Abstract
Background
The location and the number of lumbar sympathetic ganglia (LSG) vary between individuals. The aim of this study was to determine the appropriate level for a lumbar sympathetic ganglion block (LSGB), corresponding to the level at which the LSG principally aggregate.
Methods
Seventy-four consecutive subjects, including 31 women and 31 men, underwent LSGB either on the left (n = 31) or the right side (n = 43). The primary site of needle entry was randomly selected at the L3 or L4 vertebra. A total of less than 1 ml of radio opaque dye with 4% lidocaine was injected, taking caution not to traverse beyond the level of one vertebral body. The procedure was considered responsive when the skin temperature increased by more than 1℃ within 5 minutes.
Results
The median responsive level was significantly different between the left (lower third of the L4 body) and right (lower margin of the L3 body) sides (P = 0.021). However, there was no significant difference in the values between men and women. The overall median responsive level was the upper third of the L4 body. The mean responsive level did not correlate with height or BMI. There were no complications on short-term follow-up.
Conclusions
Selection of the primary target in the left lower third of the L4 vertebral body and the right lower margin of the L3 vertebral body may reduce the number of needle insertions and the volume of agents used in conventional or neurolytic LSGB and radiofrequency thermocoagulation.
Keywords: Clinical identification, Lumbar, Neurolytic, Radiofrequency, Sympathetic ganglia, Vertebral level
INTRODUCTION
Lumbar sympathetic ganglion block (LSGB) and neurolysis have been used for more than 80 years to manage patients with sympathetically maintained pain such as complex regional pain syndrome, vascular disease, and hyperhidrosis of the lower extremity [1]. Although it has been considered a safe procedure, an accurate knowledge of the relationship between the lumbar sympathetic chain and ganglia and the vertebral bodies is needed to successfully perform the procedure and to avoid complications [2,3].
In the clinical setting, the lumbar sympathetic ganglia (LSG) are commonly blocked between vertebral levels L2 and L4. In contrast to the thoracic sympathetic ganglia, the location and number of the LSG are variable. Therefore, to obtain complete sympatholytic effect, performing a multi-level LSGB appears to be more beneficial than performing a single-level LSGB [3,4]. However, multi-level LSGB can lead to complications, including lumbar nerve neuralgia, subarachnoid injection, and perforation of the aorta, inferior vena cava, bowel, lower pole of the kidney, and ureter, and may cause patient discomfort [4,5,6].
Cadaveric studies have demonstrated that an average of 3 ganglia aggregate between the lumbar vertebral levels from the lower third of the L2 vertebral body to the upper third of the L3 vertebral body, and this site is most commonly identified anterior to the L2/3 intervertebral disc [7]. It was also noted that the lumbar arteries traversed the middle of the L2 and L3 vertebrae. The use of at least 2 needles at the levels of the L2 and L3 vertebral bodies was favored by those authors. In another cadaveric study, Datta and Pai [3] reported that the ganglia are located at each level of the intervertebral discs or just paradiscal, and away from the lumbar vessels at this point. The results of a study by Rocco et al. [8] were also consistent, indicating that the LSG were most often present at the level of the L2/3 and L3/4 intervertebral disc spaces, suggesting that the needle should be approached slightly cephalad to the midpoint of the body of L3.
In clinical settings, the sympathetic function is always evaluated after sympathetic blocks. Assessing changes in skin temperature or sweating of the foot is a simple method to evaluate a successful sympathetic block [9]. A previous clinical study to investigate the appropriate block level for LSGB demonstrated that spreading local anesthetics from the lower half of the L4 vertebral body to the L4/5 interspace was most effective, and assumed this was where the LSG aggregated [10]. However in this study, a total of 3 ml of agents were utilized, followed by the spread of the agents which extended across 2 or more vertebral body levels. This resulted in difficulty of assessing the precise location of the LSG. Therefore, the authors designed a new clinical study to determine the precise lumbar vertebral level where the LSG principally aggregate.
