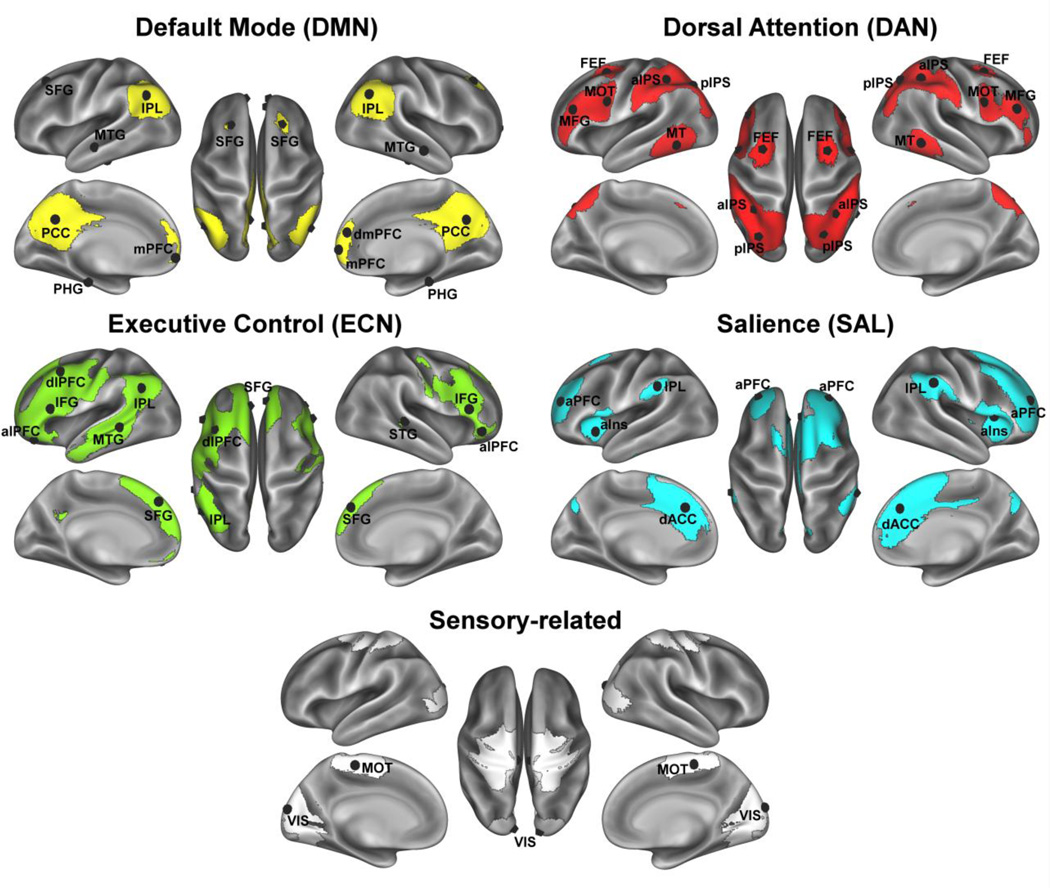
The association between age and network integrity tends to be stronger in specific networks rather than being a global phenomenon. One of the most replicable findings is an association between aging and lower FC in the default mode network (DMN; see Figure 1 and Table 1 for description of anatomical regions in the DMN) (Agosta et al., 2012; Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Meier et al., 2012; Voss et al., 2010b). Another common trend is for older adults to have poorer FC in what are commonly referred to as the Executive Control Network (ECN, see Figure 1 and Table 1) and the Salience Network (SAL, see Figure 1 and Table 1) (He et al., 2014; Meier et al., 2012; Onoda et al., 2012; Voss et al., 2010a), though there have been more mixed findings with respect to this pattern (e.g., Agosta et al., 2012; Mowinckel et al., 2012). Networks that tend to show the weakest or no differences with aging include sensory systems such as the visual, motor, and auditory systems (Andrews-Hanna et al., 2007; Mowinckel et al., 2012), though some studies have shown that the functional specificity of these systems is vulnerable to adverse effects of aging (Brier et al., 2012; Chan et al., 2014).
Figure 1.
Association networks and their ROI peaks. ROI acronyms correspond to the labels in Table 1 and are used as so throughout the paper; lateralized ROIs are visualized on the appropriate hemisphere (shown as L=L and R=R).
Table 1.
Network regions of interest (ROIs) empirically derived from the study sample using independent components analysis (ICA) based decomposition of the rsfMRI signal. Networks were identified from ICA with older and younger adults, based on knowledge of spatial activation patterns for canonical brain networks of interest. Network acronyms refer to: DMN (Default Mode Network), DAN (dorsal attention network), VIS (Visual system), MOT (Somato-motor network), SAL (Salience network), and ECN (Executive Control Network). Regions were identified based on peak Z-scores for functionally distinct regions within networks.
| Network | Region of Interest (ROI) label |
Description of anatomical region | MNI coordinates (x,y,z) |
|---|---|---|---|
| DMN | PHG | Parahippocampal/hippocampal gyrus | *L(−24,−26,−20), *R(24,−26,−20) |
| DMN | MTG | Middle temporal gyrus | *L(−52,−18,−18), R(62,−10,−16) |
| DMN | IPL | Inferior parietal lobule | L(−46,−64,32), R(50,−62,34) |
| DMN | PCC | Posterior cingulate cortex | L(−6,−54,32), R(6, −60, 32) |
| DMN | mPFC | Medial prefrontal cortex | L(−6,62,−4), R(6,62,−4) |
| DMN | dmPFC | Dorsal medial prefrontal cortex | R(6,50,20) |
| DMN | SFG | Superior frontal gyrus | L(−24,24,42), R(22,28,44) |
| DAN | pIPS | Posterior intraparietal sulcus | L(−28,−68,48), R(30,−66,48) |
| DAN | aIPS | Anterior intraparietal sulcus | L(−40,−46,46), R(38,−46,46) |
| DAN | MT | Middle temporal/visual cortex (V5) | L(−52,−60,−8), R(58,−52,−10) |
| DAN | MOT | Premotor motor cortex | L(−48,6,30), R(48,10,28) |
| DAN | FEF | Frontal eye field | L(−26,2,54), R(28,4,54) |
| DAN | MFG | Middle frontal gyrus | L(−46,34,22), R(46,34,22) |
| VIS | VIS | Occipital pole | L(−8,−90,16), R(14,−90,16) |
| MOT | MOT | Primary motor cortex | L(−6,−28,58), R(6,−26,58) |
| SAL | IPL | Inferior parietal lobule | L(−60,−40,36), R(60,−38,38) |
| SAL | aINS | Anterior insula/cingulo-operculum | L(−38,16,2), R(42,16,2) |
| SAL | dACC | Dorsal anterior cingulate cortex | L(6,32,30), R(−6,26,32) |
| SAL | aPFC | Anterior prefrontal cortex | L(−30,48,26), R(32,48,26) |
| ECN | SFG | Superior frontal gyrus | L(−6,42,44), R(6,50,38) |
| ECN | alPFC | Anterior lateral prefrontal cortex | L(−46,42,−6), R(48,36,−10) |
| ECN | dlPFC | Dorso-lateral prefrontal cortex | L(−44,12,48) |
| ECN | IFG | Inferior frontal gyrus | L(−52,20,20), R(54,26,18) |
| ECN | IPL | Inferior parietal lobule | L(−50,−60,32) |
| ECN | MTG | Middle temporal gyrus | L(−58,−38,0) |
| ECN | STG | Superior temporal gyrus | R(52,−32,0) |
Regions identified as part of network based on a previous study from our group (Voss et al., 2010a,b) finding age- and fitness-related individual differences in network membership. Note other regions identified from rsfMRI in this sample also overlap substantially with regions identified in previous study (Voss et al., 2010a,b); note from Voss et al., 2010b, previously referred to “fronto-parietal” network is referred to as DAN in the current study, and previously referred to “fronto-executive” network contains primarily regions in the SAL network in the current study.
Given these findings, it is significant that our previous research has suggested that the functional networks most sensitive to age-related decline are also sensitive to individual differences in fitness (DMN) (Voss et al., 2010b) and they show improved FC following 1 year of aerobic exercise training (DMN, SAL) (Voss et al., 2010a). However, these studies did not assess functional connectivity during a dedicated rsfMRI scan (since this was not acquired) and rather used a simulated resting state following statistical removal of signal related to basic sensory stimulation. Therefore, in addition to evaluating the independent relationships of fitness and physical activity with brain health, the current study also serves as an opportunity to replicate and extend previous results describing the relationship between cardiorespiratory fitness and FC of the DMN and other rsfMRI networks associated with aging. Based on our previous research and the extant literature, we predicted that older adults would have the most disruption in the DMN, ECN, and SAL networks compared to healthy young adults, and that individual differences in fitness and physical activity would be most strongly associated with the functional integrity of the networks most sensitive to aging. Additionally, based on the replicable benefits of physical activity on temporal and prefrontal cortex brain health (Voss et al., 2013b), we predicted the strongest associations with fitness or physical activity would occur within regions including the prefrontal and temporal cortices (Voss et al., 2010a; Voss et al., 2010b).
Regarding the distinction between fitness and physical activity, we do not have strong predictions about their independent relationships with functional brain networks. Although we have previously shown that MVPA was linked to white matter integrity even after controlling for fitness (Burzynska et al., 2014), there is still little empirical evidence for a strong prediction about their independent relationships with functional brain health.
2. Materials and Methods
2.1. Participants
MRI, physical activity, and cardiorespiratory fitness data were collected from 247 community-dwelling healthy older adults (average age of 65 years, 68% female, average of 15.6 years of education), and MRI data were collected from 59 college-age adults (average age of 22 years, 47% female, average of 15.1 years of education). Fitness and physical activity data were not collected on the young adults. The young adults are included in the current study to examine age effects on network functional connectivity within the same sample of older participants in which we examine relationships with fitness and physical activity in older adults.