MATERIALS AND METHODS
This retrospective study was approved by the Institutional Review Board and ethics committee of Gangnam Severance Hospital. Written informed consent was obtained from each patient prior to the procedure. All cases of LSGB between January 2012 and January 2015 were reviewed in our Pain Clinic. The exclusion criteria were as follows: cases without a detailed description of the procedure, fluoroscopic findings or results of LSGB; diseases that are associated with a minimal temperature change, even with a successful blockade, such as peripheral artery occlusive disease [11].
Intravenous hydration with 500 ml of normal saline or Hartmann's solution was applied before the procedure. The patients were placed in the prone or lateral position on a radiology table. Temperature probes (Marquette, GE medical systems; Wisconsin, USA) for real-time temperature monitoring were attached to the soles of both feet [12,13].
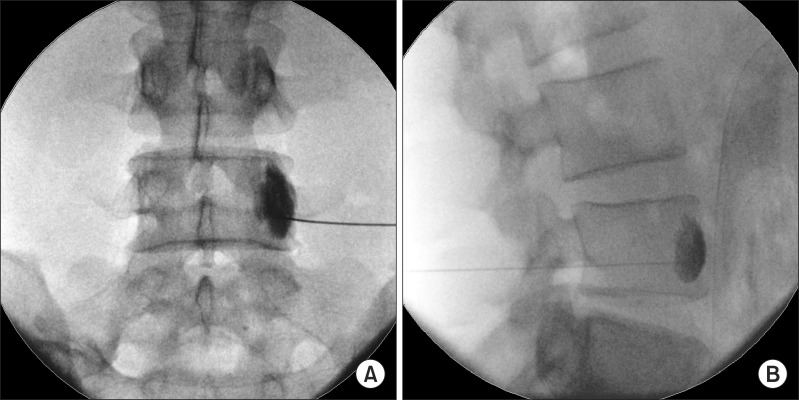
The primary site of needle entry was randomly selected at the L3 or L4 vertebra. The skin was prepared and draped in a sterile fashion. The spinous process of the L3 or L4 vertebra was identified using a fluoroscope in an antero-posterior (AP) projection. Then, the fluoroscope was rotated to a 25° oblique angle toward the corresponding side. The skin entry point was identified at the lateral edge of the vertebral body, and a local anesthetic infiltration was introduced. A 22-gauge, short bevel needle was advanced to the lateral margin of the vertebral body by means of a "tunnel vision technique" under fluoroscopic guidance [6,14]. When the needle touched the L3 or L4 vertebral body, the fluoroscope was rotated to a lateral projection, and the needle was advanced until the needle tip was just behind the anterior edge of the vertebral body. At this stage, the needle tip should lie on the anterolateral part of the vertebral body and appear to be just outside the facetal line on the AP projection (Fig. 1).
Fig. 1. Fluoroscopic view of LSGB. (A) Anteroposterior fluoroscopic view of the level of spread of the dye. (B) Lateral view of the level of spread of the dye. In this case, A and B demonstrate that the longitudinal dye spread is from point 5.5 to 7.5 on the numerical scale (see Fig. 2).
When the needle tip approached the target site, the upper or lower third of the vertebral body, aspiration was performed to check for bleeding. Less than 1 ml of a mixture of radio opaque dye (Bonorex®, 300 mgl/ml; CMS, Korea) and the same volume of 4% lidocaine was injected, taking care not to traverse one vertebral body level. The procedure was considered responsive when the skin temperature increased by more than 1℃ within 5 minutes [9,10]. When the first procedure was determined as unresponsive, the second one was repeated at the next vertebral level sequentially. Additionally, when radio opaque dye was observed in the psoas muscle or blood vessels, the procedure was repeated at another vertebral level.