Eligible older adult participants met the following criteria: (1) were between the ages of 60 and 80 years old, (2) were free from psychiatric and neurological illness and had no history of stroke, transient ischemic attack, or head trauma, (3) scored > 23 on the Mini-Mental State Exam (MMSE) and >21 on a Telephone Interview of Cognitive Status (TICS-M) questionnaire, (4) scored < 10 on the geriatric depression scale (GDS-15), (5) scored > 75% right-handedness on the Edinburgh Handedness Questionnaire, (6) demonstrated normal or corrected-to-normal vision of at least 20/40 and no color blindness, and (7) were screened for safe participation in an MRI environment (e.g., no metallic implants that could interfere with the magnetic field or cause injury and no claustrophobia). The older adults are a cross-sectional subsample from a randomized controlled exercise trial. We further excluded participants with MMSE < 27 to exclude those with possible mild cognitive impairment. Finally, our participants were screened for good quality MRI data (see below).
Eligible younger adult participants (1) were free from psychiatric and neurological illness and had no history of stroke, transient ischemic attack, or head trauma, (2) scored ≥75% right-handedness on the Edinburgh Handedness Questionnaire, (3) demonstrated normal or corrected-to-normal vision of at least 20/40 and no color blindness, and (4) were screened for safe participation in an MRI environment (e.g., no metallic implants that could interfere with the magnetic field or cause injury and no claustrophobia). The young adult sample was further trimmed to a subset based on quality of the MRI data and to achieve a comparable proportion of females and years of education between the young and older adult groups. This was done to minimize gender and other demographic variables as confounds when operationalizing network peaks (described below) and when evaluating age-related differences in functional connectivity.
The final sample of older adults included 189 community-dwelling healthy older adults (68% female) with an average age of 65 years (SD=4.4) and average education of 16 years (SD=2.9). The final sample of young adults included 36 adults (64% female) with an average age of 22 years (SD=3) and average education of 15.6 years (SD=2). See Table 2 for full descriptive statistics and more demographic and health variables.
Table 2.
Cells show mean (standard deviation) for demographic, fitness, and physical activity variables broken down by age and fitness groups. MMSE=mini-mental status exam; BMI=body mass index; SBP=systolic blood pressure; DBP=diastolic blood pressure.
| Variable | Young Adults |
Older Adults |
Older Adults LF |
Older Adults HF |
Young vs. Old (p-value) |
LF vs. HF (p-value) |
|---|---|---|---|---|---|---|
| N | 36 | 189 | 47 | 47 | ||
| Age | 22.1 (3.0) | 65.1 (4.4) | 64.6 (3.7) | 64.5 (4.1) | p < .001 | NS |
| Percent Female |
64% | 68% | 62% | 57% | NS | NS |
| MMSE | - | 28.9 (1.0) | 28.8 (1.0) | 29.0 (1.1) | NS | NS |
| Education | 15.6 (2.0) | 16.0 (2.9) | 15.7 (2.9) | 16.4 (2.7) | NS | NS |
| BMI | - | 30.8 (5.6) | 33.9 (5.9) | 27.7 (3.8) | - | p < .001 |
| SBP | - | 131.6 (13.2) |
133.4 (14.6) |
131.2 (14.0) |
- | NS |
| DBP | - | 79.4 (7.5) | 78.9 (7.4) | 79.8 (6.7) | - | NS |
| Fitness (VO2max) |
- | 19.8 (4.8) | 15.0 (3.2) | 25.0 (3.9) | - | p < .001 |
| Fitness males (VO2max) |
- | 22.48 (5.3) | 16.6 (3.0) | 27.8 (3.1) | - | p < .001 |
| Fitness females (VO2max) |
- | 18.59 (3.9) | 14.0 (2.9) | 22.9 (3.0) | - | p < .001 |
| Avg daily minutes Light |
- | 258.0 (66.0) |
254.5 (73.7) |
250.4 (52.1) | - | NS |
| Median daily minutes MVPA* |
- | 38.0 (32.9) |
38.0 (29.9) |
34.8 (36.5) | - | NS |
Median is presented for MVPA (moderate-to-vigorous physical activity) because the variable was skewed, value in parenthesis is the inter-quartile range; p-values for MVPA are based on independent samples t-tests on the natural log transformed MVPA variable. LF=lower fit and HF=higher fit, and it should be noted by the group averages that all participants in both groups are still on the bottom half of the population for cardiorespiratory fitness compared to their age and gender matched peers (ACSM, 2013). This is not surprising given the sample was screened to be relatively low in self-reported physical activity in order to enter an exercise training intervention.
2.2. Physical activity and health history assessment
Older participants were instructed to wear the GT3X ActiGraph accelerometer (ActiGraph; Pensacola, Florida) for 7 consecutive days on an elastic belt on the left hip during all waking hours, except for when bathing or swimming. The participants completed a daily log to record the time that the accelerometer was worn, and this log was used to verify the accelerometer data for processing with the ActiLife v5.6.0 software. For the purposes of this study, a valid day of data consisted of at least 10 hours of valid wear-time (Matthews et al., 2008; Troiano et al., 2008), where hours flagged as “invalid” were defined as 60 minutes of consecutive zeros. In this way, there could only be a couple minutes of registered movement within the hour, but as long as they were distributed enough to not allow 60 or more minutes of consecutive zeros then the data was considered valid. Only data for individuals with a minimum of 3 valid days were included in analyses (Hart et al., 2011).
Each valid measurement epoch (minute) was classified into light physical activity (LIPA) or moderate-to-vigorous physical activity (MVPA) based on displacement magnitude and frequency. We used activity intensity cut-off ranges appropriate for older adults (Copeland and Esliger, 2009) using MeterPlus v4.2 software (Santech, Inc.; San Diego, CA). MVPA was defined as > 1040 counts/minute and LIPA was defined as 51–1040 counts/minute. The total minutes of each intensity was divided by total valid days to yield average time in minutes spent daily in a specific physical activity intensity (see summary descriptive statistics of physical activity variables in Table 2). Observed MVPA was positively skewed so we used a natural log-transformation to improve normality of the distribution (see supplementary figure 2).
2.3. Cardiorespiratory fitness assessment
All older participants obtained physician’s approval to engage in cardiorespiratory fitness testing. Cardiorespiratory fitness was defined as maximal oxygen consumption (VO2max in mL/kg/min), measured with indirect calorimetry during a modified Balke graded maximal exercise test on a motor-driven treadmill test. Oxygen consumption (VO2) was calculated from expired air sampled at 30-s intervals until peak VO2 was reached or the test was terminated due to volitional exhaustion and/or symptom limitation. VO2max was determined after two of three criteria were met: (1) a plateau in VO2 after increase in workload; (2) a respiratory exchange ratio >1.10, and (3) a maximal heart rate within 10bpm of their age-predicted maximum.
2.4. MRI acquisition
All images were acquired during a single session on a 3T Siemens Trio Tim system with 45 mT/m gradients and 200 T/m/sec slew rates (Siemens, Erlangen, Germany). T2*-weighted resting state images were acquired with a fast echo-planar imaging (EPI) sequence with BOLD contrast (6min, TR = 2s, TE = 25ms, flip angle = 80 degrees, 3.4 × 3.4 mm2 in-plane resolution, 35 4mm-thick slices acquired in ascending order, Grappa acceleration factor = 2, 64 × 64 matrix), while the participants were asked to lay still with their eyes closed. Additionally, dual-echo gradient field maps were acquired to account for geometric distortions caused by magnetic field inhomogeneity (Jezzard and Balaban, 1995). The gradient field maps were collected as 35, 4 mm-thick slices, 3.4 × 3.4 mm2 in-plane resolution, TR = 700ms, TE = 10/12.46 ms, and flip angle = 35 degrees. Resting state and field-map images were obtained parallel to the anterior-posterior commissure plane with no inter-slice gap.
High-resolution structural MR scans were acquired using a 3D MPRAGE T1-weighted sequence (TR = 1900 ms; TE = 2.32 ms; TI: 900 ms; flip angle = 9°; matrix = 256 × 256; FOV = 230 mm; 192 slices; resolution = 0.9 × 0.9 × 0.9 mm; GRAPPA acceleration factor 2) and used as an intermediate step in registration of functional images to standard MNI space.
2.5. Data Processing
Image processing and analyses were carried out with an in-house script library using tools from FSL 5.0.4 (Functional Magnetic Resonance Imaging of the Brain’s Software Library, http://www.fmrib.ox.ac.uk/fsl), AFNI (http://afni.nimh.nih.gov/afni), FreeSurfer (http://surfer.nmr.mgh.harvard.edu), and MATLAB (The MathWorks, Natick, MA, USA). Raw DICOM images were converted to a NIfTI image using FreeSurfer’s mri_convert tool and reoriented to RPI orientation with FSL’s fslorient. FSL’s BET (Brain Extraction Technique) algorithm was then used to strip voxels containing non-brain tissue from the T1 structural images (Smith, 2002). Skull-stripped T1 images were manually inspected and corrected for errors. A 6 degree-of-freedom rigid-body head motion correction was applied to rsfMRI EPI data using AFNI’s 3dvolreg function, producing six parameters of head motion (root-mean-squares of translational and rotational movement: X, Y, Z, pitch, roll, and yaw directions) for use as regressors to partial out variance in the BOLD signal associated with motion. Gradient field map images were skull-stripped with BET and then processed for subsequent EPI unwarping using FSL’s fsl_prepare_fieldmap. The processed field maps were applied to the motion corrected EPIs using FSL’s epi_reg for simultaneous EPI distortion correction and registration to the T1. Finally, non-brain tissue was removed from the corrected rsfMRI EPI image using BET, and spatial smoothing was applied using a 6.0 mm three-dimensional Gaussian kernel of full-width at half-maximum.
AFNI’s 3dBandpass tool was then used to temporally filter the time series data to restrict the signal to a frequency band of .008 < f < 0.08 Hz; this was done to reduce sources of noise such as high frequency physiological signals (e.g., cardiac pulse) and low frequency scanner drift. This frequency band is also reflective of the spontaneous, low frequency fluctuations of the BOLD fMRI signal (Leopold et al., 2003; Salvador et al., 2005). Following temporal filtering, the mean time series was extracted from a region in deep white matter (retrolenticular portion of the left internal capsule), a region in the lateral ventricle, and global signal derived from a whole-brain mask. These were considered nuisance signals and used as covariates to further control for physiological artifacts that could confound FC measures. In addition, the six head motion parameters described above were bandpassed with the same temporal filter applied to the fMRI data and included as nuisance regressors (Hallquist et al., 2013). Together, the 9 bandpassed nuisance regressors (white matter, CSF, global, and 6 motion parameters) were entered into a multiple regression as independent variables predicting the preprocessed rsfMRI data (using FSL’s FEAT tool). The residual time series data from the nuisance regression was then evaluated for any motion-contaminated volumes (Power et al., 2012). Briefly, volumes above a frame-wise displacement threshold of 0.5 mm that coincided with BOLD signal changes were removed from subsequent FC analyses. Overall, motion scrubbing only affected 9 older participants with an average of 5.9 volumes (3%) removed from analysis.
2.6. FC analysis of resting state networks
Two FC analyses were performed on the preprocessed rsfMRI data: 1) a targeted pairwise region of interest (ROI) analysis based on correlations between pairs of ROIs and 2) a whole-brain exploratory analysis based on the correlation of selected network ROIs with all other voxels in the brain. For the targeted approach, our ROIs were derived from a group-level independent components analysis (ICA) that was applied to the pre-processed rsfMRI data using FSL’s MELODIC with automatic dimensionality estimation. The rsfMRI data were decomposed into 13 independent spatiotemporal components (IC) that were common across the young and older adults. Of the 13 ICs, we identified 4 cognitively relevant and well-replicated ICs: 1) default-mode network (DMN), 2) dorsal attention network (DAN), 3) executive control network (ECN), and 4) salience network (SAL) (see Figure 1). We also identified 2 ICs resembling sensory-related networks: visual network (VIS) and a somato-motor network (MOT) (see Figure 1). For each of these 6 networks we constructed 14-mm diameter spheres centered on the peak coordinates within the IC statistical map (listed in Table 1; illustrated in Figure 1). These target ROIs served as a set of empirically derived nodes for the analysis of pairwise correlations within and between networks of interest.
Target ROIs were each created in standard MNI (2 mm) space and then registered to native (functional) space through a multi-stage procedure. First, each participant’s EPI was registered to their high-resolution structural T1 image using the boundary-based registration (BBR) algorithm (Greve and Fischl, 2009). Registration of the participant’s high-resolution structural image to standard MNI space was carried out with FNIRT nonlinear registration using a default 10 mm warp resolution (Andersson et al., 2007a, b). The resulting two transformations were then concatenated and applied to the participant’s functional image to register their functional image to standard MNI space; a reverse transform was used to register the ROIs from standard MNI space to each participant’s native functional space.
For FC analyses with these target ROIs, the mean (preprocessed) time series was extracted from all ROIs and cross-correlated with all other ROIs using MATLAB. Cross-correlations of all ROI pairs were estimated as Pearson’s correlation coefficients and then transformed to Fisher’s Z estimates Z(r) using Fisher’s r-to-z transformation. Initial exploratory analyses of bivariate connectivity patterns as a function of age, fitness, and physical activity, but uncorrected for multiple comparisons, are presented in the supplementary materials. To address the multiple comparisons issue, we used the network-based statistic (NBS) to identify network components that have ROI-ROI pairs with greater or lesser functional connectivity as a function of age or fitness and physical activity. The NBS algorithm has been well-validated (Zalesky et al., 2012; Zalesky et al., 2010) and widely applied (Bai et al., 2012; Cocchi et al., 2012; Thompson and Fransson, 2015; Wang et al., 2013) as a tool to provide both statistical power and protection against multiple comparisons when examining effects proposed to occur across distributed sub-systems of network nodes. Based on the literature, there is evidence that there are important sub-networks or sub-systems within the broader cortical systems that are vulnerable to normal aging (e.g., Andrews-Hanna et al., 2007; Damoiseaux et al., 2008). Our studies and others have also shown that the association of fitness and fitness training with brain health is likely at the level of sub-systems rather than expressed robustly throughout a network (Voss et al., 2010a; Voss et al., 2010b). Therefore, the strengths of this approach map onto our goal of detecting effects at the level of sub-systems while still being able to examine inter-regional associations both within and between broadly distributed cortical systems.
Briefly, the NBS approach tests whether a set of multiple pairwise connections associated with a contrast or effect of interest (e.g., t-test or correlation) form a connected component of ROI-ROI pairs that would be highly unlikely to have occurred at random. The statistical significance of a connected component is determined through a combination of an initial cluster-forming probability threshold on each individual ROI-ROI link and the size of connected components. Similar to how typical statistical parametric mapping considers whether a cluster is statistically significant based on the number of contiguous voxels, the NBS approach evaluates whether a component of ROI-ROI pairs of a certain size is non-random based on comparison to a null distribution of maximal component size empirically derived from permutations of randomized data (Zalesky et al., 2010). Thus similar to how we would not interpret a single voxel within a functional cluster on an activation map, NBS is not designed for the interpretation of isolated ROI-ROI links within identified network components. All NBS statistics reported here were computed using the GraphVar toolbox (Version 5.1) (Kruschwitz et al., 2015), which is publicly available on NITRC (http://www.nitrc.org/projects/graphvar/). For all NBS analyses, we used an initial cluster-forming threshold of p<.01, generated randomized data with 1000 permutations, and considered network components statistically significant at a FWER corrected threshold of p<.01. NBS results are visualized with BrainNet Viewer (https://www.nitrc.org/projects/bnv).
For the whole-brain exploratory analyses, the mean preprocessed time series was extracted from a subset of the target ROIs above. Then a cross-correlation of the ROI’s time series with the time series of all other voxels in the brain was computed using MATLAB to generate Pearson’s correlation coefficient maps designating a value to each voxel that corresponds to the strength of correlation with the seed ROI. This map was then converted into Z-score maps with a Fisher’s r-to-z transformation, resulting in individual-subject level Fisher’s Z maps representing voxels whose resting BOLD signal have a positive correlation with the ROI seed.
For group analysis of these voxel-wise seed maps, individual-subject level seed maps were registered to standard MNI (2 mm) space through the multi-stage procedure described above. Once in standard space, the seed maps from individual subjects were concatenated to form a 4D image file (subject as the fourth dimension) and this 4D image was input to a between-subjects ordinary least-squares (OLS) regression using FSL’s flameo (Beckmann et al., 2003). Multiple comparisons for the resulting group-level statistical maps were controlled by thresholding group contrast maps at Z>2.33, with cluster correction of p < 0.05 (Worsley et al., 1992). Results for whole-brain exploratory analyses are visualized with the MRIcron toolbox (https://www.nitrc.org/projects/mricron).
3. Results
3.1. Descriptive statistics and relationships between physical activity and cardiorespiratory fitness variables
Table 2 presents descriptive statistics for the study sample, including demographics, physical activity, and cardiorespiratory fitness. Although young and older adults differed in chronological age, they did not differ in gender composition of the samples or average self-reported years of education (all p >.05). Histograms of cardiorespiratory fitness and physical activity variables are available in the supplementary materials. Within the older adults, chronological age was negatively correlated with cardiorespiratory fitness (r =−.22, p<.01) and MVPA (r=−.33, p<.001), but not LIPA (r=−.11, p>.05). Cardiorespiratory fitness was positively correlated with LIPA (r=.17, p<.05) and MVPA (r=.46, p<.001), and LIPA was correlated with MVPA (r=.48, p<.001). Although cardiorespiratory fitness and physical activity were moderately correlated, the modest correlations between fitness and physical activity variables support the notion that there is non-overlapping variance between the variables, which may be meaningfully related to brain health.
3.2. Determining age-related differences in the FC of resting state networks
In order to evaluate whether fitness or physical activity are positively associated with network characteristics that are vulnerable to aging, we first sought to characterize age-group differences in the FC of resting-state networks. To this end, we compared the target ROI-ROI correlation matrices between the younger and older participants in two ways.
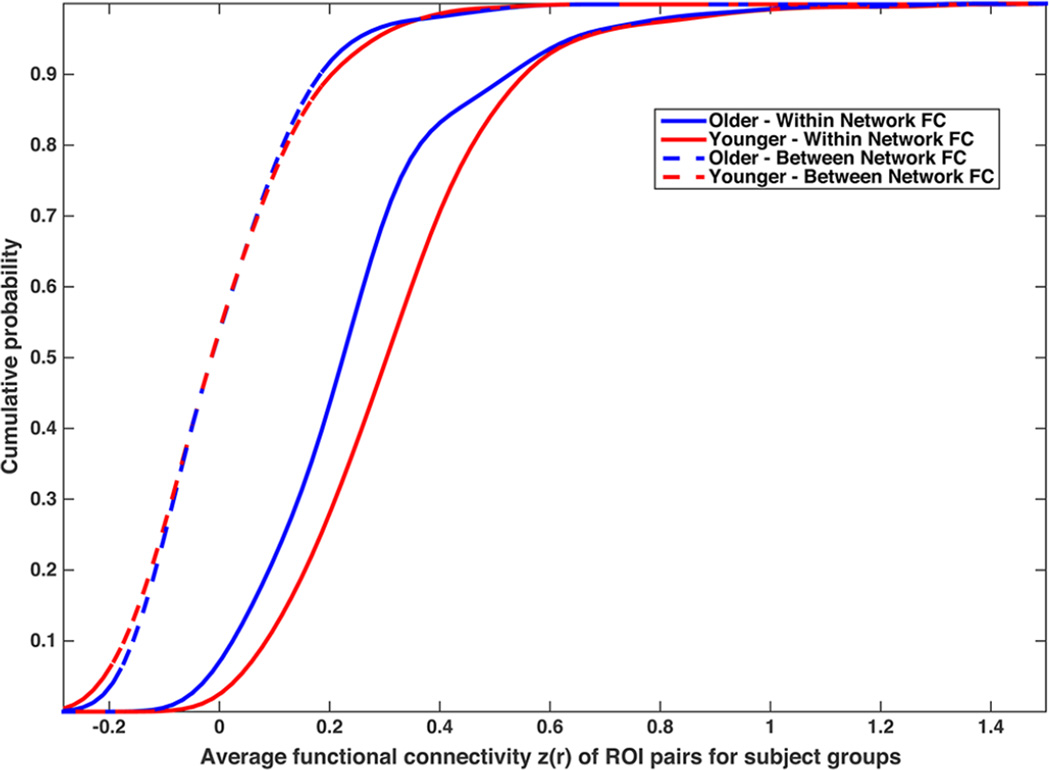
First, we compared the cumulative distributions for young and older adults, for both the average within-network correlation of all ROI pairs defined as part of the same network and the average between-network correlation of all ROI pairs defined as part of different networks. As shown in the cumulative distribution graphs in Figure 2, the within-network FC is approximately 0.10 Z(r) greater for young adults compared to older adults across a broad spectrum of the distribution. In contrast, the between-network FC is nearly identical for young and older adults across the distribution. This result helps provide a reference for meaningful differences in FC as measured by Fisher’s Z(r) correlations, and suggests that within-network FC is more vulnerable to age effects than between-network FC.