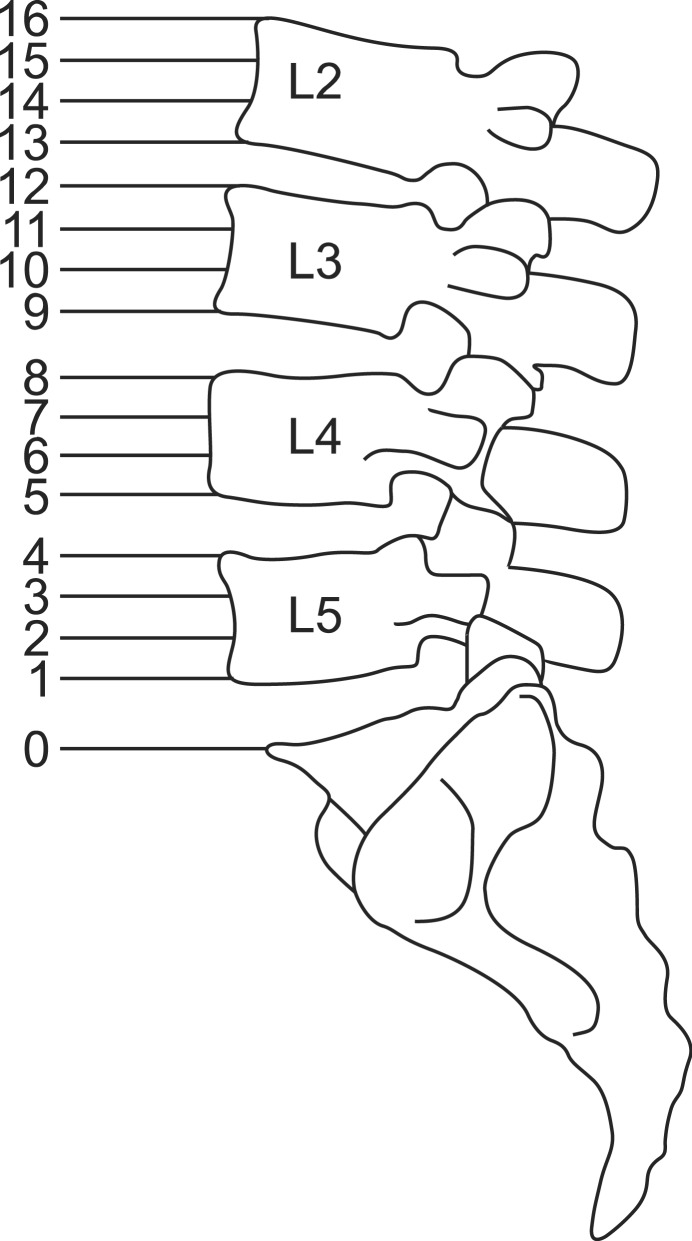
The height of the lumbar vertebral column was subdivided as follows; upper, medium, and lower lumbar body and lumbar intervertebral disc segment. The space between the upper border of the sacrum (point 0) to the upper border of the L2 vertebra (point 16) was divided into 16 segments to allow numerical description of the spread of the dye (Fig. 2).
Fig. 2. The space between upper border of sacrum and upper border of L2 vertebral body were divided into 16 segments to allow numerical description of the dye spread.
Statistical analyses were performed with the Statistical Package for the Social Sciences 21.0 (SPSS Inc., Chicago, IL, USA). All data are expressed as median (Q1-Q3) or as the number of patients. Normality tests were performed using Kolmogorov-Smirnov tests. Comparison of the demographic data between the groups was performed using the Mann-Whitney test. For inter-group comparisons, the Mann-Whitney test was used to compare the mean values of the spread level of the dye for nonparametric descriptive data. A P value < 0.05 was considered statistically significant.
RESULTS
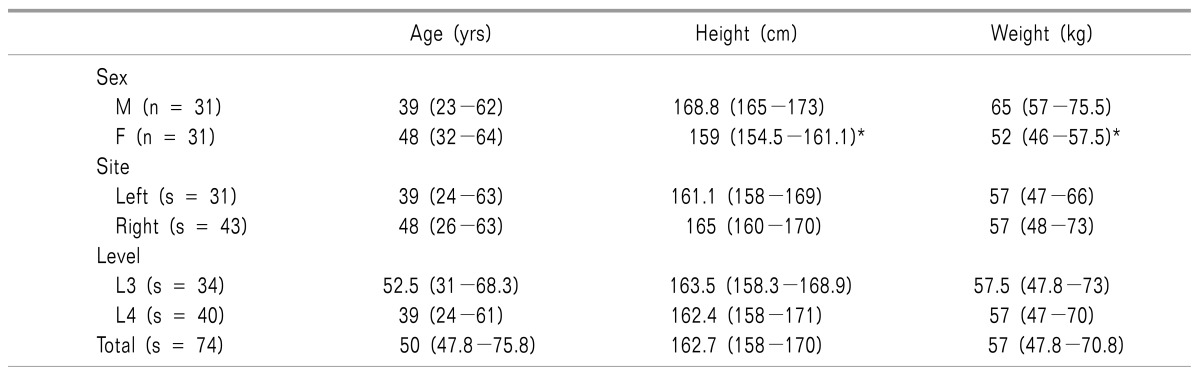
Demographic data for the 74 subjects enrolled in the study are listed in Table 1. Of the 74 consecutive subjects (31 men and 31 women) aged 20-83 years, 31 and 43 subjects were treated with LSGB on the left and the right side, respectively. LSGB was performed at the L3 and L4 vertebrae in 34 and 40 subjects, respectively. Seventeen subjects with LSGB at L3 and 13 with LSGB at L4 required an additional blockade at the next vertebral levels due to the lack of a response.
Table 1. Demographic Data.
The values are expressed as the median (Q1−Q3). n: number of patients. s: number of subjects. *P < 0.05 compared to male group.
There were 29 subjects with hyperhidrosis, 14 with lumbar postlaminectomy syndrome, 12 with lumbosacral spinal stenosis, 9 with complex regional pain syndrome (CRPS), 5 with autonomic nervous system disorder, 4 with Raynaud's disease, and 2 with frostbite.
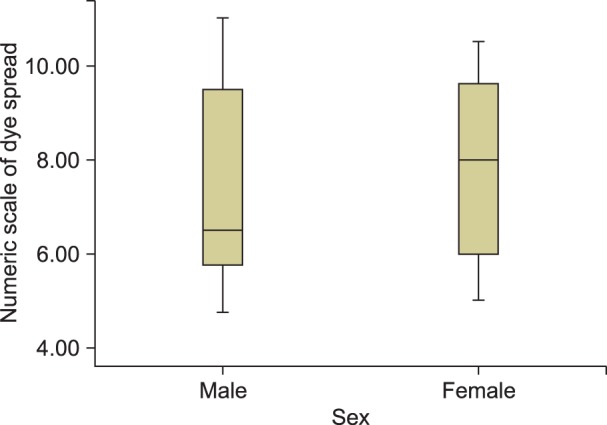
In male patients, the mean level at which a response was achieved according to the numeric scales was between 5.6 (lower third of the L4 body) and 9.5 (lower third of the L3 body), with a median value of 6.5 (upper margin of the L4 body). In female patients, the values were between 6 (middle third of the L4 body) and 9.7 (lower third of the L3 body) with a median value of 8 (upper margin of the L4 body). Although there seemed to be a tendency of a higher median spread of the dye level in female patients compared to male patients, there was no statistically significant difference between the two groups due to the similar distribution pattern of the mean responsive level (P = 0.251) (Fig. 3).
Fig. 3. The mean responsive level in male and female patients. The boxes are expressed as the median (Q1−Q3).
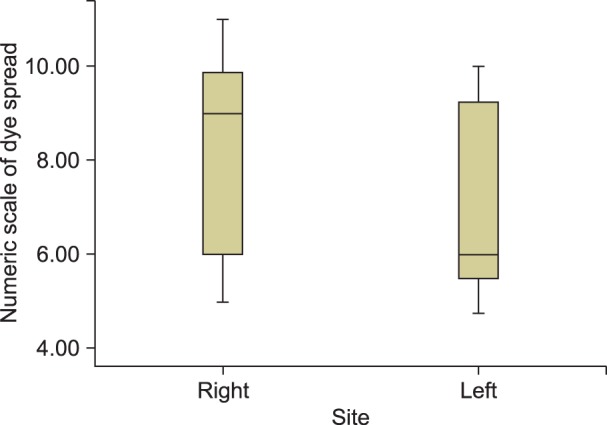
In the right LSGB group, the mean responsive level on the numerical scale was between 6 (middle third of the L4 body) and 10 (middle third of the L3 body), with a median value of 9 (lower margin of the L3 body). In the left LSGB group, the values were between 5.5 (lower third of the L4 body) and 9.3 (lower third of the L3 body) with a median value of 6 (lower third of the L4 body) (Fig. 4). The difference was statistically significant between the two groups (P = 0.021). Among the enrolled patients, the median responsive level was the upper third of the L4 body.