Figure 2.
The cumulative frequency plot visualizes the average functional connectivity for the set of ROI pairs that are either within the same network or in different networks (i.e., between), separated by young and older adults.
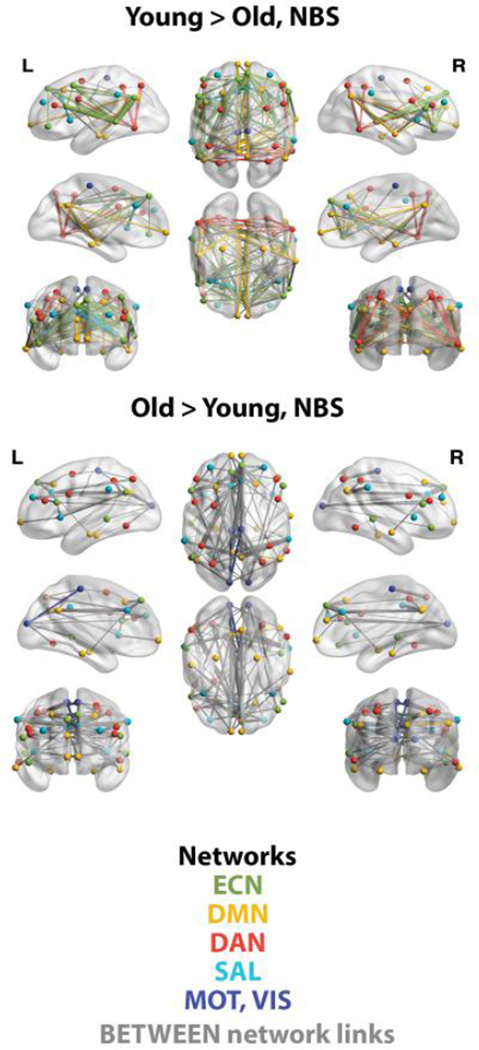
Next, we applied NBS to describe age group differences in FC within and between networks with more regional and network specificity. NBS identified one significant network component (p<.01, FWER corrected) that included the 47 target ROIs. As shown in Figure 3, component links with greater FC for young adults were primarily within networks such as the ECN and DMN. In contrast, the component links with greater FC for older adults were primarily between networks (illustrated as gray links in Figure 3). Note these results are also consistent with the pattern of age-group differences observed in the simple bivariate connectivity of ROI pairs shown in the supplemental materials (see supplementary figure 2).
Figure 3.
Visualization of age differences in FC as identified by NBS, p<.01 FWER corrected. Within-network links are illustrated with the same color as the network nodes as described in the color key in the figure; between-network links are illustrated as gray. Of all possible within-network ROI pairs for each network, those showing an effect in favor of young adults (Y>O) included 60% of ECN pairs, 22% of DMN pairs, 20% of DAN pairs, and 18% of SAL pairs. Consistent with Figure 2, young adults have greater FC primarily within networks.
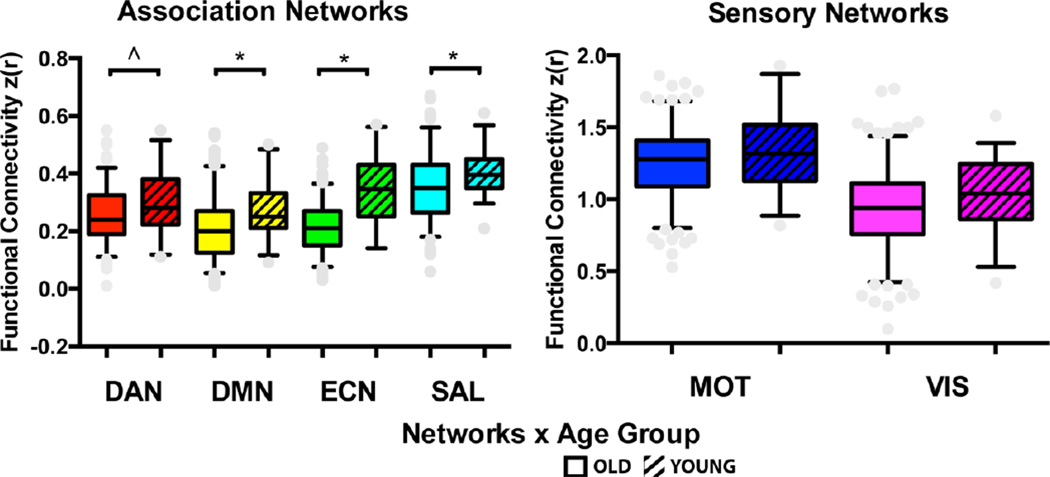
Together, these analyses justified reducing the full ROI-ROI correlation matrix by averaging within-network FC to estimate the effect size for age differences in favor of young adults for each network. Results showed age effects in favor of young adults in the DMN (t(223)=2.99, p=.003, d=.56), the ECN (t(223)=7.83, p<.001, d=1.25), and the SAL network (t(223)=2.76, p=.006, d=.56) (see Figure 4). Young adults also had greater FC in the DAN network with a p-value that equaled the alpha criterion (t(223)=2.49, p=.01, d=.43.
Figure 4.
Summary of age group comparisons for average functional network connectivity. Boxes represent the width between the 25th and 75th quartiles (interquartile range) and the whiskers represent the 5th to 95th percentiles of the data, and the middle bar represents the median. *p<.01, ^p=.01, based on two-tailed t-tests.
3.3. Are fitness and physical activity associated with network characteristics vulnerable to aging?
To determine the independent associations between fitness and physical activity with network FC in the older adults, we used multiple linear regression to create residuals for each variable of interest after regressing out (i.e., controlling for) variance associated with covariates. For instance, to determine the unique relationship between cardiorespiratory fitness and FC, we entered age, sex, MVPA, and LIPA as independent predictors in a linear regression predicting the dependent variable of cardiorespiratory fitness. Unstandardized residuals from this regression analysis represent individual differences in cardiorespiratory fitness after controlling for variance in covariates, and were then used as the variable of interest in computing the correlation between cardiorespiratory fitness and FC for each ROI-ROI pair.
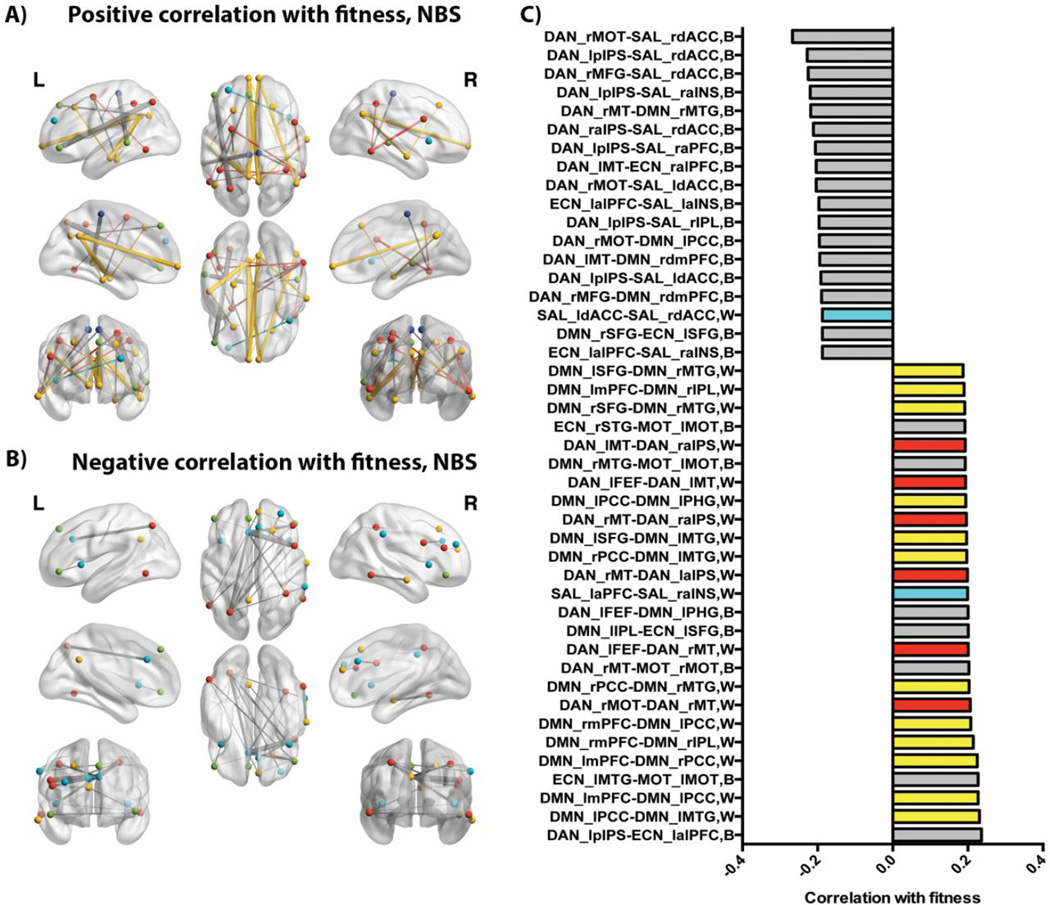
NBS identified one network component (p<.01, FWER corrected) correlated with fitness that included 34 regions, including primarily within-network links in the DMN and DAN showing a positive correlation with fitness, and primarily between-network links showing a negative correlation with fitness (see Figure 5). A full list of the regions identified in this component is provided in Table 1 of supplementary materials and a list of component links is shown in Figure 5. Results of the NBS analysis are consistent with the exploratory results of all bivariate relationships for cardiorespiratory fitness shown in the supplemental materials. Briefly, at a more strict link-wise threshold of p<.001 (uncorrected), FC between regions of the DMN were positively correlated with cardiorespiratory fitness. In particular, the DMN regions from both analyses notably included the theoretically proposed “core” of the DMN including the PCC and mPFC (Andrews-Hanna et al., 2010) (see supplementary figure 3).