Fig. 4. The mean responsive level in left- and right-sided LSGB. The boxes are expressed as the median (Q1−Q3).
There were statistically significant differences in height, weight, and BMI between the male and female patients. However the mean responsive level did not correlate with height, weight, or BMI.
There were no complications during the short-term follow-up, and the study was successfully completed in all patients.
DISCUSSION
The lumbar sympathetic ganglia lie in the anterolateral aspect of the L2, L3, and L4 vertebral bodies and anterior to the psoas muscle [8]. An LSGB using a chemical neurolytic agent requires the spreading of the agent into the retroperitoneal compartment, which is surrounded by the anterior angle of the psoas muscle and the anterolateral plane of the vertebral body. It is important to ensure that the agent does not spread toward the ureter, backward into the psoas muscle, or into blood vessels. When the spread of the dye and the rise in skin temperature both indicated an effective block without complications, the same volume of dehydrated alcohol was injected to chemically degenerate the sympathetic ganglion.
In single-needle LSGBs with large volumes (>5 ml), which have been performed in an earlier era, the tip of the needle did not necessarily need to be close to the ganglion because the injected solution would sufficiently spread along the sympathetic chain [4,15]. However, with the injection of larger volumes, there is an increase in the frequency of complications, including motor weakness, genitofemoral neuritis, lumbar neuralgia, and somatic nerve blockade, especially when neurolytic agents are used [5,11]. By placing the tip of the needle in close proximity to the ganglion, an LSGB can be performed with only a small amount of neurolytic solution and thus with little or no spread to adjacent structures.
In the past, the LSG was assumed to be most frequently located at the level of the intervertebral disc of L2 and L3 on both the right and left sides [7]. However, Kim et al. [10] reported that the LSG is located in the lower third of the L4 vertebra in patients with plantar hyperhidrosis or pain pathology of the lower extremities. This finding corresponds to the connection of foot lesions to the lower LSG [16].
Sayson et al. [11] reported that the incidence of genitofemoral nerve blocks after LSGB is greater at L4 (40%) than at L2 (0%). Feigl et al. [17] reported that there is a greater likeliness that the genitofemoral nerve will be located at the L3/4 and L4/5 levels than at the L2/3 level, based on a cadaveric study. Anatomically, the genitofemoral nerve approached the medial margin of the psoas major muscle and passed the lumbar sympathetic trunk [17]. This is thought to be one of the explanations for the lower incidence of psoas muscle injection during LSGBs at L2 compared with injections at the L3 and L4 vertebral levels [18].
On the other hand, an LSGB at the L2 vertebral level increases the potential risk of kidney and ureter injury compared with an LSGB at the L3 level due to the shorter distance from the spinous process of the L2 vertebra [5,19]. At a 10 cm distance from the spinous process at the L2 level, the rate of renal puncture was 57.5% [5]. Ureter injury from neurolytic agents during LSGBs has also been reported [20]. In addition, ureter necrosis is reported to be a serious complication in chemically induced LSGBs at the L2 vertebral level [21]. Ejaculatory dysfunction was also reported after neurolytic an LSGB at the L2 level due to the spread of agents into the thoraco-lumbar sympathetic chain (T11-L2) [22]. In a study comparing RF lumbar sympatholysis with neurolytic LSGBs at the L3 level, although not significantly different statistically, the success rates were 78% and 89%, respectively. While there were no complications reported in patients that received RF thermocoagulation, the relatively high proportion of complications and the occurrence of serious complications such as ejaculation failure is thought to be due to the large injection volume of the neurolytic agent [23].
Investigation of the location of the major vessels demonstrated that the shortest distance was found at the right side of L3, compared to the left side of L3 and L2 [24]. Middleton and Chan [5] reported that puncture of the inferior vena cava in right-sided LSGBs occurred in 20% of patients. Another study reported that the incidence of intravascular injection during LSGBs was not significantly different when the procedures were performed at the L2, L3, and L4 vertebral levels [18]. There is a possibility of retrograde ejaculatory failure in LSGBs performed at the L5 vertebral level [25]. Because the LSG at the L5 vertebral level is anatomically in close proximity to the superior hypogastric plexus which coordinates bladder sphincter control during ejaculation.