Figure 5.
Visualization of relationship between cardiovascular fitness and FC as identified by NBS, p<.01 FWER corrected, after controlling for age, gender, MVPA, and LIPA. For both positive (A) and negative (B) correlations, within-network links are illustrated with the same color as the network nodes and between-network links are illustrated as gray. The panel on the right (C) lists the ROI pair labels shown in panels A and B, where “W” is listed for within-network pairs and “B” is listed for between-network pairs; ROI pairs are listed in order of strength of their association with fitness as indicated on x-axis. Nodes, links, and bar graph elements for within-network pairs are colored with the same labeling scheme as previous figures. Of all possible within-network ROI pairs for each network, those showing a positive correlation with fitness in the identified component included 0% of ECN pairs, 15% of DMN pairs, 9% of DAN pairs, and 4% of SAL pairs.
The same regression approach was used to identify variance in MVPA and LIPA that was independent of cardiorespiratory fitness. For each of MVPA and LIPA variables we ran independent regressions where the variable of interest for associations with ROI-ROI pair FC was the dependent variable with all covariates as predictors. The residuals from each of these independent regressions were then used for computing correlations with the FC of each ROI pair. However, NBS did not identify any statistically significant network components associated with MVPA or LIPA. The simple bivariate (uncorrected) analyses shown in supplementary materials also suggest little to no within-network structure for ROI pairs positively correlated with MVPA (see supplementary figure 5, supplementary figure 6) or LIPA (see supplementary figure 7,supplementary figure 8).
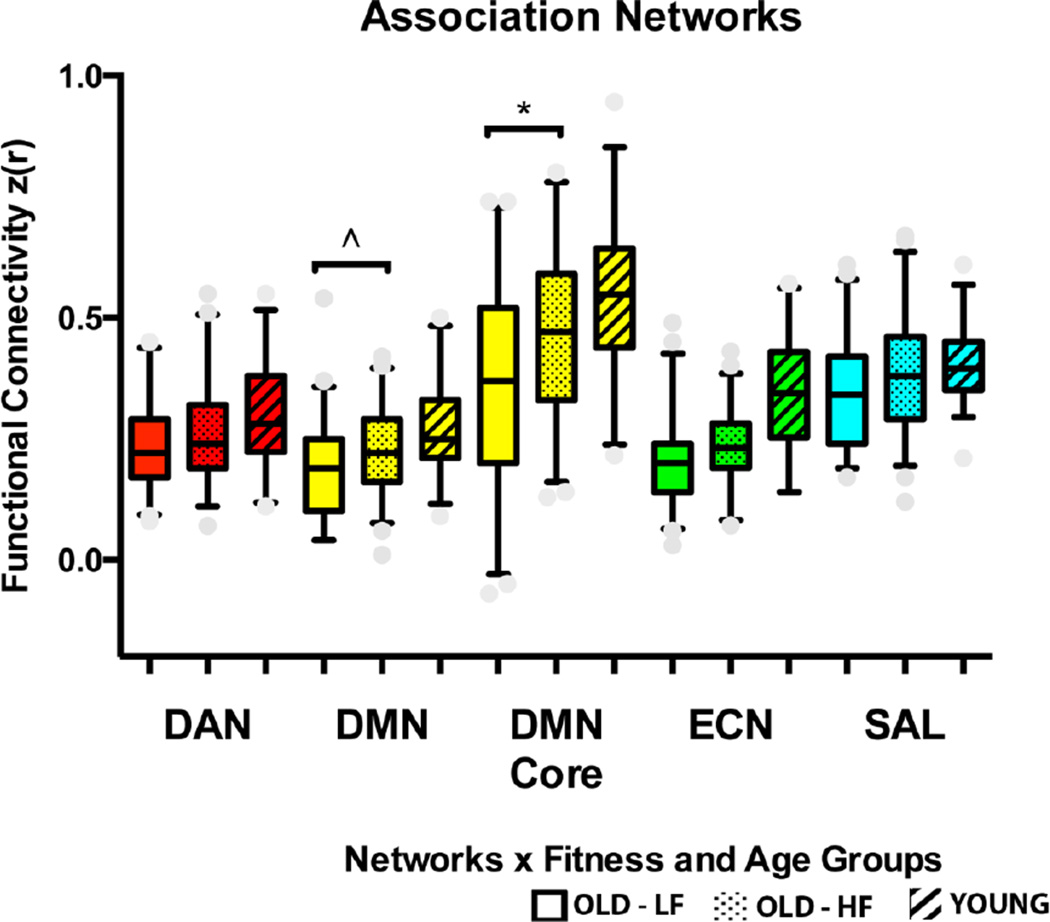
Overall, these results support the conclusion that cardiorespiratory fitness has a positive association, independent of physical activity, with FC within networks that exhibit age-related reductions in FC. To further quantify this association and because results emphasizing the DMN Core in part involved uncorrected multiple comparisons of many ROI pairs, we performed follow-up analyses with extreme fitness groups based on cardiorespiratory fitness that utilized complementary imaging analyses. Fitness groups were formed as a “lower fit” (LF) older adult group comprised of the bottom 25% of the unstandardized residuals and a “higher fit” (HF) group comprised of the top 25% of the unstandardized residuals, following the multiple linear regression approach described above to identify variance in cardiorespiratory fitness independent of physical activity. This resulted in N=47 older participants in each group, and the groups did not differ in age, gender proportion, MVPA, or LIPA (see Table 2, supplemental figure 1E and 1F). We then compared the LF and HF groups on average within-network FC of the DMN, DMN Core, DAN, ECN, and SAL with a one-directional independent samples t-test, based on our hypothesis that the HF group would have greater FC than the LF group. Results showed that compared to the LF older adults, HF older adults had greater FC selectively in the DMN (t(92)=2.24, p=.01, d=.46) and DMN Core (t(92)=2.85, p=.003, d=.59) (see Figure 6).
Figure 6.
Summary of extreme fitness groups comparisons for average functional network connectivity: OLD-LF=Older adult lower-fit group, OLD-HF=Older adult higher-fit group, YOUNG=younger adult group. Boxes represent the width between the 25th and 75th quartiles (interquartile range), whiskers represent the 5th to 95th percentiles of the data, and the middle bar represents the median. *p<.01, ^p=.01, based on one-tailed t-tests.
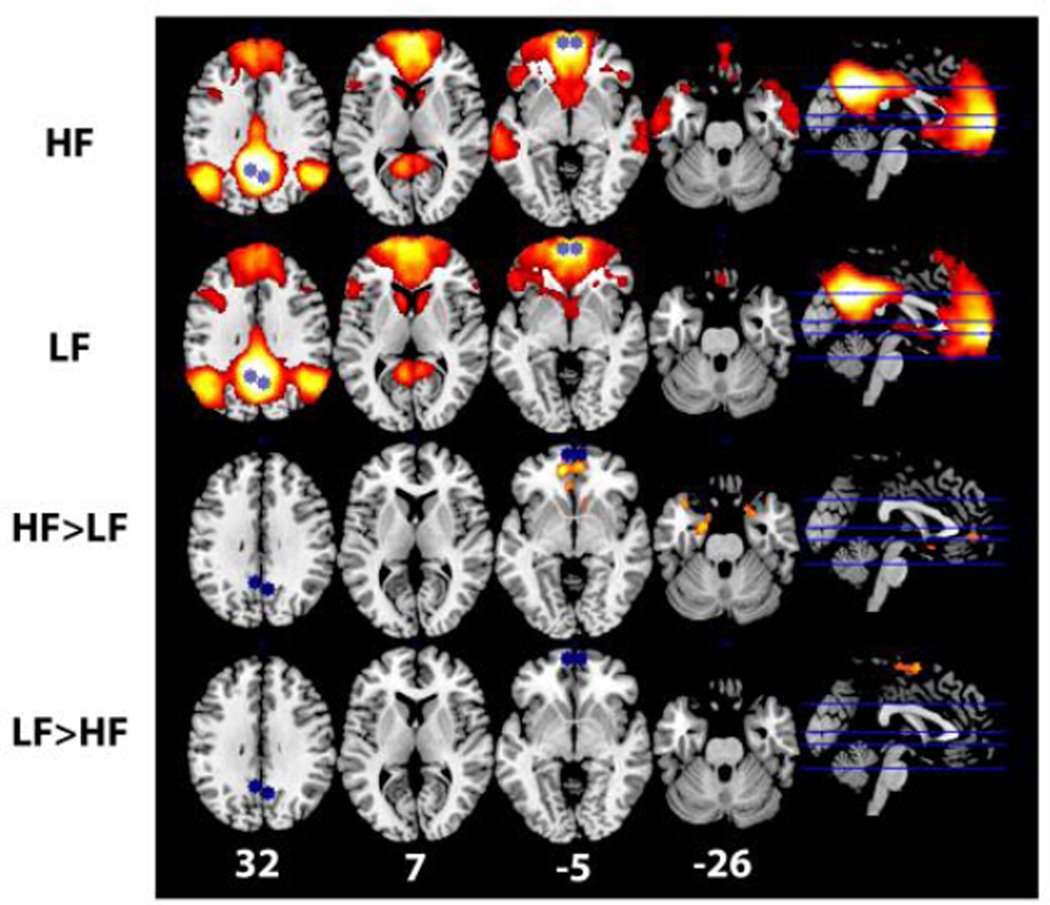
Finally, we conducted a whole-brain seed-based analysis of the DMN Core. Again, the rationale for this analysis is to add a complementary perspective for evaluating the specificity of fitness group differences in FC with the DMN Core. Given our first set of approaches utilized defined ROIs and indicated the association with fitness is strongest in the DMN Core, a seed-based approach with the DMN Core as a joint seed ROI allowed us to evaluate where the effects are strongest with these regions without imposing constraints about where in the brain effects could be realized. Results are shown in Figure 7 and are consistent and complementary with our results from other approaches. The contrast of HF greater than LF identified one cluster of 1176 voxels with a peak Z=3.67 centered in the left amygdala and extending into the right amygdala, left and right hippocampus, subcallosal cortex, and left and right ventral mPFC; the contrast of LF greater than HF identified one cluster of 828 voxels with a peak Z=3.78 centered in left dorsal precentral gyrus, extending into right precentral gyrus, left post central gyrus, and left and right supplementary motor cortex. Overall, results from the seed-based analysis showed that HF had greater FC with the mPFC of the DMN Core and in particular with the medial temporal lobe (MTL) sub-system of the DMN as conceptualized by Andrews-Hanna and colleagues (Andrews-Hanna, 2012; Andrews-Hanna et al., 2010). However, LF had greater FC with regions outside of the DMN and DMN Core (see Figure 7).
Figure 7.
Visualization of results for whole-brain seed-based analysis of the DMN core. Regions in the DMN Core (L and R mPFC and L and R PCC) were used as a joint seed and are shown in blue. Regions positively correlated with the seed for each contrast (LF mean, HF mean, HF>LF, LF>HF) are shown in hot colors. The Z-plane for each axial slice is indicated along the bottom in white in MNI coordinate space. For all images L=L/R=R.
4. Discussion and Conclusions
The current study replicates and extends previous research demonstrating a positive relationship between cardiorespiratory fitness and FC of cognitively and clinically relevant brain networks observed during the resting state and which are vulnerable to adverse effects of aging (Voss et al., 2010b). Overall, there are three important themes from our results. First, our results replicate previous studies showing that the DMN, ECN, and SAL networks are most disrupted with advanced age (e.g., Figure 4). We also show that young adults had greater FC in the DAN compared to the older adults, but that the effect was weaker for this network compared to other association networks. Importantly, results suggest that at least some of the effects related to age on the DMN are not inevitable and may be moderated by cardiorespiratory fitness. Secondly, the positive associations between cardiorespiratory fitness and FC were specific rather than global to all FC correlates of aging. Consistent with our predictions, FC in the PFC and temporal cortex showed the strongest associations with cardiorespiratory fitness (see Figures 5 and 7). A third important theme is that the association between cardiorespiratory fitness and functional correlates of brain aging was independent of lifestyle MVPA and LIPA. Research in other domains of public health has demonstrated a similar effect with respect to cardiovascular disease and mortality (e.g., Lee et al., 2011) and our results, especially with replication from follow-up prospective and training studies, may extend this finding to brain health.
The significance of the specificity of the relationships between fitness and FC, within the context of aging, is that it provides insight into the possible mechanisms through which cardiorespiratory fitness has been linked to specific and general aspects of cognition in healthy aging and better long-term cognitive outcomes in prospective studies (Barnes et al., 2003; Colcombe and Kramer, 2003; Hillman et al., 2008; Liu et al., 2012; Wendell et al., 2014). Note that because NBS resulted in one significant component comprised of multiple theorized networks for both the age and fitness analysis, we should interpret the specificity of effects on different networks cautiously from the NBS analysis alone. However, follow-up analyses of average within-network FC and the whole-brain seed-based analysis also support the idea that some networks are more sensitive to aging and individual differences in fitness than others. In our results, the DMN and particularly the DMN Core had the strongest association with cardiorespiratory fitness while also showing sensitivity to individual differences in age. Because the DMN is associated with self-referential thoughts and spatial processing and its FC has also been implicated in executive function and selective attention (Andrews-Hanna et al., 2014; Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Kelly et al., 2008; Menon and Uddin, 2010), these relationships may represent a systems-level mechanism for the association commonly observed between cardiorespiratory fitness and performance on tasks that emphasize executive function, selective attention, and cognitive control as well as general cognitive functioning (Barnes et al., 2003; Colcombe and Kramer, 2003; Colcombe et al., 2004; Hillman et al., 2008; Smith et al., 2010).
The specificity of the relationships between fitness and FC observed in the current study is significant from a public health perspective because it provides mechanistic insight into understanding the positive relationship between fitness and brain health in exercise training studies (Erickson et al., 2011; Maass et al., 2014; Pereira et al., 2007; Voss et al., 2013a). Overall, our results are most consistent with the cardiovascular fitness hypothesis, which suggests that cardiovascular (i.e., aerobic) fitness is the key physiological mediator that explains the positive relationship between physical exercise and cognitive performance, with the implication that gains in cardiovascular fitness are necessary for gains in brain health to be observed (Barnes et al., 2013; Brickman et al., 2014; Dustman et al., 1984).
One mechanism through which cardiorespiratory fitness may be linked to better brain function includes improved oxygen transport and metabolism, not only in muscle but also in the brain. This in turn is thought to enable more efficient neurotransmitter function supporting neuronal signaling, and less oxidative stress and inflammation-related neuronal injury, both resulting in improved cognitive performance (Davenport et al., 2012; Dustman et al., 1990; Dustman et al., 1984). Another, not mutually exclusive possibility, is that aerobic training directly affects the heart and central vessels, which then affect compliance and pressure of flow in end organs such as the kidney and brain; accordingly, greater vascular compliance would lower pulsatile pressure-related damage in cerebral micro-vessels that supply energy for neuronal processing (Gauthier et al., 2014; Mitchell et al., 2011; Tarumi et al., 2013). Finally, another not mutually exclusive mechanistic pathway associated with cardiorespiratory fitness could be training-related improvements in the oxidative capacity of large skeletal muscles, through both enhanced mitochondrial function and muscle capillarization. These improvements would allow for more exchange of muscle-derived circulating humoral factors that could up-regulate central expression of neurotrophic factors, such as brain-derived neurotrophic factor, in the hippocampus and neocortex (Voss et al., 2013b; Wrann et al., 2013).
Given that these mechanisms are proposed to work from adaptations initiated by physical activity or training at moderate-to-vigorous intensities, what could account for the specific association we observed between cardiorespiratory fitness and brain health independent of physical activity? One possibility is the strong genetic influence (up to 50%) over cardiorespiratory fitness and over the capacity for training-induced changes in fitness (Bouchard et al., 1999; Bouchard et al., 2011). Similarly, there may be overlapping genetic predictors of the responsiveness of central and peripheral cellular and vascular systems to regular MVPA and training. For example, increased distribution of blood flow to the muscle is a very important predictor of cardiorespiratory fitness, and this is influenced by the heart’s ability to generate more cardiac output (e.g., via increases in stroke volume) and to recruit and form new capillaries (i.e., angiogenesis) in the muscle. Another important training-related adaptation includes changes in the oxidative capacity of mitochondria in active muscle fibers. Angiogenesis and mitochondrial function also increase in the brain in response to exercise training (Marques-Aleixo et al., 2012; Voss et al., 2013b), and so it is possible that individuals that are genetically predisposed to have relatively higher levels of cardiorespiratory fitness or have greater training-induced increases in fitness will experience the most protection against adverse effects of aging on the brain from through these vascular and cellular pathways.
Interestingly, the distribution of cardiorespiratory fitness for our sample is similar to other studies that have detected significant relationships between fitness and cognitive and brain health. For instance, prospective studies evaluating the predictive value of cardiorespiratory fitness for cognitive decline and other health outcomes have generally found that the greatest benefit is in the difference between the lowest to moderate levels of cardiorespiratory fitness (Barnes et al., 2003; Berry et al., 2011; Liu et al., 2012), which is consistent with the relative differences between our LF and HF older adult groups. In addition, our sample has a similar distribution of fitness as other studies linking variation in fitness to individual differences in DMN FC (Voss et al., 2010b) and hippocampal volume in healthy older adults (Erickson et al., 2009) and early AD (Honea et al., 2009). This suggests that the mechanism linking fitness and brain health may not depend on extremely high levels of fitness, and rather is sensitive to individual differences in (or training related improvements in) fitness from even low to moderate fitness levels.
Finally, our conclusions should be interpreted within the context of several limitations. First, our sample was relatively homogenous in regard to age, health, ethnicity, and education and so it will be important for follow-up studies to generalize our findings to more diverse samples with a variety of age-related chronic conditions. Second, our cross-sectional design precludes making causal statements about the influence of cardiorespiratory fitness on brain health, so future research will be needed to test our hypotheses and extend our findings within the context of training-induced changes in cardiorespiratory fitness. In addition, we generated a number of predictions about the cognitive and clinical relevance of the specificity of our results for the DMN, and it will be important to follow these predictions with explicit tests of whether these FC measures mediate the relationships between cardiorespiratory fitness and cognitive performance and clinical outcomes. A particularly good framework for testing these relationships will be an analysis that integrates across multiple measures of brain structure and function (Hedden et al., 2014). A final limitation is that our measure of physical activity was only a brief sample of physical activity rather than a measurement over months or years of behavior. While this is standard practice (e.g., Troiano et al., 2008; Varma et al., 2014), this may add noise to the measurement of physical activity compared with the measurement of cardiorespiratory fitness, since fitness is a physiological attribute that may be affected by years of physical activity and other lifestyle choices. Thus it is possible that fitness is a marker for more long-standing physical health and activity. In addition, it should be noted that while we used a hip-worn accelerometer, accelerometers can also be worn on the wrist or ankle and each site has advantages and disadvantages for the types of activities they are sensitive to (e.g., Hildebrand et al., 2014). In turn, longitudinal studies that measure both physical activity and cardiorespiratory fitness regularly over a long duration of training for inactive adults, and studies with different or additional accelerometer sites, could further test the extent to which our results stem from matters of measurement or mechanism.