Several studies of lumbar sympathetic radiofrequency (RF) lesioning have been performed at the L2, L3, and L4 vertebral levels [23,26,27,28]. Compared to neurolytic LSGBs, in which the agent spreads to at least one or more vertebral body level, lumbar sympathetic RF thermocoagulation will only coagulate a small area surrounding the active portion of the RF needle tip. Hence, RF thermocoagulation is considered safe but less effective in terms of the success rate compared to neurolytic LSGBs. A single RF needle produced lesions with short diameters which were 4 to 5 times the needle diameter and long diameters the length of the active tip. And so 2 or 3 lesions were made at each vertebral level of L2, L3, and L4 in these studies.
An investigational in vitro study with porcine spinal tissue demonstrated development of a sufficient, wider and longer lesion with RF thermocoagulation utilizing 2 needles [29]. Bipolar probes have been introduced clinically in accordance with the development of engineering techniques and made it possible to reduce the number of needle insertion and produce longer and larger linear lesions in RF thermocoagulation [30]. These bipolar probes had overcome the shortcomings of conventional lumbar sympathetic RF lesioning in the upper and lower third of a single vertebral body. Although this dual needle placement method led to successful results in terms of patient satisfaction, multiple needle placements can lead to more complications, such as unnecessary lesioning of adjacent structures and patient discomfort [31]. Therefore, assessing the precise location of the LSG and placing the needle tip in close proximity to the ganglia is even more important in RF lesioning.
Anatomically, lumbar arteries inevitably cross the sympathetic chain, which runs vertically along the vertebral column at the middle portion of each vertebral body. Lumbar arteries were observed to cross the sympathetic chain at the level of the middle third of each vertebral body in cadaveric studies [7,8]. Recognition of the crossing point of the lumbar arteries and sympathetic chain is clinically important to avoid arterial puncture, since injury of these arteries may result in massive retroperitoneal hemorrhage, and injection of neurolytics into these arteries may cause neurologic sequelae due to vasospasm and/or spinal cord infarction [32,33].
Based on these previous studies, we chose the block level at the L3 and L4 vertebrae, and the volume of neurolytic agent was reduced to less than 1 ml, taking care not to traverse one vertebral body level with an intent to decrease complications. However, this study has several limitations. First, the results did not present a normal distribution, and the limited number of patients were unequally distributed among specific disease groups. If the volume of agents is further reduced and injected with caution not to traverse two numeric scale levels, the results are anticipated to present a normal distribution. Second, we enrolled patients with a significant rise in skin temperature without considering improvement of pain scores. A LSGB is performed to manage sympathetically maintained pain. Cutaneous toe temperatures approaching core temperature, provides a useful monitor of a lumbar sympathetic block and may predict relief of sympathetically maintained pain [13]. However, a successful LSGB does not always indicate improvement of pain and vice versa. Third, because the agents were injected with caution taken not to traverse one vertebral body level at the L3 and L4 vertebral levels, the spread of the dye did not extend beyond L2, and thus our results were not comparable to those of previous studies that insisted that the LSG aggregated at the level of the L2/3 intervertebral disc [3]. In addition, further study is required to investigate the relationship between destructive LSGBs and the long-term outcomes and to determine the shape and size of the lesions produced in lumbar sympathetic RF thermocoagulation in humans.
In conclusion, this study demonstrates that the LSG presumably aggregate at a lower level (lower third of L4) in left-sided LSGBs than in right-sided LSGBs (lower margin of L3). There was no significant difference in the spread of dye between men and women. The results may be useful to better select the primary target when performing LSGBs, thus reducing the volume of the agent used in conventional and neurolytic LSGBs and decreasing the number of needle insertions performed in LSGBs or radiofrequency thermocoagulation.
Footnotes
This research has been presented on the 60th Scientific meeting of the Korean Pain Society.