Another important set of limitations relates to factors that could be associated with individual differences in resting state network FC that also correlate with fitness, but are not reflective of the functional integrity of brain networks. The methodological steps important for detecting network measures sensitive to neuronal versus non-neuronal aspects of brain networks continue to be carefully evaluated, and there is no clear single optimal processing or covariate strategy at this time (e.g., Bright and Murphy, 2015; Zeng et al., 2014). Thus, although we have used a processing stream that is consistent with much of the published literature investigating individual differences in resting state FC in relation to aging and cognition, it will be important for future studies to replicate our findings with additional consideration for individual differences in resting heart rate and blood pressure during scanning and examining training-related changes in FC within the same individuals that are likely to have similar movement and signal-to-noise profiles during scanning (e.g., Zeng et al., 2014).
In summary, our results suggest that cardiorespiratory fitness is related to greater FC of brain networks that are relevant to age-related changes in cognition and risk for neurological diseases. Our results suggest that the positive association of fitness with brain function could occur independent of habitual physical activity. This highlights the importance of measuring both physical activity and cardiorespiratory fitness as results that show relationships with physical activity without accounting for individual differences in fitness may actually be attributable to fitness among high responders to regular physical activity. As the world’s aging population continues to grow, our results may also suggest that cardiorespiratory fitness is an important physiological attribute to modify for improved cognitive and brain health throughout the lifespan.
Supplementary Material
Highlights.
Normal aging is associated with selective disruption of large-scale brain networks
Cardiorespiratory fitness is related to the function of networks affected by aging
Fitness is related to network function independent of physical activity
Acknowledgements
This work was supported by the National Institute on Aging at the National Institutes of Health (R37 AG025667) and funding from Abbott Nutrition through the Center for Nutrition, Learning, and Memory at the University of Illinois. We would also like to thank Anya Knecht, Susan Houseworth, Nancy Dodge, Holly Tracy, and all of the Lifelong Brain and Cognition and Exercise Psychology Laboratory graduate students and staff for their help in participant recruitment and data collection. Finally, we would like to thank Merry Mani, Vincent Magnotta, and Joel Bruss for their help with development and improvement of image processing scripts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription. 9th Edition ed. Lippincott Williams & Wilkins; 2013. [Google Scholar]
- Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni G, Filippi M. Resting state fMRI in Alzheimer’s disease: beyond the default mode network. Neurobiol Aging. 2012;33:1564–1578. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB Analysis Group Technical Reports. 2007a [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation. FMRIB Analysis Group Technical Reports. 2007b [Google Scholar]
- Andrews-Hanna J. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J, Reidler J, Sepulcre J, Poulin R, Buckner R. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J, Smallwood J, Spreng R. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J, Snyder A, Vincent J, Lustig C, Head D, Raichle M, Buckner R. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar H, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- Bai F, Shu N, Yuan Y, Shi Y, Yu H, Wu D, Wang J, Xia M, He Y, Zhang Z. Topologically convergent and divergent structural connectivity patterns between patients with remitted geriatric depression and amnestic mild cognitive impairment. J Neurosci. 2012;32:4307–4318. doi: 10.1523/JNEUROSCI.5061-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D, Blackwell T, Stone K, Goldman S, Hillier T, Yaffe K. Cognition in older women: the importance of daytime movement. J Am Geriatr Soc. 2008;56:1658–1664. doi: 10.1111/j.1532-5415.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D, Santos-Modesitt W, Poelke G, Kramer A, Castro C, Middleton L, Yaffe K. The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med. 2013;173:797–804. doi: 10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D, Yaffe K, Satariano W, Tager I. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Berry J, Willis B, Gupta S, Barlow C, Lakoski S, Khera A, Rohatgi A, de Lemos J, Haskell W, Lloyd-Jones D. Lifetime risks for cardiovascular disease mortality by cardiorespiratory fitness levels measured at ages 45, 55, and 65 years in men. The Cooper Center Longitudinal Study. J Am Coll Cardiol. 2011;57:1604–1610. doi: 10.1016/j.jacc.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots E, Schultz S, Oh J, Larson J, Edwards D, Cook D, Koscik R, Dowling M, Gallagher C, Carlsson C, Rowley H, Bendlin B, LaRue A, Asthana S, Hermann B, Sager M, Johnson S, Okonkwo O. Brain Imaging Behav. 2014. Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, An P, Rice T, Skinner J, Wilmore J, Gagnon J, Pérusse L, Leon A, Rao D. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985) 1999;87:1003–1008. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Sarzynski M, Rice T, Kraus W, Church T, Sung Y, Rao D, Rankinen T. Genomic predictors of the maximal O2 uptake response to standardized exercise training programs. J Appl Physiol (1985) 2011;110:1160–1170. doi: 10.1152/japplphysiol.00973.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman A, Khan U, Provenzano F, Yeung L, Suzuki W, Schroeter H, Wall M, Sloan R, Small S. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci. 2014 doi: 10.1038/nn.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier M, Thomas J, Snyder A, Benzinger T, Zhang D, Raichle M, Holtzman D, Morris J, Ances B. Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. J Neurosci. 2012;32:8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright M, Murphy K. Is fMRI “noise” really noise? Resting state nuisance regressors remove variance with network structure. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A, Boyle P, Yu L, Shah R, Wilson R, Bennett D. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska A, Chaddock-Heyman L, Voss M, Wong C, Gothe N, Olson E, Knecht A, Lewis A, Monti J, Cooke G, Wojcicki T, Fanning J, Chung H, Awick E, McAuley E, Kramer A. Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PLoS One. 2014;9:e107413. doi: 10.1371/journal.pone.0107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casperson CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Reports. 1985;1985:126–131. [PMC free article] [PubMed] [Google Scholar]
- Prevention CfDCa., editor. CDC. The State of Aging and Health in America 2013. Atlanta, GA: US Dept of Health and Human Services; 2013. [Google Scholar]
- Chan M, Park D, Savalia N, Petersen S, Wig G. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A. 2014;111:E4997–E5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Janz K, Zhu W, Brychta R. Redefining the roles of sensors in objective physical activity monitoring. Med Sci Sports Exerc. 2012;44:S13–S23. doi: 10.1249/MSS.0b013e3182399bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L, Bramati I, Zalesky A, Furukawa E, Fontenelle L, Moll J, Tripp G, Mattos P. Altered functional brain connectivity in a non-clinical sample of young adults with attention-deficit/hyperactivity disorder. J Neurosci. 2012;32:17753–17761. doi: 10.1523/JNEUROSCI.3272-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer A. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer A, Erickson K, Scalf P, McAuley E, Cohen N, Webb A, Jerome G, Marquez D, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Esliger D. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Act. 2009;17:17–30. doi: 10.1123/japa.17.1.17. [DOI] [PubMed] [Google Scholar]
- Craft L, Zderic T, Gapstur SM, Vanlterson EH, Thomas DM, Siddique J, Hamilton MT. Evidence that women meeting physical activity guidelines do not sit less: an observational inclinometry study. International Journal of Behavioral Nutrition and Physical Activity. 2012;9:1–9. doi: 10.1186/1479-5868-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J, Beckmann C, Arigita E, Barkhof F, Scheltens P, Stam C, Smith S, Rombouts S. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Davenport M, Hogan D, Eskes G, Longman R, Poulin M. Cerebrovascular reserve: the link between fitness and cognitive function? Exerc Sport Sci Rev. 2012;40:153–158. doi: 10.1097/JES.0b013e3182553430. [DOI] [PubMed] [Google Scholar]
- Dennis E, Thompson P. Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol Rev. 2014;24:49–62. doi: 10.1007/s11065-014-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy O, Gauthier C, Fraser S, Desjardins-Crepeau L, Desjardins M, Mekary S, Lesage F, Hoge R, Pouliot P, Bherer L. Higher levels of cardiovascular fitness are associated with better executive function and prefrontal oxygenation in younger and older women. Front Hum Neurosci. 2015;9:66. doi: 10.3389/fnhum.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustman R, Emmerson R, Ruhling R, Shearer D, Steinhaus L, Johnson S, Bonekat H, Shigeoka J. Age and fitness effects on EEG, ERPs, visual sensitivity, and cognition. Neurobiol Aging. 1990;11:193–200. doi: 10.1016/0197-4580(90)90545-b. [DOI] [PubMed] [Google Scholar]
- Dustman R, Ruhling R, Russell E, Shearer D, Bonekat H, Shigeoka J, Wood J, Bradford D. Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiol Aging. 1984;5:35–42. doi: 10.1016/0197-4580(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Erickson K, Prakash R, Voss M, Chaddock L, Hu L, Morris K, White S, Wojcicki T, McAuley E, Kramer A. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K, Voss M, Prakash R, Basak C, Szabo A, Chaddock L, Kim J, Heo S, Alves H, White S, Wojcicki T, Mailey E, Vieira V, Martin S, Pence B, Woods J, McAuley E, Kramer A. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier J, Nowell P, Landers D, Sibley B. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52:119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gauthier C, Lefort M, Mekary S, Desjardins-Crepeau L, Skimminge A, Iversen P, Madjar C, Desjardins M, Lesage F, Garde E, Frouin F, Bherer L, Hoge R. Hearts and minds: linking vascular rigidity and aerobic fitness with cognitive aging. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Gordon B, Rykhlevskaia E, Brumback C, Lee Y, Elavsky S, Konopack J, McAuley E, Kramer A, Colcombe S, Gratton G, Fabiani M. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45:825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M, Srivastava G, Reiss A, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- Hart T, McClain J, Tudor-Locke C. Controlled and free-living evaluation of objective measures of sedentary and active behaviors. J Phys Act Health. 2011;8:848–857. doi: 10.1123/jpah.8.6.848. [DOI] [PubMed] [Google Scholar]
- Hayes S, Hayes J, Cadden M, Verfaellie M. A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Front Aging Neurosci. 2013;5:31. doi: 10.3389/fnagi.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Qin W, Liu Y, Zhang X, Duan Y, Song J, Li K, Jiang T, Yu C. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2014;35:3446–3464. doi: 10.1002/hbm.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Schultz A, Rieckmann A, Mormino E, Johnson K, Sperling R, Buckner R. Multiple Brain Markers are Linked to Age-Related Variation in Cognition. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand M, VAN Hees V, Hansen B, Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc. 2014;46:1816–1824. doi: 10.1249/MSS.0000000000000289. [DOI] [PubMed] [Google Scholar]
- Hillman C, Erickson K, Kramer A. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Honea R, Thomas G, Harsha A, Anderson H, Donnelly J, Brooks W, Burns J. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd M, Martorell P, Delavande A, Mullen K, Langa K. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D, Ainsworth B, Hartman T, Leon A. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban R. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Kelly A, Uddin L, Biswal B, Castellanos F, Milham M. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kruschwitz J, List D, Waller L, Rubinov M, Walter H. GraphVar: a user-friendly toolbox for comprehensive graph analyses of functional brain connectivity. J Neurosci Methods. 2015;245:107–115. doi: 10.1016/j.jneumeth.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Larson E, Wang L, Bowen J, McCormick W, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Laye M, Nielsen M, Hansen L, Knudsen T, Pedersen B. Physical activity enhances metabolic fitness independently of cardiorespiratory fitness in marathon runners. Dis Markers. 2015;2015:806418. doi: 10.1155/2015/806418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Sui X, Ortega F, Kim Y, Church T, Winett R, Ekelund U, Katzmarzyk P, Blair S. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med. 2011;45:504–510. doi: 10.1136/bjsm.2009.066209. [DOI] [PubMed] [Google Scholar]
- Leopold D, Murayama Y, Logothetis N. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex. 2003;13:422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- Liu R, Sui X, Laditka J, Church T, Colabianchi N, Hussey J, Blair S. Cardiorespiratory fitness as a predictor of dementia mortality in men and women. Med Sci Sports Exerc. 2012;44:253–259. doi: 10.1249/MSS.0b013e31822cf717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Duzel S, Goerke M, Becke A, Sobieray U, Neumann K, Lovden M, Lindenberger U, Backman L, Braun-Dullaeus R, Ahrens D, Heinze H, Muller N, Duzel E. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.114. [DOI] [PubMed] [Google Scholar]
- Makizako H, Liu-Ambrose T, Shimada H, Doi T, Park H, Tsutsumimoto K, Uemura K, Suzuki T. Moderate-Intensity Physical Activity, Hippocampal Volume, and Memory in Older Adults With Mild Cognitive Impairment. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu136. [DOI] [PubMed] [Google Scholar]
- Marques-Aleixo I, Oliveira P, Moreira P, Magalhaes J, Ascensao A. Physical exercise as a possible strategy for brain protection: evidence from mitochondrial-mediated mechanisms. Prog Neurobiol. 2012;99:149–162. doi: 10.1016/j.pneurobio.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Matthews C, Chen K, Freedson P, Buchowski M, Beech B, Pate R, Troiano R. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier T, Desphande A, Vergun S, Nair V, Song J, Biswal B, Meyerand M, Birn R, Prabhakaran V. Support vector machine classification and characterization of age-related reorganization of functional brain networks. NeuroImage. 2012;60:601–613. doi: 10.1016/j.neuroimage.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin L. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton L, Manini T, Simonsick E, Harris T, Barnes D, Tylavsky F, Brach J, Everhart J, Yaffe K. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171:1251–1257. doi: 10.1001/archinternmed.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G, van Buchem M, Sigurdsson S, Gotal J, Jonsdottir M, Kjartansson O, Garcia M, Aspelund T, Harris T, Gudnason V, Launer L. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowinckel A, Espeseth T, Westlye L. Network-specific effects of age and in-scanner subject motion: a resting-state fMRI study of 238 healthy adults. NeuroImage. 2012;63:1364–1373. doi: 10.1016/j.neuroimage.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Services UDoHaH., editor. NIA. Why population aging matters: A global perspective. 2007. [Google Scholar]
- Nyberg J, Aberg M, Schioler L, Nilsson M, Wallin A, Toren K, Kuhn H. Cardiovascular and cognitive fitness at age 18 and risk of early-onset dementia. Brain. 2014;137:1514–1523. doi: 10.1093/brain/awu041. [DOI] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, Yamaguchi S. Decreased functional connectivity by aging is associated with cognitive decline. J Cogn Neurosci. 2012;24:2186–2198. doi: 10.1162/jocn_a_00269. [DOI] [PubMed] [Google Scholar]
- Pate R, O’Neill J, Lobelo F. The evolving definition of “sedentary”. Exerc Sport Sci Rev. 2008;36:173–178. doi: 10.1097/JES.0b013e3181877d1a. [DOI] [PubMed] [Google Scholar]
- Pereira A, Huddleston D, Brickman A, Sosunov A, Hen R, McKhann G, Sloan R, Gage F, Brown T, Small S. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J, Barnes K, Snyder A, Schlaggar B, Petersen S. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Voss M, Erickson K, Lewis J, Chaddock L, Malkowski E, Alves H, Kim J, Szabo A, White S, Wojcicki T, Klamm E, McAuley E, Kramer A. Cardiorespiratory fitness and attentional control in the aging brain. Front Hum Neurosci. 2011;4:229. doi: 10.3389/fnhum.2010.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands A, Fraysse F, Catt M, Stiles V, Stanley R, Eston R, Olds T. Comparability of measured acceleration from accelerometry-based activity monitors. Med Sci Sports Exerc. 2015;47:201–210. doi: 10.1249/MSS.0000000000000394. [DOI] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman M, Pickard J, Menon D, Bullmore E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 2005;15:1332–1342. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- Seeley W, Crawford R, Zhou J, Miller B, Greicius M. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw E, Schultz A, Sperling R, Hedden T. Functional connectivity in multiple cortical networks is associated with performance across cognitive domains in older adults. Brain Connect. 2015 doi: 10.1089/brain.2014.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley-Oyen A, Lowry K, Francois S, Kohut M, Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: the “selective improvement” and “cardiovascular fitness” hypotheses. Ann Behav Med. 2008;36:280–291. doi: 10.1007/s12160-008-9064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. Physical activity, vascular health, and cognitive impairment. Arch Intern Med. 2012;172:83. doi: 10.1001/archinternmed.2011.615. author reply 84. [DOI] [PubMed] [Google Scholar]
- Smith P, Blumenthal J, Hoffman B, Cooper H, Strauman T, Welsh-Bohmer K, Browndyke J, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Fox P, Miller K, Glahn D, Fox P, Mackay C, Filippini N, Watkins K, Toro R, Laird A, Beckmann C. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Valecchi D, Bacci D, Abbate R, Gensini G, Casini A, Macchi C. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269:107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun V, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager A. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirduso W. Physical fitness, aging, and psychomotor speed: a review. J Gerontol. 1980;35:850–865. doi: 10.1093/geronj/35.6.850. [DOI] [PubMed] [Google Scholar]
- Tarumi T, Gonzales M, Fallow B, Nualnim N, Pyron M, Tanaka H, Haley A. Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. J Hypertens. 2013;31:2400–2409. doi: 10.1097/HJH.0b013e328364decc. [DOI] [PubMed] [Google Scholar]
- Thompson WH, Fransson P. The frequency dimension of fMRI dynamic connectivity: network connectivity, functional hubs and integration in the resting brain. NeuroImage in press. 2015 doi: 10.1016/j.neuroimage.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Troiano R, Berrigan D, Dodd K, Masse L, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Schuna JJ, Barreira T, Mire E, Broyles S, Katzmarzyk P, Johnson W. Normative steps/day values for older adults: NHANES 2005–2006. J Gerontol A Biol Sci Med Sci. 2013;68:1426–1432. doi: 10.1093/gerona/glt116. [DOI] [PubMed] [Google Scholar]
- Varma V, Chuang Y, Harris G, Tan E, Carlson M. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus. 2014 doi: 10.1002/hipo.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M, Heo S, Prakash R, Erickson K, Alves H, Chaddock L, Szabo A, Mailey E, Wojcicki T, White S, Gothe N, McAuley E, Sutton B, Kramer A. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp. 2013a;34:2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M, Prakash R, Erickson K, Basak C, Chaddock L, Kim J, Alves H, Heo S, Szabo A, White S, Wojcicki T, Mailey E, Gothe N, Olson E, McAuley E, Kramer A. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci 2. 2010a doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M, Vivar C, Kramer A, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013b;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Carr LJ, Clark R, Weng T. Revenge of the “sit” II: Does lifestyle impact neuronal and cognitive health through distinct mechanisms associated with sedentary behavior and physical activity? Mental Health and Physical Activity. 2014;7:9–24. [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, Kim JS, Morris KS, White SM, Wójcicki TR. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010b;48:1394–1406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zuo X, Dai Z, Xia M, Zhao Z, Zhao X, Jia J, Han Y, He Y. Disrupted functional brain connectome in individuals at risk for Alzheimer’s disease. Biol Psychiatry. 2013;73:472–481. doi: 10.1016/j.biopsych.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Voss M, Prakash R, Chaddock L, Szabo A, White S, Wojcicki T, Mailey E, McAuley E, Kramer A, Erickson K. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun. 2012;26:811–819. doi: 10.1016/j.bbi.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendell C, Gunstad J, Waldstein S, Wright J, Ferrucci L, Zonderman A. Cardiorespiratory fitness and accelerated cognitive decline with aging. J Gerontol A Biol Sci Med Sci. 2014;69:455–462. doi: 10.1093/gerona/glt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc. 2001;33:754–761. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K, Evans A, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Wrann C, White J, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin J, Greenberg M, Spiegelman B. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Nevitt M, Lui L, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- Young J, Angevaren M, Rusted… J. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. The Cochrane …. 2015;4:CD005381. doi: 10.1002/14651858.CD005381.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Cocchi L, Fornito A, Murray M, Bullmore E. Connectivity differences in brain networks. NeuroImage. 2012;60:1055–1062. doi: 10.1016/j.neuroimage.2012.01.068. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore E. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zeng L, Wang D, Fox M, Sabuncu M, Hu D, Ge M, Buckner R, Liu H. Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci U S A. 2014;111:6058–6062. doi: 10.1073/pnas.1317424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.