References
- 1.Boas RA. Sympathetic nerve blocks: in search of a role. Reg Anesth Pain Med. 1998;23:292–305. doi: 10.1016/s1098-7339(98)90058-x. [DOI] [PubMed] [Google Scholar]
- 2.Stanton-Hicks M. Complications of sympathetic blocks for extremity pain. Tech Reg Anesth Pain Manag. 2007;11:148–151. [Google Scholar]
- 3.Datta S, Pai U. Paradiscal extraforaminal technique for lumbar sympathetic block: report of a proposed new technique utilizing a cadaver study. Pain Physician. 2004;7:53–57. [PubMed] [Google Scholar]
- 4.Hong JH, Oh MJ. Comparison of multilevel with single level injection during lumbar sympathetic ganglion block: efficacy of sympatholysis and incidence of psoas muscle injection. Korean J Pain. 2010;23:131–136. doi: 10.3344/kjp.2010.23.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middleton WJ, Chan VW. Lumbar sympathetic block: a review of complications. Tech Reg Anesth Pain Manag. 1998;2:137–146. [Google Scholar]
- 6.Kim WH, Kim SK, Lee CJ, Kim TH, Sim WS. Determination of adequate entry angle of lumbar sympathetic ganglion block in Korean. Korean J Pain. 2010;23:11–17. doi: 10.3344/kjp.2010.23.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umeda S, Arai T, Hatano Y, Mori K, Hoshino K. Cadaver anatomic analysis of the best site for chemical lumbar sympathectomy. Anesth Analg. 1987;66:643–646. [PubMed] [Google Scholar]
- 8.Rocco AG, Palombi D, Raeke D. Anatomy of the lumbar sympathetic chain. Reg Anesth. 1995;20:13–19. [PubMed] [Google Scholar]
- 9.Stevens RA, Stotz A, Kao TC, Powar M, Burgess S, Kleinman B. The relative increase in skin temperature after stellate ganglion block is predictive of a complete sympathectomy of the hand. Reg Anesth Pain Med. 1998;23:266–270. doi: 10.1016/s1098-7339(98)90053-0. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Lee CO, Shin YS, Lee YW. Appropriate block level in neurolytic lumbar sympathetic ganglion block. J Korean Pain Soc. 2001;14:199–206. [Google Scholar]
- 11.Sayson SC, Ramamurthy S, Hoffman J. Incidence of genitofemoral nerve block during lumbar sympathetic block: comparison of two lumbar injection sites. Reg Anesth. 1997;22:569–574. [PubMed] [Google Scholar]
- 12.Kim YC, Bahk JH, Lee SC, Lee YW. Infrared thermographic imaging in the assessment of successful block on lumbar sympathetic ganglion. Yonsei Med J. 2003;44:119–124. doi: 10.3349/ymj.2003.44.1.119. [DOI] [PubMed] [Google Scholar]
- 13.Tran KM, Frank SM, Raja SN, El-Rahmany HK, Kim LJ, Vu B. Lumbar sympathetic block for sympathetically maintained pain: changes in cutaneous temperatures and pain perception. Anesth Analg. 2000;90:1396–1401. doi: 10.1097/00000539-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Gofeld M, Faclier G. Radiofrequency denervation of the lumbar zygapophysial joints--targeting the best practice. Pain Med. 2008;9:204–211. doi: 10.1111/j.1526-4637.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- 15.Hatangdi VS, Boas RA. Lumbar sympathectomy: a single needle technique. Br J Anaesth. 1985;57:285–289. doi: 10.1093/bja/57.3.285. [DOI] [PubMed] [Google Scholar]
- 16.Waldman SD. Atlas of interventional pain management. 3rd ed. Philadelphia (PA): WB Saunders; 2004. pp. 1314–1321. [Google Scholar]
- 17.Feigl GC, Dreu M, Ulz H, Breschan C, Maier C, Likar R. Susceptibility of the genitofemoral and lateral femoral cutaneous nerves to complications from lumbar sympathetic blocks: is there a morphological reason? Br J Anaesth. 2014;112:1098–1104. doi: 10.1093/bja/aet552. [DOI] [PubMed] [Google Scholar]
- 18.Hong JH, Kim AR, Lee MY, Kim YC, Oh MJ. A prospective evaluation of psoas muscle and intravascular injection in lumbar sympathetic ganglion block. Anesth Analg. 2010;111:802–807. doi: 10.1213/ANE.0b013e3181e9eb35. [DOI] [PubMed] [Google Scholar]
- 19.Koizuka S, Saito S, Obata H, Tobe M, Koyama Y, Takahashi A. Anatomic analysis of computed tomography images obtained during fluoroscopic computed tomography-guided percutaneous lumbar sympathectomy. J Anesth. 2008;22:373–377. doi: 10.1007/s00540-008-0663-x. [DOI] [PubMed] [Google Scholar]
- 20.Kuzmarov IW, MacIsaac SG, Sioufi J, DeDomenico I. Iatrogenic ureteral injury secondary to lumbar sympathetic ganglion blockade. Urology. 1980;16:617–619. doi: 10.1016/0090-4295(80)90574-9. [DOI] [PubMed] [Google Scholar]
- 21.Ryttov N, Boe S, Nielsen H, Jacobsen J. Necrosis of ureter as a complication to chemical lumbar sympathectomy. Report of a case. Acta Chir Scand. 1981;147:79–80. [PubMed] [Google Scholar]
- 22.Baxter AD, O'Kafo BA. Ejaculatory failure after chemical sympathectomy. Anesth Analg. 1984;63:770–771. [PubMed] [Google Scholar]
- 23.Chung YJ, Choi JB, Lee YW. Radiofrequency lumbar sympatholysis: comparison with neurolytic alcohol block. J Korean Pain Soc. 2004;17:42–46. [Google Scholar]
- 24.Koizuka S, Saito S, Masuoka S, Nakajima K, Koyama Y. Location of major vessels in prone-positioned patients undergoing percutaneous lumbar sympathectomy. Neuroradiology. 2012;54:1127–1131. doi: 10.1007/s00234-012-1007-y. [DOI] [PubMed] [Google Scholar]
- 25.Bateman DK, Millhouse PW, Shahi N, Kadam AB, Maltenfort MG, Koerner JD, et al. Anterior lumbar spine surgery: a systematic review and meta-analysis of associated complications. Spine J. 2015;15:1118–1132. doi: 10.1016/j.spinee.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 26.Rocco AG. Radiofrequency lumbar sympatholysis. The evolution of a technique for managing sympathetically maintained pain. Reg Anesth. 1995;20:3–12. [PubMed] [Google Scholar]
- 27.Racz GB, Stanton-Hicks M. Lumbar and thoracic sympathetic radiofrequency lesioning in complex regional pain syndrome. Pain Pract. 2002;2:250–256. doi: 10.1046/j.1533-2500.2002.02032.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim SH, Moon DE, Park CM, Ryu KH, Seo KS, You SH. Thoracic spinal cord stimulation and radiofrequency thermocoagulation of lumbar sympathetic ganglion in a patient with complex regional pain syndrome in the lower extremity: a case report. Korean J Pain. 2005;18:240–245. [Google Scholar]
- 29.Derby R, Lee CH. The efficacy of a two needle electrode technique in percutaneous radiofrequency rhizotomy: an investigational laboratory study in an animal model. Pain Physician. 2006;9:207–213. [PubMed] [Google Scholar]
- 30.Anfinsen OG, Kongsgaard E, Foerster A, Amlie JP, Aass H. Bipolar radiofrequency catheter ablation creates confluent lesions at larger interelectrode spacing than does unipolar ablation from two electrodes in the porcine heart. Eur Heart J. 1998;19:1075–1084. doi: 10.1053/euhj.1998.1015. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Kim DW, Sim WS. Lumbar sympathetic radiofrequency thermocoagulation using bipolar probe in the hyperhidrosis patient: a case report. Korean J Pain. 2005;18:92–95. [Google Scholar]
- 32.Boas RA. Sympathetic blocks in clinical practice. Int Anesthesiol Clin. 1978;16:149–182. doi: 10.1097/00004311-197801640-00008. [DOI] [PubMed] [Google Scholar]
- 33.Nagpal A, Eckmann M, Small S, Stevens S. Onset of spontaneous lower extremity pain after lumbar sympathetic block. Pain Physician. 2015;18:E89–E91. [PubMed] [Google Scholar]